N-Acetylcysteine Alleviates Impaired Muscular Function Resulting from Sphingosine Phosphate Lyase Functional Deficiency-Induced Sphingoid Base and Ceramide Accumulation in Caenorhabditis elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture of Nematode Strains
2.2. RNAi Experiments
2.3. Lifespan Assay
2.4. Pharyngeal Pumping Rate Assay
2.5. Body Movement Assay
2.6. Body Length Assay
2.7. Myofiber and Mitochondria Morphology Measurements
2.8. ROS Measurement
2.9. N-Acetylcysteine Treatment
2.10. Lipid Extraction
2.11. Sphingolipid Analysis
2.12. Reverse-Transcription PCR
2.13. Statistical Analysis
3. Results
3.1. Spl-1 RNA Interference Reduces Lifespan and Impairs Locomotion in Caenorhabditis elegans
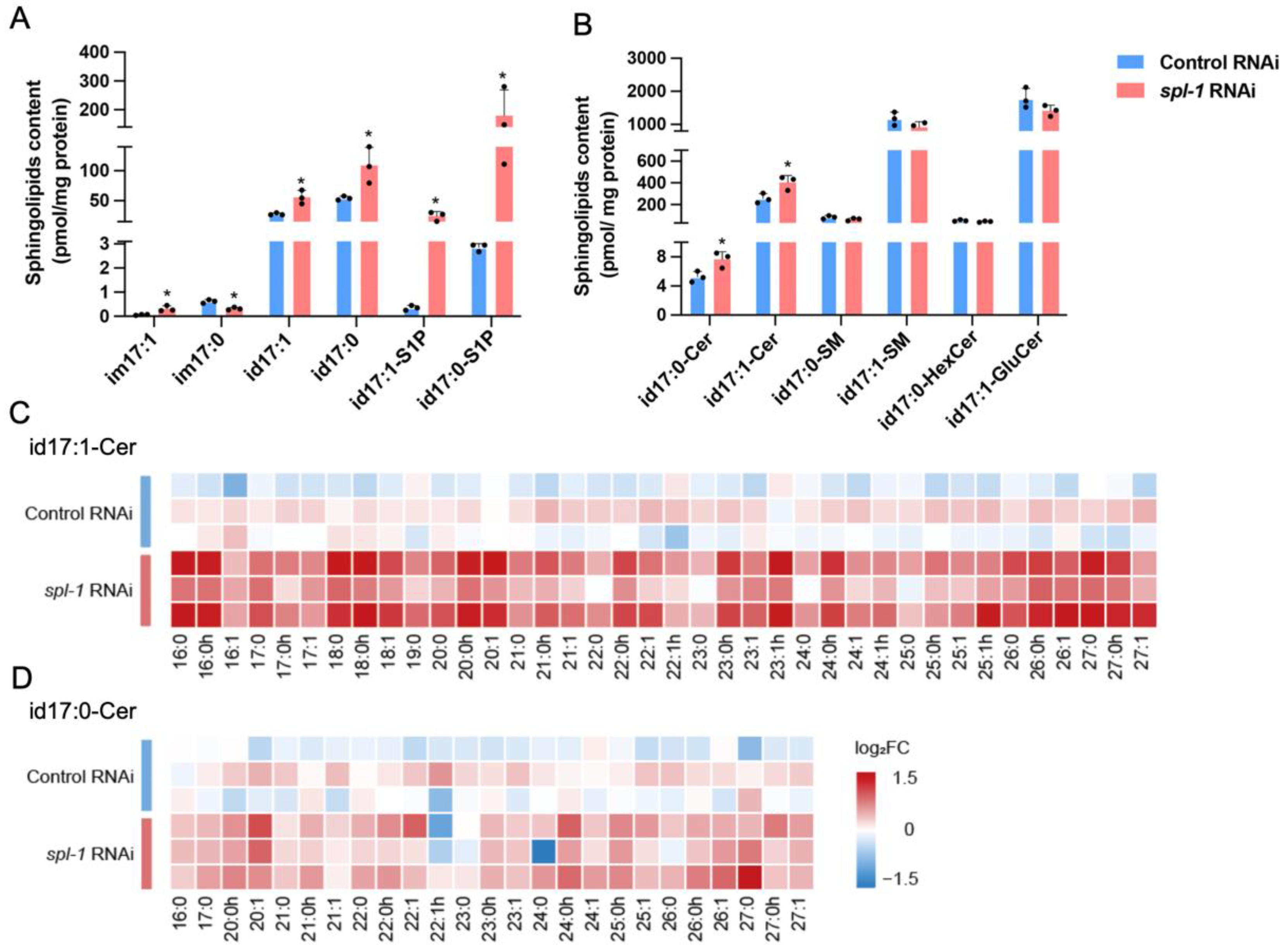
3.2. SPL Loss-of-Function Leads to Accumulations of Sphingoid Bases and Ceramides in C. elegans
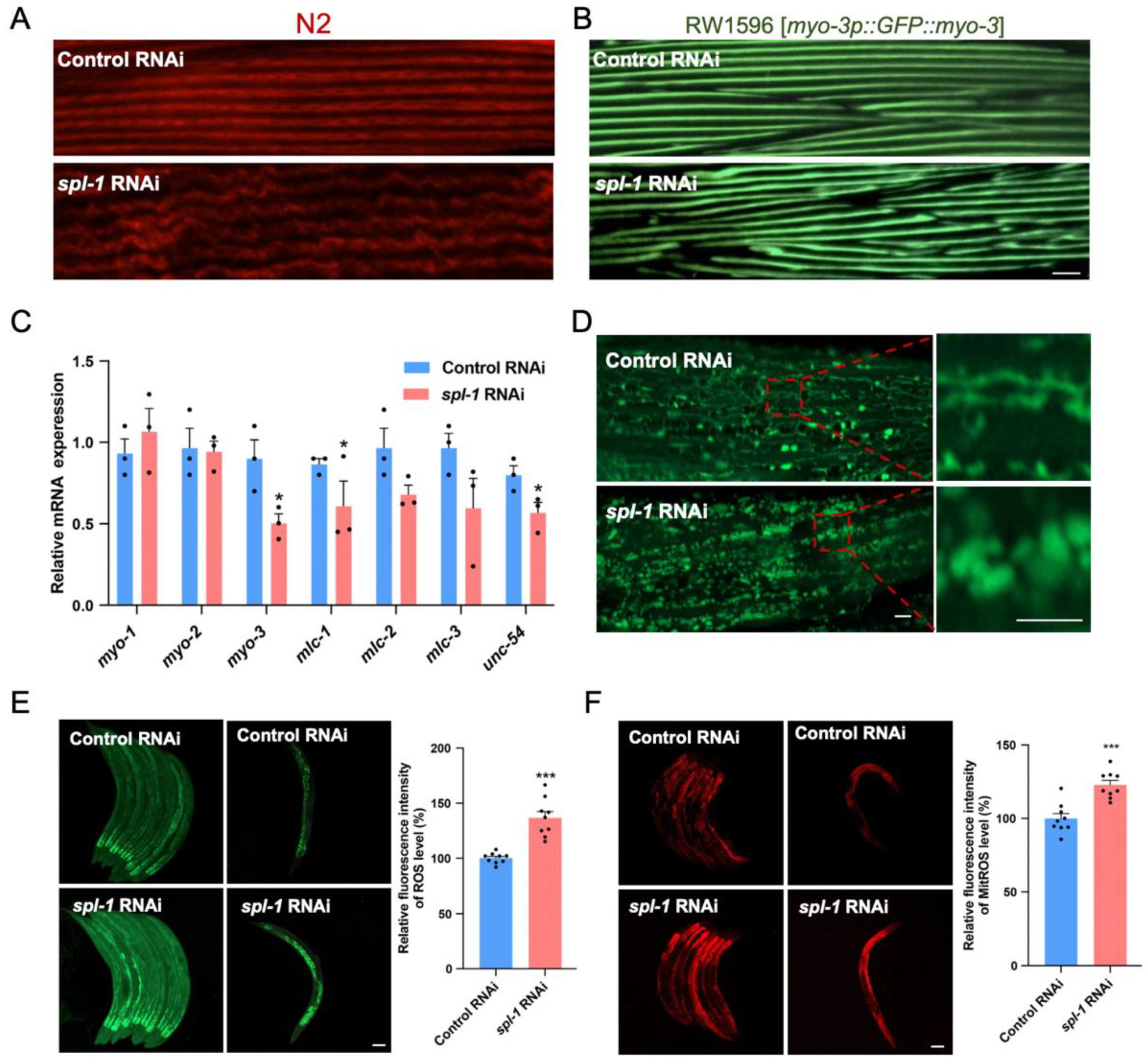
3.3. Spl-1 RNAi Disrupts Muscle Fiber Organization and Elevates ROS Levels in C. elegans
3.4. NAC Supplementation Alleviates Muscle Morphology Defects and Motor Impairment in SPL-Deficient C. elegans
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- Green, C.D.; Maceyka, M.; Cowart, L.A.; Spiegel, S. Sphingolipids in metabolic disease: The good, the bad, and the unknown. Cell Metab. 2021, 33, 1293–1306. [Google Scholar] [CrossRef] [PubMed]
- Trayssac, M.; Hannun, Y.A.; Obeid, L.M. Role of sphingolipids in senescence: Implication in aging and age-related diseases. J. Clin. Investig. 2018, 128, 2702–2712. [Google Scholar] [CrossRef] [PubMed]
- Uranbileg, B.; Kurano, M.; Kano, K.; Sakai, E.; Arita, J.; Hasegawa, K.; Nishikawa, T.; Ishihara, S.; Yamashita, H.; Seto, Y.; et al. Sphingosine 1-phosphate lyase facilitates cancer progression through converting sphingolipids to glycerophospholipids. Clin. Transl. Med. 2022, 12, e1056. [Google Scholar] [CrossRef] [PubMed]
- Weigel, C.; Hüttner, S.S.; Ludwig, K.; Krieg, N.; Hofmann, S.; Schröder, N.H.; Robbe, L.; Kluge, S.; Nierhaus, A.; Winkler, M.S.; et al. S1P lyase inhibition protects against sepsis by promoting disease tolerance via the S1P/S1PR3 axis. EBioMedicine 2020, 58, 102898. [Google Scholar] [CrossRef] [PubMed]
- Cencetti, F.; Bruno, G.; Bernacchioni, C.; Japtok, L.; Puliti, E.; Donati, C.; Bruni, P. Sphingosine 1-phosphate lyase blockade elicits myogenic differentiation of murine myoblasts acting via Spns2/S1P(2) receptor axis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158759. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gorshkova, I.A.; Berdyshev, E.; He, D.; Fu, P.; Ma, W.; Su, Y.; Usatyuk, P.V.; Pendyala, S.; Oskouian, B.; et al. Protection of LPS-induced murine acute lung injury by sphingosine-1-phosphate lyase suppression. Am. J. Respir. Cell Mol. Biol. 2011, 45, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Schwab, S.R.; Pereira, J.P.; Matloubian, M.; Xu, Y.; Huang, Y.; Cyster, J.G. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 2005, 309, 1735–1739. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, D.; Nikodinovic Glumac, J.; Asselbergh, B.; Ermanoska, B.; Blocquel, D.; Steiner, R.; Estrada-Cuzcano, A.; Peeters, K.; Ooms, T.; De Vriendt, E.; et al. Sphingosine 1-phosphate lyase deficiency causes Charcot-Marie-Tooth neuropathy. Neurology 2017, 88, 533–542. [Google Scholar] [CrossRef]
- Schwiebs, A.; Herrero San Juan, M.; Schmidt, K.G.; Wiercinska, E.; Anlauf, M.; Ottenlinger, F.; Thomas, D.; Elwakeel, E.; Weigert, A.; Farin, H.F.; et al. Cancer-induced inflammation and inflammation-induced cancer in colon: A role for S1P lyase. Oncogene 2019, 38, 4788–4803. [Google Scholar] [CrossRef]
- Herr, D.R.; Fyrst, H.; Phan, V.; Heinecke, K.; Georges, R.; Harris, G.L.; Saba, J.D. Sply regulation of sphingolipid signaling molecules is essential for Drosophila development. Development 2003, 130, 2443–2453. [Google Scholar] [CrossRef] [PubMed]
- Oskouian, B.; Sooriyakumaran, P.; Borowsky, A.D.; Crans, A.; Dillard-Telm, L.; Tam, Y.Y.; Bandhuvula, P.; Saba, J.D. Sphingosine-1-phosphate lyase potentiates apoptosis via p53- and p38-dependent pathways and is down-regulated in colon cancer. Proc. Natl. Acad. Sci. USA 2006, 103, 17384–17389. [Google Scholar] [CrossRef] [PubMed]
- Borowsky, A.D.; Bandhuvula, P.; Kumar, A.; Yoshinaga, Y.; Nefedov, M.; Fong, L.G.; Zhang, M.; Baridon, B.; Dillard, L.; de Jong, P.; et al. Sphingosine-1-phosphate lyase expression in embryonic and adult murine tissues. J. Lipid Res. 2012, 53, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Van Veldhoven, P.P.; Gijsbers, S.; Mannaerts, G.P.; Vermeesch, J.R.; Brys, V. Human sphingosine-1-phosphate lyase: cDNA cloning, functional expression studies and mapping to chromosome 10q22(1). Biochim. Biophys. Acta 2000, 1487, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Bektas, M.; Allende, M.L.; Lee, B.G.; Chen, W.; Amar, M.J.; Remaley, A.T.; Saba, J.D.; Proia, R.L. Sphingosine 1-phosphate lyase deficiency disrupts lipid homeostasis in liver. J. Biol. Chem. 2010, 285, 10880–10889. [Google Scholar] [CrossRef] [PubMed]
- Tan-Chen, S.; Guitton, J.; Bourron, O.; Le Stunff, H.; Hajduch, E. Sphingolipid Metabolism and Signaling in Skeletal Muscle: From Physiology to Physiopathology. Front. Endocrinol. 2020, 11, 491. [Google Scholar] [CrossRef] [PubMed]
- Bandet, C.L.; Tan-Chen, S.; Bourron, O.; Le Stunff, H.; Hajduch, E. Sphingolipid Metabolism: New Insight into Ceramide-Induced Lipotoxicity in Muscle Cells. Int. J. Mol. Sci. 2019, 20, 479. [Google Scholar] [CrossRef] [PubMed]
- Wohlwend, M.; Laurila, P.P.; Goeminne, L.J.E.; Lima, T.; Daskalaki, I.; Li, X.; von Alvensleben, G.; Crisol, B.; Mangione, R.; Gallart-Ayala, H.; et al. Inhibition of CERS1 in skeletal muscle exacerbates age-related muscle dysfunction. Elife 2024, 12, RP90522. [Google Scholar] [CrossRef] [PubMed]
- Tosetti, B.; Brodesser, S.; Brunn, A.; Deckert, M.; Blüher, M.; Doehner, W.; Anker, S.D.; Wenzel, D.; Fleischmann, B.; Pongratz, C.; et al. A tissue-specific screen of ceramide expression in aged mice identifies ceramide synthase-1 and ceramide synthase-5 as potential regulators of fiber size and strength in skeletal muscle. Aging Cell 2020, 19, e13049. [Google Scholar] [CrossRef]
- Lima, T.I.; Laurila, P.P.; Wohlwend, M.; Morel, J.D.; Goeminne, L.J.E.; Li, H.; Romani, M.; Li, X.; Oh, C.M.; Park, D.; et al. Inhibiting de novo ceramide synthesis restores mitochondrial and protein homeostasis in muscle aging. Sci. Transl. Med. 2023, 15, eade6509. [Google Scholar] [CrossRef]
- Laurila, P.-P.; Wohlwend, M.; Imamura de Lima, T.; Luan, P.; Herzig, S.; Zanou, N.; Crisol, B.; Bou-Sleiman, M.; Porcu, E.; Gallart-Ayala, H. Sphingolipids accumulate in aged muscle, and their reduction counteracts sarcopenia. Nature Aging 2022, 2, 1159–1175. [Google Scholar] [CrossRef] [PubMed]
- Hla, T.; Dannenberg, A.J. Sphingolipid signaling in metabolic disorders. Cell Metab. 2012, 16, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.M.; Janes, M.S.; Beckman, J.S. The selective detection of mitochondrial superoxide by live cell imaging. Nat. Protoc. 2008, 3, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Petrov, A.M.; Shalagina, M.N.; Protopopov, V.A.; Sergeev, V.G.; Ovechkin, S.V.; Ovchinina, N.G.; Sekunov, A.V.; Zefirov, A.L.; Zakirjanova, G.F.; Bryndina, I.G. Changes in Membrane Ceramide Pools in Rat Soleus Muscle in Response to Short-Term Disuse. Int. J. Mol. Sci. 2019, 20, 4860. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Q.; Abidi, P.; Afaq, F.; Schiffmann, D.; Mossman, B.T.; Kamp, D.W.; Athar, M. Glutathione redox system in oxidative lung injury. Crit. Rev. Toxicol. 1999, 29, 543–568. [Google Scholar] [CrossRef] [PubMed]
- Fyrst, H.; Saba, J.D. An update on sphingosine-1-phosphate and other sphingolipid mediators. Nat. Chem. Biol. 2010, 6, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Garris, C.S.; Blaho, V.A.; Hla, T.; Han, M.H. Sphingosine-1-phosphate receptor 1 signalling in T cells: Trafficking and beyond. Immunology 2014, 142, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Gudipaty, S.A.; Rosenblatt, J. Epithelial cell extrusion: Pathways and pathologies. Semin. Cell Dev. Biol. 2017, 67, 132–140. [Google Scholar] [CrossRef]
- Kono, M.; Allende, M.L.; Proia, R.L. Sphingosine-1-phosphate regulation of mammalian development. Biochim. Biophys. Acta 2008, 1781, 435–441. [Google Scholar] [CrossRef]
- Takuwa, Y.; Du, W.; Qi, X.; Okamoto, Y.; Takuwa, N.; Yoshioka, K. Roles of sphingosine-1-phosphate signaling in angiogenesis. World J. Biol. Chem. 2010, 1, 298–306. [Google Scholar] [CrossRef]
- Bandet, C.L.; Tan-Chen, S.; Ali-Berrada, S.; Campana, M.; Poirier, M.; Blachnio-Zabielska, A.; Pais-de-Barros, J.P.; Rouch, C.; Ferré, P.; Foufelle, F.; et al. Ceramide analog C2-cer induces a loss in insulin sensitivity in muscle cells through the salvage/recycling pathway. J. Biol. Chem. 2023, 299, 104815. [Google Scholar] [CrossRef] [PubMed]
- Bergman, B.C.; Brozinick, J.T.; Strauss, A.; Bacon, S.; Kerege, A.; Bui, H.H.; Sanders, P.; Siddall, P.; Wei, T.; Thomas, M.K.; et al. Muscle sphingolipids during rest and exercise: A C18:0 signature for insulin resistance in humans. Diabetologia 2016, 59, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Ogata, T.; Yamasaki, Y. Ultra-high-resolution scanning electron microscopy of mitochondria and sarcoplasmic reticulum arrangement in human red, white, and intermediate muscle fibers. Anat. Rec. Off. Publ. Am. Assoc. Anat. 1997, 248, 214–223. [Google Scholar] [CrossRef]
- Hammerschmidt, P.; Ostkotte, D.; Nolte, H.; Gerl, M.J.; Jais, A.; Brunner, H.L.; Sprenger, H.G.; Awazawa, M.; Nicholls, H.T.; Turpin-Nolan, S.M.; et al. CerS6-Derived Sphingolipids Interact with Mff and Promote Mitochondrial Fragmentation in Obesity. Cell 2019, 177, 1536–1552.e1523. [Google Scholar] [CrossRef] [PubMed]
- Jamil, M.; Cowart, L.A. Sphingolipids in mitochondria-from function to disease. Front. Cell Dev. Biol. 2023, 11, 1302472. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, A.; Williams, J.; Bradshaw, T.; Güran, T.; Braslavsky, D.; Casas, J.; Chan, L.; Metherell, L.; Prasad, R. Sphingosine-1-phosphate lyase (SGPL1) deficiency is associated with mitochondrial dysfunction. J. Steroid Biochem. Mol. Biol. 2020, 202, 105730. [Google Scholar] [CrossRef] [PubMed]
- Zigdon, H.; Kogot-Levin, A.; Park, J.W.; Goldschmidt, R.; Kelly, S.; Merrill, A.H., Jr.; Scherz, A.; Pewzner-Jung, Y.; Saada, A.; Futerman, A.H. Ablation of ceramide synthase 2 causes chronic oxidative stress due to disruption of the mitochondrial respiratory chain. J. Biol. Chem. 2013, 288, 4947–4956. [Google Scholar] [CrossRef]
- Redwan, A.; Kiriaev, L.; Kueh, S.; Morley, J.W.; Houweling, P.; Perry, B.D.; Head, S.I. Six weeks of N-acetylcysteine antioxidant in drinking water decreases pathological fiber branching in MDX mouse dystrophic fast-twitch skeletal muscle. Front. Physiol. 2023, 14, 1109587. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; You, Y.; Zhu, H.; Chen, Y.; Hu, Z.; Duan, J. N-Acetylcysteine Alleviates Impaired Muscular Function Resulting from Sphingosine Phosphate Lyase Functional Deficiency-Induced Sphingoid Base and Ceramide Accumulation in Caenorhabditis elegans. Nutrients 2024, 16, 1623. https://doi.org/10.3390/nu16111623
Liu M, You Y, Zhu H, Chen Y, Hu Z, Duan J. N-Acetylcysteine Alleviates Impaired Muscular Function Resulting from Sphingosine Phosphate Lyase Functional Deficiency-Induced Sphingoid Base and Ceramide Accumulation in Caenorhabditis elegans. Nutrients. 2024; 16(11):1623. https://doi.org/10.3390/nu16111623
Chicago/Turabian StyleLiu, Min, Yunfei You, Huaiyi Zhu, Yu Chen, Zhenying Hu, and Jingjing Duan. 2024. "N-Acetylcysteine Alleviates Impaired Muscular Function Resulting from Sphingosine Phosphate Lyase Functional Deficiency-Induced Sphingoid Base and Ceramide Accumulation in Caenorhabditis elegans" Nutrients 16, no. 11: 1623. https://doi.org/10.3390/nu16111623
APA StyleLiu, M., You, Y., Zhu, H., Chen, Y., Hu, Z., & Duan, J. (2024). N-Acetylcysteine Alleviates Impaired Muscular Function Resulting from Sphingosine Phosphate Lyase Functional Deficiency-Induced Sphingoid Base and Ceramide Accumulation in Caenorhabditis elegans. Nutrients, 16(11), 1623. https://doi.org/10.3390/nu16111623





