Three Strains of Lactobacillus Derived from Piglets Alleviated Intestinal Oxidative Stress Induced by Diquat through Extracellular Vesicles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical
2.2. Bacterial Isolation and Culture
2.3. Bacterial EV Isolation
2.4. Animal Experiments
2.5. Morphological Analysis
2.6. Oxidation Markers’ Assessment
2.7. 16S rRNA Sequencing
2.8. Statistical Analysis
3. Results
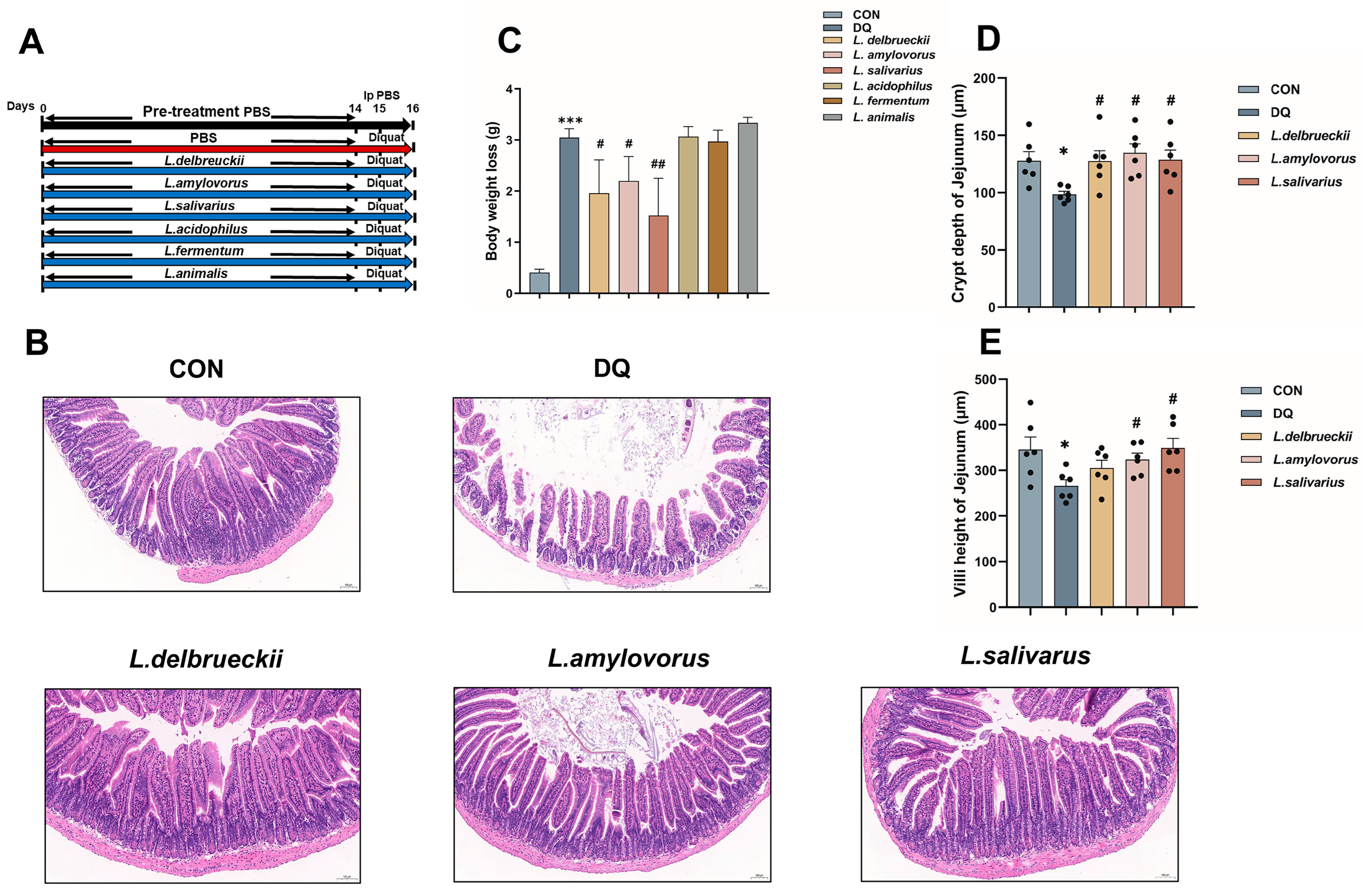
3.1. Lactobacillus Pre-Treatment Attenuates Diquat-Induced Weight Loss and Jejunal Damage
3.2. Lactobacillus Down-Regulates Serum and Jejunal Levels of Oxidative Indicators
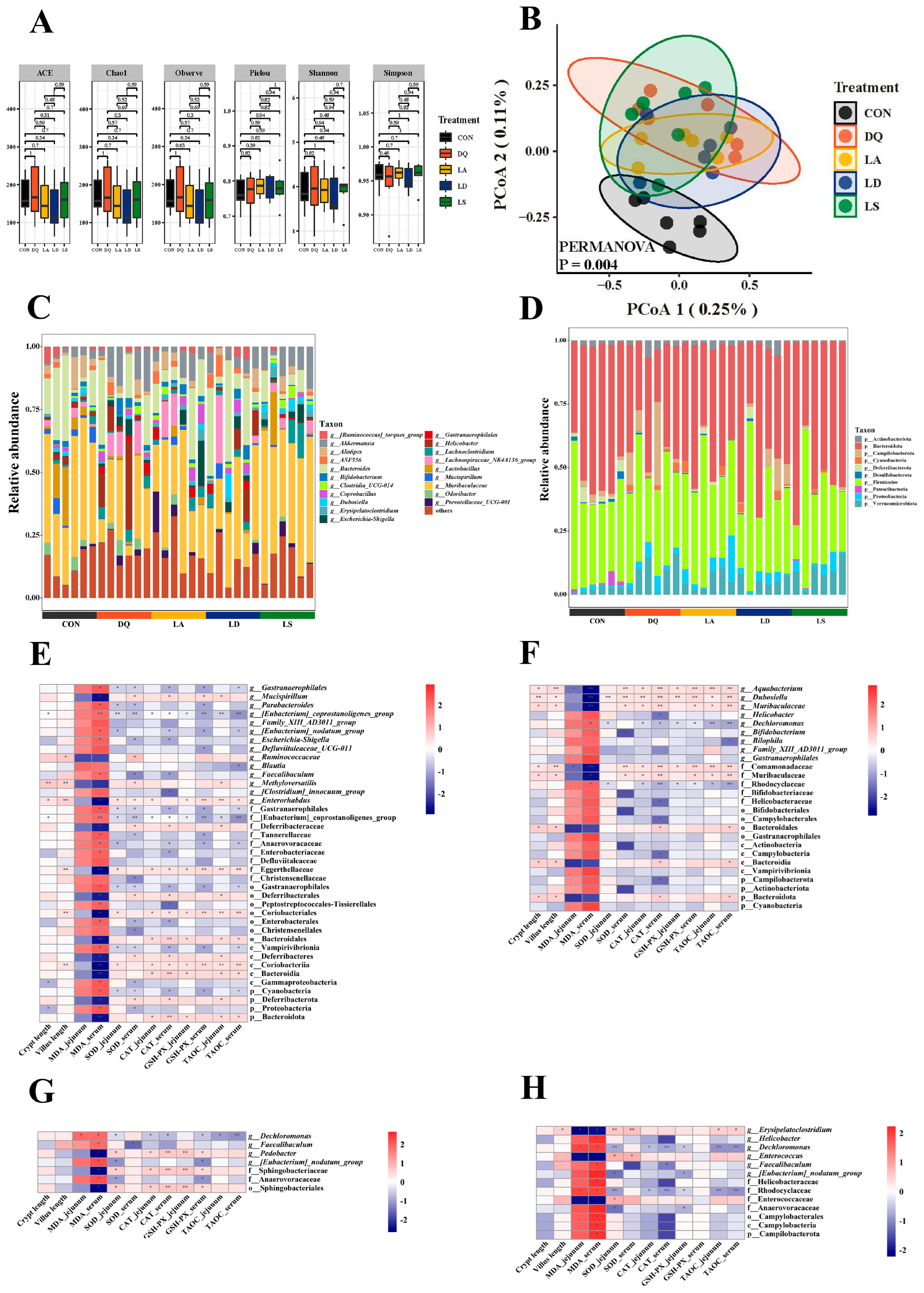
3.3. Lactobacillus Can Regulate the Disturbance Induced by Diquat to the Gut Microbiota
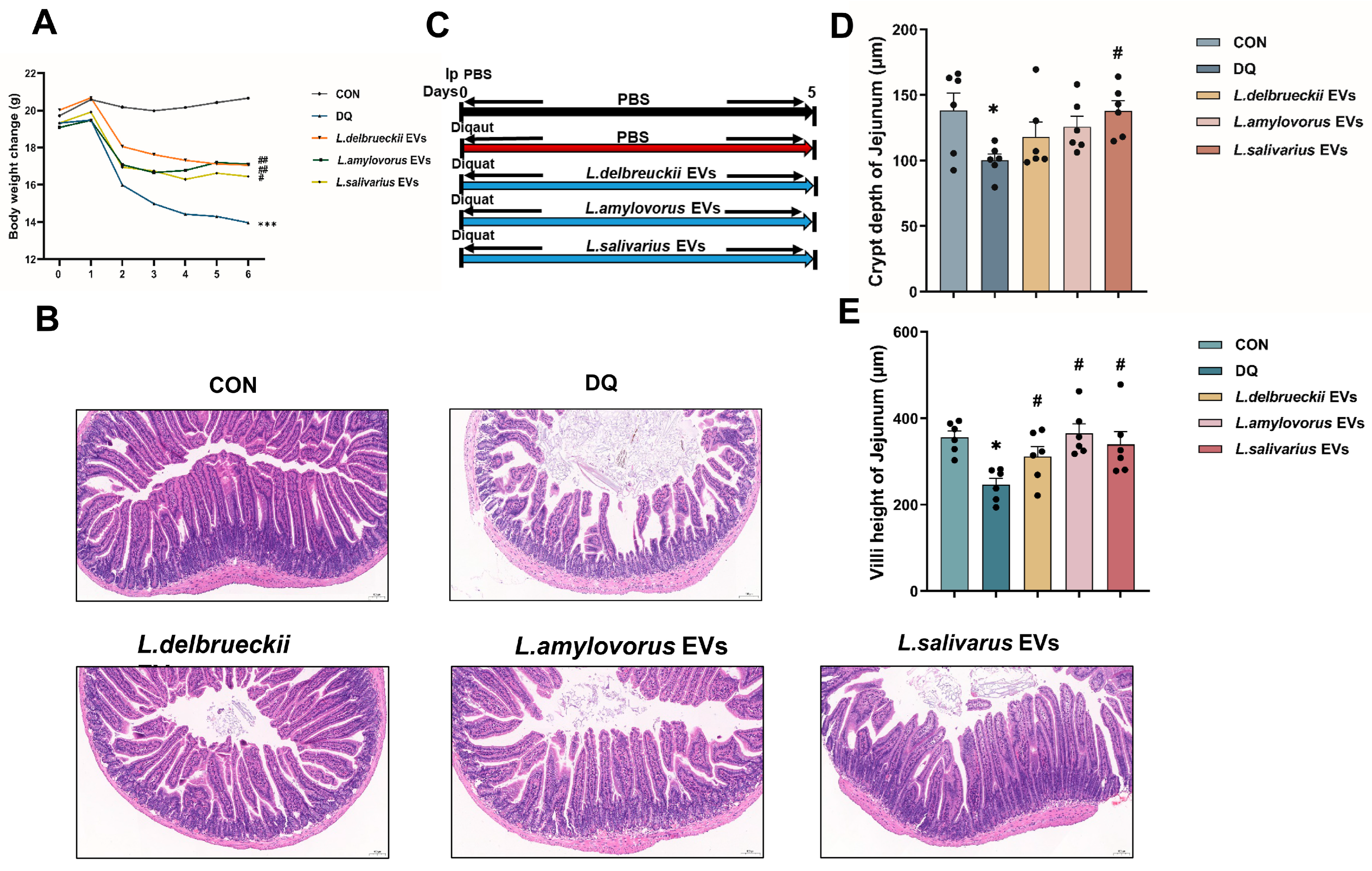
3.4. EV Treatment Ameliorates Diquat-Induced Weight Loss and Jejunal Damage
3.5. EV Treatment Reduced Oxidation Indicator Levels in the Serum and Jejunum
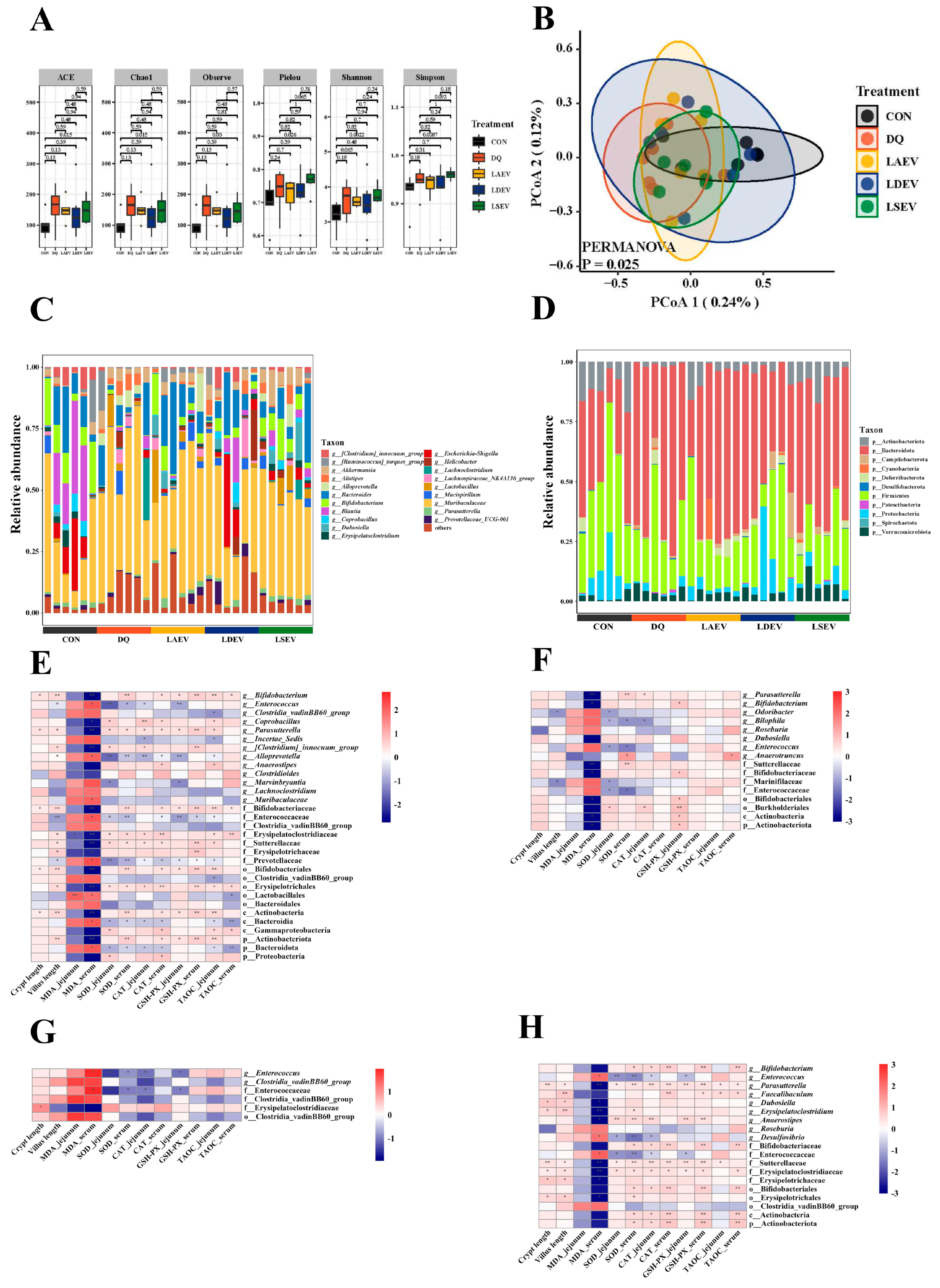
3.6. EV Therapy Modulated Diquat-Induced Disordered Gut Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, T.; Zhang, H.; Wang, S.; Xiang, Z.; Kong, H.; Xue, Q.; He, M.; Yu, X.; Li, Y.; Sun, D.; et al. A review on the advances in the extraction methods and structure elucidation of Poria cocos polysaccharide and its pharmacological activities and drug carrier applications. Int. J. Biol. Macromol. 2022, 217, 536–551. [Google Scholar] [CrossRef]
- Li, X.; Ma, L.; Zhang, L. Molecular basis for Poria cocos mushroom polysaccharide used as an antitumor drug in China. Prog. Mol. Biol. Transl. Sci. 2019, 163, 263–296. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, S.; Jiang, Y.; Wu, J.; Chen, L.; Ding, Y.; Zhou, Y.; Deng, L.; Chen, X. Poria cocos Polysaccharide Ameliorated Antibiotic-Associated Diarrhea in Mice via Regulating the Homeostasis of the Gut Microbiota and Intestinal Mucosal Barrier. Int. J. Mol. Sci. 2023, 11, 1423. [Google Scholar] [CrossRef]
- Yin, L.; Huang, G.; Khan, I.; Su, L.; Xia, W.; Law, B.Y.K.; Wong, V.K.W.; Wu, Q.; Wang, J.; Leong, W.K.; et al. Poria cocos polysaccharides exert prebiotic function to attenuate the adverse effects and improve the therapeutic outcome of 5-FU in Apc(Min/+) mice. Chin. Med. 2022, 103, 116. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Wang, L.; Chen, W.D.; Duan, Y.T.; Sun, M.J.; Huang, J.J.; Peng, D.Y.; Yu, N.J.; Wang, Y.Y.; Zhang, Y. Poria cocos polysaccharide prevents alcohol-induced hepatic injury and inflammation by repressing oxidative stress and gut leakiness. Front. Nutr. 2022, 817, 963598. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Huang, J.; Sun, M.; Jiang, Y.; Wang, S.; Wang, L.; Yu, N.; Peng, D.; Wang, Y.; Chen, W.; et al. Poria cocos polysaccharide improves intestinal barrier function and maintains intestinal homeostasis in mice. Int. J. Biol. Macromol. 2023, 728, 125953. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Fan, J.; Huang, X.; Wu, X.; Guo, C. Hepatoprotective effects exerted by Poria cocos polysaccharides against acetaminophen-induced liver injury in mice. Int. J. Biol. Macromol. 2018, 715, 137–142. [Google Scholar] [CrossRef]
- Tan, Y.Y.; Yue, S.R.; Lu, A.P.; Zhang, L.; Ji, G.; Liu, B.C.; Wang, R.R. The improvement of nonalcoholic steatohepatitis by Poria cocos polysaccharides associated with gut microbiota and NF-κB/CCL3/CCR1 axis. Phytomedicine 2022, 8, 154208. [Google Scholar] [CrossRef]
- Zhang, D.D.; Li, H.J.; Zhang, H.R.; Ye, X.C. Poria cocos water-soluble polysaccharide modulates anxiety-like behavior induced by sleep deprivation by regulating the gut dysbiosis, metabolic disorders and TNF-α/NF-κB signaling pathway. Food Funct. 2022, 720, 6648–6664. [Google Scholar] [CrossRef]
- Lan, K.; Yang, H.; Zheng, J.; Hu, H.; Zhu, T.; Zou, X.; Hu, B.; Liu, H. Poria cocos oligosaccharides ameliorate dextran sodium sulfate-induced colitis mice by regulating gut microbiota dysbiosis. Food Funct. 2023, 23, 857–873. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, D.; Huang, F.; Zhao, A.; Kuang, J.; Ren, Z.; Chen, T.; Lei, J.; Lin, J.; Wang, X.; et al. Theabrownin and Poria cocos Polysaccharide Improve Lipid Metabolism via Modulation of Bile Acid and Fatty Acid Metabolism. Front. Pharmacol. 2022, 27, 875549. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, Y.; Zeng, P.; Liu, Y.; Zhang, M.; Hao, C.; Wang, H.; Lv, Z.; Zhang, L. Molecular basis for Poria cocos mushroom polysaccharide used as an antitumour drug in China. J. Cell Mol. Med. 2019, 11, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Liu, Z.; Pu, Y.; Bao, Y. Immunomodulatory effects exerted by Poria cocos polysaccharides via TLR4/TRAF6/NF-κB signaling in vitro and in vivo. Biomed. Pharmacother. 2019, 4, 108709. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Wei, C.C.; Huang, C.C.; Chen, W.L.; Huang, H.Y. The Beneficial Effects of Lactobacillus plantarum PS128 on High-Intensity, Exercise-Induced Oxidative Stress, Inflammation, and Performance in Triathletes. Nutrients 2019, 11, 353. [Google Scholar] [CrossRef]
- Li, J.; Feng, S.; Wang, Z.; He, J.; Zhang, Z.; Zou, H.; Wu, Z.; Liu, X.; Wei, H.; Tao, S. Limosilactobacillus mucosae-derived extracellular vesicles modulates macrophage phenotype and orchestrates gut homeostasis in a diarrheal piglet model. NPJ Biofilms Microbiomes 2023, 9, 33. [Google Scholar] [CrossRef]
- Maerz, J.K.; Steimle, A.; Lange, A.; Bender, A.; Fehrenbacher, B.; Frick, J.S. Outer membrane vesicles blebbing contributes to B. vulgatus mpk-mediated immune response silencing. Gut Microbes 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Cañas, M.A.; Fábrega, M.J.; Giménez, R.; Badia, J.; Baldomà, L. Outer Membrane Vesicles From Probiotic and Commensal Escherichia coli Activate NOD1-Mediated Immune Responses in Intestinal Epithelial Cells. Front. Microbiol. 2018, 9, 498. [Google Scholar] [CrossRef]
- Seo, M.K.; Park, E.J.; Ko, S.Y.; Choi, E.W.; Kim, S. Therapeutic effects of kefir grain Lactobacillus-derived extracellular vesicles in mice with 2,4,6-trinitrobenzene sulfonic acid-induced inflammatory bowel disease. J. Dairy Sci. 2018, 101, 8662–8671. [Google Scholar] [CrossRef]
- Li, W.; Fang, K.; Yuan, H.; Li, D.; Li, H.; Chen, Y.; Luo, X.; Zhang, L.; Ye, X. Acid-induced Poria cocos alkali-soluble polysaccharide hydrogel: Gelation behaviour, characteristics, and potential application in drug delivery. Int. J. Biol. Macromol. 2023, 71 Pt 2, 124383. [Google Scholar] [CrossRef]
- Jiang, Y.; Fan, L. The effect of Poria cocos ethanol extract on the intestinal barrier function and intestinal microbiota in mice with breast cancer. J. Ethnopharmacol. 2021, 266, 113456. [Google Scholar] [CrossRef]
- Li, W.; Yu, J.; Zhao, J.; Xiao, X.; Li, W.; Zang, L.; Yu, J.; Liu, H.; Niu, X. Poria cocos polysaccharides reduces high-fat diet-induced arteriosclerosis in ApoE(-/-) mice by inhibiting inflammation. Phytother. Res. PTR 2021, 35, 2220–2229. [Google Scholar] [CrossRef]
- Ye, H.; Ma, S.; Qiu, Z.; Huang, S.; Deng, G.; Li, Y.; Xu, S.; Yang, M.; Shi, H.; Wu, C.; et al. Poria cocos polysaccharides rescue pyroptosis-driven gut vascular barrier disruption in order to alleviates non-alcoholic steatohepatitis. J. Ethnopharmacol. 2022, 296, 115457. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, L.; Zhang, G.; Peng, X. Poria cocos polysaccharides attenuate chronic nonbacterial prostatitis by targeting the gut microbiota: Comparative study of Poria cocos polysaccharides and finasteride in treating chronic prostatitis. Int. J. Biol. Macromol. 2021, 189, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.T.; Zhou, J.; Wu, C.Y.; Zhang, W.; Shen, H.; Xu, J.D.; Zhang, Y.Q.; Long, F.; Li, S.L. Protective effects of Poria cocos and its components against cisplatin-induced intestinal injury. J. Ethnopharmacol. 2021, 269, 113722. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 5, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Suboc, T.M.; Tyagi, S.; Salzman, N.; Wang, J.; Ying, R.; Tanner, M.J.; Kakarla, M.; Baker, J.E.; Widlansky, M.E. Lactobacillus plantarum 299v Supplementation Improves Vascular Endothelial Function and Reduces Inflammatory Biomarkers in Men With Stable Coronary Artery Disease. Circ. Res. 2018, 812, 1091–1102. [Google Scholar] [CrossRef]
- Lee, N.Y.; Yoon, S.J.; Han, D.H.; Gupta, H.; Youn, G.S.; Shin, M.J.; Ham, Y.L.; Kwak, M.J.; Kim, B.Y.; Yu, J.S.; et al. Lactobacillus and Pediococcus ameliorate progression of non-alcoholic fatty liver disease through modulation of the gut microbiome. Gut Microbes 2020, 7, 882–899. [Google Scholar] [CrossRef]
- Petrova, M.I.; Reid, G.; Vaneechoutte, M.; Lebeer, S. Lactobacillus iners: Friend or Foe? Trends Microbiol. 2017, 5, 182–191. [Google Scholar] [CrossRef]
- Mercer, E.M.; Arrieta, M.C. Probiotics to improve the gut microbiome in premature infants: Are we there yet. Gut Microbes 2023, 113, 2201160. [Google Scholar] [CrossRef]
- Liu, Y.; Tran, D.Q.; Rhoads, J.M. Probiotics in Disease Prevention and Treatment. J. Clin. Pharmacol. 2018, 10 (Suppl. 10), S164–S179. [Google Scholar] [CrossRef]
- Iqbal, Z.; Ahmed, S.; Tabassum, N.; Bhattacharya, R.; Bose, D. Role of probiotics in prevention and treatment of enteric infections: A comprehensive review. 3 Biotech 2021, 5, 242. [Google Scholar] [CrossRef] [PubMed]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 5, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Qin, X.; Zhou, G.; Wang, C.; Mu, C.; Liu, X.; Zhong, W.; Xu, X.; Wang, B.; Jiang, K.; et al. Lactobacillus rhamnosus GG supernatant promotes intestinal mucin production through regulating 5-HT4R and gut microbiota. Food Funct. 2022, 9, 12144–12155. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Cheng, Y.; Su, W.; Wang, C.; Lu, Z.; Jin, M.; Wang, F.; Wang, Y. Pediococcus pentosaceus ZJUAF-4 relieves oxidative stress and restores the gut microbiota in diquat-induced intestinal injury. Appl. Microbiol. Biotechnol. 2021, 105, 1657–1668. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal 2018, 910, 1727–1745. [Google Scholar] [CrossRef]
- Jin, Y.; Zhai, Z.; Jia, H.; Lai, J.; Si, X.; Wu, Z. Kaempferol attenuates diquat-induced oxidative damage and apoptosis in intestinal porcine epithelial cells. Food Funct. 2021, 12, 6889–6899. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, W.H.; Si, Z.L.; Liu, H.L.; Wang, H.; Jiang, H.; Liu, Y.F.; Alolga, R.N.; Chen, C.; Liu, S.J.; et al. The gut microbe Bacteroides fragilis ameliorates renal fibrosis in mice. Nat. Commun. 2022, 13, 6081. [Google Scholar] [CrossRef]
- Wu, S.; Zuo, J.; Cheng, Y.; Zhang, Y.; Zhang, Z.; Wu, M.; Yang, Y.; Tong, H. Ethanol extract of Sargarsum fusiforme alleviates HFD/STZ-induced hyperglycemia in association with modulation of gut microbiota and intestinal metabolites in type 2 diabetic mice. Food Res. Int. 2021, 147, 110550. [Google Scholar] [CrossRef]
- Chen, S.; Wu, X.; Yu, Z. Juglone Suppresses Inflammation and Oxidative Stress in Colitis Mice. Front. Immunol. 2021, 12, 674341. [Google Scholar] [CrossRef]
- Wei, W.; Jiang, W.; Tian, Z.; Wu, H.; Ning, H.; Yan, G.; Zhang, Z.; Li, Z.; Dong, F.; Sun, Y.; et al. Fecal g. Streptococcus and g. Eubacterium_coprostanoligenes_group combined with sphingosine to modulate the serum dyslipidemia in high-fat diet mice. Clin. Nutr. 2021, 40, 4234–4245. [Google Scholar] [CrossRef]
- Chen, M.; Cai, W.; Zhao, S.; Shi, L.; Chen, Y.; Li, X.; Sun, X.; Mao, Y.; He, B.; Hou, Y.; et al. Oxidative stress-related biomarkers in saliva and gingival crevicular fluid associated with chronic periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Garrido, N.; Badia, J.; Baldomà, L. Microbiota-derived extracellular vesicles in interkingdom communication in the gut. J. Extracell. Vesicles 2021, 10, e12161. [Google Scholar] [CrossRef]
- Diaz-Garrido, N.; Cordero, C.; Olivo-Martinez, Y.; Badia, J.; Baldomà, L. Cell-to-Cell Communication by Host-Released Extracellular Vesicles in the Gut: Implications in Health and Disease. Int. J. Mol. Sci. 2021, 22, 2213. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 816, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Shu, X.; Wang, J. Helicobacter pylori-Mediated Oxidative Stress and Gastric Diseases: A Review. Front. Microbiol. 2022, 13, 811258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, L.; Qin, S.; Yuan, J.; Xie, X.; Wang, F.; Hu, S.; Yi, Y.; Chen, M. C-phycocyanin alleviated cisplatin-induced oxidative stress and inflammation via gut microbiota-metabolites axis in mice. Front. Nutr. 2022, 9, 996614. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, S.; Liu, Y.; Xu, J.; Fan, J.; Li, J.; Wu, Z.; Sun, Y.; Xiong, W. Three Strains of Lactobacillus Derived from Piglets Alleviated Intestinal Oxidative Stress Induced by Diquat through Extracellular Vesicles. Nutrients 2023, 15, 4198. https://doi.org/10.3390/nu15194198
Feng S, Liu Y, Xu J, Fan J, Li J, Wu Z, Sun Y, Xiong W. Three Strains of Lactobacillus Derived from Piglets Alleviated Intestinal Oxidative Stress Induced by Diquat through Extracellular Vesicles. Nutrients. 2023; 15(19):4198. https://doi.org/10.3390/nu15194198
Chicago/Turabian StyleFeng, Shengkai, Yihan Liu, Jing Xu, Jinping Fan, Jingjing Li, Zhifeng Wu, Yue Sun, and Wen Xiong. 2023. "Three Strains of Lactobacillus Derived from Piglets Alleviated Intestinal Oxidative Stress Induced by Diquat through Extracellular Vesicles" Nutrients 15, no. 19: 4198. https://doi.org/10.3390/nu15194198
APA StyleFeng, S., Liu, Y., Xu, J., Fan, J., Li, J., Wu, Z., Sun, Y., & Xiong, W. (2023). Three Strains of Lactobacillus Derived from Piglets Alleviated Intestinal Oxidative Stress Induced by Diquat through Extracellular Vesicles. Nutrients, 15(19), 4198. https://doi.org/10.3390/nu15194198




