Effects of Resistance Exercise and Essential Amino Acid Intake on Muscle Quality, Myokine, and Inflammation Factors in Young Adult Males
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Participation
2.3. Measurement of Body Composition
2.4. Measurement of Muscle Quality (Grip, Trunk, and Knee Strength Test)
2.5. Hematological Analysis
2.6. Essential Amino Acids Supplement
2.7. Resistance Exercise Program
2.8. Statistical Analysis
3. Results
3.1. Changes in Body Composition
3.2. Changes in Muscle Quality
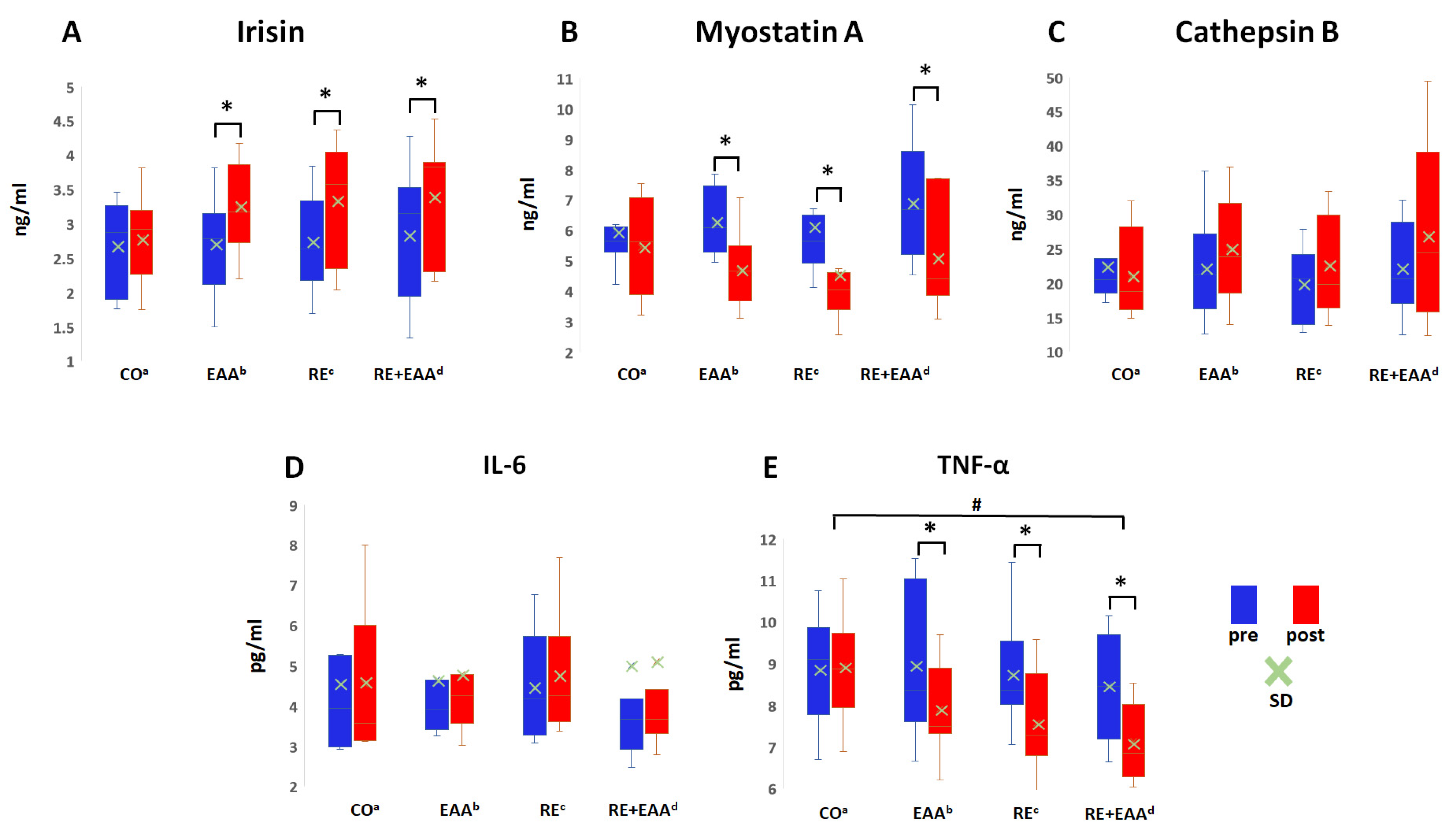
3.3. Changes in Myokine and Inflammation Factors
4. Discussion
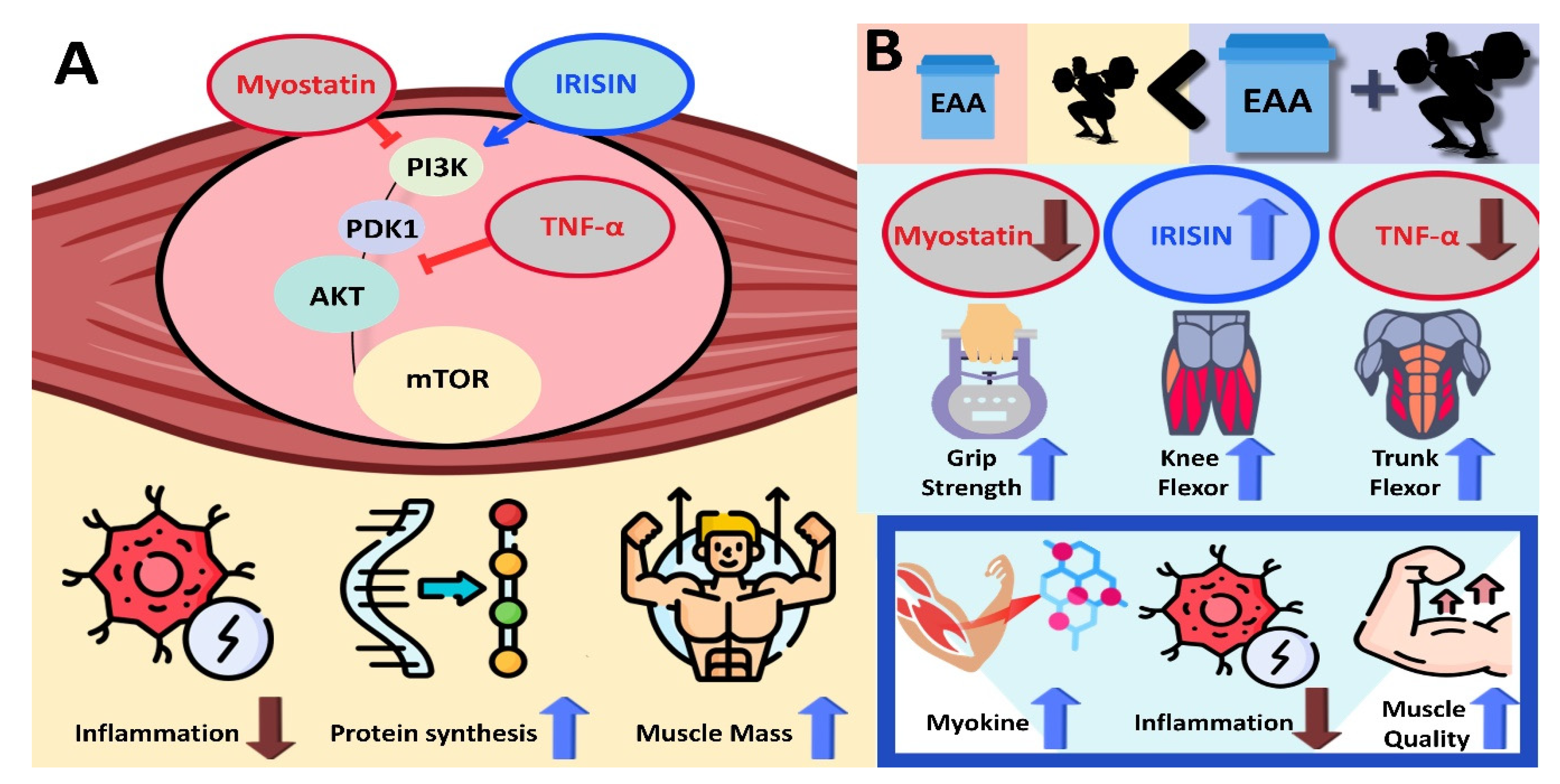
4.1. How Do Resistance Exercise and EAA Intake Treatment Affect Muscle Mass?
4.2. What Effects Do Resistance Exercise and EAA Treatment Have on Muscle Quality?
4.3. How Do Resistance Exercise, EAA, and Their Combination Affect Myokine and Inflammation Factors?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nour, T.Y.; Altintaş, K.H. Effect of the COVID-19 pandemic on obesity and its risk factors: A systematic review. BMC Public Health 2023, 23, 1018. [Google Scholar] [CrossRef]
- Katzmarzyk, P.T.; Janssen, I. The economic costs associated with physical inactivity and obesity in Canada: An update. Can. J. Appl. Physiol. 2004, 29, 90–115. [Google Scholar] [CrossRef]
- Lee, I.M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T.; Lancet Physical Activity Series Working Group. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Boveris, A.; Navarro, A. Systemic and mitochondrial adaptive responses to moderate exercise in rodents. Free Radical Biol. Med. 2008, 44, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Durstine, J.L.; Gordon, B.; Wang, Z.Z.; Luo, X.J. Chronic disease and the link to physical activity. J. Sport Health Sci. 2013, 2, 3–11. [Google Scholar] [CrossRef]
- Naci, H.; Ioannidis, J.P.A. Comparative effectiveness of exercise and drug interventions on mortality outcomes: Metaepidemiological study. BMJ Br. Med. J. 2013, 347, f5577. [Google Scholar] [CrossRef] [PubMed]
- Burd, N.A.; Tang, J.E.; Moore, D.R.; Phillips, S.M. Exercise training and protein metabolism: Influences of contraction, protein intake, and sex-based differences. J. Appl. Physiol. 2009, 106, 1692–1701. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Steensberg, A.; Fischer, C.; Keller, C.; Keller, P.; Plomgaard, P.; Febbraio, M.; Saltin, B. Searching for the exercise factor: Is IL-6 a candidate? J. Muscle Res. Cell. Motil. 2003, 24, 113–119. [Google Scholar] [CrossRef]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; Van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Goo, Y.L.; Haus, J.M.; et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef]
- Ramírez-Vélez, R.; González, A.; García-Hermoso, A.; Amézqueta, I.L.; Izquierdo, M.; Díez, J. Revisiting skeletal myopathy and exercise training in heart failure: Emerging role of myokines. Metab. Clin. Exp. 2023, 138, 155348. [Google Scholar] [CrossRef]
- Carey, A.L.; Steinberg, G.R.; Macaulay, S.L.; Thomas, W.G.; Holmes, A.G.; Ramm, G.; Prelovsek, O.; Hohnen-Behrens, C.; Watt, M.J.; James, D.E.; et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 2006, 55, 2688–2697. [Google Scholar] [CrossRef]
- Barra, N.G.; Chew, M.V.; Holloway, A.C.; Ashkar, A.A. Interleukin-15 treatment improves glucose homeostasis and insulin sensitivity in obese mice. Diabetes Obes. Metab. 2012, 14, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Steiner, S.; Zhou, Y.; Arai, H.; Roberson, E.D.; Sun, B.G.; Chen, J.; Wang, X.; Yu, G.Q.; Esposito, L.; Mucke, L.; et al. Antiamyloidogenic and neuroprotective functions of cathepsin B: Implications for Alzheimer’s disease. Neuron 2006, 51, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Korta, P.; Pochec, E.; Mazur-Bialy, A. Irisin as a Multifunctional Protein: Implications for Health and Certain Diseases. Med.-Lith. 2019, 55, 485. [Google Scholar] [CrossRef] [PubMed]
- Consitt, L.A.; Clark, B.C. The Vicious Cycle of Myostatin Signaling in Sarcopenic Obesity: Myostatin Role in Skeletal Muscle Growth, Insulin Signaling and Implications for Clinical Trials. J. Frailty Aging 2018, 7, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Church, D.D.; Hirsch, K.R.; Park, S.; Kim, I.Y.; Gwin, J.A.; Pasiakos, S.M.; Wolfe, R.R.; Ferrando, A.A. Essential Amino Acids and Protein Synthesis: Insights into Maximizing the Muscle and Whole-Body Response to Feeding. Nutrients 2020, 12, 3717. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.R.; Miller, S.L. Amino acid availability controls muscle protein metabolism. Diabetes Nutr. Metab. 1999, 12, 322–328. [Google Scholar] [PubMed]
- Moberg, M.; Apró, W.; Ekblom, B.; van Hall, G.; Holmberg, H.C.; Blomstrand, E. Activation of mTORC1 by leucine is potentiated by branched-chain amino acids and even more so by essential amino acids following resistance exercise. Am. J. Physiol. Cell Physiol. 2016, 310, C874–C884. [Google Scholar] [CrossRef] [PubMed]
- D’Antona, G.; Ragni, M.; Cardile, A.; Tedesco, L.; Dossena, M.; Bruttini, F.; Caliaro, F.; Corsetti, G.; Bottinelli, R.; Carruba, M.O.; et al. Branched-Chain Amino Acid Supplementation Promotes Survival and Supports Cardiac and Skeletal Muscle Mitochondrial Biogenesis in Middle-Aged Mice. Cell Metab. 2010, 12, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.M.; Soop, M.; Sohn, T.S.; Morse, D.M.; Schimke, J.M.; Klaus, K.A.; Nair, K.S. High Insulin Combined With Essential Amino Acids Stimulates Skeletal Muscle Mitochondrial Protein Synthesis While Decreasing Insulin Sensitivity in Healthy Humans. J. Clin. Endocrinol. Metab. 2014, 99, E2574–E2583. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Wei, L.; Chan, W.P.; Hsu, C.Y.; Huang, S.W.; Wang, H.; Lin, Y.N. Effects of protein supplementation on aerobic training-induced gains in cardiopulmonary fitness, muscle mass, and functional performance in chronic stroke: A randomized controlled pilot study. Clin. Nutr. 2020, 39, 2743–2750. [Google Scholar] [CrossRef]
- Yu, L.L.; Li, Y.M.; Zhang, Q.; Zhu, L.C.; Ding, N.; Zhang, B.Y.; Zhang, J.L.; Liu, W.J.; Li, S.Y.; Zhang, J. Association between dietary essential amino acids intake and metabolic biomarkers: Influence of obesity among Chinese children and adolescents. Amino Acids 2021, 53, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.C.; Yoshizawa, F.; Anthony, T.G.; Vary, T.C.; Jefferson, L.S.; Kimball, S.R. Leucine Stimulates Translation Initiation in Skeletal Muscle of Postabsorptive Rats via a Rapamycin-Sensitive Pathway. J. Nutr. 2000, 130, 2413–2419. [Google Scholar] [CrossRef]
- Wolfson, R.L.; Chantranupong, L.; Saxton, R.A.; Shen, K.; Scaria, S.M.; Cantor, J.R.; Sabatini, D.M. METABOLISM Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 2016, 351, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Park, M.; Kim, B.; Kang, S.H. Effect of Black Maca Supplementation on Inflammatory Markers and Physical Fitness in Male Elite Athletes. Nutrients 2023, 15, 1618. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-H.; Lim, S.-T.; Lee, J.; Kim, B.-J.; Oh, S.-B.; Kang, S.; Park, M.-H.; Lim, S.-T.; Lee, J.; Kim, B.-J. Effects of Resistance Exercise, Black Maca and Combined Treatment on Blood Muscle Fatigue Factors and Muscle Function in Racket Athletes. Exerc. Sci. 2022, 31, 459–468. [Google Scholar] [CrossRef]
- Kim, J.K.; Kang, S.; Park, K.M. Effect of resistance training and detraining on metabolic markers. J. Men’s Health 2019, 15, E40–E49. [Google Scholar] [CrossRef]
- McCaulley, G.O.; McBride, J.M.; Cormie, P.; Hudson, M.B.; Nuzzo, J.L.; Quindry, J.C.; Triplett, N.T. Acute hormonal and neuromuscular responses to hypertrophy, strength and power type resistance exercise. Eur. J. Appl. Physiol. 2009, 105, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Ratamess, N.A.; Alvar, B.A.; Evetoch, T.E.; Housh, T.J.; Ben Kibler, W.; Kraemer, W.J.; Triplett, N.T. Progression Models in Resistance Training for Healthy Adults. Med. Sci. Sports Exerc. 2009, 41, 687–708. [Google Scholar] [CrossRef] [PubMed]
- Damas, F.; Libardi, C.A.; Ugrinowitsch, C. The development of skeletal muscle hypertrophy through resistance training: The role of muscle damage and muscle protein synthesis. Eur. J. Appl. Physiol. 2018, 118, 485–500. [Google Scholar] [CrossRef]
- Wolfe, R.R. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 2006, 84, 475–482. [Google Scholar] [CrossRef]
- Kim, Y.; Park, S.; Lee, J.; Jang, J.; Jung, J.; Koh, J.H.; Choi, C.S.; Wolfe, R.R.; Kim, I. Essential Amino Acid-Enriched Diet Alleviates Dexamethasone-Induced Loss of Muscle Mass and Function through Stimulation of Myofibrillar Protein Synthesis and Improves Glucose Metabolism in Mice. Metabolites 2022, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.C.; Ellefsen, S.; Baar, K. Adaptations to endurance and strength training. Cold Spring Harbor Perspect. Med. 2018, 8, a029769. [Google Scholar] [CrossRef] [PubMed]
- Cermak, N.M.; Res, P.T.; de Groot, L.; Saris, W.H.M.; van Loon, L.J.C. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: A meta-analysis. Am. J. Clin. Nutr. 2012, 96, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Dillon, E.L.; Sheffield-Moore, M.; Paddon-Jones, D.; Gilkison, C.; Sanford, A.P.; Casperson, S.L.; Jiang, J.; Chinkes, D.L.; Urban, R.J. Amino Acid Supplementation Increases Lean Body Mass, Basal Muscle Protein Synthesis, and Insulin-Like Growth Factor-I Expression in Older Women. J. Clin. Endocrinol. Metab. 2009, 94, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Park, S.; Kim, Y.; Jung, J.; Lee, J.; Chang, Y.; Lee, S.P.; Park, B.C.; Wolfe, R.R.; Choi, C.S.; et al. Myostatin Inhibition-Induced Increase in Muscle Mass and Strength Was Amplified by Resistance Exercise Training, and Dietary Essential Amino Acids Improved Muscle Quality in Mice. Nutrients 2021, 13, 1508. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am. J. Clin. Nutr. 2005, 82, 1065–1073. [Google Scholar] [CrossRef]
- Antonio, J.; Sanders, M.S.; Ehler, L.A.; Uelmen, J.; Raether, J.B.; Stout, J.R. Effects of exercise training and amino-acid supplementation on body composition and physical performance in untrained women. Nutrition 2000, 16, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Reid, K.F.; Naumova, E.N.; Carabello, R.J.; Phillips, E.M.; Fielding, R.A. Lower extremity muscle mass predicts functional performance in mobility-limited elders. J. Nutr. Health Aging 2008, 12, 493–498. [Google Scholar] [CrossRef]
- Volpi, E.; Kobayashi, H.; Sheffield-Moore, M.; Mittendorfer, B.; Wolfe, R.R. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults2. Am. J. Clin. Nutr. 2003, 78, 250–258. [Google Scholar] [CrossRef]
- Ikeda, T.; Matsunaga, Y.; Kanbara, M.; Kamono, A.; Masuda, T.; Watanabe, M.; Nakanishi, R.; Jinno, T. Effect of exercise therapy combined with branched-chain amino acid supplementation on muscle strength in elderly women after total hip arthroplasty: A randomized controlled trial. Asia Pac. J. Clin. Nutr. 2019, 28, 720–726. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Ratamess, N.A.; Kang, J.; Falvo, M.J.; Faigenbaum, A.D. Effect of Protein Intake on Strength, Body Composition and Endocrine Changes in Strength/Power Athletes. J. Int. Soc. Sports Nutr. 2006, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, A.; Casolo, A.; Negro, F.; Scorcelletti, M.; Bazzucchi, I.; Enoka, R.; Felici, F.; Farina, D. The increase in muscle force after 4 weeks of strength training is mediated by adaptations in motor unit recruitment and rate coding. J. Physiol. 2019, 597, 1873–1887. [Google Scholar] [CrossRef] [PubMed]
- Borsheim, E.; Bui, Q.U.T.; Tissier, S.; Kobayashi, H.; Ferrando, A.A.; Wolfe, R.R. Effect of amino acid supplementation on muscle mass, strength and physical function in elderly. Clin. Nutr. 2008, 27, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jun, H.S. Role of Myokines in Regulating Skeletal Muscle Mass and Function. Front. Physiol. 2019, 10, 435092. [Google Scholar] [CrossRef] [PubMed]
- Laurens, C.; Bergouignan, A.; Moro, C. Exercise-Released Myokines in the Control of Energy Metabolism. Front. Physiol. 2020, 11, 467932. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J.; et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Weigert, C. Skeletal Muscle as an Endocrine Organ: The Role of Myokines in Exercise Adaptations. Cold Spring Harbor Perspect. Med. 2017, 7, a029793. [Google Scholar] [CrossRef]
- Tuttle, C.S.L.; Thang, L.A.N.; Maier, A.B. Markers of inflammation and their association with muscle strength and mass: A systematic review and meta-analysis. Ageing Res. Rev. 2020, 64, 101185. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Langley, B.; Thomas, M.; Bishop, A.; Sharma, M.; Gilmour, S.; Kambadur, R. Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J. Biol. Chem. 2002, 277, 49831–49840. [Google Scholar] [CrossRef]
- Elkina, Y.; von Haehling, S.; Anker, S.D.; Springer, J. The role of myostatin in muscle wasting: An overview. J. Cachexia Sarcopenia Muscle 2011, 2, 143–151. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, C.; Plummer, E.; Thomas, M.; Hennebry, A.; Ashby, M.; Ling, N.; Smith, H.; Sharma, M.; Kambadur, R. Myostatin induces cachexia by activating the ubiquitin proteolytic system through an NF-κB-independent, FoxO1-dependent. J. Cell. Physiol. 2006, 209, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.J.; Glynn, E.L.; Fry, C.S.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Essential Amino Acids Increase MicroRNA-499,-208b, and-23a and Downregulate Myostatin and Myocyte Enhancer Factor 2C mRNA Expression in Human Skeletal Muscle. J. Nutr. 2009, 139, 2279–2284. [Google Scholar] [CrossRef] [PubMed]
- Yarasheski, K.E.; Pak-Loduca, J.; Hasten, D.L.; Obert, K.A.; Brown, M.B.; Sinacore, D.R. Resistance exercise training increases mixed muscle protein synthesis rate in frail women and men≥ 76 yr old. Am. J. Physiol. Endocrinol. Metab. 1999, 277, E118–E125. [Google Scholar] [CrossRef] [PubMed]
- Waskiw-Ford, M.; Hannaian, S.; Duncan, J.; Kato, H.; Abou Sawan, S.; Locke, M.; Kumbhare, D.; Moore, D. Leucine-Enriched Essential Amino Acids Improve Recovery from Post-Exercise Muscle Damage Independent of Increases in Integrated Myofibrillar Protein Synthesis in Young Men. Nutrients 2020, 12, 1061. [Google Scholar] [CrossRef] [PubMed]
- Askari, H.; Rajani, S.F.; Poorebrahim, M.; Haghi-Aminjan, H.; Raeis-Abdollahi, E.; Abdollahi, M. A glance at the therapeutic potential of irisin against diseases involving inflammation, oxidative stress, and apoptosis: An introductory review. Pharmacol. Res. 2018, 129, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Chesley, A.; MacDougall, J.; Tarnopolsky, M.; Atkinson, S.; Smith, K. Changes in human muscle protein synthesis after resistance exercise. J. Appl. Physiol. 1992, 73, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Tipton, K.D.; Aarsland, A.; Wolf, S.E.; Wolfe, R.R. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am. J. Physiol. Endocrinol. Metab. 1997, 273, E99–E107. [Google Scholar] [CrossRef]
- Jordan, L.Y.; Melanson, E.L.; Melby, C.L.; Hickey, M.S.; Miller, B.F. Nitrogen Balance in Older Individuals in Energy Balance Depends on Timing of Protein Intake. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 1068–1076. [Google Scholar] [CrossRef]
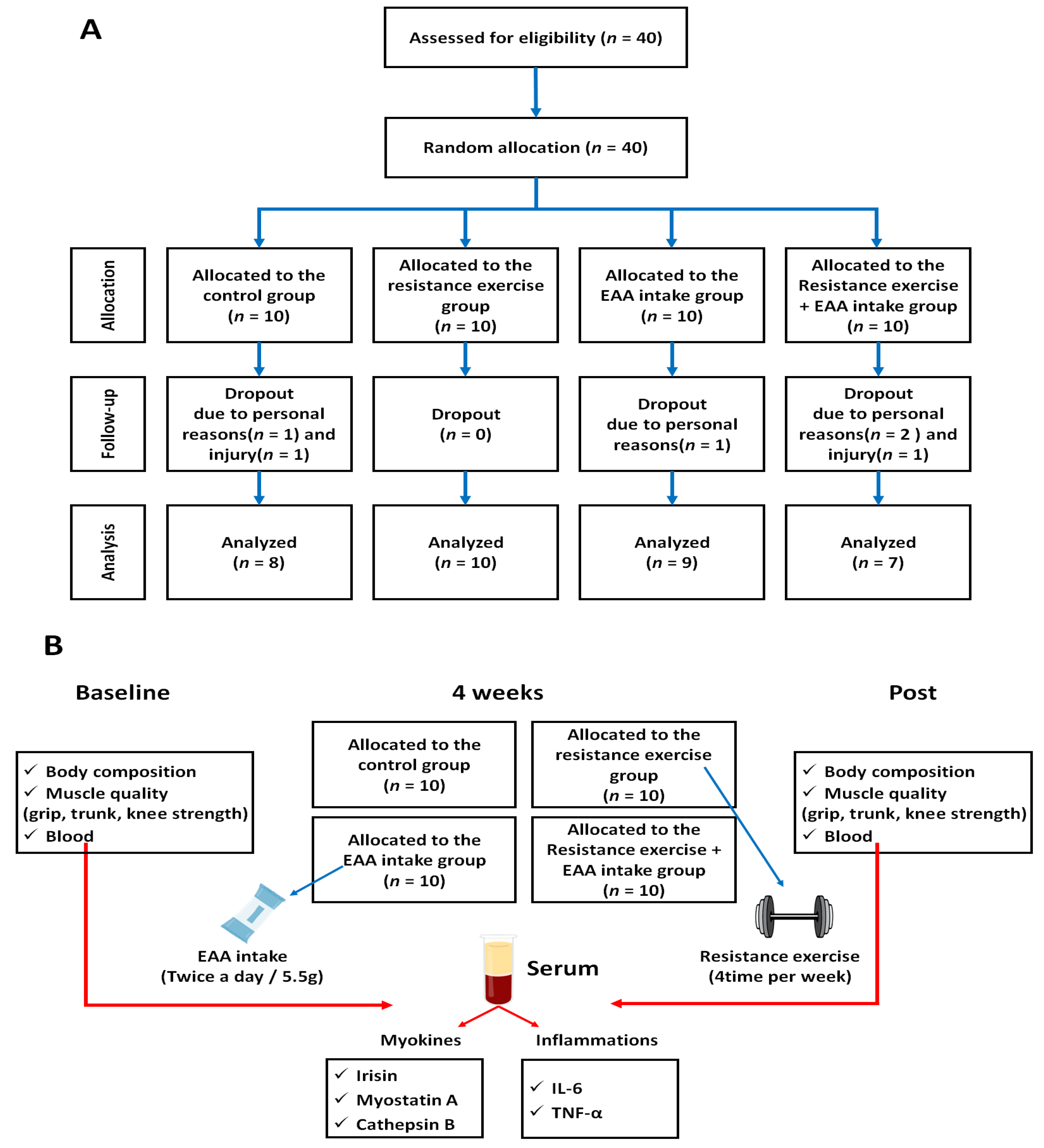
| Treatment | Age | Height (cm) | Weight (kg) | BMI (kg/m2) | Lean Body Fat (kg) | Lean Body Mass (kg) |
|---|---|---|---|---|---|---|
| CO a (n = 8) | 20.63 ± 1.06 | 174.51 ± 4.73 | 65.46 ± 4.49 | 21.54 ± 1.85 | 8.16 ± 2.44 | 32.65 ± 1.85 |
| RE b (n = 10) | 21.80 ± 1.03 | 173.98 ± 4.07 | 71.66 ± 7.04 | 23.69 ± 2.12 | 10.95 ± 2.68 | 34.48 ± 3.46 |
| EAA c (n = 9) | 21.67 ± 2.29 | 176.76 ± 4.01 | 67.20 ± 6.79 | 21.53 ± 2.20 | 11.04 ± 4.46 | 31.90 ± 1.81 |
| RE + EAA d (n = 7) | 22.57 ± 2.37 | 174.09 ± 3.56 | 75.69 ± 4.10 | 25.01 ± 1.49 | 12.41 ± 2.24 | 36.43 ± 1.93 |
| Variable | Total: 5500 mg |
|---|---|
| Sodium | 40 mg |
| Carbohydrate | 1000 mg |
| Fat | 100 mg |
| L-Leucine | 1389 mg |
| L-Valine | 357 mg |
| L-Arginine | 321 mg |
| L-Phenylalanine | 218 mg |
| L-Histidine | 53 mg |
| L-Isoleucine | 347 mg |
| L-Threonine | 304 mg |
| L-Methionine | 107 mg |
| L-Lysine Monohydrochloride | 562 mg |
| L-Tryptophan | 2 mg |
| Flavor | 700 mg |
| Composition | Exercise Program | Set | Time (min) | Intensity and Volume | |||
|---|---|---|---|---|---|---|---|
| Warm Up | Dynamic Stretching | 1 | 10 | ||||
| Resistance exercise | Mon, Thu | Part | Tue, Fri | Part | 3 | 50 | 1~2 weeks 1RM/ 60~70% 3~4 weeks 1RM/ 70~80% 300~350 kcl 4 times Per week |
| Bench Press | Chest | Squat | Leg | ||||
| Leg Extension | |||||||
| Dumbbell Press | |||||||
| Leg Curl | |||||||
| Chest Dips | |||||||
| Leg Press | |||||||
| Cable Crossover Dead Lift | Back | ||||||
| Calf Raise | |||||||
| Lat Pull-down | |||||||
| Military Press | Shoulder | ||||||
| Seated Row | |||||||
| Side Lateral raise | |||||||
| Assisted Pull-up | |||||||
| Biceps Curl | Arm | ||||||
| Back Extension | Abdominals | ||||||
| Lying triceps Extension | |||||||
| Crunch | |||||||
| Crunch | Abdominals | ||||||
| Cool down | Dynamic stretching | 1 | 10 | ||||
| Variable | Treatment | Baseline | 4 Weeks | Group | F-Value (p-Value) |
|---|---|---|---|---|---|
| Height (cm) | CO a | 174.51 ± 4.73 | 174.51 ± 4.62 | Baseline - 4 weeks - | G: 0.806 (p = 0.500) T: 1.14 (p = 0.294) G × T: 1.015 (p = 0.400) |
| RE b | 173.98 ± 4.07 | 174.17 ± 4.11 | |||
| EAA c | 176.76 ± 4.01 | 176.67 ± 3.96 | |||
| RE + EAA d | 174.09 ± 3.56 | 174.30 ± 3.71 | |||
| Weight (kg) | CO a | 65.46 ± 4.49 | 65.51 ± 5.01 | Baseline d > a, c c > a 4 weeks d > a, c b > a | G: 4.625 (p = 0.009) T: 3.272 (p = 0.081) G × T: 0.668 (p = 0.578) |
| RE b | 71.66 ± 7.04 | 71.04 ± 6.25 | |||
| EAA c | 67.20 ± 6.79 | 66.93 ± 6.10 | |||
| RE + EAA d | 75.69 ± 4.10 | 74.86 ± 3.77 | |||
| BMI (kg/m2) | CO a | 21.54 ± 1.85 | 21.55 ± 1.85 | Baseline d, c > a, b 4 weeks d, c > a, b | G: 5.976 (p = 0.003) T: 4.939 (p = 0.034) G × T: 1.146 (p = 0.347) |
| RE b | 23.69 ± 2.12 | 23.41 ± 1.77 | |||
| EAAs c | 21.53 ± 2.20 | 21.46 ± 2.04 | |||
| RE + EAA d | 25.01 ± 1.49 | 24.64 ± 1.28 | |||
| Lean body fat (kg) | CO a | 8.16 ± 2.44 | 8.56 ± 2.83 | Baseline - 4 weeks - | G: 1.925 (p = 0.147) T: 7.893 (p = 0.009) G × T: 3.585 (p = 0.025) |
| RE b | 10.95 ± 2.68 | 10.48 ± 2.34 * | |||
| EAA c | 11.04 ± 4.46 | 10.33 ± 3.88 | |||
| RE + EAA d | 12.41 ± 2.24 | 11.60 ± 2.58 * | |||
| Lean body mass (kg) | CO a | 32.65 ± 1.85 | 32.44 ± 2.08 | Baseline d > a 4 weeks d > b > a, c | G: 7.006 (p = 0.001) T: 6.136 (p = 0.019) G × T: 4.513 (p = 0.010) |
| RE b | 34.48 ± 3.46 | 34.53 ± 3.17 | |||
| EAA c | 31.90 ± 1.81 | 32.21 ± 1.63 | |||
| RE + EAA d | 36.43 ± 1.93 | 37.49 ± 1.38 * |
| Variable | Treatment | Baseline | 4 Weeks | Group | F-Value (p-Value) |
|---|---|---|---|---|---|
| Grip strength (L) (kg) | CO a | 41.63 ± 3.14 | 43.69 ± 4.43 | Baseline d > a, c 4 weeks d > a, c | G: 3.550 (p = 0.028) T: 4.457 (p = 0.044) G × T: 0.203 (p = 0.984) |
| RE b | 44.49 ± 4.51 | 45.70 ± 5.57 | |||
| EAA c | 41.50 ±6.83 | 42.36 ± 3.03 | |||
| RE + EAA d | 47.77 ± 3.03 | 48.71 ± 3.02 * | |||
| Grip strength (R) (kg) | CO a | 44.51 ± 2.35 | 45.75 ± 3.13 | Baseline - 4 weeks - | G: 1.776 (p = 0.174) T: 10.748 (p = 0.003) G × T: 0.470 (p = 0.706) |
| RE b | 46.37 ± 2.58 | 49.30 ± 4.38 * | |||
| EAA c | 45.44 ± 4.52 | 48.00 ± 5.13 | |||
| RE + EAA d | 48.97 ± 6.39 | 50.40 ± 5.39 | |||
| Trunk extension peak torque [(Newton-meter/body weight (kg)] | CO a | 321.25 ± 51.09 | 315.00 ± 35.44 | Baseline b > a, b, c 4 weeks a, b, d > c | G: 3.241 (p = 0.036) T: 0.602 (p = 0.444) G × T: 0.868 (p = 0.468) |
| RE b | 329.20 ± 58.64 | 343.00 ± 30.16 | |||
| EAA c | 287.11 ± 39.72 | 280.67 ± 41.99 | |||
| RE + EAA d | 300.14 ± 26.84 | 323.00 ± 48.30 | |||
| Trunk flexion peak torque [(Newton-meter/body weight (kg)] | CO a | 289.25 ± 16.11 | 288.63 ± 27.94 | Baseline - 4 weeks - | G: 1.161 (p = 0.341) T: 4.174 (p = 0.050) G × T: 2.962 (p = 0.048) |
| RE b | 286.20 ± 25.18 | 294.90 ± 24.62 * | |||
| EAA c | 276.56 ± 19.01 | 273.33 ± 12.63 | |||
| RE + EAA d | 279.71 ± 15.03 | 293.00 ± 17.45 * | |||
| Knee extension peak torque (R) [Newton-meter/body weight (kg)] | CO a | 280.87 ± 50.43 | 275.25 ± 55.91 | Baseline - 4 weeks - | G: 2.320 (p = 0.095) T: 0.882 (p = 0.355) G × T: 1.741 (p = 0.180) |
| RE b | 306.70 ± 44.26 | 309.40 ± 45.17 | |||
| EAA c | 261.44 ± 43.68 | 259.78 ± 22.74 | |||
| RE + EAA d | 287.29 ± 35.53 | 306.43 ± 37.06 | |||
| Knee extension peak torque (L) [Newton-meter/body weight (kg)] | CO a | 271.13 ± 46.51 | 268.12 ± 48.46 | Baseline - 4 weeks - | G: 1.700 (p = 0.188) T: 0.149 (p = 0.702) G × T: 1.419 (p = 0.257) |
| RE b | 290.30 ± 40.71 | 308.00 ± 38.62 | |||
| EAA c | 269.11 ± 47.98 | 257.89 ± 42.07 | |||
| RE + EAA d | 293.14 ± 39.99 | 298.14 ± 47.65 | |||
| Knee flexion peak torque (R) [(Newton-meter/body weight (kg)] | CO a | 140.75 ± 19.29 | 149.75 ± 22.89 | Baseline - 4 weeks - | G: 2.627 (p = 0.068) T: 2.867 (p = 0.101) G × T: 0.686 (p = 0.568) |
| RE b | 158.90 ± 10.21 | 163.70 ± 11.38 | |||
| EAA c | 143.00 ± 18.13 | 141.78 ± 18.89 | |||
| RE + EAA d | 147.29 ± 19.81 | 152.14 ± 19.00 | |||
| Knee flexion peak torque (L) [(Newton-meter/body weight (kg)] | CO a | 139.37 ± 27.24 | 139.75 ± 27.57 | Baseline - 4 weeks - | G: 1.714 (p = 0.185) T: 4.618 (p = 0.040) G × T: 0.614 (p = 0.611) |
| RE b | 151.10 ± 24.25 | 158.00 ± 20.98 | |||
| EAA c | 134.44 ± 18.48 | 139.00 ± 12.77 | |||
| RE + EAA d | 146.14 ± 17.99 | 156.71 ± 15.59 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, D.; Park, K.; Lee, J.; Choi, J.; Du, H.; Jeong, H.; Li, L.; Sakai, K.; Kang, S. Effects of Resistance Exercise and Essential Amino Acid Intake on Muscle Quality, Myokine, and Inflammation Factors in Young Adult Males. Nutrients 2024, 16, 1688. https://doi.org/10.3390/nu16111688
Jeong D, Park K, Lee J, Choi J, Du H, Jeong H, Li L, Sakai K, Kang S. Effects of Resistance Exercise and Essential Amino Acid Intake on Muscle Quality, Myokine, and Inflammation Factors in Young Adult Males. Nutrients. 2024; 16(11):1688. https://doi.org/10.3390/nu16111688
Chicago/Turabian StyleJeong, Deokhwa, Kyumin Park, Jinseok Lee, Jiye Choi, Haifeng Du, Hyeongmo Jeong, Liangliang Li, Kenji Sakai, and Sunghwun Kang. 2024. "Effects of Resistance Exercise and Essential Amino Acid Intake on Muscle Quality, Myokine, and Inflammation Factors in Young Adult Males" Nutrients 16, no. 11: 1688. https://doi.org/10.3390/nu16111688
APA StyleJeong, D., Park, K., Lee, J., Choi, J., Du, H., Jeong, H., Li, L., Sakai, K., & Kang, S. (2024). Effects of Resistance Exercise and Essential Amino Acid Intake on Muscle Quality, Myokine, and Inflammation Factors in Young Adult Males. Nutrients, 16(11), 1688. https://doi.org/10.3390/nu16111688






