Effect of Encapsulated Purple Garlic Oil on Microvascular Function and the Components of Metabolic Syndrome: A Randomized Placebo-Controlled Study—The ENDOTALLIUM Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Study Population
2.3. Investigational Product
2.4. Study Intervention and Procedures
2.5. Study Variables
2.5.1. Clinical Variables
2.5.2. Laboratory Parameters
2.5.3. Circulating Biomarkers
2.5.4. Microvascular Endothelial Function
2.5.5. Statistical Analysis
3. Results
3.1. Subjects
3.2. Changes in Microvascular Function and Circulating Biomarkers
3.3. Changes in MetS Components
3.4. Tolerability and Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial Function and Dysfunction: Testing and Clinical Relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef]
- Shimokawa, H. 2014 Williams Harvey Lecture: Importance of Coronary Vasomotion Abnormalities-from Bench to Bedside. Eur. Heart J. 2014, 35, 3180–3193. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H.C. Endothelial Dysfunction and Vascular Disease—A 30th Anniversary Update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [CrossRef] [PubMed]
- Flammer, A.J.; Anderson, T.; Celermajer, D.S.; Creager, M.A.; Deanfield, J.; Ganz, P.; Hamburg, N.M.; Lüscher, T.F.; Shechter, M.; Taddei, S.; et al. The Assessment of Endothelial Function: From Research into Clinical Practice. Circulation 2012, 126, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Cracowski, J.L.; Minson, C.T.; Salvat-Melis, M.; Halliwill, J.R. Methodological Issues in the Assessment of Skin Microvascular Endothelial Function in Humans. Trends Pharmacol. Sci. 2006, 27, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Godo, S.; Takahashi, J.; Yasuda, S.; Shimokawa, H. Endothelium in Coronary Macrovascular and Microvascular Diseases. J. Cardiovasc. Pharmacol. 2021, 78, S19–S29. [Google Scholar] [CrossRef] [PubMed]
- Seliger, S.L.; Salimi, S.; Pierre, V.; Giffuni, J.; Katzel, L.; Parsa, A. Microvascular Endothelial Dysfunction Is Associated with Albuminuria and CKD in Older Adults. BMC Nephrol. 2016, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Muris, D.M.J.; Houben, A.J.H.M.; Schram, M.T.; Stehouwer, C.D.A. Microvascular Dysfunction Is Associated with a Higher Incidence of Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 3082–3094. [Google Scholar] [CrossRef]
- Gryglewska, B.; Głuszewska, A.; Zarzycki, B.; Dzieża-Grudnik, A.; Fedyk-Łukasik, M.; Major, P.; Budzyński, A.; Gąsowski, J.; Grodzicki, T. Post-Occlusive Reactive Hyperemic Response of Skin Microcirculation among Extremely Obese Patients in the Short and Long Term after Bariatric Surgery. Microcirculation 2020, 27, e12600. [Google Scholar] [CrossRef]
- Grundy, S.M.; Hansen, B.; Smith, S.C.; Cleeman, J.I.; Kahn, R.A.; National Heart, Lung, and Blood Institute; American Diabetes Association. Clinical Management of Metabolic Syndrome: Report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association Conference on Scientific Issues Related to Management. Arterioscler. Thromb. Vasc. Biol. 2004, 24, e19–e24. [Google Scholar] [CrossRef]
- Horton, W.B.; Barrett, E.J. Microvascular Dysfunction in Diabetes Mellitus and Cardiometabolic Disease. Endocr. Rev. 2021, 42, 29–55. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.; Gerber, M. Food Processing and the Mediterranean Diet. Nutrients 2015, 7, 7925–7964. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Fatima, K.; Rashid, A.M.; Memon, U.A.A.; Fatima, S.S.; Javaid, S.S.; Shahid, O.; Zehri, F.; Obaid, M.A.; Ahmad, M.; Almas, T.; et al. Mediterranean Diet and Its Effect on Endothelial Function: A Meta-Analysis and Systematic Review. Ir. J. Med. Sci. 2023, 192, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Varade, S.; Nadella, M.; Hirake, A.; Mungase, S.B.; Ali, A.; Adela, R. Effect of Garlic on the Components of Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Ethnopharmacol. 2024, 318, 116960. [Google Scholar] [CrossRef] [PubMed]
- Geddo, F.; Querio, G.; Asteggiano, A.; Antoniotti, S.; Porcu, A.; Occhipinti, A.; Medana, C.; Gallo, M.P. Improving Endothelial Health with Food-Derived H2S Donors: An in Vitro Study with S-Allyl Cysteine and with a Black-Garlic Extract Enriched in Sulfur-Containing Compounds. Food Funct. 2023, 14, 4163–4172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shang, C.; Tian, Z.; Amin, H.K.; Kassab, R.B.; Abdel Moneim, A.E.; Zhang, Y. Diallyl Disulfide Suppresses Inflammatory and Oxidative Machineries Following Carrageenan Injection-Induced Paw Edema in Mice. Mediat. Inflamm. 2020, 2020, 8508906. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.K.; Maulik, S.K. Effect of Garlic on Cardiovascular Disorders: A Review. Nutr. J. 2002, 1, 4. [Google Scholar] [CrossRef]
- Poupelloz, J.V. Assessment Report on Allium sativum L., Bulbus; EMA/HMPC/7686/2013; EMA: Amsterdam, The Netherlands, 2020; Volume 44. [Google Scholar]
- Diretto, G.; Rubio-Moraga, A.; Argandoña, J.; Castillo, P.; Gómez-Gómez, L.; Ahrazem, O. Tissue-Specific Accumulation of Sulfur Compounds and Saponins in Different Parts of Garlic Cloves from Purple and White Ecotypes. Molecules 2017, 22, 1359. [Google Scholar] [CrossRef]
- Homroy, S.; Chopra, R.; Singh, P.K.; Dhiman, A.; Chand, M.; Talwar, B. Role of Encapsulation on the Bioavailability of Omega-3 Fatty Acids. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13272. [Google Scholar] [CrossRef]
- Leitão, R.; de Oliveira, G.V.; Rezende, C.; Volino-Souza, M.; Mesquita, J.; de Carvalho, L.L.; Alvares, T.S. Improved Microvascular Reactivity after Aged Garlic Extract Intake Is Not Mediated by Hydrogen Sulfide in Older Adults at Risk for Cardiovascular Disease: A Randomized Clinical Trial. Eur. J. Nutr. 2022, 61, 3357–3366. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and Management of the Metabolic Syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- Galisteo, A.; González-Coloma, A.; Castillo, P.; Andrés, M.F. Valorization of the Hydrolate Byproduct from the Industrial Extraction of Purple Alium Sativum Essential Oil as a Source of Nematicidal Products. Life 2022, 12, 905. [Google Scholar] [CrossRef] [PubMed]
- Amagase, H.; Petesch, B.L.; Matsuura, H.; Kasuga, S.; Itakura, Y. Intake of Garlic and Its Bioactive Components. J. Nutr. 2001, 131, 955S–962S. [Google Scholar] [CrossRef] [PubMed]
- Ansary, J.; Forbes-Hernández, T.Y.; Gil, E.; Cianciosi, D.; Zhang, J.; Elexpuru-Zabaleta, M.; Simal-Gandara, J.; Giampieri, F.; Battino, M. Potential Health Benefit of Garlic Based on Human Intervention Studies: A Brief Overview. Antioxidants 2020, 9, 619. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.S.; Blair, S.N.; Mahar, M.T.; Wier, L.T.; Ross, R.M.; Stuteville, J.E. Prediction of Functional Aerobic Capacity without Exercise Testing. Med. Sci. Sports Exerc. 1990, 22, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Fabregate-Fuente, M.; Arbeitman, C.R.; Cymberknop, L.J.; Bara-Ledesma, N.; Arriazu-Galindo, M.; Martin-Fernandez, L.; Armentano, R.L.; Saban-Ruiz, J. Characterization of Microvascular Post Occlusive Hyperemia Using Laser Doppler Flowmetry Technique in Subjects With Cardiometabolic Disorders. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2019, 2019, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Saban-Ruiz, J.; Fabregate-Fuente, M.; Fabregate-Fuente, R.; Andres-Castillo, A.; Palomino-Antolin, A.; Barrio-Carreras, D.; Martin-Fernandez, L.; Altamirano, F.; Fernandez-Fernandez, C.; Andres-Lacueva, C. Iberian Cured-Ham Consumption Improves Endothelial Function in Healthy Subjects. J. Nutr. Health Aging 2017, 21, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Settergren, M.; Böhm, F.; Rydén, L.; Pernow, J.; Kalani, M. Lipid Lowering versus Pleiotropic Effects of Statins on Skin Microvascular Function in Patients with Dysglycaemia and Coronary Artery Disease. J. Intern. Med. 2009, 266, 492–498. [Google Scholar] [CrossRef]
- Chen, J.W.; Hsu, N.W.; Wu, T.C.; Lin, S.J.; Chang, M.S. Long-Term Angiotensin-Converting Enzyme Inhibition Reduces Plasma Asymmetric Dimethylarginine and Improves Endothelial Nitric Oxide Bioavailability and Coronary Microvascular Function in Patients with Syndrome X. Am. J. Cardiol. 2002, 90, 974–982. [Google Scholar] [CrossRef]
- De Boer, M.P.; Meijer, R.I.; Newman, J.; Stehouwer, C.D.A.; Eringa, E.C.; Smulders, Y.M.; Serné, E.H. Insulin-Induced Changes in Microvascular Vasomotion and Capillary Recruitment Are Associated in Humans. Microcirculation 2014, 21, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Tucci, M.; Marino, M.; Martini, D.; Porrini, M.; Riso, P.; Del Bo’, C. Plant-Based Foods and Vascular Function: A Systematic Review of Dietary Intervention Trials in Older Subjects and Hypothesized Mechanisms of Action. Nutrients 2022, 14, 2615. [Google Scholar] [CrossRef] [PubMed]
- Ruano, J.; Lopez-Miranda, J.; Fuentes, F.; Moreno, J.A.; Bellido, C.; Perez-Martinez, P.; Lozano, A.; Gómez, P.; Jiménez, Y.; Pérez Jiménez, F. Phenolic Content of Virgin Olive Oil Improves Ischemic Reactive Hyperemia in Hypercholesterolemic Patients. J. Am. Coll. Cardiol. 2005, 46, 1864–1868. [Google Scholar] [CrossRef] [PubMed]
- Millan-Orge, M.; Torres-Peña, J.D.; Arenas-Larriva, A.; Quintana-Navarro, G.M.; Peña-Orihuela, P.; Alcala-Diaz, J.F.; Luque, R.M.; Rodriguez-Cantalejo, F.; Katsiki, N.; Lopez-Miranda, J.; et al. Influence of Dietary Intervention on Microvascular Endothelial Function in Coronary Patients and Atherothrombotic Risk of Recurrence. Sci. Rep. 2021, 11, 20301. [Google Scholar] [CrossRef] [PubMed]
- Marin, C.; Ramirez, R.; Delgado-Lista, J.; Yubero-Serrano, E.M.; Perez-Martinez, P.; Carracedo, J.; Garcia-Rios, A.; Rodriguez, F.; Gutierrez-Mariscal, F.M.; Gomez, P.; et al. Mediterranean Diet Reduces Endothelial Damage and Improves the Regenerative Capacity of Endothelium. Am. J. Clin. Nutr. 2011, 93, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Torres-Peña, J.D.; Rangel-Zuñiga, O.A.; Alcala-Diaz, J.F.; Lopez-Miranda, J.; Delgado-Lista, J. Mediterranean Diet and Endothelial Function: A Review of Its Effects at Different Vascular Bed Levels. Nutrients 2020, 12, 2212. [Google Scholar] [CrossRef] [PubMed]
- Lindstedt, S.; Wlosinska, M.; Nilsson, A.C.; Hlebowicz, J.; Fakhro, M.; Sheikh, R. Successful Improved Peripheral Tissue Perfusion Was Seen in Patients with Atherosclerosis after 12 Months of Treatment with Aged Garlic Extract. Int. Wound J. 2021, 18, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Mirzavandi, F.; Mollahosseini, M.; Salehi-Abargouei, A.; Makiabadi, E.; Mozaffari-Khosravi, H. Effects of Garlic Supplementation on Serum Inflammatory Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Melino, S.; Leo, S.; Toska Papajani, V. Natural Hydrogen Sulfide Donors from Allium Sp. as a Nutraceutical Approach in Type 2 Diabetes Prevention and Therapy. Nutrients 2019, 11, 1581. [Google Scholar] [CrossRef] [PubMed]
- Sara, J.D.S.; Prasad, M.; Zhang, M.; Lennon, R.J.; Herrmann, J.; Lerman, L.O.; Lerman, A. High-Sensitivity C-Reactive Protein Is an Independent Marker of Abnormal Coronary Vasoreactivity in Patients with Non-Obstructive Coronary Artery Disease. Am. Heart J. 2017, 190, 1–11. [Google Scholar] [CrossRef]
- Atkin, M.; Laight, D.; Cummings, M.H. The Effects of Garlic Extract upon Endothelial Function, Vascular Inflammation, Oxidative Stress and Insulin Resistance in Adults with Type 2 Diabetes at High Cardiovascular Risk. A Pilot Double Blind Randomized Placebo Controlled Trial. J. Diabetes Complicat. 2016, 30, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Koseoglu, M.; Isleten, F.; Atay, A.; Kaplan, Y.C. Effects of Acute and Subacute Garlic Supplement Administration on Serum Total Antioxidant Capacity and Lipid Parameters in Healthy Volunteers. Phytother. Res. 2010, 24, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.J.A.; Sutherland, W.H.F.; McCormick, M.P.; Yeoman, D.J.; de Jong, S.A. Aged Garlic Extract Improves Endothelial Function in Men with Coronary Artery Disease. Phytother. Res. 2005, 19, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.; Kim, C.T.; Kim, I.H.; Kim, Y. Anti-Obesity Effects of Hot Water Extract and High Hydrostatic Pressure Extract of Garlic in Rats Fed a High-Fat Diet. Food Chem. Toxicol. 2013, 55, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, S.; Ushijima, M.; Morihara, N.; Itakura, Y.; Nakata, Y. Effect of Aged Garlic Extract (AGE) on Hyperglycemia Induced by Immobilization Stress in Mice. Nihon Yakurigaku Zasshi 1999, 114, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Ohaeri, O.C. Effect of Garlic Oil on the Levels of Various Enzymes in the Serum and Tissue of Streptozotocin Diabetic Rats. Biosci. Rep. 2001, 21, 19–24. [Google Scholar] [CrossRef]
- Hattori, A.; Yamada, N.; Nishikawa, T.; Fukuda, H.; Fujino, T. Antidiabetic Effects of Ajoene in Genetically Diabetic KK-A(y) Mice. J. Nutr. Sci. Vitaminol. 2005, 51, 382–384. [Google Scholar] [CrossRef]
- Reinhart, K.M.; Coleman, C.I.; Teevan, C.; Vachhani, P.; White, C.M. Effects of Garlic on Blood Pressure in Patients with and without Systolic Hypertension: A Meta-Analysis. Ann. Pharmacother. 2008, 42, 1766–1771. [Google Scholar] [CrossRef]
- Girkantaite, Z.; Laucyte-Cibulskiene, A.; Ryliskyte, L.; Juceviciene, A.; Badariene, J. Laser Doppler Flowmetry Evaluation of Skin Microvascular Endothelial Function in Patients with Metabolic Syndrome. Microvasc. Res. 2022, 142, 104373. [Google Scholar] [CrossRef]
- Sangouni, A.A.; Alizadeh, M.; Jamalzehi, A.; Parastouei, K. Effects of Garlic Powder Supplementation on Metabolic Syndrome Components, Insulin Resistance, Fatty Liver Index, and Appetite in Subjects with Metabolic Syndrome: A Randomized Clinical Trial. Phyther. Res. 2021, 35, 4433–4441. [Google Scholar] [CrossRef]
- Choudhary, P.R.; Jani, R.D.; Sharma, M.S. Effect of Raw Crushed Garlic (Allium sativum L.) on Components of Metabolic Syndrome. J. Diet. Suppl. 2018, 15, 99–506. [Google Scholar] [CrossRef] [PubMed]
- Verma, T.; Aggarwal, A.; Dey, P.; Chauhan, A.K.; Rashid, S.; Chen, K.-T.; Sharma, R. Medicinal and Therapeutic Properties of Garlic, Garlic Essential Oil, and Garlic-Based Snack Food: An Updated Review. Front. Nutr. 2023, 10, 1120377. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Rajeevan, V.; Jain, P.; Bordia, A. Effect of Garlic (Allium sativum) Oil on Exercise Tolerance in Patients with Coronary Artery Disease. Indian J. Physiol. Pharmacol. 2005, 49, 115–118. [Google Scholar] [PubMed]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef] [PubMed]

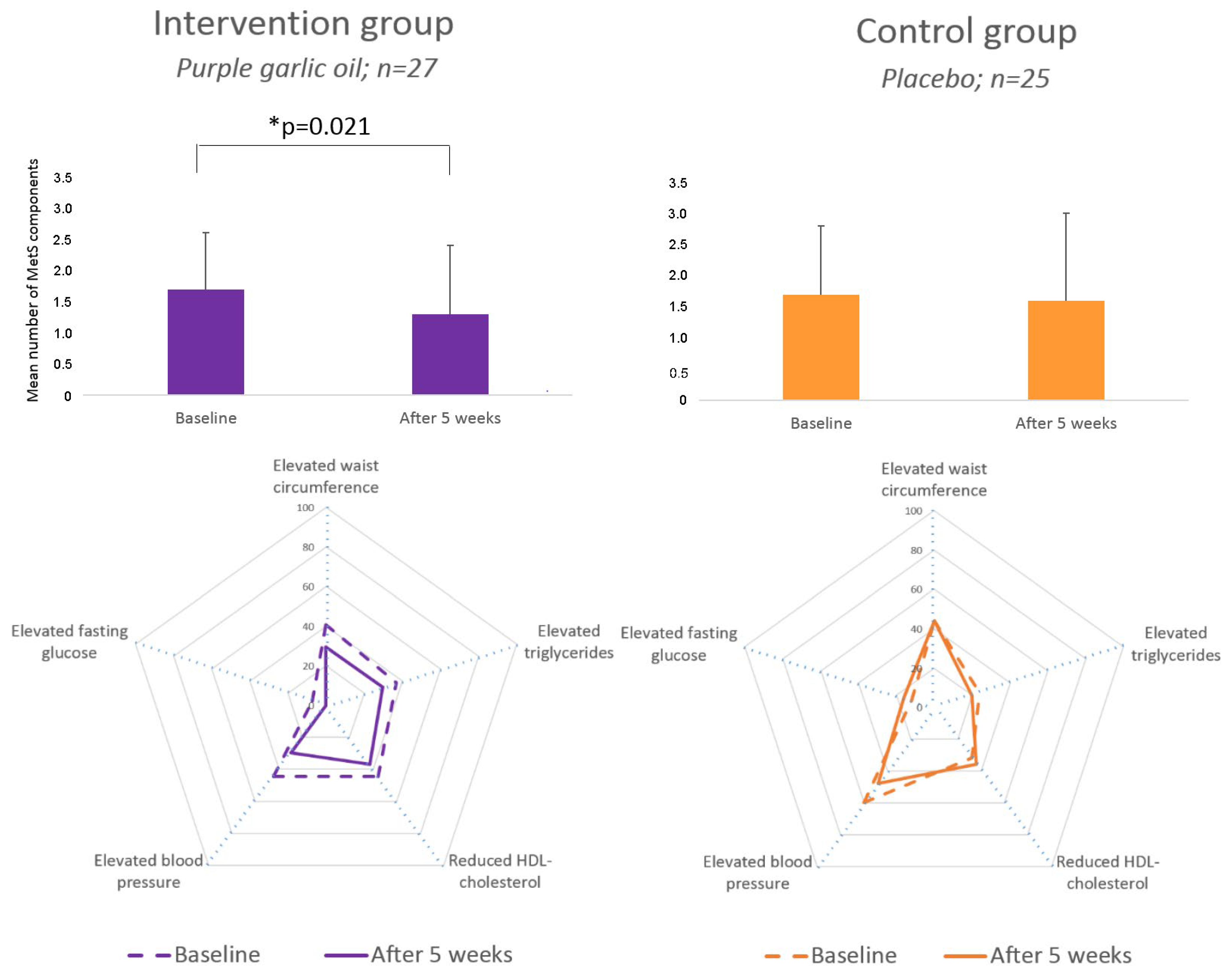
| Intervention Group Purple Garlic Oil (n = 27) | Control Group Placebo (n = 25) | |
|---|---|---|
| Demographic characteristics | ||
| Age, mean ± SD, years | 46.0 ± 12.9 | 45.7 ± 14.7 |
| Gender, n (%) | ||
| Female | 17 (63.0%) | 14 (56.0%) |
| Male | 10 (37.0%) | 11 (44.0%) |
| Clinical characteristics related to Metabolic Syndrome (NCEP/ATP III criteria) [23] | ||
| Metabolic Syndrome, n (%) | 7 (25.9%) | 5 (20.0%) |
| No. of Metabolic Syndrome components, n (%) | ||
| 1 | 14 (51.9%) | 15 (60.0%) |
| 2 | 6 (22.2%) | 5 (20.0%) |
| 3 | 7 (25.9%) | 3 (12.0%) |
| 4–5 | 0 (0.0%) | 2 (8.0%) |
| Metabolic Syndrome components, n (%) | ||
| Elevated waist circumference | 11 (40.7%) | 11 (44.0%) |
| Elevated triglycerides | 10 (37.0%) | 6 (24.0%) |
| Reduced HDL-cholesterol | 12 (44.4%) | 8 (32.0%) |
| Elevated blood pressure | 12 (44.4%) | 15 (60.0%) |
| Elevated fasting glucose | 2 (7.4%) | 3 (12.0%) |
| Lifestyle habits | ||
| Current smoker, n (%) | 6 (22.2%) | 5 (20.0%) |
| Physical activity status a, n (%) | ||
| Low physical activity | 3 (11.1%) | 5 (20.0%) |
| Moderate physical activity | 15 (55.6%) | 11 (44.0%) |
| High physical activity | 9 (33.3%) | 9 (36.0%) |
| Dietary habits, mean ± SD | ||
| Fruits and vegetables, servings/day | 3.0 ± 0.9 | 2.8 ± 1.1 |
| Nuts and dried fruits, servings/week | 3.3 ± 2.9 | 4.4 ± 4.5 |
| Garlic, cloves/week | 2.0 ± 2.2 | 3.3 ± 2.0 |
| Intervention Group Purple Garlic Oil (n = 27) | Control Group Placebo (n = 25) | Between-Group Difference [95% CI] | p-Value Between-Group Differences | |
|---|---|---|---|---|
| Skin microvascular function | ||||
| Basal flow (BF), mean ± SD, PU | ||||
| Baseline Change after 5 weeks | 14.4 ± 5.0 +2.9 ± 5.4 * | 15.6 ± 6.0 +1.2 ± 7.5 | 1.7 [−2.2 to 5.6] | 0.393 |
| Occlusion flow, mean ± SD, PU | ||||
| Baseline Change after 5 weeks | 4.9 ± 1.0 −0.3 ± 0.9 | 5.3 ± 1.5 −0.2 ± 1.9 | −0.2 [−1.1 to 0.8] | 0.722 |
| Peak flow (PF), mean ± SD, PU | ||||
| Baseline Change after 5 weeks | 61.1 ± 19.6 +11.5 ± 23.7 * | 71.7 ± 24.5 −3.9 ± 22.6 | 15.4 [1.5 to 29.4] | 0.031 |
| Half-recovery flow (HRF), mean ± SD, PU | ||||
| Baseline Change after 5 weeks | 37.9 ± 11.0 +7.2 ± 12.8 * | 43.7 ± 13.5 −1.3 ± 12.6 | 8.5 [0.9 to 16.2] | 0.029 |
| Endothelial-related circulating biomarkers | ||||
| hs-CRP, mg/L | ||||
| Baseline, median [IQR] Change after 5 weeks, mean ± SD | 2.9 [2.8] −0.5 ± 2.1 | 2.5 [1.9] +0.8 ± 2.1 | −1.3 [−2.5 to −0.0] | 0.049 |
| VCAM-1, mean ± SD, ng/mL | ||||
| Baseline Change after 5 weeks | 906.4 ± 182.2 −41.2 ± 129.4 * | 1028.5 ± 288.0 −67.5 ± 114.3 * | 26.3 [−41.9 to 94.5] | 0.443 |
| PAI-1, ng/mL | ||||
| Baseline, median [IQR] Change after 5 weeks, mean ± SD | 38.5 [41.2] +1.6 ± 19.8 | 30.9 [34.4] +5.8 ± 16.5 | −4.2 [−14.4 to 6.0] | 0.414 |
| Urinary F2-isoprostanes/creatinine, mmol/mg | ||||
| Baseline, median [IQR] Change after 5 weeks, mean ± SD | 132.3 [90.3] −6.5 ± 92.0 | 97.5 [75.1] −10.9 ± 75.0 | 4.5 [−51.8 to 42.9] | 0.851 |
| MetS Parameters | Intervention Group Purple Garlic Oil (n = 27) | Control Group Placebo (n = 25) | Between-Group Difference [95% CI] | p-Value |
|---|---|---|---|---|
| Waist circumference, mean ± SD, cm | ||||
| Baseline Change after 5 weeks | 88.9 ± 9.5 −0.4 ± 3.3 | 88.6 ± 11.0 −0.4 ± 4.6 | 0.0 [−2.2 to 2.3] | 0.977 |
| Triglycerides, mean ± SD, mg/dL | ||||
| Baseline Change after 5 weeks | 132.3 ± 54.1 −14.1 ± 40.8 | 111.6 ± 57.3 +2.9 ± 46.7 | −17.0 [−41.4 to 7.4] | 0.168 |
| HDL-cholesterol, mean ± SD, mg/dL | ||||
| Baseline Change after 5 weeks | 49.6 ± 11.2 +1.0 ± 5.6 | 52.8 ± 13.7 −1.3 ± 5.1 | 2.3 [−0.7 to 5.3] | 0.126 |
| SBP, mean ± SD, mmHg | ||||
| Baseline Change after 5 weeks | 124.2 ± 14.7 −2.2 ± 7.5 | 129.1 ± 16.0 −3.0 ± 11.5 | 0.8 [−4.6 to 6.1] | 0.773 |
| DBP, mean ± SD, mmHg | ||||
| Baseline Change after 5 weeks | 78.1 ± 8.7 −1.0 ± 4.4 | 79.7 ± 5.9 −1.0 ± 5.5 | 0.0 [−2.8 to 2.8] | 0.998 |
| Fasting glucose, mean ± SD, mg/dL | ||||
| Baseline Change after 5 weeks | 81.9 ± 8.1 −1.1 ± 7.2 | 80.6 ± 12.4 +2.6 ± 6.4 | −3.7 [−7.6 to 0.1] | 0.054 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bara-Ledesma, N.; Jimenez-Esteban, J.; Fabregate, M.; Fabregate-Fuente, R.; Cymberknop, L.J.; Castillo-Martinez, P.; Navarro-Fayos, M.T.; Gomez del Olmo, V.; Saban-Ruiz, J. Effect of Encapsulated Purple Garlic Oil on Microvascular Function and the Components of Metabolic Syndrome: A Randomized Placebo-Controlled Study—The ENDOTALLIUM Study. Nutrients 2024, 16, 1755. https://doi.org/10.3390/nu16111755
Bara-Ledesma N, Jimenez-Esteban J, Fabregate M, Fabregate-Fuente R, Cymberknop LJ, Castillo-Martinez P, Navarro-Fayos MT, Gomez del Olmo V, Saban-Ruiz J. Effect of Encapsulated Purple Garlic Oil on Microvascular Function and the Components of Metabolic Syndrome: A Randomized Placebo-Controlled Study—The ENDOTALLIUM Study. Nutrients. 2024; 16(11):1755. https://doi.org/10.3390/nu16111755
Chicago/Turabian StyleBara-Ledesma, Nuria, Judith Jimenez-Esteban, Martin Fabregate, Rosa Fabregate-Fuente, Leandro Javier Cymberknop, Purificacion Castillo-Martinez, Maria Teresa Navarro-Fayos, Vicente Gomez del Olmo, and Jose Saban-Ruiz. 2024. "Effect of Encapsulated Purple Garlic Oil on Microvascular Function and the Components of Metabolic Syndrome: A Randomized Placebo-Controlled Study—The ENDOTALLIUM Study" Nutrients 16, no. 11: 1755. https://doi.org/10.3390/nu16111755
APA StyleBara-Ledesma, N., Jimenez-Esteban, J., Fabregate, M., Fabregate-Fuente, R., Cymberknop, L. J., Castillo-Martinez, P., Navarro-Fayos, M. T., Gomez del Olmo, V., & Saban-Ruiz, J. (2024). Effect of Encapsulated Purple Garlic Oil on Microvascular Function and the Components of Metabolic Syndrome: A Randomized Placebo-Controlled Study—The ENDOTALLIUM Study. Nutrients, 16(11), 1755. https://doi.org/10.3390/nu16111755







