Correlations between Gustatory, Olfactory, Cognitive Function, and Age in Healthy Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Statistical Analyses
3. Results
3.1. Sample Characteristics
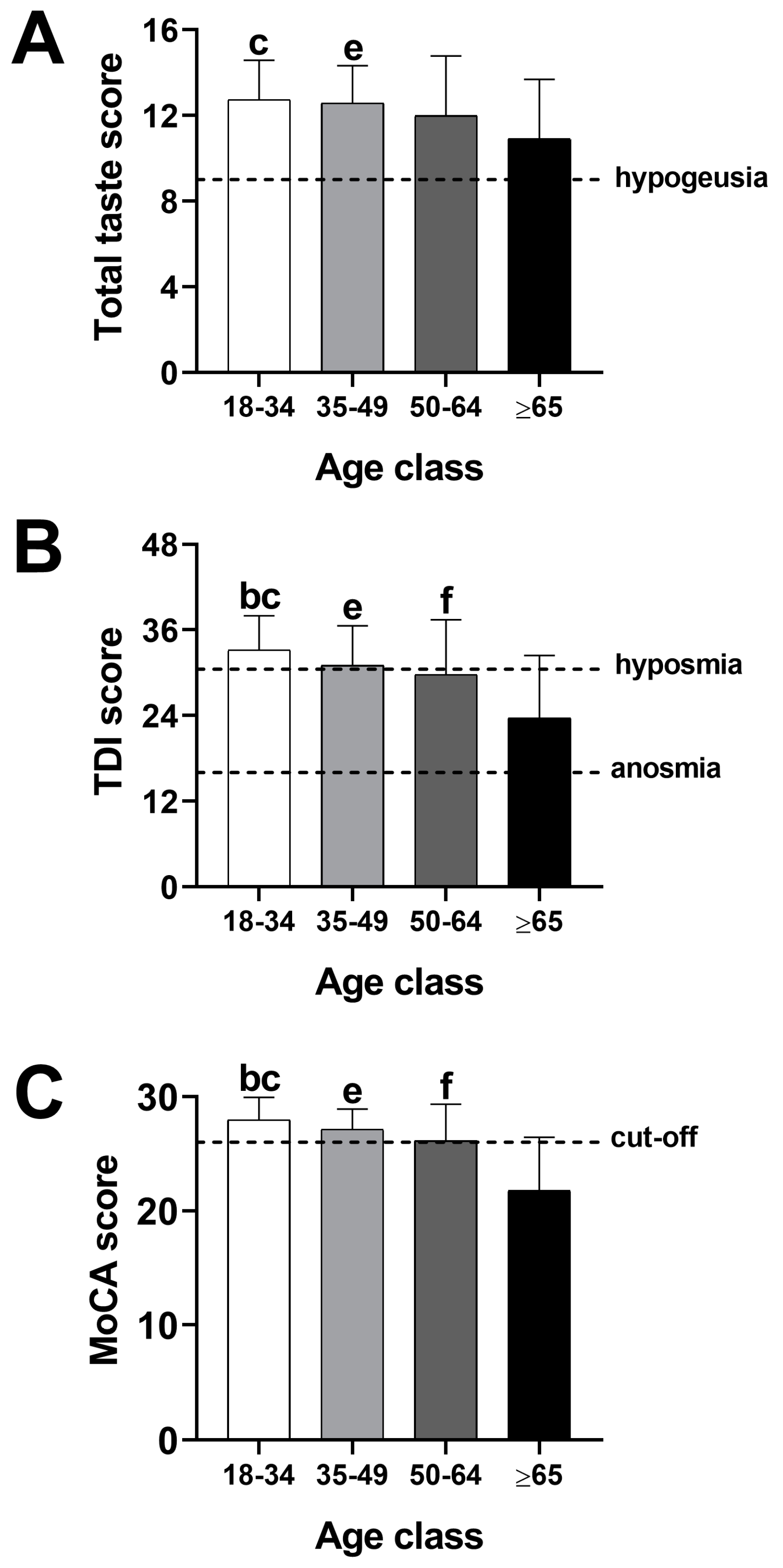
3.2. Effect of Age on Gustatory, Olfactory, and Cognitive Function
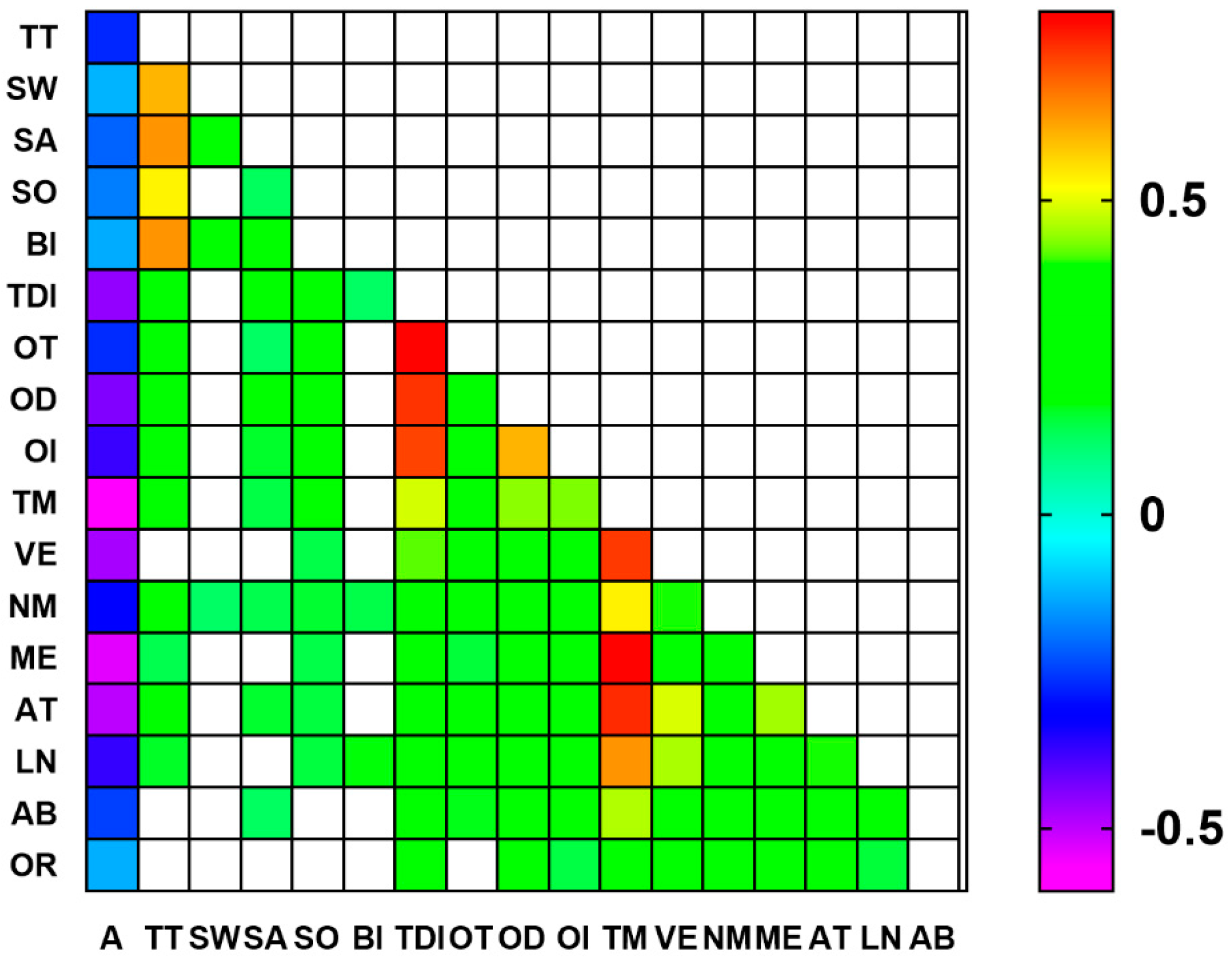
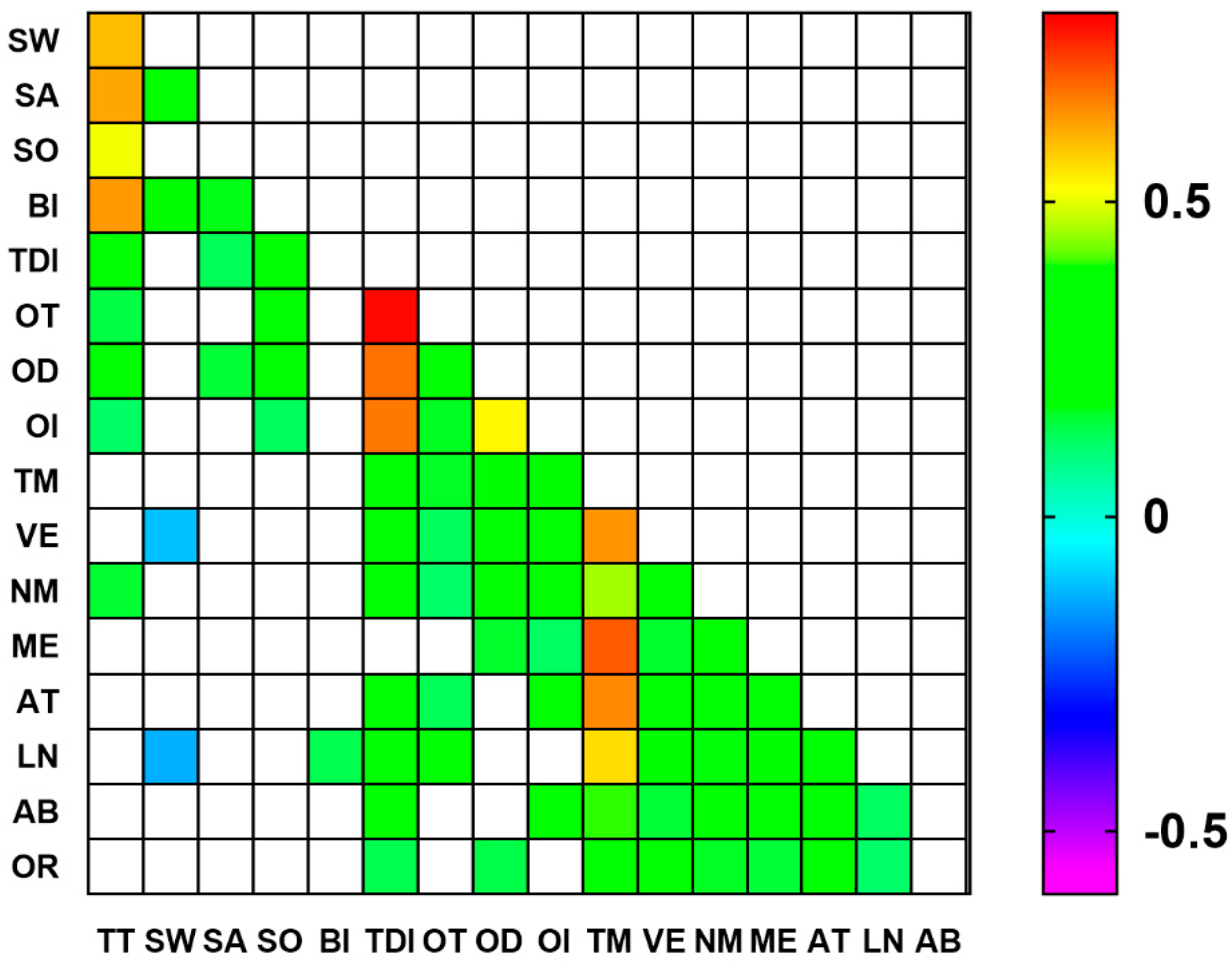
3.3. Correlations between Gustatory, Olfactory, and Cognitive Parameters
3.4. Multiple Regressions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nyberg, L.; Wåhlin, A. The many facets of brain aging. eLife 2020, 9, e56640. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Kikuta, S.; Ueha, R.; Suzukawa, K.; Yamasoba, T. Age-related olfactory dysfunction: Epidemiology, pathophysiology, and clinical management. Front. Aging Neurosci. 2020, 12, 208. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, J.K.; Ekström, I.; Larsson, M.; Nordin, S. Olfaction and aging: A review of the current state of research and future directions. Iperception 2021, 12, 20416695211020331. [Google Scholar] [CrossRef]
- Völter, C.; Thomas, J.P.; Maetzler, W.; Guthoff, R.; Grunwald, M.; Hummel, T. Sensory dysfunction in old age. Dtsch. Arztebl. Int. 2021, 118, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.M.; Wroblewski, K.E.; Huisingh-Scheetz, M.; Correia, C.; Lopez, K.J.; Chen, R.C.; Kern, D.W.; Schumm, P.L.; Dale, W.; McClintock, M.K. Global sensory impairment predicts morbidity and mortality in older U.S. adults. J. Am. Geriatr. Soc. 2017, 65, 2587–2595. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. The cingulate cortex and limbic systems for emotion, action, and memory. Brain Struct. Funct. 2019, 224, 3001–3018. [Google Scholar] [CrossRef] [PubMed]
- Vincis, R.; Fontanini, A. Central taste anatomy and physiology. Handb. Clin. Neurol. 2019, 164, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L.; Hawkes, C.H. Chemosensory dysfunction in neurodegenerative diseases. Handb. Clin. Neurol. 2019, 164, 325–360. [Google Scholar] [CrossRef] [PubMed]
- Hoskison, E. Olfaction, pheromones and life. J. Laryngol. Otol. 2013, 127, 1156–1159. [Google Scholar] [CrossRef]
- Croy, I.; Nordin, S.; Hummel, T. Olfactory disorders and quality of life- an updated review. Chem. Senses 2014, 39, 185–194. [Google Scholar] [CrossRef]
- Van Regemorter, V.; Hummel, T.; Rosenzweig, F.; Mouraux, A.; Rombaux, P.; Huart, C. Mechanisms linking olfactory impairment and risk of mortality. Front. Neurosci. 2020, 14, 140. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L. The olfactory system and its disorders. Semin. Neurol. 2009, 29, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Masala, C.; Saba, L.; Cecchini, M.P.; Solla, P.; Loy, F. Olfactory function and age: A Sniffin’ Sticks extended test study performed in Sardinia. Chemosens. Percept. 2018, 11, 19–26. [Google Scholar] [CrossRef]
- Brai, E.; Alberi, L. Olfaction, among the First Senses to Develop and Decline; Heinbockel, T., Ed.; Sensory nervous system; IntechOpen: London, UK, 2018; Volume 65, pp. 65–80. [Google Scholar] [CrossRef]
- Kovács, T. Mechanisms of olfactory dysfunction in aging and neurodegenerative disorders. Ageing Res. Rev. 2004, 3, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L. Olfactory dysfunction in neurodegenerative diseases: Is there a common pathological substrate? Lancet Neurol. 2017, 16, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Masala, C.; Solla, P.; Liscia, A.; Defazio, G.; Saba, L.; Cannas, A.; Cavazzana, A.; Hummel, T.; Haehner, A. Correlation among olfactory function, motors’ symptoms, cognitive impairment, apathy, and fatigue in patients with Parkinson’s disease. J. Neurol. 2018, 265, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Haehner, A.M.; Masala, C.; Walter, S.; Reichmann, H.; Hummel, T. Incidence of Parkinson’s disease in a large patient cohort with idiopathic smell and taste loss. J. Neurol. 2019, 266, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Berendse, H.W.; Roos, D.S.; Raijmakers, P.; Doty, R.L. Motor and non-motor correlates of olfactory dysfunction in Parkinson’s disease. J. Neurol. Sci. 2011, 310, 21–24. [Google Scholar] [CrossRef]
- Bohnen, N.I.; Kaufer, D.I.; Hendrickson, R.; Constantine, G.M.; Mathis, C.A.; Moore, R.Y. Cortical cholinergic denervation is associated with depressive symptoms in Parkinson’s disease and parkinsonian dementia. J. Neurol. Neurosurg. Psychiatr. 2007, 78, 641–643. [Google Scholar] [CrossRef]
- Postuma, R.; Gagnon, J.F. Cognition and olfaction in Parkinson’s disease. Brain 2010, 133, e160. [Google Scholar] [CrossRef]
- Sanna, F.; Loy, F.; Piras, R.; Moat, A.; Masala, C. Age-related cognitive decline and the olfactory identification deficit are associated to increased level of depression. Front. Neurosci. 2021, 15, 599593. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L.; Kamath, V. The influences of age on olfaction: A review. Front. Psychol. 2014, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Brand, G.; Millot, J.L. Sex differences in human olfaction: Between evidence and enigma. Q. J. Exp. Psychol. B Comp. Physiol. Psychol. 2001, 54B, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Cavazzana, A.; Wesarg, C.; Schriever, V.A.; Hummel, T.; Lundström, J.N.; Parma, V. A cross-cultural adaptation of the Sniffin’ Sticks olfactory Identification test for US children. Chem. Senses 2016, 42, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Chrea, C.; Valentin, D.; Sulmont-Rossé, C.; Mai, H.L.; Nguyen, D.H.; Abdi, H. Culture and odor categorization: Agreement between cultures depends upon the odors. Food Qual. Prefer. 2004, 15, 669–679. [Google Scholar] [CrossRef]
- Seiden, A.M. Postviral olfactory loss. Otolaryngol. Clin. N. Am. 2004, 37, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Whitcroft, K.L.; Andrews, P.; Altundag, A.; Cinghi, C.; Costanzo, R.M.; Damm, M.; Frasnelli, J.; Gudziol, H.; Gupta, N.; et al. Position paper on olfactory dysfunction. Rhinol. Suppl. 2017, 54, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Neiers, F.; Jarriault, D.; Menetrier, F.; Briand, L.; Heydel, J.M. The odorant metabolizing enzyme UGT2A1: Immunolocalization and impact of the modulation of its activity on the olfactory response. PLoS ONE 2021, 16, e0249029. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, H.; Kim, S.; Cha, H. The association between olfactory function and cognitive impairment in older persons with cognitive impairments: A cross-sectional study. Healthcare 2021, 9, 399. [Google Scholar] [CrossRef]
- Jacobson, P.T.; Vilarello, B.J.; Tervom, J.P.; Waring, N.A.; Gudis, D.A.; Goldberg, T.E.; Devanand, D.P.; Overdevest, J.B. Associations between olfactory dysfunction and cognition: A scoping review. J. Neurol. 2024, 271, 1170–1203. [Google Scholar] [CrossRef]
- Landis, B.N.; Welge-Luessen, A.; Brämerson, A.; Bende, M.; Mueller, C.A.; Nordin, S.; Hummel, T. Taste strips—A rapid, lateralized, gustatory bedside identification test based on impregnated filter papers. J. Neurol. 2010, 256, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Sekinger, B.; Wolf, S.R.; Pauli, E.; Kobal, G. Sniffin’ Sticks: Olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem. Senses 1997, 22, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Kobal, G.; Gudziol, H.; Mackay-Sim, A. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: An upgrade based on a group of more than 3000 subjects. Eur. Arch. Otorhinolaryngol. 2007, 264, 237–243. [Google Scholar] [CrossRef]
- Oleszkiewicz, A.; Schriever, V.A.; Croy, I.; Hähner, A.; Hummel, T. Updated Sniffin’ Sticks normative data based on an extended sample of 9139 subjects. Eur. Arch. Otorhinolaryngol. 2019, 276, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Conti, S.; Bonazzi, S.; Laiacona, M.; Masina, M.; Coralli, M.V. Montreal Cognitive Assessment (MoCA)-Italian version: Regression based norms and equivalent scores. Neurol. Sci. 2015, 36, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, M.P.; Federico, A.; Zanini, A.; Mantovani, E.; Masala, C.; Tinazzi, M.; Tamburin, S. Olfaction and taste in Parkinson’s disease: The association with mild cognitive impairment and the single cognitive domain dysfunction. J. Neural Transm. 2019, 126, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.M.; Shone, G.R. Effects of ageing on smell and taste. Postgrad. Med. J. 2006, 82, 239–241. [Google Scholar] [CrossRef]
- Masala, C.; Käehling, C.; Fall, F.; Hummel, T. Correlation between olfactory function, trigeminal sensitivity, and nasal anatomy in healthy subjects. Eur. Arch. Otorhinolaryngol. 2019, 276, 1649–1654. [Google Scholar] [CrossRef]
- Wilson, R.S.; Arnold, S.E.; Tang, Y.; Bennett, D.A. Odor identification and decline in different cognitive domains in old age. Neuroepidemiology 2006, 26, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Masala, C.; Cavazzana, A.; Sanna, F.; Cecchini, M.P.; Zanini, A.; Gasperi, F.; Menghi, L.; Endrizzi, I.; Borgogno, M.; Drago, S.; et al. Correlation between olfactory function, age, sex, and cognitive reserve index in the Italian population. Eur. Arch. Otorhinolaryngol. 2022, 279, 4943–4952. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.; Finkel, D.; Pedersen, N.L. Odor identification: Influences of age, gender, cognition, and personality. J. Gerontol. B Psychol. Sci. Soc. Sci. 2000, 55, P304–P310. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.C.; Cruz, L.A.; Hofman, J.M.; Patterson, M.Q. Taste sensitivity and aging: High incidence of decline revealed by repeated threshold measures. Chem. Senses 1995, 20, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Sergi, G.; Bano, G.; Pizzato, S.; Veronese, N.; Manzato, E. Taste loss in the elderly: Possible implications for dietary habits. Crit. Rev. Food Sci. Nutr. 2017, 57, 3684–3689. [Google Scholar] [CrossRef] [PubMed]
- Alia, S.; Aquilanti, L.; Pugnaloni, S.; Di Paolo, A.; Rappelli, G.; Vignini, A. The influence of age and oral health on taste perception in older adults: A case-control study. Nutrients 2021, 13, 4166. [Google Scholar] [CrossRef]
- Barragán, R.; Coltell, O.; Portolés, O.; Asensio, E.M.; Sorlí, J.V.; Ortega-Azorín, C.; González, J.I.; Sáiz, C.; Fernández-Carrión, R.; Ordovas, J.M.; et al. Bitter, sweet, salty, sour and umami taste perception decreases with age: Sex-specific analysis, modulation by genetic variants and taste-preference associations in 18- to 80-year-old subjects. Nutrients 2018, 10, 1539. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L. Age-related deficits in taste and smell. Otolaryngol. Clin. N. Am. 2018, 51, 815–825. [Google Scholar] [CrossRef]
- Makizako, M.; Makizako, H.; Doi, T.; Uemura, K.; Tsutsumimoto, K.; Miyaguchi, H.; Shimada, H. Olfactory identification and cognitive performance in community-dwelling older adults with mild cognitive impairment. Chem. Senses 2014, 39, 39–46. [Google Scholar] [CrossRef]
- Wang, M.C.; Chiou, J.M.; Chen, Y.C.; Chen, J.H. Association between olfactory dysfunction and cognitive impairment in dementia-free older adults: A prospective cohort study in Taiwan. J. Alzheimers Dis. 2023, 96, 1477–1488. [Google Scholar] [CrossRef]
- Kostka, J.K.; Bitzenhofer, S.H. How the sense of smell influences cognition throughout life. Neuroforum 2022, 28, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Dintica, C.S.; Marseglia, A.; Rizzuto, D.; Wang, R.; Seubert, J.; Arfanakis, K.; Bennett, D.A.; Xu, W. Impaired olfaction is associated with cognitive decline and neurodegeneration in the brain. Neurology 2019, 92, e700–e709. [Google Scholar] [CrossRef] [PubMed]
- Dahmani, L.; Patel, R.M.; Yang, Y.; Chakravarty, M.M.; Fellows, L.K.; Bohbot, V.D. An intrinsic association between olfactory identification and spatial memory in humans. Nat. Commun. 2018, 9, 4162. [Google Scholar] [CrossRef] [PubMed]
- Solla, P.; Masala, C.; Ercoli, T.; Frau, C.; Bagella, C.; Pinna, I.; Loy, F.; Defazio, G. Olfactory Impairment Correlates with Executive Functions Disorders and Other Specific Cognitive Dysfunctions in Parkinson’s Disease. Biology 2023, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, A.; Rossi, F.; Rinaldi, F.; Compostella, S.; Cosseddu, M.; Borroni, B.; Padovani, A. Exploring olfactory function and its relation with behavioral and cognitive impairment in amyotrophic lateral sclerosis patients: A cross-sectional study. Neurodegener. Dis. 2016, 16, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, Z.; Duan, S.; Zhu, X. Multiple Early Biomarkers to Predict Cognitive Decline in Dementia-Free Older Adults. J. Geriatr. Psychiatry Neurol. 2024, 9, 8919887241232650. [Google Scholar] [CrossRef]
- Pertesi, S.; Coughlan, G.; Puthusseryppady, V.; Morris, E.; Hornberger, M. Menopause, cognition and dementia—A review. Post Reprod. Health 2019, 25, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Unda, S.R.; Marciano, S.; Milner, T.A.; Marongiu, R. State-of-the-art review of the clinical research on menopause and hormone replacement therapy association with Parkinson’s disease: What meta-analysis studies cannot tell us. Front. Aging Neurosci. 2022, 14, 971007. [Google Scholar] [CrossRef]
- Stefanowski, B.; Kucharski, M.; Szeliga, A.; Snopek, M.; Kostrzak, A.; Smolarczyk, R.; Maciejewska-Jeske, M.; Duszewska, A.; Niwczyk, O.; Drozd, S.; et al. Cognitive decline and dementia in women after menopause: Prevention strategies. Maturitas 2023, 168, 53–61. [Google Scholar] [CrossRef]
- Sochocka, M.; Karska, J.; Pszczołowska, M.; Ochnik, M.; Fułek, M.; Fułek, K.; Kurpas, D.; Chojdak-Łukasiewicz, J.; Rosner-Tenerowicz, A.; Leszek, J. Cognitive Decline in Early and Premature Menopause. Int. J. Mol. Sci. 2023, 24, 6566. [Google Scholar] [CrossRef]
- Speed, L.J.; Majid, A. Grounding language in the neglected senses of touch, taste, and smell. Cogn. Neuropsychol. 2020, 37, 363–392. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M.; Jones-Gotman, M.; Zatorre, R.J.; Petrides, M.; Evans, A.C. A role for the right anterior temporal lobe in taste quality recognition. J. Neurosci. 1997, 17, 5136–5142. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, S.S.; Warwick, Z.S. Effect of flavor enhancement of foods for the elderly on nutritional status: Food intake, biochemical indices, and anthropometric measures. Physiol. Behav. 1993, 53, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, S.S. Intensification of sensory properties of foods for the elderly. J. Nutr. 2000, 130, 927S–930S. [Google Scholar] [CrossRef]
- Miquel, S.; Champ, C.; Day, J.; Aarts, E.; Bahr, B.A.; Bakker, M.; Bánáti, D.; Calabrese, V.; Cederholm, T.; Cryan, J.; et al. Poor cognitive ageing: Vulnerabilities, mechanisms and the impact of nutritional interventions. Ageing Res. Rev. 2018, 42, 40–55. [Google Scholar] [CrossRef]
| Parameter | Mean | SD | 95% C.I. |
|---|---|---|---|
| Body mass index | 23.31 | 4.47 | 22.81–23.80 |
| Beck Depression Inventory score | 8.55 | 7.38 | 7.71–9.39 |
| Total taste score | 12.28 | 2.30 | 12.03–12.54 |
| Sweet | 3.40 | 0.78 | 3.31–3.48 |
| Salty | 3.31 | 0.94 | 3.20–3.41 |
| Sour | 2.52 | 1.01 | 2.41–2.63 |
| Bitter | 3.05 | 1.08 | 2.94–3.18 |
| Total olfactory score | 30.70 | 7.08 | 29.92–31.48 |
| Odor threshold | 6.44 | 4.29 | 5.97–6.92 |
| Odor discrimination | 11.56 | 2.48 | 11.28–11.83 |
| Odor identification | 12.54 | 2.54 | 12.26–12.82 |
| Total MoCA score | 26.52 | 3.50 | 26.13–26.91 |
| Visuospatial/executive | 4.56 | 1.02 | 4.45–4.67 |
| Naming | 2.91 | 0.36 | 2.87–2.95 |
| Memory | 3.15 | 1.59 | 2.98–3.33 |
| Attention | 5.46 | 0.95 | 5.35–5.56 |
| Language | 2.43 | 0.72 | 2.35–2.51 |
| Abstraction | 1.82 | 0.44 | 1.77–1.87 |
| Orientation | 5.94 | 0.37 | 5.90–5.98 |
| Age Group (Mean ± SD) | ANOVA | Post Hoc | |||||
|---|---|---|---|---|---|---|---|
| 18–34 | 35–49 | 50–64 | ≥65 | F | p | ||
| TT | 12.74 ± 1.84 | 12.58 ± 1.75 | 12.00 ± 2.78 | 10.91 ± 2.78 | 8.94 | 0.000 | c; e |
| SW | 3.47 ± 0.73 | 3.38 ± 0.68 | 3.40 ± 0.81 | 3.18 ± 0.94 | 1.62 | 0.184 | --- |
| SA | 3.42 ± 0.79 | 3.47 ± 0.92 | 3.29 ± 1.00 | 2.79 ± 1.16 | 6.30 | 0.000 | c; e; f |
| SO | 2.71 ± 0.88 | 2.44 ± 1.16 | 2.32 ± 1.13 | 2.27 ± 1.00 | 3.84 | 0.010 | b; c |
| BI | 3.14 ± 1.04 | 3.29 ± 0.87 | 2.98 ± 1.01 | 2.67 ± 1.40 | 3.29 | 0.023 | c; e |
| TDI | 33.17 ± 4.79 | 31.02 ± 5.57 | 29.72 ± 7.69 | 23.64 ± 8.76 | 28.68 | 0.000 | b; c; e; f |
| OT | 7.46 ± 4.15 | 6.07 ± 3.85 | 5.90 ± 4.52 | 4.22 ± 3.86 | 8.27 | 0.000 | c |
| OD | 12.38 ± 1.72 | 11.69 ± 2.04 | 11.13 ± 2.83 | 9.33 ± 2.95 | 23.52 | 0.000 | b; c; e; f |
| OI | 13.12 ± 1.54 | 13.15 ± 2.15 | 12.54 ± 2.73 | 10.02 ± 3.57 | 23.54 | 0.000 | c; e; f |
| TM | 27.96 ± 1.98 | 27.13 ± 1.78 | 26.15 ± 3.19 | 21.77 ± 4.67 | 61.06 | 0.000 | b; c; e; f |
| VE | 4.83 ± 0.46 | 4.93 ± 0.25 | 4.60 ± 1.02 | 3.25 ± 1.63 | 45.64 | 0.000 | c; e; f |
| NM | 2.97 ± 0.16 | 2.96 ± 0.21 | 2.91 ± 0.28 | 2.65 ± 0.75 | 11.26 | 0.000 | c; e; f |
| ME | 3.87 ± 1.27 | 2.91 ± 1.33 | 2.81 ± 1.54 | 1.52 ± 1.46 | 38.97 | 0.000 | a; b; c; e; f |
| AT | 5.82 ± 0.46 | 5.49 ± 0.76 | 5.26 ± 0.96 | 4.52 ± 1.47 | 30.74 | 0.000 | b; c; e; f |
| LN | 2.60 ± 0.54 | 2.71 ± 0.46 | 2.34 ± 0.80 | 1.75 ± 0.86 | 24.37 | 0.000 | b; c; d; e; f |
| AB | 1.88 ± 0.36 | 1.93 ± 0.25 | 1.76 ± 0.49 | 1.58 ± 0.61 | 7.38 | 0.000 | c; e |
| OR | 5.95 ± 0.34 | 6.00 ± 0.00 | 6.00 ± 0.00 | 5.77 ± 0.69 | 4.63 | 0.003 | c; e; f |
| Age Group (Mean ± SD) | ANOVA | Post Hoc | |||||
|---|---|---|---|---|---|---|---|
| 18–34 | 35–49 | 50–64 | ≥65 | F | p | ||
| BMI | 22.18 ± 3.84 | 22.02 ± 2.85 | 25.00 ± 5.05 | 25.81 ± 5.09 | 14.45 | 0.000 | b; c; d; e |
| BDI | 8.67 ± 7.16 | 8.64 ± 6.94 | 7.15 ± 6.30 | 10.11 ± 9.92 | 1.27 | 0.283 | --- |
| Predictors | B | SD Error | Beta | t |
Significance (p Value) |
F Value | p Value | R2 |
|---|---|---|---|---|---|---|---|---|
| MoCA total score (dependent variable) | ||||||||
| OD | 0.350 | 0.087 | 0.248 | 4.00 | >0.000 | 36.58 | 0.000 | 0.258 |
| OI | 0.335 | 0.084 | 0.243 | 3.97 | >0.000 | |||
| OT | 0.128 | 0.042 | 0.157 | 3.08 | >0.002 | |||
| Visuospatial/executive subscale (dependent variable) | ||||||||
| OD | 0.097 | 0.027 | 0.234 | 3.63 | 0.000 | 25.31 | 0.000 | 0.194 |
| OI | 0.082 | 0.026 | 0.203 | 3.18 | 0.002 | |||
| OT | 0.028 | 0.013 | 0.117 | 2.20 | 0.028 | |||
| Naming (dependent variable) | ||||||||
| OD | 0.033 | 0.010 | 0.226 | 3.45 | 0.001 | 25.66 | 0.000 | 0.140 |
| OI | 0.027 | 0.009 | 0.191 | 2.92 | 0.004 | |||
| Memory (dependent variable) | ||||||||
| OD | 0.229 | 0.034 | 0.365 | 6.78 | 0.000 | 46.02 | 0.000 | 0.127 |
| Attention (dependent variable) | ||||||||
| OI | 0.117 | 0.020 | 0.313 | 5.84 | 0.000 | 27.86 | 0.000 | 0.150 |
| OT | 0.036 | 0.012 | 0.164 | 3.05 | 0.002 | |||
| Language (dependent variable) | ||||||||
| OT | 0.039 | 0.009 | 0.231 | 4.24 | 0.000 | 13.30 | 0.000 | 0.145 |
| OD | 0.048 | 0.016 | 0.165 | 3.02 | 0.003 | |||
| BI | 0.113 | 0.036 | 0.171 | 3.15 | 0.002 | |||
| SW | −0.130 | 0.050 | −0.141 | −2.61 | 0.010 | |||
| Abstraction (dependent variable) | ||||||||
| OI | 0.046 | 0.009 | 0.265 | 4.90 | 0.000 | 24.02 | 0.000 | 0.070 |
| Orientation (dependent variable) | ||||||||
| OD | 0.027 | 0.008 | 0.183 | 3.32 | 0.001 | 10.99 | 0.001 | 0.034 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanna, F.; Castelli, M.P.; Mostallino, R.; Loy, F.; Masala, C. Correlations between Gustatory, Olfactory, Cognitive Function, and Age in Healthy Women. Nutrients 2024, 16, 1731. https://doi.org/10.3390/nu16111731
Sanna F, Castelli MP, Mostallino R, Loy F, Masala C. Correlations between Gustatory, Olfactory, Cognitive Function, and Age in Healthy Women. Nutrients. 2024; 16(11):1731. https://doi.org/10.3390/nu16111731
Chicago/Turabian StyleSanna, Fabrizio, M. Paola Castelli, Rafaela Mostallino, Francesco Loy, and Carla Masala. 2024. "Correlations between Gustatory, Olfactory, Cognitive Function, and Age in Healthy Women" Nutrients 16, no. 11: 1731. https://doi.org/10.3390/nu16111731
APA StyleSanna, F., Castelli, M. P., Mostallino, R., Loy, F., & Masala, C. (2024). Correlations between Gustatory, Olfactory, Cognitive Function, and Age in Healthy Women. Nutrients, 16(11), 1731. https://doi.org/10.3390/nu16111731









