Circulating Omega-3 Polyunsaturated Fatty Acids Levels in Coronary Heart Disease: Pooled Analysis of 36 Observational Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Quality Assessment
2.3. Exposures and Outcomes
2.4. Data Extraction
2.5. Data Processing and Statistical Analysis
2.6. Subgroup Analysis
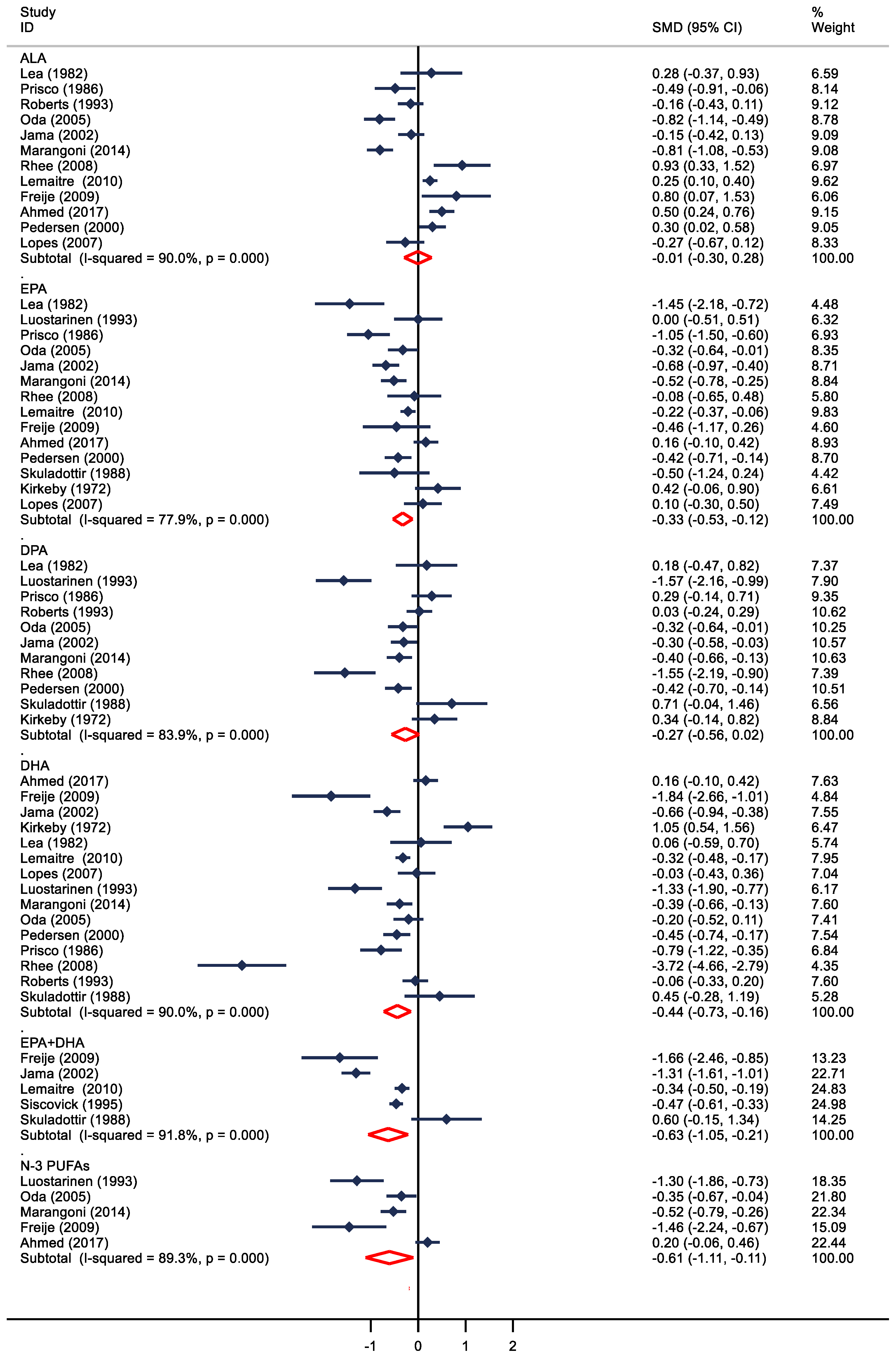
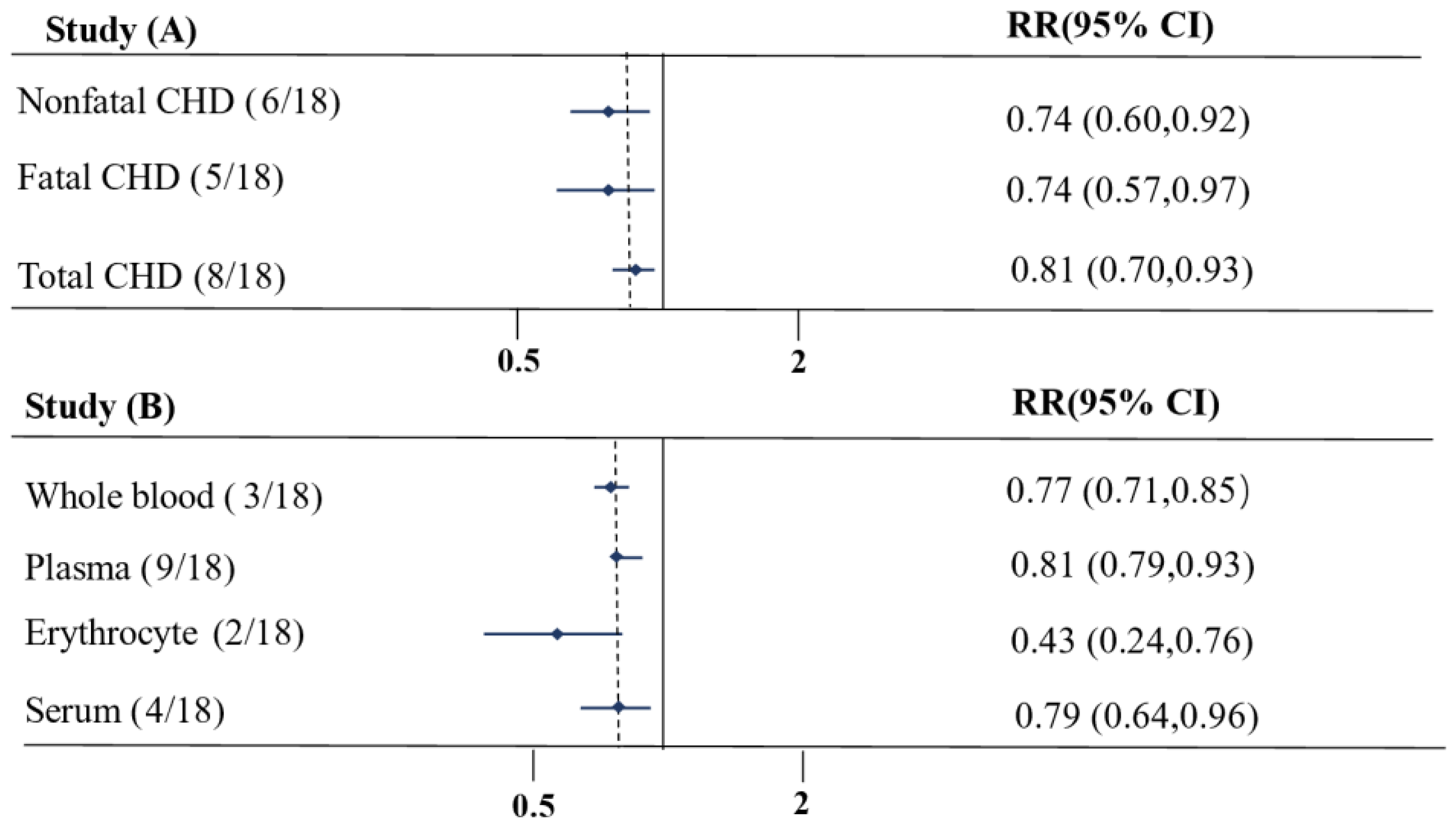
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dyerberg, J.; Bang, H.O. Hæmostatic Function and Platelet Polyunsaturated Fatty Acids in Eskimos. Lancet 1979, 314, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P.; Zock, P.L.; Kester, A.D. and Katan, M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 2003, 77, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.L. Dietary n−6 and n−3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 2009, 77, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Weylandt, K.H.; Chiu, C.Y.; Gomolka, B.; Waechter, S.F. and Wiedenmann, B. l. Omega-3 fatty acids and their lipid mediators: Towards an understanding of resolvin and protectin formation. Prostaglandins Other Lipid Mediat. 2012, 97, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish Consumption, Fish Oil, Omega-3 Fatty Acids, and Cardiovascular Disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Investigators, G.P. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Lancet 1999, 354, 447–455. [Google Scholar] [CrossRef]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H. and Kita, T. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle Jr, R.T.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef]
- Harris, W.S.; Tintle, N.L.; Etherton, M.R.; Vasan, R.S. Erythrocyte long-chain omega-3 fatty acid levels are inversely associated with mortality and with incident cardiovascular disease: The Framingham Heart Study. J. Clin. Lipidol. 2018, 12, 718–727.e6. [Google Scholar] [CrossRef]
- Kromhout, D.; Giltay, E.J.; Geleijnse, J.M. n–3 Fatty Acids and Cardiovascular Events after Myocardial Infarction. N. Engl. J. Med. 2010, 363, 2015–2026. [Google Scholar] [CrossRef] [PubMed]
- Investigators, O.T. n–3 Fatty Acids and Cardiovascular Outcomes in Patients with Dysglycemia. N. Engl. J. Med. 2012, 367, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine n−3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.; Koenig, W.; McGuire, D.K.; et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA 2020, 324, 2268–2280. [Google Scholar] [CrossRef] [PubMed]
- Aung, T.; Halsey, J.; Kromhout, D.; Gerstein, H.C.; Marchioli, R.; Tavazzi, L.; Geleijnse, J.M.; Rauch, B.; Ness, A.; Galan, P.; et al. Associations of Omega-3 Fatty Acid Supplement Use with Cardiovascular Disease Risks: Meta-analysis of 10 Trials Involving 77 917 Individuals. JAMA Cardiol. 2018, 3, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Hu, F.B.; Manson, J.E. Marine Omega-3 Supplementation and Cardiovascular Disease: An Updated Meta-Analysis of 13 Randomized Controlled Trials Involving 127 477 Participants. J. Am. Heart Assoc. 2019, 8, e013543. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Gong, C.; Jin, K.; Zhou, L.; Xiao, Y.; Ma, L. Omega-3 Fatty Acid Supplementation and Coronary Heart Disease Risks: A Meta-Analysis of Randomized Controlled Clinical Trials. Front. Nutr. 2022, 9, 809311. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.; AlAbdulghafoor, F.K.; Summerbell, C.D.; Worthington, H.V. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 11, CD003177. [Google Scholar] [CrossRef] [PubMed]
- Lattka, E.; Illig, T.; Heinrich, J.; Koletzko, B. Do FADS genotypes enhance our knowledge about fatty acid related phenotypes? Clin. Nutr. 2010, 29, 277–287. [Google Scholar] [CrossRef]
- de Lorgeril, M.; Salen, P.; Defaye, P.; Rabaeus, M. Recent findings on the health effects of omega-3 fatty acids and statins, and their interactions: Do statins inhibit omega-3? BMC Med. 2013, 11, 5. [Google Scholar] [CrossRef]
- Reigada, L.C.; Storch, B.; Alku, D.; Hazeltine, D.B.; Heppelmann, P.G.; Polokowski, A.R. Assessment of polyunsaturated fatty acids: A self-report and biomarker assessment with a racially and ethnically diverse sample of women. Prostaglandins Leukot. Essent. Fat. Acids 2021, 164, 102214. [Google Scholar]
- Harris, W.S.; Tintle, N.L.; Imamura, F.; Qian, F.; Korat, A.V.A.; Marklund, M.; Djoussé, L.; Bassett, J.K.; Carmichael, P.H.; Chen, Y.Y. Blood n-3 fatty acid levels and total and cause-specific mortality from 17 prospective studies. Nat. Commun. 2021, 12, 2329. [Google Scholar] [CrossRef] [PubMed]
- Del Gobbo, L.C.; Imamura, F.; Aslibekyan, S.; Marklund, M.; Virtanen, J.K.; Wennberg, M.; Yakoob, M.Y.; Chiuve, S.E.; Dela Cruz, L.; Frazier-Wood, A.C. ω-3 Polyunsaturated Fatty Acid Biomarkers and Coronary Heart Disease: Pooling Project of 19 Cohort Studies. JAMA Intern. Med. 2016, 176, 1155–1166. [Google Scholar] [CrossRef]
- Pan, A.; Chen, M.; Chowdhury, R.; Wu, J.H.; Sun, Q.; Campos, H.; Mozaffarian, D.; Hu, F.B. α-Linolenic acid and risk of cardiovascular disease: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 96, 1262–1273. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, K.F. What’s the Relative Risk? A Method of Correcting the Odds Ratio in Cohort Studies of Common Outcomes. JAMA 1998, 280, 1690–1691. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629. [Google Scholar] [CrossRef]
- Sun, Q.; Ma, J.; Campos, H.; Rexrode, K.M.; Albert, C.M.; Mozaffarian, D.; Hu, F.B. Blood concentrations of individual long-chain n–3 fatty acids and risk of nonfatal myocardial infarction1. Am. J. Clin. Nutr. 2008, 88, 216–223. [Google Scholar] [CrossRef]
- Sun, Y.; Koh, W.P.; Yuan, J.M.; Choi, H.; Su, J.; Ong, C.N.; van Dam, R.M. Plasma α-Linolenic and Long-Chain ω-3 Fatty Acids Are Associated with a Lower Risk of Acute Myocardial Infarction in Singapore Chinese Adults123. J. Nutr. 2016, 146, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, R.N.; King, I.B.; Mozaffarian, D.; Kuller, L.H.; Tracy, R.P.; Siscovick, D.S. n−3 Polyunsaturated fatty acids, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: The Cardiovascular Health Study. Am. J. Clin. Nutr. 2003, 77, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Matthan, N.R.; Ooi, E.M.; Van Horn, L.; Neuhouser, M.L.; Woodman, R.; Lichtenstein, A.H. Plasma Phospholipid Fatty Acid Biomarkers of Dietary Fat Quality and Endogenous Metabolism Predict Coronary Heart Disease Risk: A Nested Case-Control Study within the Women’s Health Initiative Observational Study. J. Am. Heart Assoc. 2014, 3, e000764. [Google Scholar] [CrossRef] [PubMed]
- Khaw, K.T.; Friesen, M.D.; Riboli, E.; Luben, R.; Wareham, N. Plasma phospholipid fatty acid concentration and incident coronary heart disease in men and women: The EPIC-Norfolk prospective study. PLoS Med. 2012, 9, e1001255. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Otto, M.C.; Wu, J.H.; Baylin, A.; Vaidya, D.; Rich, S.S.; Tsai, M.Y.; Jacobs, D.R., Jr.; Mozaffarian, D. Circulating and Dietary Omega-3 and Omega-6 Polyunsaturated Fatty Acids and Incidence of CVD in the Multi-Ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 2013, 2, e000506. [Google Scholar] [CrossRef] [PubMed]
- de Goede, J.; Verschuren, W.M.; Boer, J.M.; Verberne, L.D.; Kromhout, D.; Geleijnse, J.M. N-6 and N-3 Fatty Acid Cholesteryl Esters in Relation to Fatal CHD in a Dutch Adult Population: A Nested Case-Control Study and Meta-Analysis. PLoS ONE 2013, 8, e59408. [Google Scholar] [CrossRef] [PubMed]
- Guallar, E.; Hennekens, C.H.; Sacks, F.M.; Willett, W.C.; Stampfer, M.J. A prospective study of plasma fish oil levels and incidence of myocardial infarction in U.S. male physicians. J. Am. Coll. Cardiol. 1995, 25, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Lemaitre, R.N.; King, I.B.; Song, X.; Huang, H.; Sacks, F.M.; Rimm, E.B.; Wang, M.; Siscovick, D.S. Plasma phospholipid long-chain ω-3 fatty acids and total and cause-specific mortality in older adults: A cohort study. Ann. Intern. Med. 2013, 158, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Hamazaki, K.; Iso, H.; Eshak, E.S.; Ikehara, S.; Ikeda, A.; Iwasaki, M.; Hamazaki, T.; Tsugane, S.; Tsugane, S.; Sawada, N. Plasma levels of n-3 fatty acids and risk of coronary heart disease among Japanese: The Japan Public Health Center-based (JPHC) study. Atherosclerosis 2018, 272, 226–232. [Google Scholar] [CrossRef]
- Liu, Q.; Matthan, N.R.; Manson, J.E.; Howard, B.V.; Tinker, L.F.; Neuhouser, M.L.; Van Horn, L.V.; Rossouw, J.E.; Allison, M.A.; Martin, L.W. Plasma Phospholipid Fatty Acids and Coronary Heart Disease Risk: A Matched Case-Control Study within the Women’s Health Initiative Observational Study. Nutrients 2019, 11, 1672. [Google Scholar] [CrossRef]
- Simon, J.A.; Hodgkins, M.L.; Browner, W.S.; Neuhaus, J.M.; Bernert Jr, J.T.; Hulley, S.B. Serum Fatty Acids and the Risk of Coronary Heart Disease. Am. J. Epidemiol. 1995, 142, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Erkkilä, A.T.; Lehto, S.; Pyörälä, K.; Uusitupa, M.I. n−3 Fatty acids and 5-y risks of death and cardiovascular disease events in patients with coronary artery disease. Am. J. Clin. Nutr. 2003, 78, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Zelniker, T.A.; Morrow, D.A.; Scirica, B.M.; Furtado, J.D.; Guo, J.; Mozaffarian, D.; Sabatine, M.S.; O’Donoghue, M.L. Plasma Omega-3 Fatty Acids and the Risk of Cardiovascular Events in Patients After an Acute Coronary Syndrome in MERLIN-TIMI 36. J. Am. Heart Assoc. 2021, 10, e017401. [Google Scholar] [CrossRef] [PubMed]
- Chei, C.L.; Yamagishi, K.; Kitamura, A.; Kiyama, M.; Sankai, T.; Okada, T.; Imano, H.; Ohira, T.; Cui, R.; Umesawa, M. Serum fatty acid and risk of coronary artery disease―Circulatory risk in communities study (CIRCS). Circ. J. 2018, 82, 3013–3020. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Reid, K.J.; Sands, S.A.; Spertus, J.A. Blood Omega-3 and Trans Fatty Acids in Middle-Aged Acute Coronary Syndrome Patients. Am. J. Cardiol. 2007, 99, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Block, R.C.; Harris, W.S.; Reid, K.J.; Sands, S.A.; Spertus, J.A. EPA and DHA in blood cell membranes from acute coronary syndrome patients and controls. Atherosclerosis 2008, 197, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Albert, C.M.; Campos, H.; Stampfer, M.J.; Ridker, P.M.; Manson, J.E.; Willett, W.C.; Ma, J. Blood Levels of Long-Chain n–3 Fatty Acids and the Risk of Sudden Death. N. Engl. J. Med. 2002, 346, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, C.; Sala-Vila, A.; Galié, S.; Muralidharan, J.; Estruch, R.; Fitó, M.; Razquin, C.; Corella, D.; Ros, E.; Timiraos, J. Association between Fatty Acids of Blood Cell Membranes and Incidence of Coronary Heart Disease. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 819–825. [Google Scholar] [CrossRef]
- Freije, A. Fatty acid profile of the erythrocyte membranes of healthy Bahraini citizens in comparison with coronary heart disease patients. J. Oleo Sci. 2009, 58, 379–388. [Google Scholar] [CrossRef]
- Lea, E.J.A.; Jones, S.P.; Hamilton, D.V. The fatty acids of erythrocytes of myocardial infarction patients. Atherosclerosis 1982, 41, 363–369. [Google Scholar] [CrossRef]
- Lemaitre, R.N.; King, I.B.; Sotoodehnia, N.; Knopp, R.H.; Mozaffarian, D.; McKnight, B.; Rea, T.D.; Rice, K.; Friedlander, Y.; Lumley, T.S. Endogenous red blood cell membrane fatty acids and sudden cardiac arrest. Metabolism 2010, 59, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Prisco, D.; Rogasi, P.G.; Matucci, M.; Abbate, R.; Gensini, G.F.; Serneri, G.G.N. Increased thromboxane a2 generation and altered membrane fatty acid composition in platelets from patients with active angina pectoris. Thromb. Res. 1986, 44, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Siscovick, D.S.; Raghunathan, T.; King, I.; Weinmann, S.; Wicklund, K.G.; Albright, J.; Bovbjerg, V.; Arbogast, P.; Smith, H.; Kushi, L.H. Dietary Intake and Cell Membrane Levels of Long-Chain n-3 Polyunsaturated Fatty Acids and the Risk of Primary Cardiac Arrest. JAMA 1995, 274, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- Hadj Ahmed, S.; Kaoubaa, N.; Kharroubi, W.; Zarrouk, A.; Najjar, M.F.; Batbout, F.; Gamra, H.; Lizard, G.; Hammami, M. Association of plasma fatty acid alteration with the severity of coronary artery disease lesions in Tunisian patients. Lipids Health Dis. 2017, 16, 154. [Google Scholar] [CrossRef] [PubMed]
- Rhee, Y.; Paik, M.J.; Kim, K.R.; Ko, Y.G.; Kang, E.S.; Cha, B.S.; Lee, H.C.; Lim, S.K. Plasma free fatty acid level patterns according to cardiovascular risk status in postmenopausal women. Clin. Chim. Acta 2008, 392, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Yli-Jama, P.; Meyer, H.; Ringstad, J.; Pedersen, J. Serum free fatty acid pattern and risk of myocardial infarction: A case-control study. J. Intern. Med. 2002, 251, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Kirkeby, K.; Ingvaldsen, P.; Bjerkedal, I. Fatty acid composition of serum lipids in men with myocardial infarction. Acta Medica Scand. 1972, 192, 513–519. [Google Scholar] [CrossRef]
- Oda, E.; Hatada, K.; Katoh, K.; Kodama, M.; Nakamura, Y.; Aizawa, Y. A case-control pilot study on n-3 polyunsaturated fatty acid as a negative risk factor for myocardial infarction. Int. Heart J. 2005, 46, 583–591. [Google Scholar] [CrossRef]
- Skuladottir, G.; Benediktsdottir, E.; Hardarson, T.; Hallgrimsson, J.; Oddsson, G.; Sigfusson, N.; Gudbjarnason, S. Arachidonic Acid Level of Non-esterified Fatty Acids and Phospholipids in Serum and Heart Muscle of Patients with Fatal Myocardial Infarction. Acta Medica Scand. 1988, 223, 233–238. [Google Scholar] [CrossRef]
- Marangoni, F.; Novo, G.; Perna, G.; Filardi, P.P.; Pirelli, S.; Ceroti, M.; Querci, A.; Poli, A. Omega-6 and omega-3 polyunsaturated fatty acid levels are reduced in whole blood of Italian patients with a recent myocardial infarction: The AGE-IM study. Atherosclerosis 2014, 232, 334–338. [Google Scholar] [CrossRef]
- Pedersen, J.; Ringstad, J.; Almendingen, K.; Haugen, T.; Stensvold, I.; Thelle, D. Adipose tissue fatty acids and risk of myocardial infarction—A case-control study. Eur. J. Clin. Nutr. 2000, 54, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.; Wood, D.; Riemersma, R.; Gallagher, P.; Lampe, F. Linoleic acid and risk of sudden cardiac death. Br. Heart J. 1993, 70, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.; Aro, A.; Azevedo, A.; Ramos, E.; Barros, H. Intake and Adipose Tissue Composition of Fatty Acids and Risk of Myocardial Infarction in a Male Portuguese Community Sample. J. Am. Diet. Assoc. 2007, 107, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Luostarinena, R.; Boberg, M.; Saldeen, T. Fatty acid composition in total phospholipids of human coronary arteries in sudden cardiac death. Atherosclerosis 1993, 99, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, A.A.; Lavie, C.J.; Milani, R.V.; Laukkanen, J.A. Omega-3 Benefits Remain Strong Post-STRENGTH. Mayo Clin. Proc. 2021, 96, 1371–1372. [Google Scholar] [CrossRef] [PubMed]
- Rizos, E.C.; Markozannes, G.; Tsapas, A.; Mantzoros, C.S.; Ntzani, E.E. Omega-3 supplementation and cardiovascular disease: Formulation-based systematic review and meta-analysis with trial sequential analysis. Heart 2021, 107, 150. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.; Sun, J.; Ge, Y.; Wang, C. Effect of omega-3 fatty acids supplementation on the prognosis of coronary artery disease: A meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Group, A.S.C. Effects of n−3 Fatty Acid Supplements in Diabetes Mellitus. N. Engl. J. Med. 2018, 379, 1540–1550. [Google Scholar]
- Manson, J.E.; Bassuk, S.S.; Lee, I.M.; Cook, N.R.; Albert, M.A.; Gordon, D.; Zaharris, E.; MacFadyen, J.G.; Danielson, E.; Lin, J.; et al. The Vitamin D and OmegA-3 TriaL (VITAL): Rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp. Clin. Trials 2012, 33, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Kastelein, J.J.; Maki, K.C.; Susekov, A.; Ezhov, M.; Nordestgaard, B.G.; Machielse, B.N.; Kling, D.; Davidson, M.H. Omega-3 free fatty acids for the treatment of severe hypertriglyceridemia: The EpanoVa fOr Lowering Very high triglyceridEs (EVOLVE) trial. J. Clin. Lipidol. 2014, 8, 94–106. [Google Scholar] [CrossRef]
- Khan, S.U.; Lone, A.N.; Khan, M.S.; Virani, S.S.; Blumenthal, R.S.; Nasir, K.; Miller, M.; Michos, E.D.; Ballantyne, C.M.; Boden, W.E. Effect of omega-3 fatty acids on cardiovascular outcomes: A systematic review and meta-analysis. EClinicalMedicine 2021, 38, 100997. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, Q.; Liao, X.; Elbelt, U.; Weylandt, K.H. The effects of omega-3 fatty acids in type 2 diabetes: A systematic review and meta-analysis. Prostaglandins Leukot. Essent. Fat. Acids 2022, 182, 102456. [Google Scholar] [CrossRef]
- Harris, W.S.; Bulchandani, D. Why do omega-3 fatty acids lower serum triglycerides? Curr. Opin. Lipidol. 2006, 17, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.G.; Stone, N.J. Antiatherosclerotic and Antithrombotic Effects of Omega-3 Fatty Acids. Am. J. Cardiol. 2006, 98 (Suppl. 1), 39–49. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, M.; Artiach, G.; Arnardottir, H.; Bäck, M. The resolution of inflammation through omega-3 fatty acids in atherosclerosis, intimal hyperplasia, and vascular calcification. Semin. Immunopathol. 2019, 41, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Weylandt, K.-H. Docosapentaenoic acid derived metabolites and mediators—The new world of lipid mediator medicine in a nutshell. Eur. J. Pharmacol. 2016, 785, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Bäck, M. Omega-3 fatty acids in atherosclerosis and coronary artery disease. Future Sci. OA 2017, 3, FSO236. [Google Scholar] [CrossRef]
- Koh, A.S.; Pan, A.; Wang, R.; Odegaard, A.O.; Pereira, M.A.; Yuan, J.M.; Koh, W.P. The association between dietary omega-3 fatty acids and cardiovascular death: The Singapore Chinese Health Study. Eur. J. Prev. Cardiol. 2015, 22, 364–372. [Google Scholar] [CrossRef] [PubMed]
- de Goede, J.; Verschuren, W.M.; Boer, J.M.; Kromhout, D.; Geleijnse, J.M. Alpha-linolenic acid intake and 10-year incidence of coronary heart disease and stroke in 20,000 middle-aged men and women in the Netherlands. PLoS ONE 2011, 6, e17967. [Google Scholar] [CrossRef]
- Barceló-Coblijn, G.; Murphy, E.J. Alpha-linolenic acid and its conversion to longer chain n−3 fatty acids: Benefits for human health and a role in maintaining tissue n−3 fatty acid levels. Prog. Lipid Res. 2009, 48, 355–374. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Lemaitre, R.N.; King, I.B.; Song, X.; Spiegelman, D.; Sacks, F.M.; Rimm, E.B.; Siscovick, D.S. Circulating Long-Chain ω-3 Fatty Acids and Incidence of Congestive Heart Failure in Older Adults: The Cardiovascular Health Study. Ann. Intern. Med. 2011, 155, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S. The omega-3 index as a risk factor for coronary heart disease1. Am. J. Clin. Nutr. 2008, 87, 1997S–2002S. [Google Scholar] [CrossRef] [PubMed]
- Metherel, A.H.; Stark, K.D. The stability of blood fatty acids during storage and potential mechanisms of degradation: A review. Prostaglandins Leukot. Essent. Fat. Acids 2016, 104, 33–43. [Google Scholar] [CrossRef] [PubMed]

| Cohort | Total N | Study | Year | Country | N | SEX (Male%) | Design | Age, y | Follow-Up, y | Lipid Pool | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SCHS | 63,257 | Sun | 2016 | Singapore | 744/744 | 64.79 | PC, NCC | 66.1 | 11 | Plasma | AMI |
| CHS I | 5201 | Lemaitre | 2003 | USA | 179/179 | 57.4 | PC, NCC | 79.1 | 7 | Plasma | MI |
| WHI-OS I | 93,676 | Matthan | 2014 | USA | 1224/1224 | 0 | PC, NCC | 67.8 | 4.5 | Plasma | CHD |
| MRFIT | / | Simon | 1995 | USA | 94/94 | 100% | PC, NCC | 49.8 | 3 | Serum | CHD |
| EPIC | 25,639 | Khaw | 2012 | UK | 2424/4930 | 81 | PC, NCC | 64.9 | 4 | Plasma | CHD |
| PREDIMED | 7447 | Papandreou | 2019 | Spain | 136/272 | 61.3 | PC, NCC | 67.8 | 7 | Whole blood | CHD |
| EUROASPIRE | / | Erkkilä | 2003 | Finland | 334/493 | 80 | PC, NCC | 59 | 3 | Serum | AMI |
| MERLIN TIMI 36 | / | Zelniker | 2021 | USA | 528/1612 | 73.8 | PC | 66.3 | / | Serum | CHD |
| CIRCS | 12,840 | Chei | 2018 | Japan | 152/456 | 61 | PC, NCC | / | 8 | Serum | CHD |
| MESA | / | Otto | 2013 | USA | 736/2837 | 46.8 | PC, NCC | 61.5 | 11 | Plasma | CHD |
| MORGEN | 35,475 | Goede | 2013 | Netherlands | 279/279 | 70 | PC, NCC | 50.5 | 8 | Plasma | Fatal CHD |
| PHS I | 14916 | Guallar | 1995 | USA | 213/213 | 100 | PC, NCC | 58.7 | 5 | Plasma | MI |
| NHS | 32,826 | Sun | 2008 | USA | 146/288 | / | PC, NCC | 60.3 | 6 | Erythrocyte | Non-fatal MI |
| CHS II | 30,829 | Mozaffarian | 2013 | USA | 630/2692 | 36.3 | PC | 74 | / | Plasma | Fatal CHD |
| FHS | 2500 | Harris | 2018 | USA | 119/2500 | 43 | PC | 66 | 7.3 | Erythrocyte | CHD |
| ACS-Missouri I | / | Harris | 2007 | USA | 94/94 | 54.3 | PC, NCC | 46.4 | / | Whole blood | ACS |
| ACS-Missouri II | / | Block | 2008 | USA | 768/768 | 66 | PC, NCC | 61 | / | Whole blood | ACS, MI |
| PHS II | 22,071 | Albert | 2002 | USA | 94/184 | / | PC, NCC | 58.5 | 17 | Whole blood | SCA |
| JPHC | 116,896 | Hamazaki | 2018 | Japan | 209/418 | 63.6 | PC, NCC | 57.1 | 16 | Plasma | CHD |
| WHI-OS II | 93,676 | Liu | 2019 | USA | 1214/1214 | 0 | PC, NCC | 67.8 | / | Plasma | CHD |
| Study | Year | Country | N | SEX (M)% | Design | Age, y | Biomarker | Disease |
|---|---|---|---|---|---|---|---|---|
| Lea | 1982 | Britain | 20/17 | / | CC | / | Erythrocyte | MI |
| Luostarinen | 1993 | Sweden | 30/29 | / | CC | 40 | Tissue | SCD |
| Prisco | 1986 | Italy | 42/45 | 57 | CC | 51.2 | Erythrocyte | CHD |
| Roberts | 1993 | USA | 66/292 | 100 | CC | 25–64 | Adipose tissue | SCD |
| Oda | 2005 | Japan | 73/84 | 84 | CC | 65 | Serum | AMI |
| Jama | 2002 | Norway | 103/104 | 71.9 | CC | 62.7 | Serum | MI |
| Marangoni | 2014 | Italy | 119/103 | / | CC | 55.9 | Whole blood | MI |
| Rhee | 2008 | Korea | 30/20 | 0 | CC | 35.5 | Plasma | CHD |
| Lemaitre | 2009 | USA | 265/415 | 81.3 | CC | 58.4 | Erythrocyte | SCD |
| Freije | 2009 | Bahrain | 11/26 | 45.5 | CC | 18–57 | Erythrocyte | CHD |
| Ahmed | 2017 | Tunisia | 111/120 | 58 | CC | 60.8 | Plasma | CAD |
| Pedersen | 2000 | Norway | 100/98 | 72 | CC | 62.4 | Adipose tissue | MI |
| Skuladottir | 1988 | Iceland | 12/14 | / | CC | 66.3 | Serum | CHD, Fatal MI |
| Kirkeby | 1972 | Norway | 36/32 | 100 | CC | 59.1 | Serum | MI |
| Siscovick | 1995 | USA | 82/108 | 80 | CC | 59 | Erythrocyte | SCD |
| Lopes | 2007 | Portugal | 49/49 | 100 | CC | 56.6 | Adipose tissue | MI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Chen, Y.; Pietzner, A.; Elbelt, U.; Fan, Z.; Weylandt, K.H. Circulating Omega-3 Polyunsaturated Fatty Acids Levels in Coronary Heart Disease: Pooled Analysis of 36 Observational Studies. Nutrients 2024, 16, 1610. https://doi.org/10.3390/nu16111610
Xiao Y, Chen Y, Pietzner A, Elbelt U, Fan Z, Weylandt KH. Circulating Omega-3 Polyunsaturated Fatty Acids Levels in Coronary Heart Disease: Pooled Analysis of 36 Observational Studies. Nutrients. 2024; 16(11):1610. https://doi.org/10.3390/nu16111610
Chicago/Turabian StyleXiao, Yanan, Yifang Chen, Anne Pietzner, Ulf Elbelt, Zhimin Fan, and Karsten H. Weylandt. 2024. "Circulating Omega-3 Polyunsaturated Fatty Acids Levels in Coronary Heart Disease: Pooled Analysis of 36 Observational Studies" Nutrients 16, no. 11: 1610. https://doi.org/10.3390/nu16111610
APA StyleXiao, Y., Chen, Y., Pietzner, A., Elbelt, U., Fan, Z., & Weylandt, K. H. (2024). Circulating Omega-3 Polyunsaturated Fatty Acids Levels in Coronary Heart Disease: Pooled Analysis of 36 Observational Studies. Nutrients, 16(11), 1610. https://doi.org/10.3390/nu16111610






