Fish Oil Supplementation with Resistance Exercise Training Enhances Physical Function and Cardiometabolic Health in Postmenopausal Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Experimental Design
2.2. Resistance Exercise Training
2.3. Fish Oil Supplement
2.4. Physical Function Assessment
2.5. Blood Pressure Assessment
2.6. Blood Collection and Analysis of Systemic Biomarkers
2.7. Statistical Analysis
3. Results
3.1. Physical Function and Blood Pressure
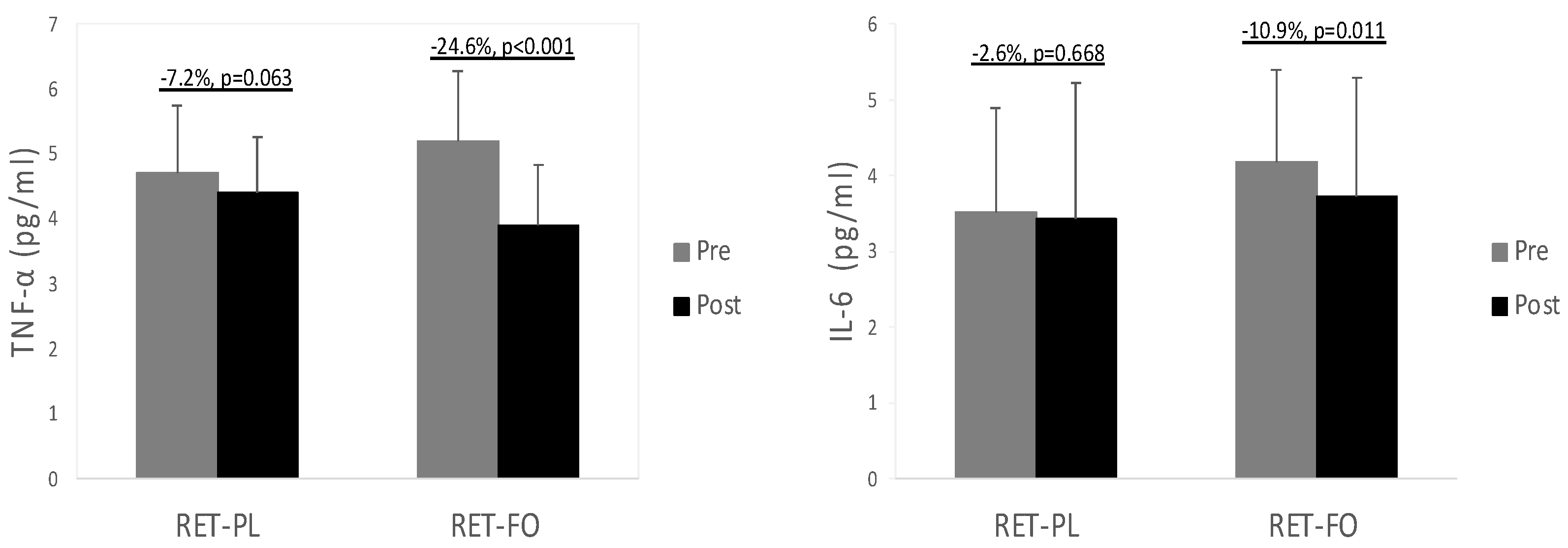
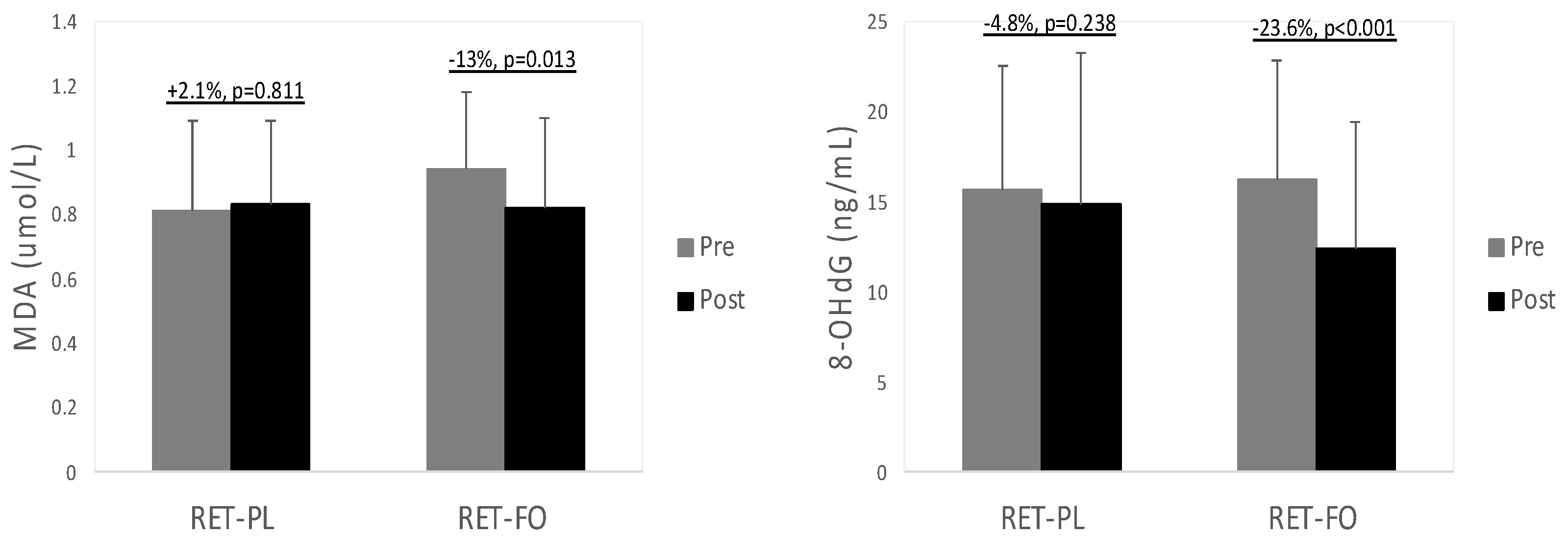
3.2. Biochemical Data
4. Discussion
4.1. Physical Function
4.2. Blood Pressure
4.3. Biochemical Biomarkers Associated with Cardiometabolic Health
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giampaoli, S.; Ferrucci, L.; Cecchi, F.; Lo Noce, C.; Poce, A.; Dima, F.; Santaquilani, A.; Vescio, M.F.; Menotti, A. Hand-grip strength predicts incident disability in non-disabled older men. Age Ageing 1999, 28, 283–288. [Google Scholar] [CrossRef]
- Rantanen, T.; Guralnik, J.M.; Foley, D.; Masaki, K.; Leveille, S.; Curb, J.D.; White, L. Midlife hand grip strength as a predictor of old age disability. JAMA 1999, 281, 558–560. [Google Scholar] [CrossRef]
- Lobo, R.A.; Davis, S.R.; De Villiers, T.J.; Gompel, A.; Henderson, V.W.; Hodis, H.N.; Lumsden, M.A.; Mack, W.J.; Shapiro, S.; Baber, R.J. Prevention of diseases after menopause. Climacteric 2014, 17, 540–556. [Google Scholar] [CrossRef] [PubMed]
- Mesalic, L.; Tupkovic, E.; Kendic, S.; Balic, D. Correlation between hormonal and lipid status in women in menopause. Bosn. J. Basic Med. Sci. 2008, 8, 188–192. [Google Scholar] [CrossRef]
- Bian, A.L.; Hu, H.Y.; Rong, Y.D.; Wang, J.; Wang, J.X.; Zhou, X.Z. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-alpha. Eur. J. Med. Res. 2017, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Roubenoff, R.; Parise, H.; Payette, H.A.; Abad, L.W.; D’Agostino, R.; Jacques, P.F.; Wilson, P.W.; Dinarello, C.A.; Harris, T.B. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: The Framingham Heart Study. Am. J. Med. 2003, 115, 429–435. [Google Scholar] [CrossRef]
- Vasilaki, A.; Jackson, M.J. Role of reactive oxygen species in the defective regeneration seen in aging muscle. Free Radic. Biol. Med. 2013, 65, 317–323. [Google Scholar] [CrossRef]
- Swiatkiewicz, I.; Wroblewski, M.; Nuszkiewicz, J.; Sutkowy, P.; Wroblewska, J.; Wozniak, A. The Role of Oxidative Stress Enhanced by Adiposity in Cardiometabolic Diseases. Int. J. Mol. Sci. 2023, 24, 6382. [Google Scholar] [CrossRef]
- Moylan, J.S.; Reid, M.B. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve 2007, 35, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.N.; Heffron, S.P.; Patel, P.N.; Ferguson, J.; Shah, R.D.; Hinkle, C.C.; Krishnamoorthy, P.; Shah, R.; Tabita-Martinez, J.; Terembula, K.; et al. A human model of inflammatory cardio-metabolic dysfunction; a double blind placebo-controlled crossover trial. J. Transl. Med. 2012, 10, 124. [Google Scholar] [CrossRef]
- Mehta, N.N.; McGillicuddy, F.C.; Anderson, P.D.; Hinkle, C.C.; Shah, R.; Pruscino, L.; Tabita-Martinez, J.; Sellers, K.F.; Rickels, M.R.; Reilly, M.P. Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes 2010, 59, 172–181. [Google Scholar] [CrossRef]
- Argiles, J.M.; Busquets, S.; Felipe, A.; Lopez-Soriano, F.J. Molecular mechanisms involved in muscle wasting in cancer and ageing: Cachexia versus sarcopenia. Int. J. Biochem. Cell Biol. 2005, 37, 1084–1104. [Google Scholar] [CrossRef] [PubMed]
- Baracos, V.E. Cancer-associated cachexia and underlying biological mechanisms. Annu. Rev. Nutr. 2006, 26, 435–461. [Google Scholar] [CrossRef]
- Gullett, N.; Rossi, P.; Kucuk, O.; Johnstone, P.A. Cancer-induced cachexia: A guide for the oncologist. J. Soc. Integr. Oncol. 2009, 7, 155–169. [Google Scholar]
- Kim, J.S.; Kosek, D.J.; Petrella, J.K.; Cross, J.M.; Bamman, M.M. Resting and load-induced levels of myogenic gene transcripts differ between older adults with demonstrable sarcopenia and young men and women. J. Appl. Physiol. 2005, 99, 2149–2158. [Google Scholar] [CrossRef]
- Parkington, J.D.; LeBrasseur, N.K.; Siebert, A.P.; Fielding, R.A. Contraction-mediated mTOR, p70S6k, and ERK1/2 phosphorylation in aged skeletal muscle. J. Appl. Physiol. 2004, 97, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Stitt, T.N.; Drujan, D.; Clarke, B.A.; Panaro, F.; Timofeyva, Y.; Kline, W.O.; Gonzalez, M.; Yancopoulos, G.D.; Glass, D.J. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 2004, 14, 395–403. [Google Scholar] [CrossRef]
- Ogawa, K.; Sanada, K.; Machida, S.; Okutsu, M.; Suzuki, K. Resistance exercise training-induced muscle hypertrophy was associated with reduction of inflammatory markers in elderly women. Mediators Inflamm. 2010, 2010, 171023. [Google Scholar] [CrossRef] [PubMed]
- Greiwe, J.S.; Cheng, B.; Rubin, D.C.; Yarasheski, K.E.; Semenkovich, C.F. Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. FASEB J. 2001, 15, 475–482. [Google Scholar] [CrossRef]
- Tucker, L.A.; Silvester, L.J. Strength training and hypercholesterolemia: An epidemiologic study of 8499 employed men. Am. J. Health Promot. 1996, 11, 35–41. [Google Scholar] [CrossRef]
- Treuth, M.S.; Hunter, G.R.; Kekes-Szabo, T.; Weinsier, R.L.; Goran, M.I.; Berland, L. Reduction in intra-abdominal adipose tissue after strength training in older women. J. Appl. Physiol. 1995, 78, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Vincent, K.R.; Braith, R.W.; Bottiglieri, T.; Vincent, H.K.; Lowenthal, D.T. Homocysteine and lipoprotein levels following resistance training in older adults. Prev. Cardiol. 2003, 6, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Kohl, H.W., 3rd; Gordon, N.F.; Scott, C.B.; Vaandrager, H.; Blair, S.N. Musculoskeletal strength and serum lipid levels in men and women. Med. Sci. Sports Exerc. 1992, 24, 1080–1087. [Google Scholar] [CrossRef]
- Halminski, M.A.; Marsh, J.B.; Harrison, E.H. Differential effects of fish oil, safflower oil and palm oil on fatty acid oxidation and glycerolipid synthesis in rat liver. J. Nutr. 1991, 121, 1554–1561. [Google Scholar] [CrossRef]
- Fetterman, J.W., Jr.; Zdanowicz, M.M. Therapeutic potential of n-3 polyunsaturated fatty acids in disease. Am. J. Health Syst. Pharm. 2009, 66, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, D.S.; Cecchini, R.; El Kadri, M.Z.; Rodriguez, M.A.; Burini, R.C.; Dichi, I. Decreased oxidative stress in patients with ulcerative colitis supplemented with fish oil omega-3 fatty acids. Nutrition 2003, 19, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Julliand, S.; Reeds, D.N.; Sinacore, D.R.; Klein, S.; Mittendorfer, B. Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am. J. Clin. Nutr. 2015, 102, 115–122. [Google Scholar] [CrossRef]
- Roelants, M.; Delecluse, C.; Verschueren, S.M. Whole-body-vibration training increases knee-extension strength and speed of movement in older women. J. Am. Geriatr. Soc. 2004, 52, 901–908. [Google Scholar] [CrossRef]
- Skulas-Ray, A.C.; Wilson, P.W.F.; Harris, W.S.; Brinton, E.A.; Kris-Etherton, P.M.; Richter, C.K.; Jacobson, T.A.; Engler, M.B.; Miller, M.; Robinson, J.G.; et al. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory From the American Heart Association. Circulation 2019, 140, e673–e691. [Google Scholar] [CrossRef]
- Hayashi, N.; Tashiro, T.; Yamamori, H.; Takagi, K.; Morishima, Y.; Otsubo, Y.; Sugiura, T.; Furukawa, K.; Nitta, H.; Nakajima, N.; et al. Effect of intravenous omega-6 and omega-3 fat emulsions on nitrogen retention and protein kinetics in burned rats. Nutrition 1999, 15, 135–139. [Google Scholar] [CrossRef]
- Rousseau, J.H.; Kleppinger, A.; Kenny, A.M. Self-reported dietary intake of omega-3 fatty acids and association with bone and lower extremity function. J. Am. Geriatr. Soc. 2009, 57, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Frison, E.; Boirie, Y.; Peuchant, E.; Tabue-Teguo, M.; Barberger-Gateau, P.; Feart, C. Plasma fatty acid biomarkers are associated with gait speed in community-dwelling older adults: The Three-City-Bordeaux study. Clin. Nutr. 2017, 36, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Abbatecola, A.M.; Cherubini, A.; Guralnik, J.M.; Andres Lacueva, C.; Ruggiero, C.; Maggio, M.; Bandinelli, S.; Paolisso, G.; Ferrucci, L. Plasma polyunsaturated fatty acids and age-related physical performance decline. Rejuvenation Res. 2009, 12, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Reinders, I.; Murphy, R.A.; Song, X.; Visser, M.; Cotch, M.F.; Lang, T.F.; Garcia, M.E.; Launer, L.J.; Siggeirsdottir, K.; Eiriksdottir, G.; et al. Polyunsaturated fatty acids in relation to incident mobility disability and decline in gait speed; the Age, Gene/Environment Susceptibility-Reykjavik Study. Eur. J. Clin. Nutr. 2015, 69, 489–493. [Google Scholar] [CrossRef]
- Reinders, I.; Song, X.; Visser, M.; Eiriksdottir, G.; Gudnason, V.; Sigurdsson, S.; Aspelund, T.; Siggeirsdottir, K.; Brouwer, I.A.; Harris, T.B.; et al. Plasma phospholipid PUFAs are associated with greater muscle and knee extension strength but not with changes in muscle parameters in older adults. J. Nutr. 2015, 145, 105–112. [Google Scholar] [CrossRef]
- Delmonico, M.J.; Ferrell, R.E.; Meerasahib, A.; Martel, G.F.; Roth, S.M.; Kostek, M.C.; Hurley, B.F. Blood pressure response to strength training may be influenced by angiotensinogen A-20C and angiotensin II type I receptor A1166C genotypes in older men and women. J. Am. Geriatr. Soc. 2005, 53, 204–210. [Google Scholar] [CrossRef]
- Martel, G.F.; Hurlbut, D.E.; Lott, M.E.; Lemmer, J.T.; Ivey, F.M.; Roth, S.M.; Rogers, M.A.; Fleg, J.L.; Hurley, B.F. Strength training normalizes resting blood pressure in 65- to 73-year-old men and women with high normal blood pressure. J. Am. Geriatr. Soc. 1999, 47, 1215–1221. [Google Scholar] [CrossRef]
- Wood, R.H.; Reyes, R.; Welsch, M.A.; Favaloro-Sabatier, J.; Sabatier, M.; Matthew Lee, C.; Johnson, L.G.; Hooper, P.F. Concurrent cardiovascular and resistance training in healthy older adults. Med. Sci. Sports Exerc. 2001, 33, 1751–1758. [Google Scholar] [CrossRef]
- Anton, M.M.; Cortez-Cooper, M.Y.; DeVan, A.E.; Neidre, D.B.; Cook, J.N.; Tanaka, H. Resistance training increases basal limb blood flow and vascular conductance in aging humans. J. Appl. Physiol. 2006, 101, 1351–1355. [Google Scholar] [CrossRef]
- Bercea, C.I.; Cottrell, G.S.; Tamagnini, F.; McNeish, A.J. Omega-3 polyunsaturated fatty acids and hypertension: A review of vasodilatory mechanisms of docosahexaenoic acid and eicosapentaenoic acid. Br. J. Pharmacol. 2021, 178, 860–877. [Google Scholar] [CrossRef]
- Das, U.N. Long-chain polyunsaturated fatty acids interact with nitric oxide, superoxide anion, and transforming growth factor-beta to prevent human essential hypertension. Eur. J. Clin. Nutr. 2004, 58, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, H.; Aarhus, L.L.; Vanhoutte, P.M. Dietary omega 3 polyunsaturated fatty acids augment endothelium-dependent relaxation to bradykinin in coronary microvessels of the pig. Br. J. Pharmacol. 1988, 95, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, C.; Schini, V.B.; Hendrickson, H.; Vanhoutte, P.M. Chronic exposure of cultured endothelial cells to eicosapentaenoic acid potentiates the release of endothelium-derived relaxing factor(s). Br. J. Pharmacol. 1990, 99, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Villalpando, D.M.; Navarro, R.; Del Campo, L.; Largo, C.; Munoz, D.; Tabernero, M.; Baeza, R.; Otero, C.; Garcia, H.S.; Ferrer, M. Effect of Dietary Docosahexaenoic Acid Supplementation on the Participation of Vasodilator Factors in Aorta from Orchidectomized Rats. PLoS ONE 2015, 10, e0142039. [Google Scholar] [CrossRef]
- Niazi, Z.R.; Silva, G.C.; Ribeiro, T.P.; Leon-Gonzalez, A.J.; Kassem, M.; Mirajkar, A.; Alvi, A.; Abbas, M.; Zgheel, F.; Schini-Kerth, V.B.; et al. EPA:DHA 6:1 prevents angiotensin II-induced hypertension and endothelial dysfunction in rats: Role of NADPH oxidase- and COX-derived oxidative stress. Hypertens. Res. 2017, 40, 966–975. [Google Scholar] [CrossRef]
- Ormsbee, M.J.; Choi, M.D.; Medlin, J.K.; Geyer, G.H.; Trantham, L.H.; Dubis, G.S.; Hickner, R.C. Regulation of fat metabolism during resistance exercise in sedentary lean and obese men. J. Appl. Physiol. 2009, 106, 1529–1537. [Google Scholar] [CrossRef]
- Fahlman, M.M.; Boardley, D.; Lambert, C.P.; Flynn, M.G. Effects of endurance training and resistance training on plasma lipoprotein profiles in elderly women. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, B54–B60. [Google Scholar] [CrossRef]
- Ng, C.L.; Goh, S.Y.; Malhotra, R.; Ostbye, T.; Tai, E.S. Minimal difference between aerobic and progressive resistance exercise on metabolic profile and fitness in older adults with diabetes mellitus: A randomised trial. J. Physiother. 2010, 56, 163–170. [Google Scholar] [CrossRef]
- Phillips, B.E.; Williams, J.P.; Greenhaff, P.L.; Smith, K.; Atherton, P.J. Physiological adaptations to resistance exercise as a function of age. JCI Insight 2017, 2, e95581. [Google Scholar] [CrossRef]
- Green, C.J.; Pramfalk, C.; Charlton, C.A.; Gunn, P.J.; Cornfield, T.; Pavlides, M.; Karpe, F.; Hodson, L. Hepatic de novo lipogenesis is suppressed and fat oxidation is increased by omega-3 fatty acids at the expense of glucose metabolism. BMJ Open Diabetes Res. Care 2020, 8, e000871. [Google Scholar] [CrossRef]
- Kuszewski, J.C.; Wong, R.H.X.; Wood, L.G.; Howe, P.R.C. Effects of fish oil and curcumin supplementation on cerebrovascular function in older adults: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 625–633. [Google Scholar] [CrossRef]
- Messier, V.; Rabasa-Lhoret, R.; Barbat-Artigas, S.; Elisha, B.; Karelis, A.D.; Aubertin-Leheudre, M. Menopause and sarcopenia: A potential role for sex hormones. Maturitas 2011, 68, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Roubenoff, R. Origins and clinical relevance of sarcopenia. Can. J. Appl. Physiol. 2001, 26, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Conen, D.; Ridker, P.M. Clinical significance of high-sensitivity C-reactive protein in cardiovascular disease. Biomark. Med. 2007, 1, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, A.G.; Burke, G.L.; Owusu, J.A.; Carnethon, M.R.; Vaidya, D.; Barr, R.G.; Jenny, N.S.; Ouyang, P.; Rotter, J.I. Inflammation and the incidence of type 2 diabetes: The Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2010, 33, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Bucova, M.; Bernadic, M.; Buckingham, T. C-reactive protein, cytokines and inflammation in cardiovascular diseases. Bratisl. Lek. Listy 2008, 109, 333–340. [Google Scholar]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Di Minno, A.; Turnu, L.; Porro, B.; Squellerio, I.; Cavalca, V.; Tremoli, E.; Di Minno, M.N. 8-Hydroxy-2-Deoxyguanosine Levels and Cardiovascular Disease: A Systematic Review and Meta-Analysis of the Literature. Antioxid. Redox. Signal 2016, 24, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Radovanovic, S.; Savic-Radojevic, A.; Pljesa-Ercegovac, M.; Djukic, T.; Suvakov, S.; Krotin, M.; Simic, D.V.; Matic, M.; Radojicic, Z.; Pekmezovic, T.; et al. Markers of oxidative damage and antioxidant enzyme activities as predictors of morbidity and mortality in patients with chronic heart failure. J. Card. Fail. 2012, 18, 493–501. [Google Scholar] [CrossRef]
- Romuk, E.; Wojciechowska, C.; Jachec, W.; Zemla-Woszek, A.; Momot, A.; Buczkowska, M.; Rozentryt, P. Malondialdehyde and Uric Acid as Predictors of Adverse Outcome in Patients with Chronic Heart Failure. Oxid. Med. Cell Longev. 2019, 2019, 9246138. [Google Scholar] [CrossRef]
- Eriksson, J.W. Metabolic stress in insulin’s target cells leads to ROS accumulation—A hypothetical common pathway causing insulin resistance. FEBS Lett. 2007, 581, 3734–3742. [Google Scholar] [CrossRef]
- Lamb, R.E.; Goldstein, B.J. Modulating an oxidative-inflammatory cascade: Potential new treatment strategy for improving glucose metabolism, insulin resistance, and vascular function. Int. J. Clin. Pract. 2008, 62, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, H.Y.; Bae, S.; Lim, Y.H.; Hong, Y.C. Diethylhexyl phthalates is associated with insulin resistance via oxidative stress in the elderly: A panel study. PLoS ONE 2013, 8, e71392. [Google Scholar] [CrossRef] [PubMed]
- Gelaleti, R.B.; Damasceno, D.C.; Lima, P.H.; Salvadori, D.M.; Calderon Ide, M.; Peracoli, J.C.; Rudge, M.V. Oxidative DNA damage in diabetic and mild gestational hyperglycemic pregnant women. Diabetol. Metab. Syndr. 2015, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Hevner, K.; Abetew, D.; Enquobahrie, D.A.; Williams, M.A. Oxidative DNA damage in early pregnancy and risk of gestational diabetes mellitus: A pilot study. Clin. Biochem. 2011, 44, 804–808. [Google Scholar] [CrossRef]
- Gammone, M.A.; Riccioni, G.; Parrinello, G.; D’Orazio, N. Omega-3 Polyunsaturated Fatty Acids: Benefits and Endpoints in Sport. Nutrients 2018, 11, 46. [Google Scholar] [CrossRef]
- Ghorbanihaghjo, A.; Safa, J.; Alizadeh, S.; Argani, H.; Rashtchizadeh, N.; Taghinia, M.V.; Abbasi, M.M. Protective effect of fish oil supplementation on DNA damage induced by cigarette smoking. J. Health Popul. Nutr. 2013, 31, 343–349. [Google Scholar] [CrossRef]
- Buonocore, D.; Verri, M.; Giolitto, A.; Doria, E.; Ghitti, M.; Dossena, M. Effect of 8-week n-3 fatty-acid supplementation on oxidative stress and inflammation in middle- and long-distance running athletes: A pilot study. J. Int. Soc. Sports Nutr. 2020, 17, 55. [Google Scholar] [CrossRef]
| RET-PL | RET-FO | |
|---|---|---|
| Age (years) | 65.4 ± 2.3 | 65.9 ± 4.3 |
| Height (cm) | 162.4 ± 4.9 | 166.7 ± 5.3 |
| Weight (kg) | 64.2 ± 5.6 | 66.3 ± 4.2 |
| Body mass index (kg/m2) | 24.4 ± 3.0 | 23.9 ± 1.5 |
| RET-PL | RET-FO | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Handgrip strength (kg) | 20.4 ± 4.0 | 20.8 ± 4.3 | 20.9 ± 3.9 | 22.1 ± 4.0 * |
| 5X-STS (second) | 7.4 ± 0.9 | 6.8 ± 0.8 * | 7.3 ± 0.7 | 6.6 ± 0.7 * |
| TUG (second) | 6.3 ± 0.4 | 5.8 ± 0.5 * | 6.2 ± 0.5 | 5.5 ± 0.3 * |
| 6MW (second) | 3.8 ± 0.3 | 3.5 ± 0.4 * | 3.6 ± 0.3 | 3.2 ± 0.3 * |
| 30S-STS (repetition) | 19.1 ± 1.7 | 21.2 ± 1.8 * | 19.4 ± 1.8 | 21.8 ± 1.4 * |
| SBP (mm Hg) | 124.9 ± 4.9 | 123.7 ± 6.8 | 123.8 ± 5.1 | 118.3 ± 8.4 * |
| DBP (mm Hg) | 81.5 ± 2.6 | 82.2 ± 3.2 | 81.0 ± 4.6 | 77.5 ± 4.3 * |
| MAP (mm Hg) | 95.6 ± 3.2 | 96.0 ± 3.8 | 95.3 ± 4.6 | 91.1 ± 5.1 * |
| TG (mg/dL) | 104.7 ± 12.6 | 101.7 ± 12 | 114 ± 22.4 | 99 ± 21.4 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-R.; Directo, D. Fish Oil Supplementation with Resistance Exercise Training Enhances Physical Function and Cardiometabolic Health in Postmenopausal Women. Nutrients 2023, 15, 4516. https://doi.org/10.3390/nu15214516
Lee S-R, Directo D. Fish Oil Supplementation with Resistance Exercise Training Enhances Physical Function and Cardiometabolic Health in Postmenopausal Women. Nutrients. 2023; 15(21):4516. https://doi.org/10.3390/nu15214516
Chicago/Turabian StyleLee, Sang-Rok, and Dean Directo. 2023. "Fish Oil Supplementation with Resistance Exercise Training Enhances Physical Function and Cardiometabolic Health in Postmenopausal Women" Nutrients 15, no. 21: 4516. https://doi.org/10.3390/nu15214516
APA StyleLee, S.-R., & Directo, D. (2023). Fish Oil Supplementation with Resistance Exercise Training Enhances Physical Function and Cardiometabolic Health in Postmenopausal Women. Nutrients, 15(21), 4516. https://doi.org/10.3390/nu15214516






