Effects of Hydrolyzed Collagen as a Dietary Supplement on Fibroblast Activation: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Inclusion and Exclusion Criteria
2.2. Search Strategy
2.3. Methodological Quality
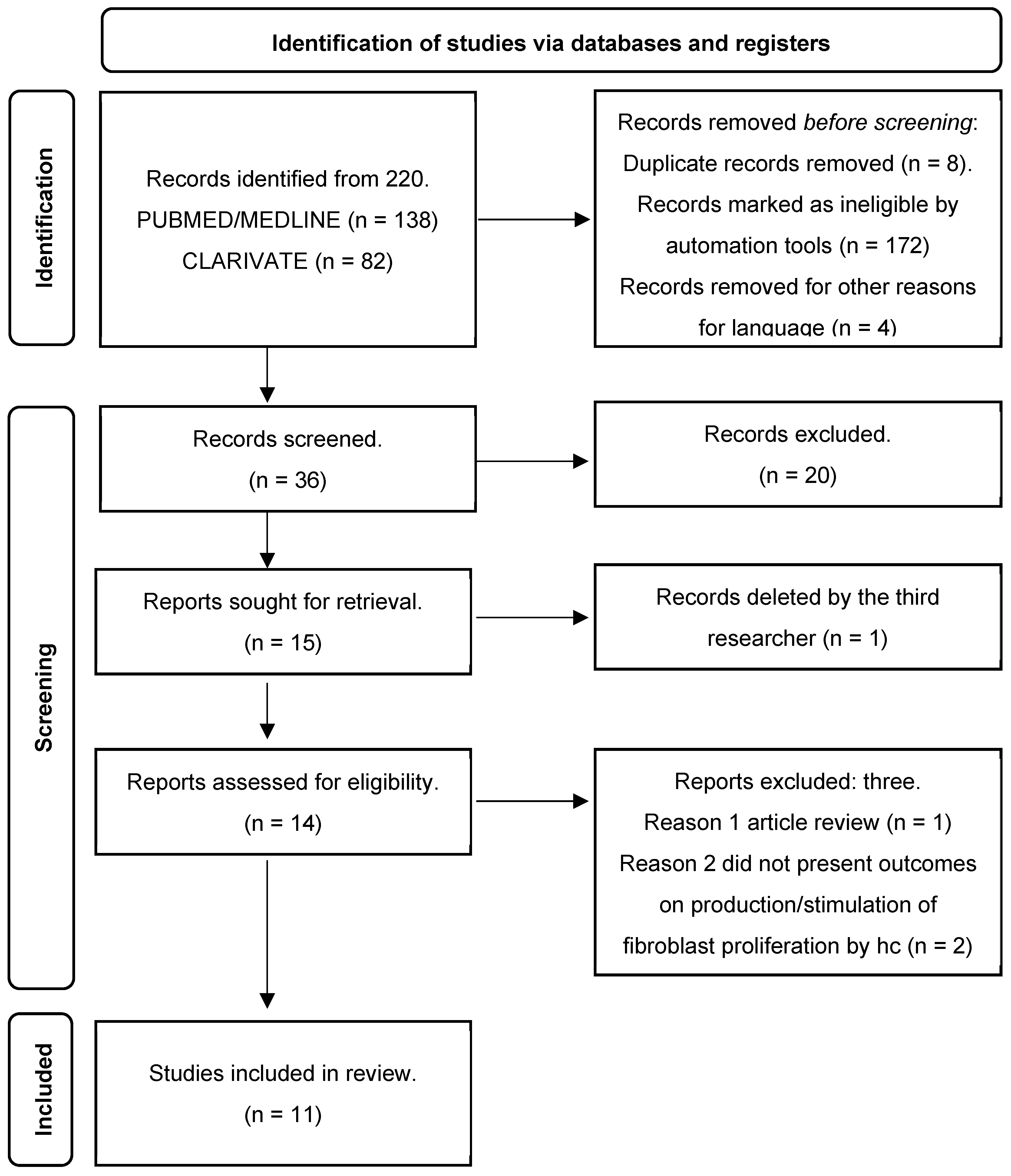
3. Results
3.1. Study Coding
3.2. Article Retrieval
3.3. Characteristics of the Studies
3.4. Methodological Quality
- Was the study hypothesis/goal/objective clearly described?
- Were the main results clearly measured and described in the Introduction or Methods section?
- Were the characteristics of the cell types used and controls described?
- Were the interventions of interest clearly described? Treatments and placebos (when relevant) to be compared should be clearly described.
- Was a list of key confounding factors provided, along with how cellular environments and processes were managed, and described clearly?
- Were the main conclusions of the study clearly described? Simple outcome data (including denominators and numerators) should be reported for all main findings so that the reader can verify the main analyses and conclusions. (This question does not cover statistical test data, which are considered below.)
- For non-normally distributed data, was the interquartile range of results reported? For normally distributed data, standard error, standard deviation, or confidence intervals should be reported.
- Did the authors clearly report the limitations of their findings? If yes, consider 1; if not, consider 0.
- Were appropriate statistical tests used to assess the main results? The statistical techniques used should be appropriate to the data.
- Were actual probability values reported (e.g., 0.035 instead of <0.05) for the main results, except where the probability value is less than 0.001?
4. Discussion
5. Limitations and Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, H. A Review of the Effects of Collagen Treatment in Clinical Studies. Polymers 2021, 13, 3868. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gordon, M.K.; Hahn, R.A. Collagens. Cell Tissue Res. 2010, 339, 247–257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, S.; Zhao, Y.; Song, W.; Zhang, C.; Wang, Q.; Li, R.; Shen, Y.; Gong, S.; Li, M.; Sun, L. Improving the Sustainability of Processing By-Products: Extraction and Recent Biological Activities of Collagen Peptides. Foods 2023, 12, 1965. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Figueres Juher, T.; Basés Pérez, E. Revisión de los efectos beneficiosos de la ingesta de colágeno hidrolizado sobre la salud osteoarticular y el envejecimiento dérmico [An overview of the beneficial effects of hydrolysed collagen intake on joint and bone health and on skin ageing]. Nutr. Hosp. 2015, 32 (Suppl. 1), 62–66. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Zague, V. A new view concerning the effects of collagen hydrolysate intake on skin properties. Arch. Dermatol. Res. 2008, 300, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Hou, H.; Zhao, X.; Zhang, Z.; Li, B. Effects of collagen and collagen hydrolysate from jellyfish (Rhopilema esculentum) on mice skin photoaging induced by UV irradiation. J. Food Sci. 2009, 74, H183–H188. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Datta, P.; Adhikari, B.; Dhara, S.; Ghosh, K.; Das Mohapatra, P.K. Collagen scaffolds derived from fresh water fish origin and their biocompatibility. J. Biomed. Mater. Res. A 2012, 100, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Pyun, H.B.; Kim, M.; Park, J.; Sakai, Y.; Numata, N.; Shin, J.Y.; Shin, H.J.; Kim, D.U.; Hwang, J.K. Effects of Collagen Tripeptide Supplement on Photoaging and Epidermal Skin Barrier in UVB-exposed Hairless Mice. Prev. Nutr. Food Sci. 2012, 17, 245–253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bi, C.; Li, X.; Xin, Q.; Han, W.; Shi, C.; Guo, R.; Shi, W.; Qiao, R.; Wang, X.; Zhong, J. Effect of extraction methods on the preparation of electrospun/electrosprayed microstructures of tilapia skin collagen. J. Biosci. Bioeng. 2019, 128, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.R.; Duarte, L.P.; Schmidt, M.M.; Cansian, R.L.; Fernandes, I.A.; de Oliveira Mello, R.; Demiate, I.M.; Dornelles, R.C.P. Extraction and characterization of collagen from sheep slaughter by-products. Waste Manag. 2020, 102, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Nekliudov, A.D.; Berdutina, A.V.; Ivankin, A.N.; Mitaleva, S.I.; Evstaf’eva, E.A. Kollagenovye fraktsii, poluchaemy vodno-solevoĭ ékstrakts’eĭ iz syr’ia zhivotnogo proiskhozhdeniia [Collagen fractions, obtained by water-salt extraction from animal fats]. Prikl. Biokhim. Mikrobiol. 2003, 39, 483–488. (In Russian) [Google Scholar] [PubMed]
- Vargas-Muñoz, D.P.; Kurozawa, L.E. Influence of combined hydrolyzed collagen and maltodextrin as carrier agents in spray drying of cocona pulp. Braz. J. Food Technol. 2020, 23, e2019254. [Google Scholar] [CrossRef]
- López-Morales, C.A.; Vázquez-Leyva, S.; Vallejo-Castillo, L.; Carballo-Uicab, G.; Muñoz-García, L.; Herbert-Pucheta, J.E.; Zepeda-Vallejo, L.G.; Velasco-Velázquez, M.; Pavón, L.; Pérez-Tapia, S.M.; et al. Determination of Peptide Profile Consistency and Safety of Collagen Hydrolysates as Quality Attributes. J. Food Sci. 2019, 84, 430–439. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burdock, G.A.; Carabin, I.G. Generally recognized as safe (GRAS): History and description. Toxicol. Lett. 2004, 150, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Brandao-Rangel, M.A.R.; Oliveira, C.R.; da Silva Olímpio, F.R.; Aimbire, F.; Mateus-Silva, J.R.; Chaluppe, F.A.; Vieira, R.P. Hydrolyzed Collagen Induces an Anti-Inflammatory Response That Induces Proliferation of Skin Fibroblast and Keratinocytes. Nutrients 2022, 14, 4975. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chotphruethipong, L.; Sukketsiri, W.; Aluko, R.E.; Sae-Leaw, T.; Benjakul, S. Effect of hydrolyzed collagen from defatted Asian sea bass (Lates calcarifer) skin on fibroblast proliferation, migration and antioxidant activities. J. Food Sci. Technol. 2021, 58, 541–551. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Offengenden, M.; Chakrabarti, S.; Wu, J. Chicken collagen hydrolysates differentially mediate anti-inflammatory activity and type I collagen synthesis on human dermal fibroblasts. Food Sci. Hum. Wellness 2018, 7, 138–147. [Google Scholar] [CrossRef]
- Molenaar, J.C. From the library of the Netherlands Journal of Medicine. Rudolf Virchow: Die Cellularpathologie in ihrer Begrundung auf physiologische und pathologische Gewebelehre; 1858. Ned. Tijdschr. Geneeskd. 2003, 147, 2236–2244. [Google Scholar] [PubMed]
- Plikus, M.V.; Wang, X.; Sinha, S.; Forte, E.; Thompson, S.M.; Herzog, E.L.; Driskell, R.R.; Rosenthal, N.; Biernaskie, J.; Horsley, V. Fibroblasts: Origins, definitions, and functions in health and disease. Cell 2021, 184, 3852–3872. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chai, H.J.; Li, J.H.; Huang, H.N.; Li, T.L.; Chan, Y.L.; Shiau, C.Y.; Wu, C.J. Effects of sizes and conformations of fish-scale collagen peptides on facial skin qualities and transdermal penetration efficiency. J. Biomed. Biotechnol. 2010, 2010, 757301. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chotphruethipong, L.; Aluko, R.E.; Benjakul, S. Hydrolyzed collagen from porcine lipase-defatted seabass skin: Antioxidant, fibroblast cell proliferation, and collagen production activities. J. Food Biochem. 2019, 43, e12825. [Google Scholar] [CrossRef] [PubMed]
- Chotphruethipong, L.; Sukketsiri, W.; Battino, M.; Benjakul, S. Conjugate between hydrolyzed collagen from defatted seabass skin and epigallocatechin gallate (EGCG): Characteristics, antioxidant activity and in vitro cellular bioactivity. RSC Adv. 2021, 11, 2175–2184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wauquier, F.; Boutin-Wittrant, L.; Bouvret, E.; Le Faouder, J.; Roux, V.; Macian, N.; Pickering, G.; Wittrant, Y. Benefits of Circulating Human Metabolites from Fish Cartilage Hydrolysate on Primary Human Dermal Fibroblasts, an Ex Vivo Clinical Investigation for Skin Health Applications. Nutrients 2022, 14, 5027. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Zhang, C.L.; Zhang, Q.; Li, P. Composite electrospun nanomembranes of fish scale collagen peptides/chito-oligosaccharides: Antibacterial properties and potential for wound dressing. Int. J. Nanomed. 2011, 6, 667–676. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chotphruethipong, L.; Hutamekalin, P.; Nilsuwan, K.; Sukketsiri, W.; Aluko, R.E.; Abdul, N.R.; Benjakul, S. Combined Effects of Defatted Hydrolyzed Collagen from Salmon Skin and Vitamin C on Proliferation and Migration of Human Fibroblast Cell. Fishes 2022, 7, 265. [Google Scholar] [CrossRef]
- Lin, P.; Hua, N.; Hsu, Y.C.; Kan, K.W.; Chen, J.H.; Lin, Y.H.; Lin, Y.H.; Kuan, C.M. Oral Collagen Drink for Antiaging: Antioxidation, Facilitation of the Increase of Collagen Synthesis, and Improvement of Protein Folding and DNA Repair in Human Skin Fibroblasts. Oxid. Med. Cell Longev. 2020, 2020, 8031795. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Benjakul, S.; Karnjanapratum, S.; Visessanguan, W. Hydrolysed collagen from Lates calcarifer skin: Its acute toxicity and impact on cell proliferation and collagen production of fibroblasts. Food Sci. Technol. 2018, 53, 1871–1879. [Google Scholar] [CrossRef]
| Article | Type of Collagen and Molecular Weight (MW) in Kda | Intervention on Fibroblast | Control/Comparasion | Outcomes |
|---|---|---|---|---|
| Chai et al., 2010 [23] | Collagen peptides from fish. | Human and mouse embryonic skin fibroblast lineage. | Fibroblast without collagen stimulation. | The doses of 0.4–200 μg/mL induced fibroblast proliferation in a dose-dependent manner. |
| Chotphruethipong et al., 2019 [24] | Sea bass skin hydrolysate and collagen with the application of porcine pancreatic lipase. | Mouse fibroblasts from subcutaneous connective tissue lineage (L929). | Fibroblasts without the addition of hydrolyzed collagen (HC) and solutions with HC concentrations of 50, 100, 200, and 250 μg/mL. | The cellular proliferation of fibroblasts, mainly between concentrations 100 and 250 μg/mL. |
| Chotphruethipong et al., 2021 [17] | Hydrolyzed collagen from defatted sea bass skin. MW = 406 to 16,120 Da. | MRC-5 fibroblast cell line (human fetal lung). | Fibroblasts with the additions of hydrolyzed collagen at the concentrations of 25, 50, 100, 500, and 1000 μg/mL. | Concentration-dependent fibroblast proliferation with no significant difference between the concentrations of 500 and 1000 μg/mL (p > 0.05). |
| Chotphruethipong et al., 2021 [25] | Hydrolyzed collagen (HC) from degreased sea bass skin and collagen (HC) + EGCG epigallocatechin gallate. | MRC-5 fibroblast cell line (human fetal lung). | Fibroblasts without addition and with the additions of hydrolyzed collagen (HC), and HC + EGCG. | HC promoted cell proliferation more effectively than HC-conjugated EGCG. |
| Wauquier et al., 2022 [26] | Fish cartilage hydrolysate (FCH). Low MW (under 3000 Da). | Human primary dermal fibroblasts | Human I seruINAIVE or human serum enriched with circulating metabolites resulting from FCH | FCH stimulates human dermal fibroblast growth |
| Brandao-Rangel et al., 2022 [16] | Hydrolyzed collagen (types I and III of hydrolyzed collagen) Total MW < 3 oderientesibroblasts (CCD72Sk). | (1) odetodernly medium stimulated), (2) lipopolysaccharide (LPS 10 ng/mL), (3) collagen 2.5 mg/mL, (4) collagen 5 mg/mL, (5) collagen 10 mg/mL, (6) LPS + collagen 2.5 mg/mL, (7) LPS + collagen 5 mg/mL, and (8) LPS + collagen 10 mg/mL. LPS was added for 1 h, with the subsequent addition of collagen. | … | Collagen supplementation (2.5 mg/mL, 5 mg/mL, 10 mg/mL) increased the proliferation of human’s fibroblasts. p < 0.001. |
| Offengenden et al., 2018 [18] | Chicken collagen hydrolysate (CCH). | Cultured human dermal fibroblasts (HDF). | A total of 4 dual-enzyme generated hydrolysates. | Only 2 out of the 4 enzyme-hydrolyzed collagen stimulate fibroblast proliferation, p < 0.01, p < 0.001. |
| Wang et al., 2011 [27] | Fish scale collagen peptide (FSCP) MW 800 Da. | Cultured human dermal fibroblasts (HDF). | PVA (polyvinyl alcohol) and E NFM (lectrospun nanofibrous membranes) served as the control. | FSCP treatments both showed good in vitro biocompatibility and supported the proliferation of fibroblasts. |
| Chotphruethipong et al., 2022 [28] | Hydrolyzed collagen from salmon skin and vitamin C (Vit C) Mw 102 Da to 10,175 Da. | Cultured human dermal fibroblasts (HDF). | HC (0, 50, 100, 200, 400, and 800 μg/mL) and Vit C (0.01, 0.1, 1, 10, and 100 μg/mL) control (without any treatment). | Hydrolyzed collagen (HC) at various levels on the proliferation of HDF cells, there was no difference in the cell proliferation of HC at the levels of 50 and 100 μg/mL, p > 0.05, cytotoxicity, and 800 µg/mL which showed a slightly decreased proliferation of the cell as compared to the control p < 0.05. Between the two protocols (hc) and vit c, there was no significant difference in relation to the increase in fibroblast proliferation p > 0.05, and when combined, greater proliferation in relation to the isolated methods p < 0.05. |
| Lin et al., 2020 [29] | Elastin collagen peptide complex juice srink (YAMII, Zhejiang Kazman Biotechnology) ingredients: water, fish collageoderide powder (12% oderana powder, apple essence, cherry essence, γ-aminobutyric acoderape powoderlma powodercai powder, perodereed powder, eoderrry powder, boderice powder, honey, vitamin C, erythritol, citric acid, fructose syrup, sucrose, flavor). | Human skin fibroblast. | Concentrations of collagen drinks (with/without UVA irradiation). 0.125%, 0.25%, and 0.5%, Control 0%. | In comparison with the control group, 0.125%, 0.25%, and 0.5% of the collagen drinks could increase the mitochondrial activities of fibroblasts by 30.6%, 16.4%, and 36.1%, respectively. Regarding photoaging improvement, the mitochondrial activities of the UVA-treated cells were improved by 100.9%, 110.2%, and 105.4%, respectively, followed by treatment with corresponding 0.125%, 0.25%, and 0.5% of the collagen drinks. The collagen drinks slightly enhanced the cell proliferation capability in comparison with the control group. |
| Benjakul et al., 2018 [30] | Hydrolysed collagen from seabass (Lates calcarifer) and HC + Vit C. Molecular weight ranges from 1050 to 1330 Da. | L929 mouse fibroblast, subcutaneous connective tissue. | HC powder was dissolved in distilled water and dosed orally at 2000 and 5000 mg kg body weight. The treated doses were calculated based on the body weights of rats after fasting. The rats in the control group were dosed with distilled water at the equivolume to the treatment group. | HC promoted cell growth L929. The greatest proliferation was evidenced in combined models of HC + Vit c in a 2:1 ratio at the dosages of 150–200 μg/mL. |
| Article | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Total Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chai et al., 2010 [23] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 8 |
| Chotphruethipong 2019 [24] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 8 |
| Chotphruethipong 2021 [17] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 8 |
| Chotphruethipong et al., 2021 [25] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 8 |
| Wauquier et al., 2022 [26] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 8 |
| Brandao-Rangel et al., 2022 [16] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 9 |
| Offengenden et al., 2018 [18] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 7 |
| Wang et al., 2011 [27] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 7 |
| Chotphruethipong et al., 2022 [28] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 8 |
| Lin et al., 2020 [29] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 7 |
| Benjakul et al., 2018 [30] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inacio, P.A.Q.; Chaluppe, F.A.; Aguiar, G.F.; Coelho, C.d.F.; Vieira, R.P. Effects of Hydrolyzed Collagen as a Dietary Supplement on Fibroblast Activation: A Systematic Review. Nutrients 2024, 16, 1543. https://doi.org/10.3390/nu16111543
Inacio PAQ, Chaluppe FA, Aguiar GF, Coelho CdF, Vieira RP. Effects of Hydrolyzed Collagen as a Dietary Supplement on Fibroblast Activation: A Systematic Review. Nutrients. 2024; 16(11):1543. https://doi.org/10.3390/nu16111543
Chicago/Turabian StyleInacio, Pedro Augusto Querido, Felipe Augusto Chaluppe, Gerson Ferreira Aguiar, Carly de Faria Coelho, and Rodolfo P. Vieira. 2024. "Effects of Hydrolyzed Collagen as a Dietary Supplement on Fibroblast Activation: A Systematic Review" Nutrients 16, no. 11: 1543. https://doi.org/10.3390/nu16111543
APA StyleInacio, P. A. Q., Chaluppe, F. A., Aguiar, G. F., Coelho, C. d. F., & Vieira, R. P. (2024). Effects of Hydrolyzed Collagen as a Dietary Supplement on Fibroblast Activation: A Systematic Review. Nutrients, 16(11), 1543. https://doi.org/10.3390/nu16111543







