Abstract
Vitamin D is a crucial micronutrient, critical to human health, and influences many physiological processes. Oral and skin-derived vitamin D is hydroxylated to form calcifediol (25(OH)D) in the liver, then to 1,25(OH)2D (calcitriol) in the kidney. Alongside the parathyroid hormone, calcitriol regulates neuro-musculoskeletal activities by tightly controlling blood-ionized calcium concentrations through intestinal calcium absorption, renal tubular reabsorption, and skeletal mineralization. Beyond its classical roles, evidence underscores the impact of vitamin D on the prevention and reduction of the severity of diverse conditions such as cardiovascular and metabolic diseases, autoimmune disorders, infection, and cancer. Peripheral target cells, like immune cells, obtain vitamin D and 25(OH)D through concentration-dependent diffusion from the circulation. Calcitriol is synthesized intracellularly in these cells from these precursors, which is crucial for their protective physiological actions. Its deficiency exacerbates inflammation, oxidative stress, and increased susceptibility to metabolic disorders and infections; deficiency also causes premature deaths. Thus, maintaining optimal serum levels above 40 ng/mL is vital for health and disease prevention. However, achieving it requires several times more than the government’s recommended vitamin D doses. Despite extensive published research, recommended daily intake and therapeutic serum 25(OH)D concentrations have lagged and are outdated, preventing people from benefiting. Evidence suggests that maintaining the 25(OH)D concentrations above 40 ng/mL with a range of 40–80 ng/mL in the population is optimal for disease prevention and reducing morbidities and mortality without adverse effects. The recommendation for individuals is to maintain serum 25(OH)D concentrations above 50 ng/mL (125 nmol/L) for optimal clinical outcomes. Insights from metabolomics, transcriptomics, and epigenetics offer promise for better clinical outcomes from vitamin D sufficiency. Given its broader positive impact on human health with minimal cost and little adverse effects, proactively integrating vitamin D assessment and supplementation into clinical practice promises significant benefits, including reduced healthcare costs. This review synthesized recent novel findings related to the physiology of vitamin D that have significant implications for disease prevention.
- Definitions of vitamin D that are used in this review:
- (a).
- Hypovitaminosis D = Insufficient 25(OH)D levels
Hypovitaminosis D, also known as vitamin D insufficiency (an ambiguous term), occurs when there are inadequate (sub-optimal) levels of vitamin D in the circulation to support its intended physiological and biological functions—defined as serum 25-hydroxyvitamin D (25(OH)D) concentration of less than 40 ng/mL. Because hypovitaminosis D exacerbates common disorders and increases vulnerability to other disorders, such as infections, it cannot be considered within the physiological range.
- (b).
- Severe vitamin D deficiency
Vitamin D deficiency is defined as having significantly low 25(OH)D in the circulation—a concentration of less than 12 ng/mL. Persons will present with signs and symptoms of vitamin D deficiency, mainly from the neuromuscular and skeletal systems. It significantly worsens most diseases—and increases complications and deaths from cardiovascular disorders, cancer, infections, and septicemia.
- (c).
- Vitamin D sufficiency:
Achieving vitamin D sufficiency is crucial for overall health and robust bodily functions. A serum 25(OH)D concentration above 50 ng/mL is necessary to overcome prevalent common disorders and diseases—the physiological range is between 40 and 80 ng/mL. From the public health point of view, the minimum serum 25(OH)D concentration for the population (or community vitamin D sufficiency) is recognized as 40 ng/mL.
1. Introduction
Most aspects of human health and well-being and its multifaceted role in disease prevention and overcoming infections depend on vitamin D’s biological and physiological functions [1]. Vitamin D is a unique nutrient that is supposed to be obtained through sunlight exposure, dietary sources, and supplements. It has become an essential component of public health. Both the synthesized and ingested vitamin D undergo metabolic processes in the body, ultimately generating its most active form, calcitriol (1,25(OH)2D) [2]. It regulates the body’s calcium and phosphorus requirements.
In addition to its well-understood functions in bone health, emerging research suggests that vitamin D has established broader implications for overall health and disease prevention. Vitamin D/calcitriol receptors (VDR = CTR) are present in most tissues and cells in the body, indicating its numerous physiological processes outside bone metabolism. The knowledge related to an area that has significantly advanced over the past decade is the immune system [1]. Vitamin D metabolites profoundly modulate immune function to facilitate defense against infections [3].
Vitamin D deficiency increases the susceptibility to certain infections, especially tuberculosis [4] and respiratory tract infections [5]. Maintaining sufficient levels of 25(OH)D leads to reduced risks of infectious diseases and downregulating inflammatory responses [6,7]. Vitamin D has also been implicated in preventing chronic diseases such as cardiovascular disease [8], diabetes [9], cancer [10], and autoimmune disorders [3,11,12].
Research has also shown a strong association between vitamin D deficiency and an increased risk of developing the conditions mentioned above. However, the mechanism varies between tissues/disease entities [13,14]. Overall, understanding the physiology of vitamin D and its implications for disease prevention encompasses its diverse roles in bone health, immune function, and various physiological processes throughout the body [6,15]. Ensuring adequate vitamin D status through sunlight exposure, dietary intake, and supplementation contributes to overall health and well-being.
1.1. The Rationale for This Study
In recent years, many publications have focused on vitamin D, primarily its extra-skeletal benefits. Given the broadness of the topic, the author searched several research databases using keywords related to vitamin D, physiology, biology, mechanisms, disease prevention, and vulnerability. Combining keywords narrowed the number of relevant manuscripts to a manageable quantity. The search encompassed databases such as PubMed, Medline, Web of Science, and EMBASE, focusing on clinical studies, randomized controlled clinical trials (RCTs), prospective clinical studies, and original and review articles. The author followed a similar methodology of systematic and narrative reviews [16], meticulously selecting pertinent references and justifying their inclusion based on their relevance to the topic. These were incorporated into the manuscript after a thorough review. The review aims to present recent findings related to the extracellular functions of vitamin D concerning the proactive use of vitamin D supplements for disease prevention.
1.2. Importance of Vitamin D for Human Health
Vitamin D deficiency is a widespread problem that affects individuals of all ages and ethnic backgrounds, yet it remains largely overlooked by global health authorities [17]. The primary vitamin D source for humans is exposure to ultraviolet B (UVB) rays from sunlight [18,19]. However, a sizable portion of the population worldwide lacks adequate exposure, leading to sub-optimal concentrations of 25(OH)D in the circulation (i.e., less than 40 ng/mL). Studies indicate that more than half of the global population has insufficient vitamin D levels, surpassing the prevalence of iron deficiency as the most common micronutrient deficiency [19,20]. This deficiency is particularly prevalent among individuals residing far from the equator, where sunlight is limited, and those living within 500 km of the equator due to sun avoidance behaviors [21,22,23,24].
The consequences of vitamin D deficiency can be profound, impacting various aspects of health and contributing to the development of numerous health conditions [20]. The lack of ultraviolet-B (UVB) rays (i.e., higher latitudes—living far from the equator) and the behavioral issue—avoiding sunlight (within 500 km of the equator due to harsh conditions) are the two most typical causes for global vitamin D deficiency. Addressing this issue requires increased awareness, education, and public health initiatives to promote safe sun exposure, dietary sources of vitamin D, and supplementation where necessary.
1.3. Clinical Signs and Symptoms of Vitamin D Deficiency
Unlike other deficiency statuses, clinical signs and symptoms of vitamin D deficiency do not manifest until the levels fall below 12 ng/mL [6,25]. Such very low circulatory levels of vitamin D and 25(OH)D are below the effective threshold for active transportation of these precursors into renal tubular cells. However, this inefficiency is partly compensated by increased 1α-hydroxylase activity, attempting to produce a more hormonal form of calcitriol [2,26,27]. As seen in the case of rickets in children and osteomalacia in adults, when the generation of calcitriol lags, signs and symptoms of deficiency begin to manifest [28,29,30].
Symptoms include lethargy, difficulty rising from a seated position or the bed, limited ability to raise arms above shoulder level (i.e., shoulder-girdle myopathy), muscle and joint pain, increased falls [25,31], and a tendency to develop hypothermia [32]. Vitamin D deficiency contributes to generalized weakness and fatigue, impacting overall energy levels [25]. Common clinical signs and symptoms of vitamin D deficiency include muscle weakness (and accumulation of osteoid tissues in bone), impaired calcium absorption, bone pain [33,34,35], skeletal abnormalities such as deformities (rickets) in children, weakened bones (osteomalacia, scoliosis) in adults [25,36], and fractures that can be prevented with vitamin D [35]. Additionally, individuals may experience bone pain, especially in the back, hips, and legs [25], and are at an increased risk of fractures due to reduced bone density caused by impaired calcium absorption [35,37].
Studies have also reported a link between low vitamin D levels and symptomatic depression, impaired wound healing [38], thinning of hair and hair loss [39,40], atrophy of type II muscle fibers [41,42], hypocalcemic seizures in children [43], disruptions in the regulation of energy metabolism [44] and the immune system [12], and poor health [37]. It is worth noting that many of these symptoms are nonspecific and may overlap with other health conditions such as rheumatic disorders, fibromyalgia, and hypothyroidism [34]. Additionally, the extraskeletal body systems do not exhibit specific symptomatology; instead, they present with progressively increased risks or worsening of metabolic disorders (such as diabetes and obesity), cardiovascular and other systemic disorders, and heightened vulnerability to cancer and infections [45,46,47,48].
1.4. Current Recommendations and Vitamin D Status
Inadequate exposure to sunlight and sun avoidance behavior are the two predominant factors causing vitamin D deficiency worldwide. Inefficient conversion of 7-dehydrocholesterol into pre-vitamin D and then to vitamin D in the skin can be caused by (i) deficient intake of dietary vitamin D or not taking supplements, (ii) gastrointestinal issues leading to impairment of vitamin D absorption, (iii) increased catabolism of vitamin D following intakes of medications that enhance the activity of some cytochrome P450 enzymes, (iv) insufficient expression or activity of CTR (e.g., due to lack of cofactors) or genetic abnormalities of vitamin D receptors, (v) activation failure of vitamin D and its metabolites as in liver cell or renal tubular cell impairment, and (vi) rare genetic disorders.
Vitamin D deficiency has emerged as a global pandemic affecting individuals across all age groups and ethnic backgrounds, yet it remains largely overlooked by leading global health agencies. The human body relies on UVB rays from sunlight to synthesize vitamin D. However, insufficient exposure has resulted in more than half of the population having insufficient levels of 25(OH)D [49,50]. The consequences of vitamin D deficiency can have significant health implications, underscoring the importance of addressing this issue through increased awareness, education, and public health initiatives. Encouraging safe sun exposure practices, promoting dietary sources of vitamin D, and considering supplementation where necessary are crucial steps in combating this pervasive health concern [19].
Based on published data, the author estimated a global prevalence of vitamin D deficiency affecting approximately 4.9 billion people sometime during the year [51,52]. Unsurprisingly, older studies using an outdated definition of 20 ng/mL as vitamin D deficiency by the Institution of Medicine (IoM) [53] estimated the prevalence as over one billion [54,55]. The signs and symptoms associated with vitamin D deficiency can be alleviated by ensuring the appropriate dose of vitamin D is taken regularly, at the right frequency. For musculoskeletal diseases, improvements are observed when serum 25(OH)D concentrations reach around 20 ng/mL [56], which was the basis for the IoM report [53]. However, metabolic disorders require higher circulatory levels exceeding 40 ng/mL [57,58]. Examples include infections, autoimmunity [15,59], and cancers [60,61,62], which may require even levels above 50 ng/mL [6,15,63,64].
1.5. Vitamin D Dose Recommendations
Little vitamin D is present in natural food; thus, the dietary intake is minimal and cannot be relied upon for the majority [65,66]. In the absence of regular exposure to daily direct sunlight, casual exposure to the sun is inadequate for raising and sustaining the concentration of serum 25(OH)D [67,68]. Most governments and their appointed committees like the Scientific Advisory Committee (SCAN) in the UK, IoM, Food and Nutrition Board (FNNB), and USPTO in the USA, etc., continue to recommend doses of vitamin D of between 400 and 1000 IU/day, with 20 ng/mL as the minimum sufficient level. These low doses fail to raise serum 25(OH)D concentration by more than 6 ng/mL after vitamin supplementation [6]. This is grossly insufficient for those with vitamin D deficiency [69]. Therefore, such doses consistently fail to raise serum 25(OH)D concentrations above the minimum therapeutic level recommended above [69].
Raising serum 25(OH)D concentration above 20 ng/mL benefits the musculoskeletal system but not others [53,70]. Governments and their appointed committees’ recommended doses of vitamin D are primarily (of less than 1000 IU/day) aimed to prevent rickets in children and osteomalacia in adults [14,67,71]. However, this does not help any other body systems or disease conditions. Therefore, it is paramount to use adequate doses of vitamin D (preferably body-weight-based doses) [49,72] to increase serum 25(OH)D to the desired concentrations.
For busy healthcare workers, it is difficult to remember the different doses of vitamin D and serum 25(OH)D concentrations needed for various diseases. Therefore, irrespective of age and body weight, when laboratory measurements are affordable and available, it is rational to maintain serum 25(OH)D concentrations above 40 ng/mL [73,74], preferably above 50 ng/mL—a range between 50 and 80 ng/mL [56,69].
Notably, the administered vitamin D vs. dose–response and serum 25(OH)D concentration is not linear [75,76,77]. Clinical research published on extra-skeletal disorders over the past decade is more favorable for having a higher minimum serum 25(OH)D concentration than 30 ng/mL recommended by the American Endocrine Society [78]. The primary rationale is that maintaining higher circulatory serum 25(OH)D concentrations leads to better short-term responses and longer-term clinical outcomes for more diseases [58,75].
Most scientific societies’ recommendations typically advocate maintaining serum 25(OH)D concentrations above 30 ng/mL [15], which is still insufficient [49,56,73,79,80]. This contrasts with the government’s recommendation of 20 ng/mL as adequate [53,81]. In contrast, higher vitamin D intakes, and serum 25(OH)D concentrations are necessary to overcome conditions such as cancer [60,61,62], autoimmune diseases [12,63], and infections to achieve and sustain serum levels above 50 ng/mL (discussed below) [56]. This highlights the potential inadequacy of current recommendations, which may be outdated in light of recent research [15].
A broad search of databases using keywords of vitamin D, clinical studies, and RCTs illustrated that while most studies have reported significant benefits from vitamin D supplementation, approximately 15% of clinical publications have inconsistent or negative results—i.e., lack of beneficial effects. Detailed evaluations revealed that most negative findings often stem from design flaws [49,56]. This is vivid in those studies funded by pharmaceutical companies, where vitamin D was administered as a drug intervention or piggybacked on a pharmaceutical RCT. Notably, most clinical research studies have focused on using vitamin D as a treatment rather than for disease prevention. Vitamin D’s primary benefit is that it is a micronutrient, yet its prophylactic use to prevent diseases was neglected. Therefore, future research should emphasize the preventive aspect of vitamin D supplementation to harness its health-promoting effects fully.
2. Generation of Vitamin D
The portion of calcitriol synthesized and secreted to the blood from the kidneys acts as a hormone that regulates calcium and phosphorus metabolism. It plays a crucial role in muscular skeletal health. It enhances the absorption of calcium and phosphorus from the intestines, promoting deposition in bones and maintaining bone mineralization [82]. Meanwhile, gross vitamin D deficiency leads to conditions like rickets in children and osteomalacia in adults, characterized by weakened and brittle bones [35,37,68].
2.1. Synthesis of Vitamin D
While diet contributes some vitamin D3 (cholecalciferol) and D2 (ergocalciferol), the amount is typically insufficient [83]. Evolutionarily, most human vitamin D requirements are anticipated to be met through skin synthesis. However, as deliberate sun exposure has declined, the necessity for increased supplement intake has become unavoidable [2]. Skin exposure to ultraviolet B spectrum (290–315 nm) (UVB) causes a photolytic conversion of the 7-dehydrocholesterol (7-DHC) to pre-vitamin D3, which then undergoes a thermally induced isomerization to form pre-vitamin D [84] (Figure 1).
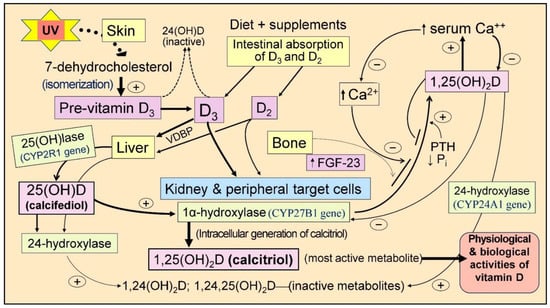
Figure 1.
Illustrates the pathway for the generation of vitamin D3 from 7-dehydrocholesterol (7-DHC) following exposure to UVB rays. The activation of vitamin D to its metabolites, 25(OH)D (calcifediol) and 1,25(OH)2D (calcitriol), is highlighted, including its 24-hydroxy, inactive catabolic metabolites. Critical organs responsible for vitamin D generation/metabolism and the parathyroid hormone (PTH)-mediated regulation of serum ionized calcium (Ca2+) levels are illustrated. The typical activation route for skin-derived and oral/dietary vitamin D forming 1,25(OH)2D is depicted. While 25-hydroxylase activity occurs mainly in the liver, the conversion of vitamin D and 25(OH)D to 1,25(OH)2D via the 1α-hydroxylase enzyme occurs in renal tubules and peripheral target cells of vitamin D. The figure also demonstrates the control of serum Ca2+ levels through intestinal absorption, bone turnover, and PTH-mediated renal handling (+ upregulation and − downregulation) (Fibroblast growth factor-23 = FGF-23; UVB = Ultraviolet B rays; VDBP = Vitamin D binding protein).
Most pre-vitamin D3 is synthesized in the epidermis near the dermal capillary bed. Therefore, the skin surface temperature and its changes are unlikely to impact the formation rate of vitamin D3 in the skin [85]. The efficiency of this process depends on various factors, including skin melanin content, UVB exposure (duration, intensity, time of day, and season), UV-blocking products or clothing use, skin conditions such as scars, and age-related factors [86]. Figure 1 illustrates the sequence of vitamin D metabolite generation.
In individuals with fair skin, approximately 30–60 min of exposure, with about one-third of the upper body exposed to direct sunlight (while protecting the eyes and face from UV/sunlight), between 10:30 A.M. and 1:30 P.M., can yield up to 10,000 IU of vitamin D [87]. It is important to note that the skin has a built-in feedback mechanism preventing excessive vitamin D from sun exposure from entering the bloodstream, thereby avoiding hypervitaminosis D or hypercalcemia [88]. Besides synthesizing vitamin D, sunlight offers other advantages to human health [88]. However, minimal or no vitamin D synthesis occurs during early mornings, late afternoons, winter months, indoor sun exposure through double-glazed glass windows, or when clothing or sunscreen covers the skin [37,71,89,90].
2.2. Vitamin D Supplementation and Its Benefits
Except for sun-exposed mushrooms and fatty fish, food has little vitamin D. Therefore, the optimal way to obtain vitamin D3 is through daily safe exposure to ultraviolet sun rays. When direct sun exposure is not feasible, daily or weekly, supplementation can maintain physiological vitamin D concentrations in the circulation [49,56]. For communities with a high prevalence of vitamin D deficiency, targeted food fortification is a cost-effective way to alleviate it [71]. These approaches can ensure an adequate supply of vitamin D3 to maintain optimal vitamin D levels in the population—mean serum 25(OH)D concentrations above 40 ng/mL [84,91]. In individuals, maintaining levels above 50 ng/mL can bolster immunity, reduce illnesses and absenteeism, and enhance productivity. Moreover, a robust population immunity can help limit the spread of pathogenic microbial infections, including viral epidemics and pandemics such as SARS-CoV-2, consequently reducing hospitalizations and fatalities [92,93].
Large datasets and emerging evidence strongly support the diverse physiological functions of vitamin D mediated by calcitriol. These findings indicate that vitamin D should be utilized as a preventative and adjunct therapy in various common disorders, including sepsis and COVID-19 infection. Despite this, vitamin D is seldom included in clinical protocols or guidelines by leading health authorities or government recommendations to promote public health [20]. Furthermore, recommendations from medical and scientific societies often lack clarity and are contradictory and outdated [6,94].
However, public awareness regarding vitamin D and its beneficial effects on the immune system has increased since the COVID-19 pandemic [95,96,97,98]. This can be attributed partly to the persistent efforts of small groups of scientists despite negative publicity from pharmaceutical companies and health agencies. Notable examples include the clinical guidelines provided by the Front-Line COVID-19 Critical Care Alliance and informative articles on platforms like Substack and websites such as covid19criticalcare.com [99,100].
3. Physiology of Vitamin D
Over the past two decades, numerous non-classical actions of vitamin D beyond the musculoskeletal system have been documented [20,101,102]. However, peripheral target cells, like immune cells, rely on maintaining adequate vitamin D and 25(OH)D levels in circulation via diffusion of the two mentioned precursor molecules. For biological actions to occur, peripheral target cells must synthesize calcitriol intracellularly, as circulating calcitriol levels are relatively low (approximately 900-fold less than 25(OH)D) and thus do not enter these cells [6,56].
In addition, intracellular calcitriol magnesium and other cofactors are utilized during metabolic activities, immune cell activation, and signaling processes [6,103]. However, the government-recommended vitamin D of 400 to 800 IU/day is insufficient to maintain circulatory levels of D3 and 25(OH)D [56]. The entry of vitamin D3 and 25(OH)D from the circulation to peripheral target cells, like immune cells needing levels beyond 50 ng/mL [49,104,105], is necessary to maintain a robust immune system [56,63,106]. No evidence suggests that circulatory calcitriol enters target cells, such as immune cells, as the levels required are about two orders of magnitude lower for diffusion [49,63,107,108,109].
3.1. Non-Classical Actions of Calcitriol
Calcitriol engages in various regulatory and physiological homeostatic activities. Having physiological levels of two precursors of calcitriol ensures the efficient functioning of immune cells through genomic and autocrine intracrine mechanisms [104,105,106,110], thereby reducing the risks of cytokine storms and complications such as acute lung injury leading to ARDS from SARS-CoV-2 infection [111,112,113,114,115]. Individuals with severe vitamin D deficiency are particularly susceptible to such complications.
Vitamin D (a vitamin) and 25(OH)D (a metabolite of vitamin D) are not hormones [2,7]. Meanwhile, 25(OH)D hydroxylation produces calcitriol in renal tubular cells, which enter the circulation; it exhibits hormonal properties in the muscular-skeletal target tissues [15,103]. Both vitamin D and 25(OH)D diffuse from the circulation into other target cells and occur primarily through a concentration-dependent diffusion [108,109]. These target cells contain enzymes, 1α-hydroxylase and 25-hydroxylase, responsible for generating calcitriol and calcitriol (vitamin D) receptors (CTR/VDR) [6].
Vitamin D signaling intracellularly (autocrine/intracrine) is crucial for multiple physiological activities, including stimulating and synthesizing intrinsic defensive compounds against microorganisms such as cathelicidin [116] and defensins [117], which have crucial anti-microbial activities [118]. In addition to directly binding to and destroying pathogens, cathelicidin also acts as a secondary messenger, enhancing vitamin D-mediated reduction in inflammation during infection [119]. Calcitriol also stabilizes tight junctions of epithelial cells of the respiratory tract and vascular system. This protects fluid leakage and viral dissemination into soft tissues [119,120]. Figure 2 illustrates fundamental differences between the hormonal and non-hormonal forms of calcitriol and the related generation of calcitriol.
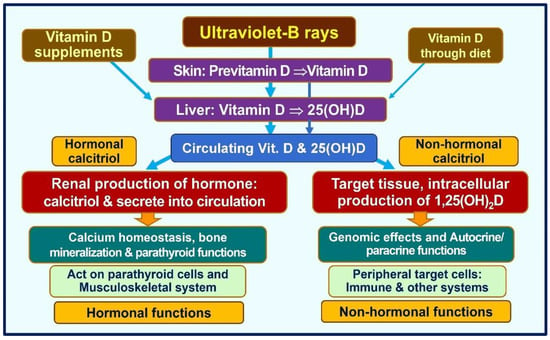
Figure 2.
The essential pathways of acquiring vitamin D in humans are illustrated. The figure demonstrates key functional differences between the circulatory hormonal form of calcitriol (the hormonal form) that is generated in renal tubular cells vs. the intracellularly generated calcitriol in peripheral target cells, such as immune cells (modified from Wimalawansa, SJ., 2023 [7]).
3.2. Activation of Vitamin D/CTR
The steroid receptor CTR is present in nearly all cell types of the human body, especially in immune cells [121]. When calcitriol binds to the CTR in the cytosol, it forms a heterodimer with the retinoid-X receptor. This complex then translocates into the nucleus, binding to DNA [122]. This binding initiates the recruitment of coactivator or corepressor proteins and transcription factors, ultimately regulating gene expression. This process can influence the transcription of numerous genes, estimated to be over 1200 [123].
The intracellular generation of calcitriol in target cells (immune cells, colon cells, breast cells, etc.) is crucial for vitamin D-related physiological functions [124,125]. This process is essential for autocrine and paracrine functions of vitamin D and its DNA interactions—genomic actions [126,127]. These intracellularly generated calcitriol gets metabolized and does not enter the circulation. One exception is the calcitriol synthesized within overactivated macrophages in granulomatous tissues, such as sarcoidosis and granulomatous tuberculosis, which can spill over to circulation [128]. This overflow of calcitriol into the bloodstream could lead to hypercalcemia, although this is uncommon.
3.3. Genetic Influences on Vitamin D/CTR
Genetics plays a role in determining certain aspects of skeletal development and the potential peak bone mass. However, vitamin D, dietary calcium, physical activity, and hormonal status also significantly influence and modulate peak bone mass, bone density, and skeletal mineral content accrual [129,130]. Vitamin D deficiency triggers increased parathyroid hormone (PTH) secretion, leading to secondary hyperparathyroidism; this is for maintaining serum ionized calcium concentration [131].
Elevated PTH levels resulting from low dietary calcium or, more commonly, vitamin D deficiency contribute to heightened bone turnover and gradual loss of bone mineral content [132], increasing fracture risk [133]. In addition, insufficient circulatory calcitriol reduces intestinal calcium absorption, while urinary calcium excretion is increased due to secondary hyperparathyroidism [131,134].
3.4. Vitamin D, 25(OH)D, and 1,25(OH)2D
Serum 25(OH)D concentration reflects both cutaneous production of vitamin D from ultraviolet B exposure and dietary intake from food and supplements. While it stands as the best indicator of vitamin D status, it does not directly reflect the amount of vitamin D stored in the body, which can vary depending on factors like muscle and fat mass. In epidemiological studies and clinical practice, serum 25(OH)D levels have become the primary marker for assessing vitamin D status. With daily administration of D3, measurement of cholecalciferol would be better [135], but its availability is limited. However, there is a risk of misclassifying individuals’ vitamin D status due to differences in half-lives—approximately one day for vitamin D compared to two to three weeks for 25(OH)D [136]. Some researchers advocate measuring both serum vitamin D and 25(OH)D concentrations in epidemiological studies, although this approach significantly increases costs [135].
In contrast, with a few hours of circulatory half-life, circulating serum 1,25(OH)2D concentrations do not reflect vitamin D status or the amount of vitamin D or 25(OH)D stored in the body and thus should not be used as a measure to assess vitamin D status. Serum calcitriol concentrations may be lower in individuals with renal failure, due to the progressive failure of renal cells to convert 25(OH)D to 1,25(OH)2D. Additionally, rare genetic disorders with abnormalities in the CYP2R1 gene, which is involved in vitamin D metabolism, can result in a relative deficiency of the 25-hydroxylase enzyme, leading to low calcitriol levels [137]. These conditions are typically associated with low 25(OH)D concentrations, hypocalcemia, and secondary hyperparathyroidism. Figure 3 illustrates the major organs involved in vitamin D metabolism in conjunction with PTH-mediated serum ionized calcium homeostasis.
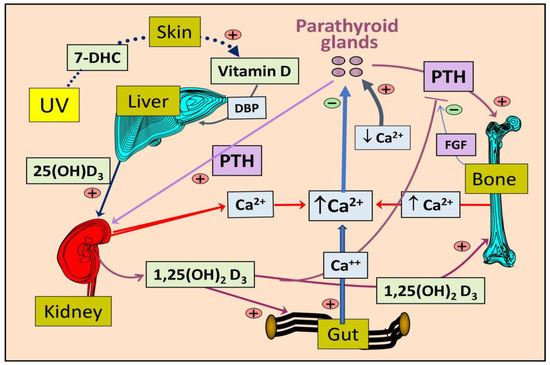
Figure 3.
Illustrates the pathways for the synthesis of vitamin D and its activation into 25(OH)D in the liver and 1,25(OH)2D in the kidneys, as well as the role of parathyroid hormone (PTH) in the maintenance of ionized calcium (Ca++) in the circulation (7-DHC, 7-dehydrocholesterol).
3.5. Intracellular Synthesis of Vitamin D and Binding to CTR
Vitamin D is crucial in maintaining calcium and phosphate homeostasis [138] and skeletal mineralization, essential for overall human health [139,140]. There are two primary isoforms of vitamin D: vitamin D2 and vitamin D3. Vitamin D3, the primary source in humans, is synthesized in the skin upon exposure to ultraviolet B (UVB) rays. Through a series of enzymatic reactions [83], 7-dehydrocholesterol in the skin is converted to pre-vitamin D, which is then isomerized to form vitamin D [141,142].
Fat-soluble vitamin D binds to vitamin D-binding protein (VDBP) before entering the bloodstream via the skin capillary system. Once in the bloodstream, it undergoes 25-hydroxylation in the liver via 25-hydroxylase enzymes (from the CYP2R1 gene) [143] to form 25(OH)D [144], also known as calcifediol. Subsequently, 25(OH)D is further hydroxylated at the 1α-position by the 1α-hydroxylase enzyme (CYP27B1), predominantly within renal tubular cells [145,146], forming the hormonal form of vitamin D 1,25(OH)2D or calcitriol.
However, calcitriol is also synthesized in peripheral target cells, including immune, colon, breast, and prostate [147]. This local production is influenced by various signals from several sources, such as cell-surface Toll-like receptors [148,149]. Depending on the tissue and the signaling received, the genomic interaction of 1,25(OH)2D with its receptor, the calcitriol/vitamin D receptor (CTR), modulates (enhances or suppresses) the transcription of over 1200 essential genes [7,150]. In mammals, hepatic P450 cytochrome enzymes catalyze the formation of calcifediol via 25-hydroxylation and calcitriol via 1α-hydroxylase in microsomes [151] (Figure 1). The CYP24A1 gene expresses a 24-hydroxylase enzyme responsible for inactivating vitamin D and its metabolites [152].
Inclusion of other micronutrients, such as magnesium, zinc, and selenium, vitamins K2, A, and C, resveratrol, and, combined with essential fatty acids, such as omega-3, would enable the maintenance of a robust immune system [153,154,155,156] and health; these are crucial to overcoming infections [56]. Maintenance of the circulatory 25(OH)D concentrations above 50 ng/mL facilitates the sufficient generation of calcitriol intracellularly in immune cells (and other peripheral target cells) that enhance the expression of a series of anti-microbial peptides and neutralizing antibodies and signaling molecules [56]. These steps require proper concentrations of intracellular calcitriol significantly higher than in circulation. Calcitriol synthesis requires the availability of its precursors, vitamin D and/or 25(OH)D, within target cells [49]. This initiates autocrine/intracrine and paracrine signaling, in addition to multiple genomic mechanisms, enhancing the expression of anti-inflammatory cytokines from lymphocytes and macrophages and suppressing inflammatory cytokines, enabling the subduing of inflammation and oxidative stress, and preventing cytokine storms [97,157].
3.6. Binding of Calcitriol to Its Receptors (CTR)
The binding of calcitriol to the CTR triggers the activation of heterodimerization between the receptor and the retinoid X receptor (RXR) [145,158]. This heterodimer complex then translocates into the nucleus, binds to vitamin D response elements, and initiates modulation and transcription of genes. In addition to this classical genomic pathway, calcitriol modulates second messenger systems. It affects the host’s and neighboring cells’ biological functions through non-genomic pathways, such as membrane effects and autocrine/intracrine and paracrine signaling. Furthermore, calcitriol influences growth factors, cytokines, and the renin–angiotensin axis [159,160].
The functional calcitriol–CTR–RXR complex binds to the vitamin D response element in the promoter region of its target genes [145,158]. Subsequent downstream actions recruit transcription factors, coactivators, or corepressors, thereby regulating mRNA expression from target genes and modulating their functions. These functions include calcium and phosphate metabolism, neurotransmission, immunoregulation [1], and hormone secretion in target endocrine cells. For instance, this pathway is responsible for the slower genomic effects of increasing phase 2 insulin secretion in glucose-stimulated insulin secretory responses [161], thereby linking hypovitaminosis D to relative insulin deficiency. This example underscores the intricate mechanisms associated with the actions of vitamin D.
3.7. Vitamin D Is a Crucial Regulator of Calcium Homeostasis
Serum 25(OH)D measurement is crucial for determining an individual’s vitamin D status. Circulating 25(OH)D serves as the substrate to produce the hormonal form of calcitriol in renal tubules and calcitriol in extra-renal target tissues, which is essential for genomic and autocrine/paracrine signaling [49,125]. However, the precise quantities involved remain unknown [162]. There is no evidence to suggest that this component of calcitriol synthesizes in peripheral tissue cells and enters circulation [56], except in cases of overactive macrophages in granulomatous cells [6,163].
Therefore, the hormonal actions of calcitriol are exclusively dependent on renal tubular cell synthesis. Consequently, impaired renal function can significantly negatively affect the musculoskeletal system and calcium homeostasis due to the deterioration of renal tubular cell functions. Therefore, individuals with impaired renal functions must be supplemented with calcitriol or 1α-vitamin D analogs. The regulation of 1α-hydroxylase in renal cells is modulated by parathyroid hormone (PTH) but not in peripheral target cells [6]. In contrast, 25(OH)D and 1,25(OH)2D are inactivated through the 24-hydroxylase enzyme expressed from the CYP24A1 gene.
The coordinated actions of 1,25(OH)2D in conjunction with PTH and fibroblast growth factor-23 (FGF-23) tightly regulate serum ionized calcium (Ca2+) concentrations and phosphate (Pi) homeostasis [143,164,165]. This tight control of serum ionized calcium is crucial for numerous physiological functions, including enzymatic activities, hormone synthesis and release from endocrine glands (e.g., PTH and insulin), intestinal calcium absorption, DNA repair, mitochondrial energy generation, and the promotion of skeletal mineralization and microarchitectural integrity [165,166]. Fluctuations in ionized calcium in the blood can have detrimental effects on survival.
3.8. Maintenace of Calcium Homeostasis
Blood calcium homeostasis is maintained through various mechanisms, including promoting intestinal calcium absorption, calcium mobilization from bones (mediated by parathyroid hormone and osteoclasts), and calcium conservation by the kidneys. These biological actions, which involve osteoclasts and osteoblasts, help regulate mineralization and bone turnover to stabilize serum-ionized calcium and phosphorus concentrations [83,146,167]. Additionally, calcitriol and serum ionized calcium levels rapidly adjust intestinal fractional calcium absorption in local cells. This adjustment also affects concentrations of calcium-binding proteins like calbindin, reflecting the fine-tuning actions of 1,25(OH)2D [88,168,169].
Circulating PTH and hormonal calcitriol, directly and indirectly, influence the expression of genes like CYP2R1, which encodes 25-hydroxylase, and CYP24A1, which encodes 24-hydroxylase enzyme, to regulate serum ionized calcium levels [6]. Conversely, ionized calcium levels also impact the synthesis and release of PTH and the activity of 24-hydroxylase [170,171]. Consequently, vitamin D deficiency disrupts calcium homeostasis and phosphate metabolism at various stages. Moreover, chronic hypovitaminosis D can delay skeletal maturation and calcification, accumulating unmineralized bone tissue known as osteoid [170,172,173,174,175,176]. Clinically, this presents as rickets in children and osteomalacia in adults [146,167].
4. Key Physiological Functions of Vitamin D
Vitamin D is crucial in facilitating the intestinal absorption of calcium and phosphorus, promoting skeletal mineralization, enhancing resistance against bacteria and viruses, modulating inflammation and the immune system, and controlling cell growth. Over the past two decades, there has been exponential growth in our understanding of the additional biological and physiological functions of the vitamin D axis.
4.1. Tissue-Specific Regulation of CYP27B1
The molecular structures of cloned CYP27B1 are identical in renal and extra-renal tissues [177,178]. Functionally, the activity of CYP27B1 in target cells regulates processes such as cell proliferation, differentiation, immune functions, and hormone secretion, but it is not directly involved in bone mineral metabolism. The latter depends on the endocrine effects of 1,25(OH)2D. Consequently, the serum concentrations of 25(OH)D required for bone mineral metabolism are lower. This suggests the essential physiological roles of extra-renal tissue activation of calcitriol. However, the regulation of CYP27B1 expression and activity in extra-renal target tissues differs from that of CYP27B1 in the kidney.
One of the most abundant drug-metabolizing P450 cytochrome enzymes, CYP3A4, is present in the liver. It is constitutively expressed in hepatocytes and small intestines [179] and is crucial in metabolizing various toxins and pharmaceutical agents [6,180]. Interestingly, 1,25(OH)2D plays a feedback role in enhancing the transcription of CYP3A4 through a mechanism mediated by the calcitriol receptor (CTR) [181,182].
Serum 25(OH)D concentration necessary to reduce disease risks varies depending on the tissue or the specific disease [20]. For instance, to resolve conditions like rickets and osteomalacia, serum 25(OH)D concentrations between 15 and 20 ng/mL are typically sufficient, as the CTR in skeletal tissues is highly sensitive to activated vitamin D metabolites [182]. However, for the prevention of cardiovascular diseases, autoimmune disorders [12,63], and cancer [60,61,62], and the reduction of all-cause mortality [183,184], higher concentrations of serum 25(OH)D are generally required [20,183,185].
4.2. Tissue-Specific Thresholds of Vitamin D
Until a few years ago, the clinical importance of circulating vitamin D, 25(OH)D, and 1,25(OH)2D concentrations was underestimated [51]. While calcitriol has the most potent biological functions, all three metabolites participate in different physiological activities [6]. For example, vitamin D directly stabilizes epithelial and endothelium [56]. Vitamin D and 25(OH)D enter renal cells and all peripheral target cells to form calcitriol [6]. Figure 4 illustrates the differences between vitamin D and its two metabolites.
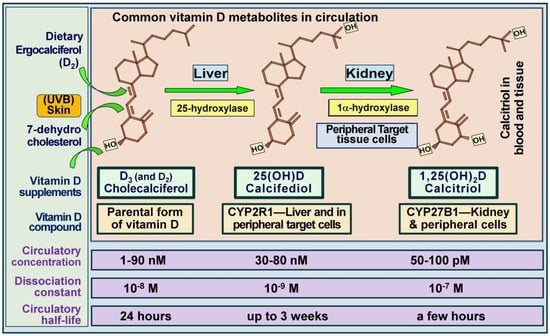
Figure 4.
The figure depicts the structures of the three most common vitamin D metabolites, highlighting sites of generation, specific hydroxylating enzymes, and their average concentrations in the bloodstream. With daily supplementation or sun exposure, D3 and 25(OH)D concentrations remain similar and in equilibrium. Notably, while circulatory concentrations of D3 and 25(OH)D3 are in the nanomolar range, 1,25(OH)2D (calcitriol) is present in picomolar amounts—approximately 900-fold lower (modified from Bickel, D [186] and Wimalawansa, 2022 [49]).
Due to the unfamiliarity with the biology of vitamin D, some study investigators neglected to measure baseline 25(OH)D concentrations before enrolling participants into clinical trials. Consequently, vitamin D-sufficient individuals may inadvertently be included in study groups [15], representing a significant and recurrent design flaw in randomized controlled trials (RCTs) [187]. Furthermore, aside from diagnosing hypovitaminosis D, low serum 25(OH)D could be a marker for poor health and nutritional status [188].
Many studies have reported that elevated circulating 1,25(OH)2D levels or a lower ratio of 25(OH)D to 1,25(OH)2D serve as predictors of poor health outcomes in individuals with cardiovascular diseases (CVD) [189,190], particularly regarding vascular calcification [191] and heart failure, leading to premature death [13,192,193]. Additionally, circulating 25(OH)D concentrations show an inverse association with the severity of various disorders, including metabolic syndrome [194,195], immunity [196,197], autoimmune disorders [12,198], the ability to combat infections [95,96,98,199], and the progression of calcified plaque in coronary arteries [200].
4.3. Other Beneficial Effects of Vitamin D
Vitamin D status during pregnancy is crucial in clinical outcomes, including the risk of pre-eclampsia [201]. For instance, dysfunctions and abnormalities in vitamin D metabolism observed in trophoblasts and placental endothelial cells (involving enzymes CYP2R1, CYP27B1, CYP24A1, and proteins VDBP and CTR have been proposed as significant pathological factors contributing to pre-eclampsia [201]. Therefore, distinct tissue-specific expressions and metabolic irregularities of vitamin D are associated with certain human disorders and conditions.
In addition, various endocrine glands and cells, including pancreatic islets and parathyroid cells, also express CYP2B1 [202,203]. The autocrine/intracrine and paracrine effects (signaling) of 1,25(OH)2D in peripheral target cells present a significant therapeutic opportunity for developing new drugs to modulate physiological processes related to vitamin D. However, the success of such therapies hinges on a comprehensive understanding of the clinically relevant 25(OH)D concentrations needed in different target tissues for optimal health [204]. Achieving stable serum concentrations of 25(OH)D is essential for long-term optimal physiological functions.
Another potential explanation for tissue- and organ-specific effects, as well as ethnic differences in responses, could be the availability of “free” (unbound) vitamin D. A study reported similar concentrations of bioavailable, free 25(OH)D in whites and blacks despite significantly lower concentrations of total 25(OH)D and VDBP in the African Americans [205]. The authors propose that differences in the prevalence of common genetic polymorphisms between racial groups may account for this disparity [206].
Concentrations of free 25(OH)D in the circulation and tissue fluids are regulated by vitamin D binding protein levels. In contrast, in target tissues and cells, such concentrations are regulated by local autocrine needs and intracellular 1α-hydroxylase activity. Figure 5 summarizes the essential physiological functions of hormonal calcitriol synthesized in renal tubular cells and non-hormonal calcitriol in peripheral target cells.
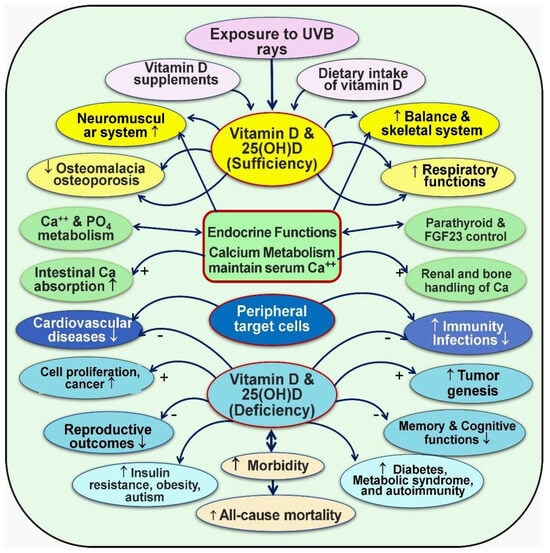
Figure 5.
Broader functions of vitamin D (1,25(OH)2D): The figure illustrates both endocrine and non-endocrine functions that affect various cells and tissues in the body (modified from Wimalawansa, SJ, 2023; [15]) ↑ = Increased activity; ↓ = Decreased activity; − = Negative (reduced); + = positive (enhanced).
The PTH and the FGF23 modulate the renal production of calcitriol–Klotho endocrine system via the kidneys. Extra-renal synthesis of calcitriol is determined by the concentration of substrate, 25(OH)D, 1α-hydroxylase activity in target tissue cells, and by the catabolic enzyme 24-hydroxylase. Functional anomalies of vitamin D occur through various mechanisms, including CTR gene polymorphism and abnormalities of its CYP family of conversion enzymes.
4.4. Clinical Consequences of CTR, CYP27B1, and CYP2R1 Mutations
While 25-hydroxylase deficiency can cause rickets, such occurrences are rare [207,208,209]. Rickets can arise from mutations affecting the CTR gene encoding the 1α,25-dihydroxyvitamin D receptor, the gene encoding the vitamin D 1α-hydroxylase (CYP27B1; located on 12q13.1), and a microsomal vitamin D 25-hydroxylase (CYP2R1; located on 11p15.2) [210]. Mutations in CYP27B1 lead to 1α-hydroxylase deficiency, known as vitamin D-dependent rickets type 1 or hereditary pseudo-vitamin D-deficient rickets, while mutations in CYP2R1 result in 25-hydroxylase deficiency [137,211,212]. Despite the genetic differences, the phenotypic outcomes remain consistent.
CYP2R1 serves as the primary 25-hydroxylase enzyme in humans. Mutations in the CYP2R1 gene can result in genetically driven vitamin D deficiency, with inheritance patterns observed in some cases. Notably, mutations such as L99P and K242N in exon 2 of the CYP2R1 gene have been documented [209,213]. Such mutations can cause a significant reduction or complete loss of 25-hydroxylase activity [213], leading to a condition known as atypical vitamin D-deficient rickets (or vitamin D-dependent rickets type 1B) [213].
4.5. Health Economics of Vitamin D—Costs to Maintain Physiological Serum 25(OH)D Concentrations
Vitamin D is a readily available generic micronutrient that is accessible worldwide without the need for prescriptions. Among its various forms, D3 stands out as the preferred choice for supplementation, as it offers a cost-effective option. In the Western market, D3 supplements are typically priced at less than USD 8 per year’s supply. It can be utilized as an adjunct therapy in infections and metabolic disorders. For example, when used in SARS-CoV-2 infection, the cost is about USD 2 per person, compared to over USD 700 with anti-viral agents (https://c19early.org; accessed on 28 March 2024) [100,209,214]. Maintaining optimal circulatory levels of 25(OH)D, above 50 ng/mL, can be achieved through regular vitamin D supplements (e.g., 70–90 IU/kg body weight for a non-obese person [49] or through safe sun exposure. This could lead to sunlight potentially reducing the prevalence and severity of several common chronic diseases and certain acute illnesses like infections.
On average, addressing vitamin D deficiency incurs a cost of less than 0.1% of the expenses associated with investigating and treating exacerbating comorbidities and complications linked to vitamin D deficiency, such as those observed in COVID-19 [215]. For instance, the typical expenses for managing diseases associated with vitamin D deficiency, including diabetes, obesity, multiple sclerosis, and related complications, range from USD 5000 to USD 18,000 annually [216]. The cost–benefit ratio of prophylactic vitamin D averages approximately 1:10,000, indicating substantial potential benefits relative to costs.
The cost-effectiveness of addressing vitamin D deficiency enables individuals to enjoy longer, healthier lives with fewer illnesses and reduced expenses. Consequently, various entities and individuals with conflicts of interest, philanthropic organizations, prominent health organizations, and government-appointed committees persistently undermine the significance of vitamin D. This lack of acknowledgment is unsurprising, given their dependence on funding and benefits from pharmaceutical companies.
4.6. Repeated Supra-Pharmacologic Doses Are Unphysiological and Can Be Harmful
Large fluctuations in serum 25(OH)D concentration, particularly following high-dose therapy, could negatively affect bodily functions [125]. Supra-pharmacologic doses of oral vitamin D can lead to high peak serum 25(OH)D concentrations, accompanied by fluctuations in target tissue and intracellular concentrations. Similar fluctuations may occur when a large bolus of vitamin D is administered parenterally and cleared from the circulation within a few weeks [125], resulting in a peak followed by a rapid decline in serum concentration.
This phenomenon occurs partly due to the rapid absorption of vitamin D after administering large doses and the relatively shorter half-life of vitamin D compared to 25(OH)D in the circulation. As a result, there can be fluctuations in the bioavailable intracellular 25(OH)D concentration, as observed within prostatic cells [51,87]. This fluctuation could be one reason for the reported U-shaped curve observed with vitamin D therapy in prostate cancer patients.
Infrequent administration of high doses of vitamin D (e.g., 300,000 to 600,000 IU) administered at 6- to 12-month intervals [217] is not physiological and may lead to adverse clinical outcomes. Large doses of vitamin D administered biannually or annually [218] are unphysiological and thus not recommended [219]. One explanation is that higher transient serum 25(OH)D concentrations obtained with intermittent bolus high doses induce the 24-hydroxylase enzyme [220], causing a rapid decline of active forms in the circulation, 25(OH)D and 1,25(OH)2D concentrations over time [221].
Supra-pharmacologic doses of vitamin D administered at intervals of less than a month [222,223,224] could lead to negative clinical outcomes, such as increased falls and resulting fractures, as demonstrated in some improperly designed clinical trials [217,225]. Therefore, vitamin D supplements should be given at less-than-monthly intervals and preferably administered daily, especially in RCTs [226].
4.7. Common Adverse Effects Following an Overdose of Vitamin D
Adverse effects following vitamin D overdose are infrequent. Toxicity may occur when serum 25(OH)D concentration exceeds 150 ng/mL [49,56,227]. Most reported cases were due to mistaken or inappropriate doses taken for prolonged periods [228,229,230,231,232,233,234]. Signs and symptoms of vitamin D toxicity are primarily related to elevated blood-ionized calcium levels and hypercalciuria. The former include loss of appetite and weight loss, nausea and vomiting, constipation, weakness, fatigue, disorientation and mental cloudiness, cardiac arrhythmia, and possibly increased mortality [231,235].
Hypercalcemia-associated hypercalciuria leads to excessive urination, thirst, dehydration, and the formation of renal stones. Vitamin D can interact with drugs at high doses, particularly those that modulate cytochrome P450-3A4 (CYP3A4) [230,232]. Additionally, vitamin D can interfere with anti-convulsant and statin drugs, thiazide diuretics, verapamil, and digitalis agents [6].
4.8. Sustained Serum 25(OH)D Concentrations Are Necessary for Optimum Outcomes
Preventing vitamin D deficiency-associated diseases requires achieving and sustaining adequate serum 25(OH)D concentrations [236,237]. Moreover, obtaining clinical benefits necessitates different serum 25(OH)D concentrations for different diseases [20,90,238], including cancer [239] and type 2 diabetes mellitus [240,241], as well as reducing the risk of all-cause mortality [238,242,243]. It is necessary to sustain circulatory 25(OH)D concentrations above 40 ng/mL to mitigate the risks and severity of certain extra-skeletal disorders [20,73,244,245,246,247].
Much of the data supporting these findings are derived from ecological and observational studies. However, mounting evidence from recent RCTs report that elevated serum 25(OH)D concentrations are associated with increased health benefits [248]. Nevertheless, there is a lack of well-designed RCTs on vitamin D-deficient subjects, with vitamin D as a primary intervention to test specified diseases and vitamin D status, and that rely on serum 25(OH)D concentrations believed to be needed for the reduction of the risk of a specific disease. Endocrine functions and interactions with various diseases and disorders are illustrated in Figure 6.
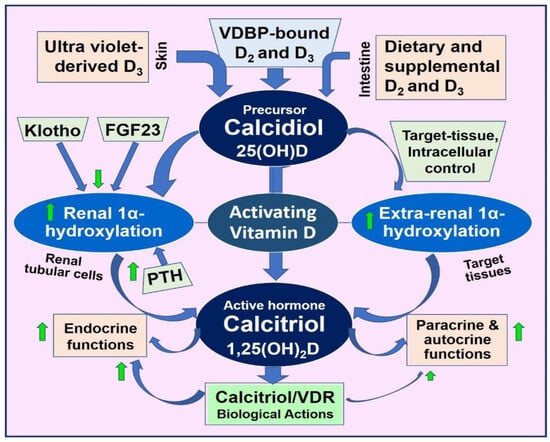
Figure 6.
The illustration highlights the key activation steps and intricate yet vital interactions of vitamin D and its active metabolites, as well as their inherent feedback control systems. These mechanisms maintain circulatory ionized calcium concentrations, representing vitamin D’s fundamental endocrine function (Up arrow = up-regulated (increased activity); Down arrow = down-regulated (reduced functions)).
4.9. Example Conditions Requiring Higher Serum 25(OH)D Concentrations
As with other physiological approaches, vitamin D supplements aim to achieve optimal serum 25(OH)D concentrations to maximize benefits while minimizing or avoiding adverse effects [20]. For most musculoskeletal disorders, including bone mineralization, a serum concentration above 20 ng/mL seems adequate [53,249]. In contrast, the current data support maintaining serum 25(OH)D concentrations between 40 and 80 ng/mL as optimal for most other disorders [20,83,90,238]. There are exceptions that certain conditions would improve by maintaining a higher serum 25(OH)D concentration [6].
Examples of these include infection [49,56,105], septicemia [250], and SARS-CoV-2 [93], which require maintenance of serum 25(OH)D concentrations above 50 ng/mL. In addition, sleep disturbances, chronic fatigue, post-COVID syndromes (with similar fundamentals), and chronic pain are managed better by maintaining serum 25(OH)D concentration above 50 ng/mL [106,251,252,253,254]. Metabolic disorders like diabetes [255,256], obesity [257], and osteoporosis, as well as autoimmune disorders such as multiple sclerosis [258], rheumatoid arthritis [259], and psoriasis [260], and certain types of cancers [243,248,261,262,263] as well as reduced all-cause mortality [183] require the maintenance of serum 25(OH)D concentrations above 60 ng/mL.
4.10. How Much Vitamin D Intake Is Necessary?
Even with a daily administration of 5000 IU/day of vitamin D3 in a vitamin D-deficient non-obese (~70 kg) adult, it could take from weeks to several months to raise serum 25(OH)D concentrations to therapeutic levels [264] and restoring robust immune functions. Doses below 3000 IU/day are unlikely to achieve the necessary therapeutic levels of serum 25(OH)D concentrations, even after one year, in those with vitamin D deficiency [6].
Even daily administration of a D3 dose of 5000 IU/day takes several months to increase serum 25(OH)D concentrations to the therapeutic levels in vitamin D-deficient persons to restore robust immune functions. Doses of less than 4000 IU/day in 70 kg adults with vitamin D deficiency would not raise serum 25(OH)D concentrations to therapeutic levels, even in the longer term. Likewise, although a bolus or upfront loading dose of 300,000 IU takes three to four days to raise serum 25(OH)D concentrations, it cannot maintain 25(OH)D levels for more than three months, and while the counter-regulatory effects, such as large boluses, are known to be effective for at least three months, such large bolus doses alone are unlikely to be effective in treating acute situations such as severe COVID-19 illness.
In addition to its role in adaptive immunity, hypovitaminosis D weakens the generation of neutralizing antibodies, impairs cytotoxic immune cell function, diminishes the effectiveness of memory cells and macrophages, and reduces immune responses following vaccination. Individuals with compromised immune systems often have severe chronic vitamin D deficiency and are particularly vulnerable to adverse effects from SARS-CoV-2 infection or immunization. This susceptibility may result in autoimmune reactions [12,63], systemic hyper-inflammation, and pathological oxidative stress, leading to severe complications and mortality. The high prevalence of hypovitaminosis D among older individuals contributed significantly to the pandemic’s impact in 2020, with COVID-19 disproportionately affecting those with severe vitamin D deficiency [52,216].
The recommendation of sub-optimal (standard-outdated) vitamin D doses for everyone, irrespective of their body weight (including obese individuals) and other factors affecting their serum 25(OH)D concentrations or the lack of serum 25(OH)D-based calculation of appropriate doses for individuals [49,56], has resulted in the administration of pediatric doses of vitamin D to adults, without benefiting the recipients. This manuscript also illustrates examples and circumstances where the efficacy and clinical necessity of rapidly increasing serum 25(OH)D levels (such as in emergencies like sepsis, SARS-CoV-2 infection, in ICU settings, etc.) and target serum 25(OH)D concentration required to address underlying illnesses such as infection or cancer are demonstrated.
4.11. Doses of Vitamin D Needed to Boost Serum 25(OH)D Concentration
The required vitamin D doses vary depending on the individual’s vitamin D status and whether the condition is acute or chronic. The latter is based on serum 25(O)H)D concentrations and/or the body weight (or body mass index–fat mass) [56,72]. The specific calculations have been published [49].
4.11.1. Vitamin D Requirements for Chronic Conditions
In a person with vitamin D deficiency, the body-weight-based doses mentioned in Section 4.5 could take from weeks to months to raise serum 25(OH)D concentrations to the desired therapeutic levels [6]. In these situations, as described below, it is helpful to give an upfront loading dose (a bolus dose) to increase serum 25(OH)D concentrations within a few days instead of months [49,265].
Chronic long-term maintenance of circulatory 25(OH)D concentrations is crucial for the intracellular generation of calcitriol D3 [108,266,267]. If the serum 25(OH)D concentration is too low or the person has conditions requiring higher daily doses (unless presented with taking higher doses), they would benefit from one-time administration of high doses of oral vitamin D (a stat dose or split over a few days using 50,000 IU capsules, taken after a meal to facilitate absorption) to replenish vitamin D stores in the body [6,268,269]. When using high doses of D3, it is important to prevent triggering upregulation of 24-hydroxylase and downregulating intracellular signaling [6,108,270,271].
Fixed higher doses of vitamin D replacement, in the form of 50,000 IU D3 capsules, are widely available and economical for clinical use; they are also standardized and have satisfactory gastrointestinal absorption when taken after eating. The 50,000 IU capsules (60,000 IU in India) are consumed as a single dose or in divided doses to achieve doses ranging from 100,000 to 400,000 IU [6]. For most people (with an average weight of 70 kg), administering vitamin D requires a dose of 200,000 IU (increase or decrease based on body weight). This approach suits non-urgent, outpatient, and community setups to boost serum 25(OH)D concentrations in those with vitamin D deficiency. They are also useful in emergencies when serum 25(OH)D concentrations are not known [49,56]. Evidence supports the efficacy of high-dose vitamin D supplementation in raising serum 25(OH)D levels within a few days [6,64,272,273,274,275,276,277].
While a bolus or upfront loading dose like 300,000 IU can raise serum 25(OH)D concentrations within three to four days, it will not sustain therapeutic levels beyond three months. Furthermore, the counter-regulatory effects triggered by such large bolus doses could reduce the level faster, thus, with a shorter period of effective levels, if repeated higher doses are administered [278,279,280], making them even less effective in treating acute conditions such as severe COVID-19 illness [6]. Thus, it is important to continue daily or weekly doses of vitamin D after a bolus dose.
4.11.2. Rapidly Increasing Serum 25(OH)D to Boost the Immune System in a Day
In emergencies like COVID-19, sepsis/infection, and other acute illnesses, it is vital to immediately increase the circulating D3 and/or 25(OH)D concentrations [49,125] to diffuse into peripheral target tissues for intracellular generation of calcitriol [49,281,282].
From generation in the skin or ingestion (Figure 1), vitamin D3 and D2 undergo 25-hydroxylation in the liver to form 25(OH)D (calcifediol), typically taking about 3–4 days. In contrast, calcifediol is already 25-hydroxylated and bypasses the liver, becoming available in circulation within four hours of administration [283]. This rapid availability offers several benefits, including rapidly boosting the immune system and enhancing other protective bodily functions within a day. As a result, calcifediol proves particularly valuable in medical emergencies such as COVID-19, sepsis, and acute conditions like Kawasaki disease, multisystem inflammatory syndrome, acute respiratory distress syndrome (ARDS), burns, and vitamin D deficiency during pregnancy.
The recommended oral dose of calcifediol is 0.014 mg/kg body weight [49]. With a single dose of calcifediol, it is advisable to administer a loading dose of vitamin D3 concurrently with or within the first week of calcifediol administration, which would allow a longer duration of raised serum 25(OH)D [56]. As with other regimens, it is essential to prescribe an appropriate daily or weekly dose of vitamin D to maintain serum 25(OH)D concentrations within the therapeutic range.
5. Discussion
The benefits of vitamin D extend beyond its well-established roles in calcium and phosphate homeostasis and the prevention and treatment of conditions such as rickets, osteomalacia, and bone loss. Based on recent data, vitamin D deficiency is defined by serum 25(OH)D concentrations below 40 ng/mL, a threshold below which various disorders may develop or worsen. The optimal physiological range is between 40 and 80 ng/mL, effective against 99.7% of these disorders. Concentrations below 40 ng/mL are associated with the worsening of many extra-skeletal conditions, including higher risks of falls, fractures, metabolic disorders, cardiovascular diseases, cancers, and increased all-cause mortality [183]. This occurs even in healthy individuals, highlighting the crucial role of vitamin D in public health and disease prevention [183]. The optimal functioning of vitamin D in genomic, endocrine, paracrine, and autocrine systems is essential for numerous physiological processes that keep people healthy.
Based on recent data, vitamin D deficiency is defined by serum 25(OH)D concentrations below 40 ng/mL, below which certain disorders are initiated and worsened. The physiological (effective) range is between 40 and 80 ng/mL, effective against 99.7% of the disorders [6]. Concentrations below are associated with several extra-skeletal disorders and conditions like higher risk of falls, fractures, various illnesses, and increased all-cause mortality, even in healthy individuals [183], illustrating its crucial role in disease prevention—Public Health. The optimal functioning of the vitamin D endocrine, paracrine, and autocrine systems is crucial for numerous physiological processes. Furthermore, the beneficial effects of vitamin D extend beyond its established roles in calcium and phosphate homeostasis, as well as the prevention and treatment of conditions like rickets, osteomalacia, and bone loss.
Vitamin D adequacy can be accurately assessed only by measuring serum 25(OH)D levels; concentrations of 1,25(OH)2D do not reliably indicate vitamin D status. Recent data from a variety of studies support a decreased incidence of non-skeletal disorders, such as hypertension [255], diabetes [256,284], multiple sclerosis [258], rheumatoid arthritis [259], osteoporosis [285,286], certain types of cancers [243,261,263], all-cause mortality [183,184], and infections when serum 25(OH)D levels are maintained above 50 ng/mL [243,255,256,261,263,284,287,288,289]. However, it is worth noting that despite the presence of physiologic concentrations of calcitriol, increased risks of illnesses and reduced longevity can still occur, suggesting the involvement of other factors in optimal health. However, it is essential to acknowledge that not all researchers agree on the reported multiple non-classical benefits of vitamin D [287,288,289].
Implementing a public health strategy to raise the mean population serum 25(OH)D concentration above 30 ng/mL would incur costs less than 0.01% of the expenses associated with investigating and managing diseases like diabetes. However, the mere existence of policies is insufficient; these policies must be accompanied by effective measures to ensure individuals reach the target vitamin D status. This includes recommendations for safe sun exposure, food fortification strategies, and vitamin D supplementation guidelines [14,19,290,291,292]. Adhering to practical public health guidelines makes eliminating vitamin D deficiency cost-effectively feasible.
The typical daily dose of vitamin D for a non-obese 70 kg adult ranges between 4000 and 7000 IU/day, or 50,000 IU once or twice a month, based on the target blood levels and the body weight. These dosages enable approximately 97% of individuals to maintain their serum 25(OH)D concentrations above 40 ng/mL [83,90]. Given the biology of vitamin D and the need to maintain a steady state of D3 or 25(OH)D in the circulation [125], daily administration of vitamin D3 is preferred to infrequent administration as a preventative measure. In cases where adequate sunlight exposure is lacking, most individuals may need a daily oral intake of vitamin D supplement ranging from 5000 to 7000 IU/day for the maintenance of circulatory 25(OH)D concentrations above 50 ng/mL (125 nmol/L) to have a meaningful positive impact on health. Sustaining stable serum 25(OH)D concentration over the long term is essential for reducing disease incidence and all-cause mortality.
Funding
This research received no funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest. This study did not receive funding from any sources, or assistance in writing from AI or other sources.
Abbreviations
| 1,25(OH)2D | 1,25-dihydroxyvitamin D |
| 25(OH)D | 25-hydroxy vitamin D |
| 7-DHC | 7-dehydrocholesterol |
| CTR | Calcitriol receptor (synonymous with vitamin D receptor; VDR) |
| MR | Mendelian randomization |
| PTH | Parathyroid hormone |
| RAS | Renin–angiotensin system |
| RCT | Randomized controlled clinical trial |
| RDA | Recommended dietary allowance |
| RXR | Retinoid X receptor |
| UVB | Ultraviolet B |
References
- Carlberg, C.; Velleuer, E. Vitamin D and aging: Central role of immunocompetence. Nutrients 2024, 16, 398. [Google Scholar] [CrossRef] [PubMed]
- Vieth, R. Why “Vitamin D” is not a hormone, and not a synonym for 1,25-dihydroxy-vitamin D, its analogs or deltanoids. J. Steroid Biochem. Mol. Biol. 2004, 89-90, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.R.; Thacher, T.D. Vitamin D: Immune function, inflammation, infections and auto-immunity. Paediatr. Int. Child. Health 2023, 43, 29–39. [Google Scholar] [CrossRef]
- Middelkoop, K.; Stewart, J.; Walker, N.; Delport, C.; Jolliffe, D.A.; Coussens, A.K.; Nuttall, J.; Tang, J.C.Y.; Fraser, W.D.; Griffiths, C.J.; et al. Vitamin D supplementation to prevent tuberculosis infection in South African schoolchildren: Multicenter phase 3 double-blind randomized placebo-controlled trial (ViDiKids). Int. J. Infect. Dis. 2023, 134, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Ganmaa, D.; Enkhmaa, D.; Nasantogtokh, E.; Sukhbaatar, S.; Tumur-Ochir, K.E.; Manson, J.E. Vitamin D, respiratory infections, and chronic disease: Review of meta-analyses and randomized clinical trials. J. Intern. Med. 2022, 291, 141–164. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Physiological basis for using vitamin D to improve health. Biomedicines 2023, 11, 1542. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Controlling chronic diseases and acute infections with vitamin D sufficiency. Nutrients 2023, 15, 3623. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Tomaschitz, A.; Marz, W.; Drechsler, C.; Ritz, E.; Zittermann, A.; Cavalier, E.; Pieber, T.R.; Lappe, J.M.; Grant, W.B.; et al. Vitamin D, cardiovascular disease and mortality. Clin. Endocrinol. 2011, 75, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Duan, J.W.; Lu, D.H.; Zhang, F.; Liu, H.L. Association between vitamin D status and cardiometabolic risk factors in adults with type 2 diabetes in Shenzhen, China. Front. Endocrinol. 2024, 15, 1346605. [Google Scholar] [CrossRef]
- Cheema, H.A.; Fatima, M.; Shahid, A.; Bouaddi, O.; Elgenidy, A.; Rehman, A.U.; Oussama Kacimi, S.E.; Hasan, M.M.; Lee, K.Y. Vitamin D supplementation for the prevention of total cancer incidence and mortality: An updated systematic review and meta-analysis. Heliyon 2022, 8, e11290. [Google Scholar] [CrossRef]
- Akdere, G.; Efe, B.; Sisman, P.; Yorulmaz, G. The relationship between vitamin D level and organ-specific autoimmune disorders in newly diagnosed type I diabetes mellitus. Bratisl. Lek. Listy 2018, 119, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Sirbe, C.; Rednic, S.; Grama, A.; Pop, T.L. An Update on the Effects of Vitamin D on the Immune System and Autoimmune Diseases. Int. J. Mol. Sci. 2022, 23, 9784. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D and cardiovascular diseases: Causality. J. Steroid Biochem. Mol. Biol. 2018, 175, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Haq, A.; Wimalawansa, S.J.; Pludowski, P.; Anouti, F.A. Clinical practice guidelines for vitamin D in the United Arab Emirates. J. Steroid Biochem. Mol. Biol. 2018, 175, 4–11. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Infections and autoimmunity-The immune system and vitamin D: A Systematic Review. Nutrients 2023, 15, 3842. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Makin, G.; Lohnes, D.; Byford, V.; Ray, R.; Jones, G. Target cell metabolism of 1,25-dihydroxyvitamin D3 to calcitroic acid. Evidence for a pathway in kidney and bone involving 24-oxidation. Biochem. J. 1989, 262, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Haq, A.; Wimalawansa, S.J.; Carlberg, C. Highlights from the 5th International Conference on Vitamin D Deficiency, Nutrition and Human Health, Abu Dhabi, United Arab Emirates, March 24-25, 2016. J. Steroid Biochem. Mol. Biol. 2018, 175, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Non-musculoskeletal benefits of vitamin D. J. Steroid Biochem. Mol. Biol. 2018, 175, 60–81. [Google Scholar] [CrossRef]
- van Schoor, N.M.; Lips, P. Worldwide vitamin D status. Best. Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Eggemoen, A.R.; Knutsen, K.V.; Dalen, I.; Jenum, A.K. Vitamin D status in recently arrived immigrants from Africa and Asia: A cross-sectional study from Norway of children, adolescents and adults. BMJ Open 2013, 3, e003293. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Gorham, E.D.; Mohr, S.B.; Garland, F.C. Vitamin D for cancer prevention: Global perspective. Ann. Epidemiol. 2009, 19, 468–483. [Google Scholar] [CrossRef] [PubMed]
- Hilger, J.; Friedel, A.; Herr, R.; Rausch, T.; Roos, F.; Wahl, D.A.; Pierroz, D.D.; Weber, P.; Hoffmann, K. A systematic review of vitamin D status in populations worldwide. Br. J. Nutr. 2014, 111, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Resurrection of vitamin D deficiency and rickets. J. Clin. Investig. 2006, 116, 2062–2072. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.R.; Spector, S.A. Hormonally active vitamin D3 (1alpha,25-dihydroxycholecalciferol) triggers autophagy in human macrophages that inhibits HIV-1 infection. J. Biol. Chem. 2011, 286, 18890–18902. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, D.; Quinkler, M.; Eardley, K.S.; Bland, R.; Lepenies, J.; Hughes, S.V.; Raymond, N.T.; Howie, A.J.; Cockwell, P.; Stewart, P.M.; et al. Reduction of the vitamin D hormonal system in kidney disease is associated with increased renal inflammation. Kidney Int. 2008, 74, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Goldring, S.R.; Krane, S.; Avioli, L.V. Disorders of calcification: Osteomalacia and rickets. In Endocrinology, 3rd ed.; DeGroot, L.J., Besser, M., Burger, H.G., Jameson, J.L., Loriaux, D.L., Marshall, J.C., Eds.; WB Saunders: Philadelphia, PA, USA, 1995; pp. 1204–1227. [Google Scholar]
- Banajeh, S.M. Nutritional rickets and vitamin D deficiency--association with the outcomes of childhood very severe pneumonia: A prospective cohort study. Pediatr. Pulmonol. 2009, 44, 1207–1215. [Google Scholar] [CrossRef]
- Weisman, Y. Vitamin D deficiency rickets and osteomalacia in Israel. Isr. Med. Assoc. J. 2003, 5, 289–290. [Google Scholar]
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Willett, W.C.; Staehelin, H.B.; Bazemore, M.G.; Zee, R.Y.; Wong, J.B. Effect of Vitamin D on falls: A meta-analysis. JAMA 2004, 291, 1999–2006. [Google Scholar] [CrossRef]
- Hahn, T.J. Drug-induced disorders of vitamin D and mineral metabolism. Clin. Endocrinol. Metab. 1980, 9, 107–127. [Google Scholar] [CrossRef] [PubMed]
- Gloth, F.M., 3rd; Greenough, W.B., 3rd. Vitamin D deficiency as a contributor to multiple forms of chronic pain. Mayo Clin. Proc. 2004, 79, 696, 699; author reply 699. [Google Scholar] [PubMed]
- Gloth, F.M., 3rd; Lindsay, J.M.; Zelesnick, L.B.; Greenough, W.B., 3rd. Can vitamin D deficiency produce an unusual pain syndrome? Arch. Intern. Med. 1991, 151, 1662–1664. [Google Scholar] [CrossRef]
- Chapuy, M.C.; Arlot, M.E.; Duboeuf, F.; Brun, J.; Crouzet, B.; Arnaud, S.; Delmas, P.D.; Meunier, P.J. Vitamin D3 and calcium to prevent hip fractures in elderly women. N. Engl. J. Med. 1992, 327, 1637–1642. [Google Scholar] [CrossRef] [PubMed]
- Sahay, M.; Sahay, R. Rickets-vitamin D deficiency and dependency. Indian J. Endocrinol. Metab. 2012, 16, 164–176. [Google Scholar] [CrossRef]
- Holick, M.F. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin. Proc. 2006, 81, 353–373. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Tu, C.L.; Menendez, A.; Nguyen, T.; Bikle, D.D. Vitamin D and calcium regulation of epidermal wound healing. J. Steroid Biochem. Mol. Biol. 2016, 164, 379–385. [Google Scholar] [CrossRef]
- Lee, S.; Kim, B.J.; Lee, C.H.; Lee, W.S. Increased prevalence of vitamin D deficiency in patients with alopecia areata: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, R.; Anwar, M.I. Vitamin D deficiency in alopecia areata. J. Coll. Physicians Surg. Pak. JCPSP 2017, 27, 200–202. [Google Scholar]
- Ceglia, L.; Harris, S.S. Vitamin D and its role in skeletal muscle. Calcif. Tissue Int. 2013, 92, 151–162. [Google Scholar] [CrossRef]
- Bollen, S.E.; Bass, J.J.; Wilkinson, D.J.; Hewison, M.; Atherton, P.J. The impact of genetic variation within the vitamin D pathway upon skeletal muscle function: A systematic review. J. Steroid Biochem. Mol. Biol. 2023, 229, 106266. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.D.; Hogler, W.; Craig, M.E.; Verge, C.F.; Walker, J.L.; Piper, A.C.; Woodhead, H.J.; Cowell, C.T.; Ambler, G.R. The re-emerging burden of rickets: A decade of experience from Sydney. Arch. Dis. Child. 2006, 91, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Gogulothu, R.; Nagar, D.; Gopalakrishnan, S.; Garlapati, V.R.; Kallamadi, P.R.; Ismail, A. Disrupted expression of genes essential for skeletal muscle fibre integrity and energy metabolism in vitamin D deficient rats. J. Steroid Biochem. Mol. Biol. 2020, 197, 105525. [Google Scholar] [CrossRef]
- Najada, A.S.; Habashneh, M.S.; Khader, M. The frequency of nutritional rickets among hospitalized infants and its relation to respiratory diseases. J. Trop. Pediatr. 2004, 50, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Sahay, T.; Ananthakrishnan, A.N. Vitamin D deficiency is associated with community-acquired clostridium difficile infection: A case-control study. BMC Infect. Dis. 2014, 14, 661. [Google Scholar] [CrossRef]
- Zasloff, M. Fighting infections with vitamin D. Nat. Med. 2006, 12, 388–390. [Google Scholar] [CrossRef]
- Zanetti, M. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 2004, 75, 39–48. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Rapidly increasing serum 25(OH)D boosts the immune system, against infections-sepsis and COVID-19. Nutrients 2022, 14, 2997. [Google Scholar] [CrossRef]
- Polonowita, A.; Wimalawansa, S.J. Impact of Withholding Cost-Effective Early Treatments, such as vitamin D, on COVID-19: An analysis using an innovative logical paradigm. World J. Adanced Pharma. Life Sci. 2023, 5, 13–34. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Biology of vitamin D. J. Steroids Horm. Sci. 2019, 10, 1–8. [Google Scholar]
- Sunil, J.W. Commonsense approaches to minimizing risks from COVID-19. Open J. Pulmonol. Respir. Med. 2020, 2, 28–37. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, L.M.; Catov, J.M.; Simhan, H.N.; Holick, M.F.; Powers, R.W.; Roberts, J.M. Maternal vitamin D deficiency increases the risk of preeclampsia. J. Clin. Endocrinol. Metab. 2007, 92, 3517–3522. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Hughes, B.; Heaney, R.P.; Holick, M.F.; Lips, P.; Meunier, P.J.; Vieth, R. Estimates of optimal vitamin D status. Osteoporos. Int. 2005, 16, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S. Overcoming infections including COVID-19, by maintaining circulating 25(OH)D concentrations above 50 ng/mL. Pathol. Lab. Med. Int. 2022, 14, 37–60. [Google Scholar] [CrossRef]
- Grant, W.B. An estimate of the global reduction in mortality rates through doubling vitamin D levels. Eur. J. Clin. Nutr. 2011, 65, 1016–1026. [Google Scholar] [CrossRef]
- Shen, Y. Role of nutritional vitamin D in chronic kidney disease-mineral and bone disorder: A narrative review. Medicine 2023, 102, e33477. [Google Scholar] [CrossRef] [PubMed]
- Bellan, M.; Andreoli, L.; Mele, C.; Sainaghi, P.P.; Rigamonti, C.; Piantoni, S.; De Benedittis, C.; Aimaretti, G.; Pirisi, M.; Marzullo, P. Pathophysiological role and therapeutic implications of vitamin D in autoimmunity: Focus on chronic autoimmune diseases. Nutrients 2020, 12, 789. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Alonso, P.; Boughanem, H.; Canudas, S.; Becerra-Tomas, N.; Fernandez de la Puente, M.; Babio, N.; Macias-Gonzalez, M.; Salas-Salvado, J. Circulating vitamin D levels and colorectal cancer risk: A meta-analysis and systematic review of case-control and prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2023, 63, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.C.; Chen, Y.H.; Liang, F.W.; Wu, Y.C.; Wang, J.J.; Lim, S.W.; Ho, C.H. Determinants of cancer incidence and mortality among people with vitamin D deficiency: An epidemiology study using a real-world population database. Front. Nutr. 2023, 10, 1294066. [Google Scholar] [CrossRef]
- Lawler, T.; Warren Andersen, S. Serum 25-hydroxyvitamin D and cancer risk: A systematic review of mendelian randomization studies. Nutrients 2023, 15, 422. [Google Scholar] [CrossRef]
- Gallo, D.; Baci, D.; Kustrimovic, N.; Lanzo, N.; Patera, B.; Tanda, M.L.; Piantanida, E.; Mortara, L. How does vitamin D affect immune cells crosstalk in autoimmune diseases? Int. J. Mol. Sci. 2023, 24, 4689. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Prophylactic use of vitamin D to maintain a robust immune system against infections like SARS-CoV-2. Glob. J. Endocrinol. Metab. GJEM 2023, 3, 1–9. [Google Scholar]
- Aji, A.S.; Yerizel, E.; Desmawati, D.; Lipoeto, N.I. Low maternal vitamin D and calcium food intake during pregnancy associated with place of residence: A coss-sectional study in West Sumatran women, Indonesia. Open Access Maced. J. Med. Sci. 2019, 7, 2879–2885. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; O’Dea, R. Exploration of strategic food vehicles for vitamin D fortification in low/lower-middle income countries. J. Steroid Biochem. Mol. Biol. 2019, 195, 105479. [Google Scholar] [CrossRef] [PubMed]
- Binkley, N.; Lewiecki, E.M. Vitamin D and common sense. J. Clin. Densitom. 2011, 14, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J.; Razzaque, M.S.; Al-Daghri, N.M. Calcium and vitamin D in human health: Hype or real? J. Steroid Biochem. Mol. Biol. 2018, 180, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Giovannucci, E.; Willett, W.C.; Dietrich, T.; Dawson-Hughes, B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am. J. Clin. Nutr. 2006, 84, 18–28. [Google Scholar] [CrossRef]
- Wimalawansa, S. Rational food fortification programs to alleviate micronutrient deficiencies. J. Food Process. Technol. 2013, 4, 257–267. [Google Scholar] [CrossRef]
- Grant, W.B.; Holick, M.F.; Wimalawansa, S.J. Vitamin D supplements and reasonable solar UVB should be recommended to prevent escalating incidence of chronic diseases. BMJ 2015, 350, h321. [Google Scholar]
- Ekwaru, J.P.; Zwicker, J.D.; Holick, M.F.; Giovannucci, E.; Veugelers, P.J. The importance of body weight for the dose response relationship of oral vitamin D supplementation and serum 25-hydroxyvitamin D in healthy volunteers. PLoS ONE 2014, 9, e111265. [Google Scholar] [CrossRef]
- McDonnell, S.L.; Baggerly, C.; French, C.B.; Baggerly, L.L.; Garland, C.F.; Gorham, E.D.; Lappe, J.M.; Heaney, R.P. Serum 25-Hydroxyvitamin D Concentrations >/=40 ng/ml Are Associated with >65% Lower Cancer Risk: Pooled Analysis of Randomized Trial and Prospective Cohort Study. PLoS ONE 2016, 11, e0152441. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, S.L.; Baggerly, K.A.; Baggerly, C.A.; Aliano, J.L.; French, C.B.; Baggerly, L.L.; Ebeling, M.D.; Rittenberg, C.S.; Goodier, C.G.; Mateus Nino, J.F.; et al. Maternal 25(OH)D concentrations >/=40 ng/mL associated with 60% lower preterm birth risk among general obstetrical patients at an urban medical center. PLoS ONE 2017, 12, e0180483. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr. Rev. 2014, 72, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Baggerly, C.A.; Cuomo, R.E.; French, C.B.; Garland, C.F.; Gorham, E.D.; Grant, W.B.; Heaney, R.P.; Holick, M.F.; Hollis, B.W.; McDonnell, S.L.; et al. Sunlight and vitamin D: Necessary for public health. J. Am. Coll. Nutr. 2015, 34, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Song, A.; Jin, Y.; Xia, Q.; Song, G.; Xing, X. A dose-response meta-analysis between serum concentration of 25-hydroxy vitamin D and risk of type 1 diabetes mellitus. Eur. J. Clin. Nutr. 2021, 75, 1010–1023. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J. Clin. Endocrinol. Metab. 2012, 97, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Al Anouti, F.; Boucher, B.J.; Fakhoury, H.M.A.; Moukayed, M.; Pilz, S.; Al-Daghri, N.M. Evidence That Increasing Serum 25(OH)D Concentrations to 30 ng/mL in the Kingdom of Saudi Arabia and the United Arab Emirates Could Greatly Improve Health Outcomes. Biomedicines 2023, 11, 994. [Google Scholar] [CrossRef] [PubMed]
- Maghbooli, Z.; Sahraian, M.A.; Ebrahimi, M.; Pazoki, M.; Kafan, S.; Tabriz, H.M.; Hadadi, A.; Montazeri, M.; Nasiri, M.; Shirvani, A.; et al. Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. PLoS ONE 2020, 15, e0239799. [Google Scholar] [CrossRef]
- Rosen, C.J.; Gallagher, J.C. The 2011 IOM report on vitamin D and calcium requirements for north America: Clinical implications for providers treating patients with low bone mineral density. J Clin Densitom 2011, 14, 79–84. [Google Scholar] [CrossRef]
- Malczewska-Lenczowska, J.; Surala, O.; Granda, D.; Szczepanska, B.; Czaplicki, A.; Kubacki, R. The relationship between bone health parameters, vitamin D and iron status, and dietary calcium intake in young males. Nutrients 2024, 16, 215. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Vitamin D: Everything You Need to Know (Book); Karunaratne & Sons: Homagama, Sri Lanka, 2012; Volume 1, ISBN 978-955-9098-94-2. [Google Scholar]
- Vieth, R. Vitamin D supplementation: Cholecalciferol, calcifediol, and calcitriol. Eur. J. Clin. Nutr. 2020, 74, 1493–1497. [Google Scholar] [CrossRef]
- Holick, M.F. The cutaneous photosynthesis of previtamin D3: A unique photoendocrine system. J. Investig. Dermatol. 1981, 77, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P.; Vieth, R.; Hollis, B.W. Vitamin D efficacy and safety. Arch. Intern. Med. 2011, 171, 266; author reply 267. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Sunlight, UV-radiation, vitamin D and skin cancer: How much sunlight do we need? Adv. Exp. Med. Biol. 2008, 624, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A global perspective for health. Derm. Endocrinol. 2013, 5, 51–108. [Google Scholar] [CrossRef] [PubMed]
- Mithal, A.; Wahl, D.A.; Bonjour, J.P.; Burckhardt, P.; Dawson-Hughes, B.; Eisman, J.A.; El-Hajj Fuleihan, G.; Josse, R.G.; Lips, P.; Morales-Torres, J. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos. Int. 2009, 20, 1807–1820. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D in the new millennium. Curr. Osteoporo Res. 2012, 10, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Achieving population vitamin D sufficiency will markedly reduce healthcare costs. Eur. J. Biomed. Pharm. Sci. 2020, 7, 136–141. [Google Scholar]
- Wimalawansa, S.J. Maintaining optimum health requires longer-term stable vitamin D concentrations. Int. J. Regen. Med. 2020, 3, 1–5. [Google Scholar] [CrossRef]
- Wimalawansa, S.J.; Polonowita, A. Boosting immunity with vitamin D for preventing complications and deaths from COVID-19. In Proceedings of the COVID 19: Impact, Mitigation, Opportunities and Building Resilience “From Adversity to Serendipity”, Perspectives of Global Relevance Based on Research, Experience and Successes in Combating COVID-19, Colombo, Sri Lanka, 27–28 January 2021; pp. 171–198. [Google Scholar]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- McCartney, D.M.; Byrne, D.G. Optimisation of vitamin D status for enhanced immuno-protection against COVID-19. Ir. Med. J. 2020, 113, 58. [Google Scholar] [PubMed]
- Zhou, Y.F.; Luo, B.A.; Qin, L.L. The association between vitamin D deficiency and community-acquired pneumonia: A meta-analysis of observational studies. Medicine 2019, 98, e17252. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [PubMed]
- Tsujino, I.; Ushikoshi-Nakayama, R.; Yamazaki, T.; Matsumoto, N.; Saito, I. Pulmonary activation of vitamin D(3) and preventive effect against interstitial pneumonia. J. Clin. Biochem. Nutr. 2019, 65, 245–251. [Google Scholar] [CrossRef] [PubMed]
- FLCCC. Critical Treatment Protocols. Available online: https://covid19criticalcare.com/understanding-vitamin-d/ (accessed on 13 May 2023).
- c19early. Vitamin D for COVID-19. Available online: https://c19vitamind.com/ (accessed on 25 January 2023).
- Lapillonne, A. Vitamin D deficiency during pregnancy may impair maternal and fetal outcomes. Med. Hypotheses 2010, 74, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D. Nonclassic actions of vitamin D. J. Clin. Endocrinol. Metab. 2009, 94, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S. Part 3: Understanding the Forms of Vitamin D: Vitamin D3, 25(OH)D, and 1,25(OH)2D. Available online: https://grassrootshealth.org/blog/understanding-forms-vitamin-d-vitamin-d3-25ohd-125oh2d/ (accessed on 25 January 2023).
- Quraishi, S.A.; Bittner, E.A.; Blum, L.; Hutter, M.M.; Camargo, C.A., Jr. Association between preoperative 25-hydroxyvitamin D level and hospital-acquired infections following Roux-en-Y gastric bypass surgery. JAMA Surg. 2014, 149, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, S.A.; De Pascale, G.; Needleman, J.S.; Nakazawa, H.; Kaneki, M.; Bajwa, E.K.; Camargo, C.A., Jr.; Bhan, I. Effect of cholecalciferol supplementation on vitamin D status and cathelicidin levels in sepsis: A randomized, placebo-controlled trial. Crit. Care Med. 2015, 43, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Borsche, L.; Glauner, B.; von Mendel, J. COVID-19 Mortality Risk Correlates Inversely with Vitamin D3 Status, and a Mortality Rate Close to Zero Could Theoretically Be Achieved at 50 ng/mL 25(OH)D3: Results of a Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3596. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M.; Shin, E.A. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Hewison, M.; Burke, F.; Evans, K.N.; Lammas, D.A.; Sansom, D.M.; Liu, P.; Modlin, R.L.; Adams, J.S. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J. Steroid Biochem. Mol. Biol. 2007, 103, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Chen, H.; Chun, R.; Ren, S.; Wu, S.; Gacad, M.; Nguyen, L.; Ride, J.; Liu, P.; Modlin, R.; et al. Substrate and enzyme trafficking as a means of regulating 1,25-dihydroxyvitamin D synthesis and action: The human innate immune response. J. Bone Miner. Res. 2007, 22 (Suppl. 2), V20–V24. [Google Scholar] [CrossRef]
- Kurylowicz, A.; Bednarczuk, T.; Nauman, J. The influence of vitamin D deficiency on cancers and autoimmune diseases development. Endokrynol. Pol. 2007, 58, 140–152. [Google Scholar] [PubMed]
- Hughes, D.A.; Norton, R. Vitamin D and respiratory health. Clin. Exp. Immunol. 2009, 158, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sriramula, S.; Xia, H.; Moreno-Walton, L.; Culicchia, F.; Domenig, O.; Poglitsch, M.; Lazartigues, E. Clinical Relevance and Role of Neuronal AT(1) Receptors in ADAM17-Mediated ACE2 Shedding in Neurogenic Hypertension. Circ. Res. 2017, 121, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Hanff, T.C.; Harhay, M.O.; Brown, T.S.; Cohen, J.B.; Mohareb, A.M. Is There an Association Between COVID-19 Mortality and the Renin-Angiotensin System? A Call for Epidemiologic Investigations. Clin. Infect. Dis. 2020, 71, 870–874. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, J.; Chen, J.; Luo, Q.; Zhang, Q.; Zhang, H. Vitamin D alleviates lipopolysaccharide induced acute lung injury via regulation of the renin-angiotensin system. Mol. Med. Rep. 2017, 16, 7432–7438. [Google Scholar] [CrossRef] [PubMed]
- Kaparianos, A.; Argyropoulou, E. Local renin-angiotensin II systems, angiotensin-converting enzyme and its homologue ACE2: Their potential role in the pathogenesis of chronic obstructive pulmonary diseases, pulmonary hypertension and acute respiratory distress syndrome. Curr. Med. Chem. 2011, 18, 3506–3515. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Silwal, P.; Kim, I.; Modlin, R.L.; Jo, E.K. Vitamin D-cathelicidin axis: At the crossroads between protective immunity and pathological inflammation during Infection. Immune Netw. 2020, 20, e12. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S. Vitamin D as a defensin. J. Musculoskelet. Neuronal Interact. 2006, 6, 344–346. [Google Scholar]
- Mangin, M.; Sinha, R.; Fincher, K. Inflammation and vitamin D: The infection connection. Inflamm. Res. 2014, 63, 803–819. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.S.; Dombrowski, Y.; Koglin, S.; Ruzicka, T.; Schauber, J. Impact of vitamin D3 on cutaneous immunity and antimicrobial peptide expression. Derm. Endocrinol. 2011, 3, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lu, R.; Zhang, Y.G.; Sun, J. Vitamin D receptor deletion Leads to the destruction of tight and adheren junctions in lungs. Tissue Barriers 2018, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Booth, D.R.; Ding, N.; Parnell, G.P.; Shahijanian, F.; Coulter, S.; Schibeci, S.D.; Atkins, A.R.; Stewart, G.J.; Evans, R.M.; Downes, M.; et al. Cistromic and genetic evidence that the vitamin D receptor mediates susceptibility to latitude-dependent autoimmune diseases. Genes Immun. 2016, 17, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Racz, A.; Barsony, J. Hormone-dependent translocation of vitamin D receptors is linked to transactivation. J. Biol. Chem. 1999, 274, 19352–19360. [Google Scholar] [CrossRef] [PubMed]
- Seuter, S.; Neme, A.; Carlberg, C. Epigenome-wide effects of vitamin D and their impact on the transcriptome of human monocytes involve CTCF. Nucleic Acids Res. 2016, 44, 4090–4104. [Google Scholar] [CrossRef]
- Karlgren, M.; Miura, S.; Ingelman-Sundberg, M. Novel extrahepatic cytochrome P450s. Toxicol. Appl. Pharmacol. 2005, 207, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W.; Wagner, C.L. Clinical review: The role of the parent compound vitamin D with respect to metabolism and function: Why clinical dose intervals can affect clinical outcomes. J. Clin. Endocrinol. Metab. 2013, 98, 4619–4628. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.N.; Young, M.V.; Persons, K.S.; Wang, L.; Mathieu, J.S.; Whitlatch, L.W.; Holick, M.F.; Chen, T.C. Vitamin D metabolism in human prostate cells: Implications for prostate cancer chemoprevention by vitamin D. Anticancer Res. 2006, 26, 2567–2572. [Google Scholar]
- Zhu, J.; DeLuca, H.F. Vitamin D 25-hydroxylase—Four decades of searching, are we there yet? Arch. Biochem. Biophys. 2012, 523, 30–36. [Google Scholar] [CrossRef]
- Rahman, F.Z.; Hayee, B.; Chee, R.; Segal, A.W.; Smith, A.M. Impaired macrophage function following bacterial stimulation in chronic granulomatous disease. Immunology 2009, 128, 253–259. [Google Scholar] [CrossRef]
- Sunyecz, J.A. The use of calcium and vitamin D in the management of osteoporosis. Ther. Clin. Risk Manag. 2008, 4, 827–836. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Barrile, G.C.; Cavioni, A.; Mansueto, F.; Mazzola, G.; Oberto, L.; Patelli, Z.; Pirola, M.; Tartara, A.; et al. Nutrition, physical activity, and dietary supplementation to prevent bone mineral density loss: A food pyramid. Nutrients 2021, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Wang, M.; Miao, C.; Jin, D.; Wang, H. Mechanism of calcitriol regulating parathyroid cells in secondary hyperparathyroidism. Front. Pharmacol. 2022, 13, 1020858. [Google Scholar] [CrossRef] [PubMed]
- Lips, P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: Consequences for bone loss and fractures and therapeutic implications. Endocr. Rev. 2001, 22, 477–501. [Google Scholar] [CrossRef] [PubMed]
- Kota, S.; Jammula, S.; Kota, S.; Meher, L.; Modi, K. Correlation of vitamin D, bone mineral density and parathyroid hormone levels in adults with low bone density. Indian J. Orthop. 2013, 47, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Seely, E.W.; Wood, R.J.; Brown, E.M.; Graves, S.W. Lower serum ionized calcium and abnormal calciotropic hormone levels in preeclampsia. J. Clin. Endocrinol. Metab. 1992, 74, 1436–1440. [Google Scholar] [CrossRef]
- Jorde, R.; Grimnes, G. Serum cholecalciferol may be a better marker of vitamin D status than 25-hydroxyvitamin D. Med. Hypotheses 2018, 111, 61–65. [Google Scholar] [CrossRef]
- Fan, H.; Hui, L.; Yan, X.; Hou, W.; Bai, E.; Wang, L.; Yu, X. Serum 25 hydroxyvitamin D levels and affecting factors among preconception fertile women. BMC Womens Health 2020, 20, 146. [Google Scholar] [CrossRef]
- Miller, W.L. Genetic disorders of Vitamin D biosynthesis and degradation. J. Steroid Biochem. Mol. Biol. 2017, 165, 101–108. [Google Scholar] [CrossRef]
- Plum, L.A.; DeLuca, H.F. Vitamin D, disease and therapeutic opportunities. Nat. Rev. Drug Discov. 2010, 9, 941–955. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D and bone. Curr. Osteoporos. Rep. 2012, 10, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Laird, E.; Ward, M.; McSorley, E.; Strain, J.J.; Wallace, J. Vitamin D and bone health: Potential mechanisms. Nutrients 2010, 2, 693–724. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Chen, T.C.; Lu, Z.; Sauter, E. Vitamin D and skin physiology: A D-lightful story. J. Bone Miner. Res. 2007, 22 (Suppl. 2), V28–V33. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.; Genehr, T.; Knuschke, P.; Pietzsch, J.; Meurer, M. UVB-induced conversion of 7-dehydrocholesterol to 1alpha,25-dihydroxyvitamin D3 in an in vitro human skin equivalent model. J. Investig. Dermatol. 2001, 117, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Kagi, L.; Bettoni, C.; Pastor-Arroyo, E.M.; Schnitzbauer, U.; Hernando, N.; Wagner, C.A. Regulation of vitamin D metabolizing enzymes in murine renal and extrarenal tissues by dietary phosphate, FGF23, and 1,25(OH)2D3. PLoS ONE 2018, 13, e0195427. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Sarandeses, L.A.; Allewaert, K.; Zhao, J.; Mascarenas, J.L.; Mourino, A.; Vrielynck, S.; de Clercq, P.; Vandewalle, M. Biologic activity of dihydroxylated 19-nor-(pre)vitamin D3. J. Bone Miner. Res. 1993, 8, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Schuetz, E.G.; Xu, Y.; Thummel, K.E. Interplay between vitamin D and the drug metabolizing enzyme CYP3A4. J. Steroid Biochem. Mol. Biol. 2013, 136, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D: An essential component for skeletal health. Ann. NYAS 2012, 1240, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Associations of vitamin D with insulin resistance, obesity, type 2 diabetes, and metabolic syndrome. J. Steroid Biochem. Mol. Biol. 2018, 175, 177–189. [Google Scholar] [CrossRef]
- Adamczak, D.M. The role of Toll-Like receptors and vitamin D in cardiovascular diseases-A review. Int. J. Mol. Sci. 2017, 18, 2252. [Google Scholar] [CrossRef]
- Valdes-Lopez, J.F.; Velilla, P.; Urcuqui-Inchima, S. Vitamin D modulates the expression of Toll-like receptors and pro-inflammatory cytokines without affecting Chikungunya virus replication, in monocytes and macrophages. Acta Trop. 2022, 232, 106497. [Google Scholar] [CrossRef]
- Haussler, M.R.; Whitfield, G.K.; Haussler, C.A.; Hsieh, J.C.; Thompson, P.D.; Selznick, S.H.; Dominguez, C.E.; Jurutka, P.W. The nuclear vitamin D receptor: Biological and molecular regulatory properties revealed. J. Bone Miner. Res. 1998, 13, 325–349. [Google Scholar] [CrossRef]
- Wikvall, K. Cytochrome P450 enzymes in the bioactivation of vitamin D to its hormonal form (review). Int. J. Mol. Med. 2001, 7, 201–209. [Google Scholar] [CrossRef]
- Norman, A.W. From vitamin D to hormone D: Fundamentals of the vitamin D endocrine system essential for good health. Am. J. Clin. Nutr. 2008, 88, 491S–499S. [Google Scholar] [CrossRef]
- Biesalski, H.K.; Aggett, P.J.; Anton, R.; Bernstein, P.S.; Blumberg, J.; Heaney, R.P.; Henry, J.; Nolan, J.M.; Richardson, D.P.; van Ommen, B.; et al. 26th Hohenheim Consensus Conference, September 11, 2010 Scientific substantiation of health claims: Evidence-based nutrition. Nutrition 2011, 27, S1–S20. [Google Scholar] [CrossRef] [PubMed]
- Tsai, F.; Coyle, W.J. The microbiome and obesity: Is obesity linked to our gut flora? Curr. Gastroenterol. Rep. 2009, 11, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Zhu, X.; Manson, J.E.; Song, Y.; Li, X.; Franke, A.A.; Costello, R.B.; Rosanoff, A.; Nian, H.; Fan, L.; et al. Magnesium status and supplementation influence vitamin D status and metabolism: Results from a randomized trial. Am. J. Clin. Nutr. 2018, 108, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; O’Keefe, J.H. Magnesium and vitamin D deficiency as a potential cause of immune dysfunction, cytokine storm and disseminated intravascular coagulation in covid-19 patients. Mo. Med. 2021, 118, 68–73. [Google Scholar]
- Adams, J.S.; Modlin, R.L.; Diz, M.M.; Barnes, P.F. Potentiation of the macrophage 25-hydroxyvitamin D-1-hydroxylation reaction by human tuberculous pleural effusion fluid. J. Clin. Endocrinol. Metab. 1989, 69, 457–460. [Google Scholar] [CrossRef]
- Dong, D.; Noy, N. Heterodimer formation by retinoid X receptor: Regulation by ligands and by the receptor’s self-association properties. Biochemistry 1998, 37, 10691–10700. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1alpha,25(OH)(2)vitamin D(3): Genomic and non-genomic mechanisms. Best. Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. ACE inhibitors and angiotensin receptor blockers reduce the complications associated with COVID-19 infection. World J. Pharma Res. 2021, 10, 2579–2600. [Google Scholar] [CrossRef]
- Fu, Z.; Gilbert, E.R.; Liu, D. Regulation of Insulin Synthesis and Secretion and Pancreatic Beta-Cell Dysfunction in Diabetes. Curr. Diabetes Rev. 2013, 9, 25–53. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Hewison, M. Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Arch. Biochem. Biophys. 2012, 523, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Taniguchi, M.; Tsuruya, K.; Iida, M. Recurrent sarcoidosis with psoas muscle granuloma and hypercalcaemia in a patient on chronic haemodialysis. Nephrology 2009, 14, 452–453. [Google Scholar] [CrossRef]
- Khan, H.; Kunutsor, S.; Franco, O.H.; Chowdhury, R. Vitamin D, type 2 diabetes and other metabolic outcomes: A systematic review and meta-analysis of prospective studies. Proc. Nutr. Soc. 2013, 72, 89–97. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D and bone health. J. Nutr. 1996, 126, 1159S–1164S. [Google Scholar] [CrossRef]
- Kroll, M.H.; Bi, C.; Garber, C.C.; Kaufman, H.W.; Liu, D.; Caston-Balderrama, A.; Zhang, K.; Clarke, N.; Xie, M.; Reitz, R.E.; et al. Temporal relationship between vitamin D status and parathyroid hormone in the United States. PLoS ONE 2015, 10, e0118108. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Heaney, R.P.; Dowell, M.S.; Hale, C.A.; Bendich, A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J. Am. Coll. Nutr. 2003, 22, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Mark, K.A.; Dumas, K.J.; Bhaumik, D.; Schilling, B.; Davis, S.; Oron, T.R.; Sorensen, D.J.; Lucanic, M.; Brem, R.B.; Melov, S.; et al. Vitamin D Promotes Protein Homeostasis and Longevity via the Stress Response Pathway Genes skn-1, ire-1, and xbp-1. Cell Rep. 2016, 17, 1227–1237. [Google Scholar] [CrossRef]
- Steingrimsdottir, L.; Gunnarsson, O.; Indridason, O.S.; Franzson, L.; Sigurdsson, G. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA 2005, 294, 2336–2341. [Google Scholar] [CrossRef]
- Wahl, D.A.; Cooper, C.; Ebeling, P.R.; Eggersdorfer, M.; Hilger, J.; Hoffmann, K.; Josse, R.; Kanis, J.A.; Mithal, A.; Pierroz, D.D.; et al. A global representation of vitamin D status in healthy populations. Arch. Osteoporos. 2012, 7, 155–172. [Google Scholar] [CrossRef]
- Dahlquist, D.T.; Dieter, B.P.; Koehle, M.S. Plausible ergogenic effects of vitamin D on athletic performance and recovery. J. Int. Soc. Sports Nutr. 2015, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Gerster, H. The role of vitamin C in athletic performance. J. Am. Coll. Nutr. 1989, 8, 636–643. [Google Scholar] [CrossRef]
- Lips, P.; Binkley, N.; Pfeifer, M.; Recker, R.; Samanta, S.; Cohn, D.A.; Chandler, J.; Rosenberg, E.; Papanicolaou, D.A. Once-weekly dose of 8400 IU vitamin D(3) compared with placebo: Effects on neuromuscular function and tolerability in older adults with vitamin D insufficiency. Am. J. Clin. Nutr. 2010, 91, 985–991. [Google Scholar] [CrossRef]
- Blain, H.; Jaussent, A.; Thomas, E.; Micallef, J.P.; Dupuy, A.M.; Bernard, P.L.; Mariano-Goulart, D.; Cristol, J.P.; Sultan, C.; Rossi, M.; et al. Appendicular skeletal muscle mass is the strongest independent factor associated with femoral neck bone mineral density in adult and older men. Exp. Gerontol. 2010, 45, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Okuno, J.; Tomura, S.; Yanagi, H.; Kim, M.J.; Okura, T.; Tanaka, K. Evaluation of the association between impaired renal function and physical function among community-dwelling Japanese frail elderly based on the estimated glomerular filtration rate (eGFR). Nihon Ronen Igakkai Zasshi 2009, 46, 63–70. [Google Scholar] [CrossRef]
- Fuchtbauer, L.; Brusgaard, K.; Ledaal, P.; Frost, M.; Frederiksen, A.L. Case report: Vitamin D-dependent rickets type 1 caused by a novel CYP27B1 mutation. Clin. Case Rep. 2015, 3, 1012–1016. [Google Scholar] [CrossRef]
- Hu, W.W.; Ke, Y.H.; He, J.W.; Fu, W.Z.; Wang, C.; Zhang, H.; Yue, H.; Gu, J.M.; Zhang, Z.L. A novel compound mutation of CYP27B1 in a Chinese family with vitamin D-dependent rickets type 1A. J. Pediatr. Endocrinol. Metab. JPEM 2014, 27, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Watkins, P.B.; Wrighton, S.A.; Schuetz, E.G.; Molowa, D.T.; Guzelian, P.S. Identification of glucocorticoid-inducible cytochromes P-450 in the intestinal mucosa of rats and man. J. Clin. Investig. 1987, 80, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Cytochrome P-450 3A4: Regulation and role in drug metabolism. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Dragt, B.S.; Porte, R.J.; Groothuis, G.M. Regulation of VDR expression in rat and human intestine and liver--consequences for CYP3A expression. Toxicol. Vitr. 2010, 24, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Valdivielso, J.M. The physiology of vitamin D receptor activation. Contrib. Nephrol. 2009, 163, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Kim, J.J.; Mohr, S.B.; Gorham, E.D.; Grant, W.B.; Giovannucci, E.L.; Baggerly, L.; Hofflich, H.; Ramsdell, J.W.; Zeng, K.; et al. Meta-analysis of all-cause mortality according to serum 25-hydroxyvitamin D. Am. J. Public. Health 2014, 104, e43–e50. [Google Scholar] [CrossRef] [PubMed]
- Rostand, S.G. Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension 1979, 30, 150–156. [Google Scholar]
- Thomas, G.N.; o Hartaigh, B.; Bosch, J.A.; Pilz, S.; Loerbroks, A.; Kleber, M.E.; Fischer, J.E.; Grammer, T.B.; Bohm, B.O.; Marz, W. Vitamin D levels predict all-cause and cardiovascular disease mortality in subjects with the metabolic syndrome: The Ludwigshafen Risk and Cardiovascular Health (LURIC) Study. Diabetes Care 2012, 35, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D regulation of immune function during covid-19. Rev. Endocr. Metab. Disord. 2022, 23, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Boucher, B.J.; Bhattoa, H.P.; Lahore, H. Why vitamin D clinical trials should be based on 25-hydroxyvitamin D concentrations. J. Steroid Biochem. Mol. Biol. 2018, 177, 266–269. [Google Scholar] [CrossRef]
- Ray, J.A.; Meikle, A.W. D-light: Vitamin D and good health. MLO Med. Lab. Obs. 2010, 42, 32, 34–38. [Google Scholar]
- Gruson, D.; Ferracin, B.; Ahn, S.A.; Zierold, C.; Blocki, F.; Hawkins, D.M.; Bonelli, F.; Rousseau, M.F. 1,25-Dihydroxyvitamin D to PTH(1-84) Ratios Strongly Predict Cardiovascular Death in Heart Failure. PLoS ONE 2015, 10, e0135427. [Google Scholar] [CrossRef] [PubMed]
- Bislev, L.S.; Langagergaard Rodbro, L.; Bech, J.N.; Pedersen, E.B.; Kjaergaard, A.D.; Ladefoged, S.A.; Rolighed, L.; Sikjaer, T.; Rejnmark, L. The effect of vitamin D3 supplementation on markers of cardiovascular health in hyperparathyroid, vitamin D insufficient women: A randomized placebo-controlled trial. Endocrine 2018, 62, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Albaaj, F.; Hutchison, A. Hyperphosphataemia in renal failure: Causes, consequences and current management. Drugs 2003, 63, 577–596. [Google Scholar] [CrossRef] [PubMed]
- Umehara, K.; Mukai, N.; Hata, J.; Hirakawa, Y.; Ohara, T.; Yoshida, D.; Kishimoto, H.; Kitazono, T.; Hoka, S.; Kiyohara, Y.; et al. Association between serum vitamin D and all-cause and cause-specific death in a general Japanese population- The Hisayama Study. Circ. J. 2017, 81, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Ernst, J.B.; Pilz, S.; Dreier, J.; Kuhn, J.; Knabbe, C.; Gummert, J.F.; Morshuis, M.; Milting, H. Calciotropic and phosphaturic hormones in end-stage heart failure patients supported by a left-ventricular assist device. PLoS ONE 2016, 11, e0164459. [Google Scholar] [CrossRef] [PubMed]
- Giovinazzo, S.; Alibrandi, A.; Campenni, A.; Trimarchi, F.; Ruggeri, R.M. Correlation of cardio-metabolic parameters with vitamin D status in healthy premenopausal women. J. Endocrinol. Investig. 2017, 40, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Vigna, L.; Cassinelli, L.; Tirelli, A.S.; Felicetta, I.; Napolitano, F.; Tomaino, L.; Mutti, M.; Barberi, C.E.; Riboldi, L. 25(OH)D Levels in Relation to Gender, Overweight, Insulin Resistance, and Inflammation in a Cross-Sectional Cohort of Northern Italian Workers: Evidence in Support of Preventive Health Care Programs. J. Am. Coll. Nutr. 2017, 36, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.P.; D’Vaz, N.; Meldrum, S.; Palmer, D.J.; Zhang, G.; Prescott, S.L. 25-hydroxyvitamin D3 status is associated with developing adaptive and innate immune responses in the first 6 months of life. Clin. Exp. Allergy 2015, 45, 220–231. [Google Scholar] [CrossRef]
- Israel, A.; Cicurel, A.; Feldhamer, I.; Stern, F.; Dror, Y.; Giveon, S.M.; Gillis, D.; Strich, D.; Lavie, G. Vitamin D deficiency is associated with higher risks for SARS-CoV-2 infection and COVID-19 severity: A retrospective case-control study. Intern. Emerg. Med. 2022, 17, 1053–1063. [Google Scholar] [CrossRef]
- Amital, H.; Szekanecz, Z.; Szucs, G.; Danko, K.; Nagy, E.; Csepany, T.; Kiss, E.; Rovensky, J.; Tuchynova, A.; Kozakova, D.; et al. Serum concentrations of 25-OH vitamin D in patients with systemic lupus erythematosus (SLE) are inversely related to disease activity: Is it time to routinely supplement patients with SLE with vitamin D? Ann. Rheum. Dis. 2010, 69, 1155–1157. [Google Scholar] [CrossRef]
- Konstantakis, C.; Tselekouni, P.; Kalafateli, M.; Triantos, C. Vitamin D deficiency in patients with liver cirrhosis. Ann. Gastroenterol. 2016, 29, 297–306. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ichiyama, T.; Ohsaki, A.; Hasegawa, S.; Shiraishi, M.; Furukawa, S. Anti-inflammatory effect of 1alpha,25-dihydroxyvitamin D(3) in human coronary arterial endothelial cells: Implication for the treatment of Kawasaki disease. J. Steroid Biochem. Mol. Biol. 2009, 113, 134–138. [Google Scholar] [CrossRef]
- Ma, R.; Gu, Y.; Zhao, S.; Sun, J.; Groome, L.J.; Wang, Y. Expressions of vitamin D metabolic components VDBP, CYP2R1, CYP27B1, CYP24A1, and VDR in placentas from normal and preeclamptic pregnancies. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E928–E935. [Google Scholar] [CrossRef]
- Rondini, E.A.; Pant, A.; Kocarek, T.A. Transcriptional regulation of cytosolic sulfotransferase 1C2 by intermediates of the cholesterol biosynthetic pathway in primary cultured rat hepatocytes. J. Pharmacol. Exp. Ther. 2015, 355, 429–441. [Google Scholar] [CrossRef]
- Lohr, M.; Hummel, F.; Faulmann, G.; Ringel, J.; Saller, R.; Hain, J.; Gunzburg, W.H.; Salmons, B. Microencapsulated, CYP2B1-transfected cells activating ifosfamide at the site of the tumor: The magic bullets of the 21st century. Cancer Chemother. Pharmacol. 2002, 49 (Suppl. 1), S21–S24. [Google Scholar] [CrossRef]
- Morris, H.A.; Anderson, P.H. Autocrine and paracrine actions of vitamin D. Clin. Biochem. Rev. 2010, 31, 129–138. [Google Scholar]
- Powe, C.E.; Karumanchi, S.A.; Thadhani, R. Vitamin D-binding protein and vitamin D in blacks and whites. N. Engl. J. Med. 2014, 370, 880–881. [Google Scholar] [CrossRef]
- Powe, C.E.; Evans, M.K.; Wenger, J.; Zonderman, A.B.; Berg, A.H.; Nalls, M.; Tamez, H.; Zhang, D.; Bhan, I.; Karumanchi, S.A.; et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N. Engl. J. Med. 2013, 369, 1991–2000. [Google Scholar] [CrossRef]
- Feldman, D.; P, J.M. Mutations in the vitamin D receptor and hereditary vitamin D-resistant rickets. BoneKEy Rep. 2014, 3, 510. [Google Scholar] [CrossRef]
- Koren, R. Vitamin D receptor defects: The story of hereditary resistance to vitamin D. Pediatr. Endocrinol. Rev. 2006, 3 (Suppl. 3), 470–475. [Google Scholar]
- Al Mutair, A.N.; Nasrat, G.H.; Russell, D.W. Mutation of the CYP2R1 vitamin D 25-hydroxylase in a Saudi Arabian family with severe vitamin D deficiency. J. Clin. Endocrinol. Metab. 2012, 97, E2022–E2025. [Google Scholar] [CrossRef] [PubMed]
- Molin, A.; Wiedemann, A.; Demers, N.; Kaufmann, M.; Do Cao, J.; Mainard, L.; Dousset, B.; Journeau, P.; Abeguile, G.; Coudray, N.; et al. Vitamin D-dependent rickets Type 1B (25-hydroxylase deficiency): A rare condition or a misdiagnosed condition? J. Bone Miner. Res. 2017, 32, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Thacher, T.D.; Fischer, P.R.; Singh, R.J.; Roizen, J.; Levine, M.A. CYP2R1 mutations impair generation of 25-hydroxyvitamin D and cause an atypical form of vitamin D seficiency. J. Clin. Endocrinol. Metab. 2015, 100, E1005–E1013. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef] [PubMed]
- Thacher, T.D.; Levine, M.A. CYP2R1 mutations causing vitamin D-deficiency rickets. J. Steroid Biochem. Mol. Biol. 2017, 173, 333–336. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Decoding the paradox: Understanding elevated hospitalization and reduced mortality in SARS-CoV-2 variants. Int. J. Front. Sci. Technol. Res. 2024, 6, 1–20. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Vitamin D deficiency: Effects on oxidative stress, epigenetics, gene regulation, and aging. Biology 2019, 8, 30. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Reducing risks from COVID-19: Cost-effective ways of strengthening individual’s and the population immunity with vitamin D. J. Endocrinol. Sci. 2020, 2, 1–13. [Google Scholar] [CrossRef]
- Diamond, T.H.; Ho, K.W.; Rohl, P.G.; Meerkin, M. Annual intramuscular injection of a megadose of cholecalciferol for treatment of vitamin D deficiency: Efficacy and safety data. Med. J. Aust. 2005, 183, 10–12. [Google Scholar] [CrossRef]
- Sanders, K.M.; Stuart, A.L.; Williamson, E.J.; Simpson, J.A.; Kotowicz, M.A.; Young, D.; Nicholson, G.C. Annual high-dose oral vitamin D and falls and fractures in older women: A randomized controlled trial. JAMA 2010, 303, 1815–1822. [Google Scholar] [CrossRef]
- Heaney, R.P.; Armas, L.A. Quantifying the vitamin D economy. Nutr. Rev. 2015, 73, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Vieth, R. How to optimize vitamin D supplementation to prevent cancer, based on cellular adaptation and hydroxylase enzymology. Anticancer Res. 2009, 29, 3675–3684. [Google Scholar] [PubMed]
- Ketha, H.; Thacher, T.D.; Oberhelman, S.S.; Fischer, P.R.; Singh, R.J.; Kumar, R. Comparison of the effect of daily versus bolus dose maternal vitamin D3 supplementation on the 24,25-dihydroxyvitamin D3 25-hydroxyvitamin D3 ratio. Bone 2018, 110, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Rooney, M.R.; Harnack, L.; Michos, E.D.; Ogilvie, R.P.; Sempos, C.T.; Lutsey, P.L. Trends in Use of High-Dose Vitamin D Supplements Exceeding 1000 or 4000 International Units Daily, 1999–2014. JAMA 2017, 317, 2448–2450. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, D.M.; Cardona, A.; Urcuqui-Inchima, S. High-dose of vitamin D supplement is associated with reduced susceptibility of monocyte-derived macrophages to dengue virus infection and pro-inflammatory cytokine production: An exploratory study. Clin. Chim. Acta 2017, 478, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Behjat Sasan, S.; Zandvakili, F.; Soufizadeh, N.; Baybordi, E. The Effects of Vitamin D Supplement on Prevention of Recurrence of Preeclampsia in Pregnant Women with a History of Preeclampsia. Obstet. Gynecol. Int. 2017, 2017, 8249264. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Optimum duration and safety of long-term use of potent anti-resorptive medications in osteoporosis. Expert. Rev. Endocrinol. Metab. 2016, 11, 329–348. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J.; Whittle, R. Vitamin D: A single initial dose is not bogus if followed by an appropriate maintenance intake. JBMR Plus 2022, 6, e10606. [Google Scholar] [CrossRef]
- Cannell, J.J.; Vieth, R.; Willett, W.; Zasloff, M.; Hathcock, J.N.; White, J.H.; Tanumihardjo, S.A.; Larson-Meyer, D.E.; Bischoff-Ferrari, H.A.; Lamberg-Allardt, C.J.; et al. Cod liver oil, vitamin A toxicity, frequent respiratory infections, and the vitamin D deficiency epidemic. Ann. Otol. Rhinol. Laryngol. 2008, 117, 864–870. [Google Scholar] [CrossRef]
- Shamim, N.; Majid, H.; Khemani, S.; Salim, M.; Muneer, S.; Khan, A.H. Inappropriate supplementation of vitamin D can result in toxicity: A cross-sectional study of paediatrics population. JPMA J. Pak. Med. Assoc. 2023, 73, 500–504. [Google Scholar] [CrossRef]
- Aljehani, F.; Qashqari, M.B.; Alghamdi, M.K.; Saadi, A.I.; Alreasini, M.Y.; Alsolami, E.; Alfawaz, M. Prevalence of iatrogenic vitamin D toxicity among the Saudi population of vitamin D users due to overcorrection. Cureus 2023, 15, e37521. [Google Scholar] [CrossRef] [PubMed]
- Asif, A.; Farooq, N. Vitamin D Toxicity. In StatPearls (Internet); Natl Cntr Biotechnol Info: Treasure Island, FL, USA, 2024. [Google Scholar]
- Lim, K.; Thadhani, R. Vitamin D Toxicity. J. Bras. Nefrol. 2020, 42, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Vieth, R. Vitamin D toxicity, policy, and science. J. Bone Miner. Res. 2007, 22 (Suppl. 2), V64–V68. [Google Scholar] [CrossRef] [PubMed]
- Koutkia, P.; Chen, T.C.; Holick, M.F. Vitamin D intoxication associated with an over-the-counter supplement. N. Engl. J. Med. 2001, 345, 66–67. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Holick, M.F.; Alfonso, B.D.; Charlap, E.; Romero, C.M.; Rizk, D.; Newman, L.G. Vitamin D intoxication with severe hypercalcemia due to manufacturing and labeling errors of two dietary supplements made in the United States. J. Clin. Endocrinol. Metab. 2011, 96, 3603–3608. [Google Scholar] [CrossRef] [PubMed]
- Jones, G. Pharmacokinetics of vitamin D toxicity. Am. J. Clin. Nutr. 2008, 88, 582S–586S. [Google Scholar] [CrossRef] [PubMed]
- Wyon, M.A.; Koutedakis, Y.; Wolman, R.; Nevill, A.M.; Allen, N. The influence of winter vitamin D supplementation on muscle function and injury occurrence in elite ballet dancers: A controlled study. J. Sci. Med. Sport. 2014, 17, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R.; Whiteman, D.C.; Kimlin, M.G.; Janda, M.; Clarke, M.W.; Lucas, R.M.; Neale, R.E. Effect of solar ultraviolet radiation exposure on serum 25(OH)D concentration: A pilot randomised controlled trial. Photochem. Photobiol. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Armas, L.A.; Hollis, B.W.; Heaney, R.P. Vitamin D2 is much less effective than vitamin D3 in humans. J. Clin. Endocrinol. Metab. 2004, 89, 5387–5391. [Google Scholar] [CrossRef]
- Lappe, J.; Watson, P.; Travers-Gustafson, D.; Recker, R.; Garland, C.; Gorham, E.; Baggerly, K.; McDonnell, S.L. Effect of vitamin D and calcium supplementation on cancer incidence in older women: A randomized clinical trial. JAMA 2017, 317, 1234–1243. [Google Scholar] [CrossRef]
- Perez-Lopez, F.R. Vitamin D and its implications for musculoskeletal health in women: An update. Maturitas 2007, 58, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Nasri, H.; Behradmanesh, S.; Ahmadi, A.; Rafieian-Kopaei, M. Impact of oral vitamin D (cholecalciferol) replacement therapy on blood pressure in type 2 diabetes patients; a randomized, double-blind, placebo controlled clinical trial. J. Nephropathol. 2014, 3, 29–33. [Google Scholar] [CrossRef]
- Gaksch, M.; Jorde, R.; Grimnes, G.; Joakimsen, R.; Schirmer, H.; Wilsgaard, T.; Mathiesen, E.B.; Njolstad, I.; Lochen, M.L.; Marz, W.; et al. Vitamin D and mortality: Individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS ONE 2017, 12, e0170791. [Google Scholar] [CrossRef] [PubMed]
- Tretli, S.; Schwartz, G.G.; Torjesen, P.A.; Robsahm, T.E. Serum levels of 25-hydroxyvitamin D and survival in Norwegian patients with cancer of breast, colon, lung, and lymphoma: A population-based study. Cancer Causes Control 2012, 23, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.Y.; Kim, M.K.; Jung, S.; Shin, J.; Choi, B.Y. The cross-sectional relationships of dietary and serum vitamin D with cardiometabolic risk factors: Metabolic components, subclinical atherosclerosis, and arterial stiffness. Nutrition 2016, 32, 1048–1056.e1. [Google Scholar] [CrossRef] [PubMed]
- Souberbielle, J.C.; Body, J.J.; Lappe, J.M.; Plebani, M.; Shoenfeld, Y.; Wang, T.J.; Bischoff-Ferrari, H.A.; Cavalier, E.; Ebeling, P.R.; Fardellone, P.; et al. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: Recommendations for clinical practice. Autoimmun. Rev. 2010, 9, 709–715. [Google Scholar] [CrossRef]
- Park, S.K.; Garland, C.F.; Gorham, E.D.; BuDoff, L.; Barrett-Connor, E. Plasma 25-hydroxyvitamin D concentration and risk of type 2 diabetes and pre-diabetes: 12-year cohort study. PLoS ONE 2018, 13, e0193070. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Cross, H.S.; Garland, C.F.; Gorham, E.D.; Moan, J.; Peterlik, M.; Porojnicu, A.C.; Reichrath, J.; Zittermann, A. Estimated benefit of increased vitamin D status in reducing the economic burden of disease in western Europe. Prog. Biophys. Mol. Biol. 2009, 99, 104–113. [Google Scholar] [CrossRef]
- McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Baggerly, L.L.; Garland, C.F.; Gorham, E.D.; Hollis, B.W.; Trump, D.L.; Lappe, J.M. Breast cancer risk markedly lower with serum 25-hydroxyvitamin D concentrations >/=60 vs <20 ng/mL (150 vs 50 nmol/L): Pooled analysis of two randomized trials and a prospective cohort. PLoS ONE 2018, 13, e0199265. [Google Scholar] [CrossRef]
- Heaney, R.P.; Holick, M.F. Why the IOM recommendations for vitamin D are deficient. J. Bone Miner. Res. 2011, 26, 455–457. [Google Scholar] [CrossRef]
- Pender, M.P. CD8+ T-cell deficiency, Epstein-Barr virus infection, vitamin D deficiency, and steps to autoimmunity: A unifying hypothesis. Autoimmune Dis. 2012, 2012, 189096. [Google Scholar] [CrossRef] [PubMed]
- Gominak, S.C. Vitamin D deficiency changes the intestinal microbiome reducing B vitamin production in the gut. The resulting lack of pantothenic acid adversely affects the immune system, producing a “pro-inflammatory” state associated with atherosclerosis and autoimmunity. Med. Hypotheses 2016, 94, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.L.; Dahl, L.; Olson, G.; Thornton, D.; Graff, I.E.; Froyland, L.; Thayer, J.F.; Pallesen, S. Fish consumption, sleep, daily functioning, and heart rate variability. J. Clin. Sleep. Med. 2014, 10, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Shah, S.; Long, Q.; Crankshaw, A.K.; Tangpricha, V. Improvement of pain, sleep, and quality of life in chronic pain patients with vitamin D supplementation. Clin. J. Pain. 2013, 29, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Dudenkov, D.V.; Yawn, B.P.; Oberhelman, S.S.; Fischer, P.R.; Singh, R.J.; Cha, S.S.; Maxson, J.A.; Quigg, S.M.; Thacher, T.D. Changing Incidence of Serum 25-hydroxyvitamin D values above 50 ng/mL: A 10-year population-based study. Mayo Clin. Proc. 2015, 90, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Brashear, M.M.; Johnson, W.D. Prediabetes and prehypertension in healthy adults are associated with low vitamin D levels. Diabetes Care 2011, 34, 658–660. [Google Scholar] [CrossRef] [PubMed]
- Hamed, E.A.; Abu Faddan, N.H.; Adb Elhafeez, H.A.; Sayed, D. Parathormone—25(OH)-vitamin D axis and bone status in children and adolescents with type 1 diabetes mellitus. Pediatr. Diabetes 2011, 12, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, A.; Kamali Sarvestani, H.; Rafat, Z.; Ghaderkhani, S.; Mahmoudi-Aliabadi, M.; Jafarzadeh, B.; Mehrtash, V. The association between the level of serum 25(OH) vitamin D, obesity, and underlying diseases with the risk of developing COVID-19 infection: A case-control study of hospitalized patients in Tehran, Iran. J. Med. Virol. 2021, 93, 2359–2364. [Google Scholar] [CrossRef]
- Munger, K.L.; Zhang, S.M.; O’Reilly, E.; Hernan, M.A.; Olek, M.J.; Willett, W.C.; Ascherio, A. Vitamin D intake and incidence of multiple sclerosis. Neurology 2004, 62, 60–65. [Google Scholar] [CrossRef]
- Merlino, L.A.; Curtis, J.; Mikuls, T.R.; Cerhan, J.R.; Criswell, L.A.; Saag, K.G.; Iowa Women’s Health, S. Vitamin D intake is inversely associated with rheumatoid arthritis: Results from the Iowa Women’s Health Study. Arthritis Rheum. 2004, 50, 72–77. [Google Scholar] [CrossRef]
- Dai, Q.; Zhang, Y.; Liu, Q.; Zhang, C. Efficacy and safety of vitamin D supplementation on psoriasis: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0294239. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, D.A.; Prindiville, S.; Weiss, D.G.; Willett, W.; Group, V.A.C.S. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA 2003, 290, 2959–2967. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Boucher, B.J. Randomized controlled trials of vitamin D and cancer incidence: A modeling study. PLoS ONE 2017, 12, e0176448. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.L.; Robertson, A.S.; Rodriguez, C.; Jacobs, E.J.; Chao, A.; Carolyn, J.; Calle, E.E.; Willett, W.C.; Thun, M.J. Calcium, vitamin D, dairy products, and risk of colorectal cancer in the cancer prevention study II nutrition cohort (United States). Cancer Causes Control 2003, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, V.; Vieth, R. Three-year follow-up of serum 25-hydroxyvitamin D, parathyroid hormone, and bone mineral density in nursing home residents who had received 12 months of daily bread fortification with 125 mug of vitamin D(3). Nutr. J. 2013, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J.; Polonowita, A.K. Logical analysis of response of health officials’ worldwide, to cost-effective early remedies for COVID-19. World J. Adv. Pharma Life Sci. 2023, 5, 13–34. [Google Scholar] [CrossRef]
- BishopL, E.; Ismailova, A.; Dimeloe, S.; Hewison, M.; White, J.H. Vitamin D and immune regulation: Antibacterial, antiviral, anti-inflammatory. JBMR Plus 2021, 5, e10405. [Google Scholar] [CrossRef] [PubMed]
- Bilezikian, J.P.; Bikle, D.; Hewison, M.; Lazaretti-Castro, M.; Formenti, A.M.; Gupta, A.; Madhavan, M.V.; Nair, N.; Babalyan, V.; Hutchings, N.; et al. Mechanisms in Endocrinology: Vitamin D and COVID-19. Eur. J. Endocrinol. 2020, 183, R133–R147. [Google Scholar] [CrossRef] [PubMed]
- Grenade, N.; Kosar, C.; Steinberg, K.; Avitzur, Y.; Wales, P.W.; Courtney-Martin, G. Use of a Loading Dose of Vitamin D for Treatment of Vitamin D Deficiency in Patients with Intestinal Failure. JPEN J. Parenter. Enter. Nutr. 2017, 41, 512–516. [Google Scholar] [CrossRef]
- Sainaghi, P.P.; Bellan, M.; Nerviani, A.; Sola, D.; Molinari, R.; Cerutti, C.; Pirisi, M. Superiority of a high loading dose of cholecalciferol to correct hypovitaminosis d in patients with inflammatory/autoimmune rheumatic diseases. J. Rheumatol. 2013, 40, 166–172. [Google Scholar] [CrossRef]
- Griffin, G.; Hewison, M.; Hopkin, J.; Kenny, R.A.; Quinton, R.; Rhodes, J.; Subramanian, S.; Thickett, D. Preventing vitamin D deficiency during the COVID-19 pandemic: UK definitions of vitamin D sufficiency and recommended supplement dose are set too low. Clin. Med. 2021, 21, e48–e51. [Google Scholar] [CrossRef]
- van Driel, M.; Koedam, M.; Buurman, C.J.; Hewison, M.; Chiba, H.; Uitterlinden, A.G.; Pols, H.A.; van Leeuwen, J.P. Evidence for auto/paracrine actions of vitamin D in bone: 1alpha-hydroxylase expression and activity in human bone cells. FASEB J. 2006, 20, 2417–2419. [Google Scholar] [CrossRef]
- Bader, D.A.; Abed, A.; Mohammad, B.A.; Aljaberi, A.; Sundookah, A.; Habash, M.; Alsayed, A.R.; Abusamak, M.; Al-Shakhshir, S.; Abu-Samak, M. The Effect of Weekly 50,000 IU Vitamin D(3) Supplements on the Serum Levels of Selected Cytokines Involved in Cytokine Storm: A Randomized Clinical Trial in Adults with Vitamin D Deficiency. Nutrients 2023, 15, 1188. [Google Scholar] [CrossRef]
- Binkley, N.; Gemar, D.; Engelke, J.; Gangnon, R.; Ramamurthy, R.; Krueger, D.; Drezner, M.K. Evaluation of ergocalciferol or cholecalciferol dosing, 1,600 IU daily or 50,000 IU monthly in older adults. J. Clin. Endocrinol. Metab. 2011, 96, 981–988. [Google Scholar] [CrossRef]
- Binkley, N.; Gemar, D.; Engelke, J.; Gangnon, R.; Ramamurthy, R.; Krueger, D. Dosing with ergocalciferol or cholecalciferol, 1,600 IU daily or 50,000 IU monthly, is safe but does not assure vitamin D adequacy (Abst.). J. Bone Miner. Res. 2009, 24 (Suppl. 1). [Google Scholar]
- Havens, P.L.; Mulligan, K.; Hazra, R.; Flynn, P.; Rutledge, B.; Van Loan, M.D.; Lujan-Zilbermann, J.; Kapogiannis, B.G.; Wilson, C.M.; Stephensen, C.B.; et al. Serum 25-hydroxyvitamin D response to vitamin D3 supplementation 50,000 IU monthly in youth with HIV-1 infection. J. Clin. Endocrinol. Metab. 2012, 97, 4004–4013. [Google Scholar] [CrossRef]
- Mazahery, H.; Stonehouse, W.; von Hurst, P.R. The effect of monthly 50,000 IU or 100,000 IU vitamin D supplements on vitamin D status in premenopausal Middle Eastern women living in Auckland. Eur. J. Clin. Nutr. 2015, 69, 367–372. [Google Scholar] [CrossRef]
- Middleton, L.; Stack, B.C., Jr.; Riggs, A.T.; Bodenner, D.L. Vitamin D deficiency: A simple algorithm employing weekly administration of 50,000 IU of vitamin D. Am. J. Otolaryngol. 2014, 35, 85–88. [Google Scholar] [CrossRef]
- Manaseki-Holland, S.; Maroof, Z.; Bruce, J.; Mughal, M.Z.; Masher, M.I.; Bhutta, Z.A.; Walraven, G.; Chandramohan, D. Effect on the incidence of pneumonia of vitamin D supplementation by quarterly bolus dose to infants in Kabul: A randomised controlled superiority trial. Lancet 2012, 379, 1419–1427. [Google Scholar] [CrossRef]
- Rothen, J.P.; Rutishauser, J.; Walter, P.N.; Hersberger, K.E.; Arnet, I. Vitamin D oral intermittent treatment (DO IT) study, a randomized clinical trial with individual loading regimen. Sci. Rep. 2021, 11, 18746. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.T.; Cui, Q.Q.; Hong, Y.M.; Yao, W.G. A meta-analysis of high dose, intermittent vitamin D supplementation among older adults. PLoS ONE 2015, 10, e0115850. [Google Scholar] [CrossRef] [PubMed]
- Chauss, D.; Freiwald, T.; McGregor, R.; Yan, B.; Wang, L.; Nova-Lamperti, E.; Kumar, D.; Zhang, Z.; Teague, H.; West, E.E.; et al. Autocrine vitamin D signaling switches off pro-inflammatory programs of T(H)1 cells. Nat. Immunol. 2022, 23, 62–74. [Google Scholar] [CrossRef]
- Chau, A.S.; Weber, A.G.; Maria, N.I.; Narain, S.; Liu, A.; Hajizadeh, N.; Malhotra, P.; Bloom, O.; Marder, G.; Kaplan, B. The Longitudinal Immune Response to Coronavirus Disease 2019: Chasing the Cytokine Storm. Arthritis Rheumatol. 2021, 73, 23–35. [Google Scholar] [CrossRef]
- Suñé Negre, J.; Azpitarte, I.O.; Barrios, P.D.A.; Hernández Herrero, G. Calcifediol Soft Capsules. Available online: https://patents.google.com/patent/WO2016124724A1/en?oq=WO+2016124724 (accessed on 7 April 2024).
- Chiu, K.C.; Chu, A.; Go, V.L.; Saad, M.F. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am. J. Clin. Nutr. 2004, 79, 820–825. [Google Scholar] [CrossRef]
- Feskanich, D.; Willett, W.C.; Colditz, G.A. Calcium, vitamin D, milk consumption, and hip fractures: A prospective study among postmenopausal women. Am. J. Clin. Nutr. 2003, 77, 504–511. [Google Scholar] [CrossRef]
- Meier, C.; Woitge, H.W.; Witte, K.; Lemmer, B.; Seibel, M.J. Supplementation with oral vitamin D3 and calcium during winter prevents seasonal bone loss: A randomized controlled open-label prospective trial. J. Bone Miner. Res. 2004, 19, 1221–1230. [Google Scholar] [CrossRef]
- Theodoratou, E.; Tzoulaki, I.; Zgaga, L.; Ioannidis, J.P. Vitamin D and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ 2014, 348, g2035. [Google Scholar] [CrossRef]
- Autier, P.; Mullie, P.; Macacu, A.; Dragomir, M.; Boniol, M.; Coppens, K.; Pizot, C.; Boniol, M. Effect of vitamin D supplementation on non-skeletal disorders: A systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017, 5, 986–1004. [Google Scholar] [CrossRef]
- Ryan, J.W.; Anderson, P.H.; Morris, H.A. Pleiotropic Activities of Vitamin D Receptors—Adequate Activation for Multiple Health Outcomes. Clin. Biochem. Rev. 2015, 36, 53–61. [Google Scholar]
- Zipitis, C.S.; Elazabi, A.; Samanta, S. Vitamin D deficiency and guideline awareness. Arch. Dis. Child. Fetal Neonatal Ed. 2011, 96, F310. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Wimalawansa, S.J.; Holick, M.F.; Cannell, J.J.; Pludowski, P.; Lappe, J.M.; Pittaway, M.; May, P. Emphasizing the health benefits of vitamin D for those with neurodevelopmental disorders and intellectual disabilities. Nutrients 2015, 7, 1538–1564. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D adequacy and improvements of comorbidities in persons with intellectual developmental disabilities. J. Child. Dev. Disord. 2016, 2, 22–33. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).