Maternal Hypertriglyceridemia in Gestational Diabetes: A New Risk Factor?
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
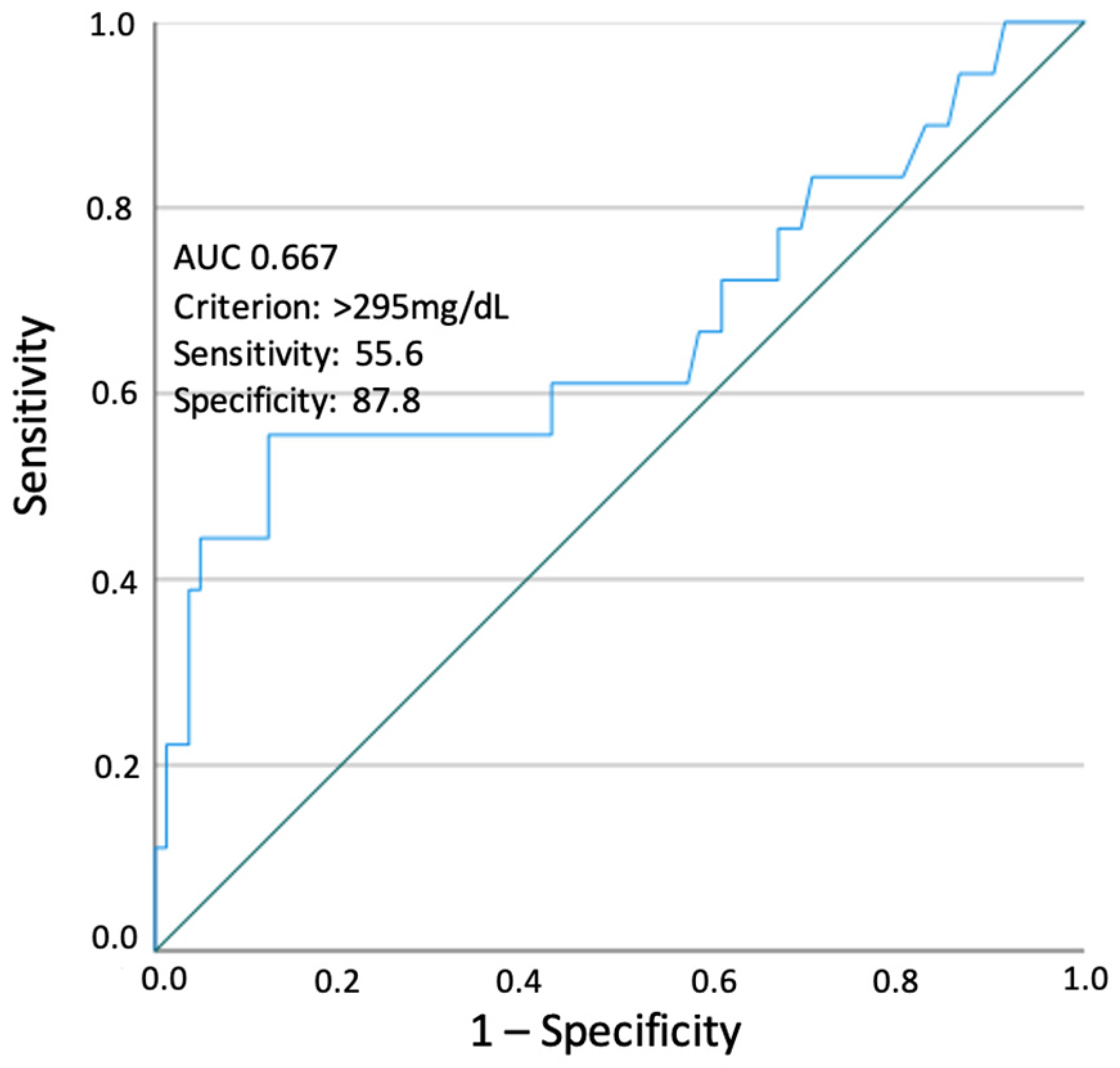
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langer, O.; Yogev, Y.; Most, O.; Xenakis, E.M.J. Gestational diabetes: The consequences of not treating. Am. J. Obstet. Gynecol. 2005, 192, 989–997. [Google Scholar] [CrossRef] [PubMed]
- The HAPO Study Cooperative Research Group. Hyperglycemia and Adverse Pregnancy Outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, J. Diabetes and pregnancy; blood sugar of newborn infants during fasting and glucose administration. Ugeskr. Laeger 1952, 114, 685. [Google Scholar]
- Herrera Martínez, A. Hyperlipidemia during gestational diabetes and its relation with maternal and offspring complications. Nutr. Hosp. 2018, 35, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.-H.; Wu, D.-D.; Zhou, C.-L.; Chen, L.; Li, J.; Li, Z.-Z.; Fan, J.-X.; Liu, X.-M.; Lin, X.-H.; Huang, H.-F. Association of high maternal triglyceride levels early and late in pregnancy with adverse outcomes: A retrospective cohort study. J. Clin. Lipidol. 2021, 15, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Adank, M.C.; Benschop, L.; Kors, A.W.; Peterbroers, K.R.; Gregoor, A.M.S.; Mulder, M.T.; Schalekamp-Timmermans, S.; Van Lennep, J.E.R.; Steegers, E.A.P. Maternal lipid profile in early pregnancy is associated with foetal growth and the risk of a child born large-for-gestational age: A population-based prospective cohort study. BMC Med. 2020, 18, 276. [Google Scholar] [CrossRef] [PubMed]
- Son, G.H.; Kwon, J.Y.; Kim, Y.H.; Park, Y.W. Maternal serum triglycerides as predictive factors for large-for-gestational age newborns in women with gestational diabetes mellitus. Acta Obstet. Gynecol. Scand. 2010, 89, 700–704. [Google Scholar] [CrossRef] [PubMed]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef]
- Sociedade Portuguesa de Diabetologia (SPD). Consenso “diabetes gestacional”: Atualização 2017. Rev. Port. Diabetes 2017, 12, 24–38. [Google Scholar]
- Dathan-Stumpf, A.; Vogel, M.; Jank, A.; Thiery, J.; Kiess, W.; Stepan, H. Reference intervals of serum lipids in the second and third trimesters of pregnancy in a Caucasian cohort: The LIFE Child study. Arch. Gynecol. Obstet. 2019, 300, 1531–1539. [Google Scholar] [CrossRef]
- Sousa-Santos, R.F.; Miguelote, R.F.; Cruz-Correia, R.J.; Santos, C.C.; Bernardes, J.F.M.A.L. Development of a birthweight standard and comparison with currently used standards. What is a 10th centile? Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 206, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, L.; Saraiva, M.; Amado, A.; Paredes, S.; Pichel, F.; Pinto, C.; Vilaverde, J.; Dores, J. Third trimester HbA1c and the association with large-for-gestational-age neonates in women with gestational diabetes. Arch. Endocrinol. Metab. 2021, 65, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Schaefer-Graf, U.M.; Kjos, S.L.; Kilavuz, O.; Plagemann, A.; Brauer, M.; Dudenhausen, J.W.; Vetter, K. Determinants of Fetal Growth at Different Periods of Pregnancies Complicated by Gestational Diabetes Mellitus or Impaired Glucose Tolerance. Diabetes Care 2003, 26, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Ricart, W.; López, J.; Mozas, J.; Pericot, A.; Sancho, M.A.; González, N.; Balsells, M.; Luna, R.; Cortázar, A.; Navarro, P.; et al. Body mass index has a greater impact on pregnancy outcomes than gestational hyperglycaemia. Diabetologia 2005, 48, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Ehrenberg, H.M.; Durnwald, C.P.; Catalano, P.; Mercer, B.M. The influence of obesity and diabetes on the risk of cesarean delivery. Am. J. Obstet. Gynecol. 2004, 191, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Lapolla, A.; Dalfrà, M.G.; Bonomo, M.; Parretti, E.; Mannino, D.; Mello, G.; Di Cianni, G. Gestational diabetes mellitus in Italy: A multicenter study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 145, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Yogev, Y.; Visser, G.H.A. Obesity, gestational diabetes and pregnancy outcome. Semin. Fetal Neonatal Med. 2009, 14, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Luo, Z.-C.; Nuyt, A.M.; Audibert, F.; Wei, S.-Q.; Abenhaim, H.A.; Bujold, E.; Julien, P.; Huang, H.; Levy, E.; et al. Large-for-Gestational-Age May Be Associated With Lower Fetal Insulin Sensitivity and β-Cell Function Linked to Leptin. J. Clin. Endocrinol. Metab. 2018, 103, 3837–3844. [Google Scholar] [CrossRef] [PubMed]
- Whyte, K.; Kelly, H.; O’Dwyer, V.; Gibbs, M.; O’Higgins, A.; Turner, M.J. Offspring birth weight and maternal fasting lipids in women screened for gestational diabetes mellitus (GDM). Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 67–70. [Google Scholar] [CrossRef]
- Barrett, H.L.; Gatford, K.L.; Houda, C.M.; De Blasio, M.J.; McIntyre, H.D.; Callaway, L.K.; Dekker Nitert, M.; Coat, S.; Owens, J.A.; Hague, W.M.; et al. Maternal and neonatal circulating markers of metabolic and cardiovascular risk in the metformin in gestational diabetes (MiG) trial: Responses to maternal metformin versus insulin treatment. Diabetes Care 2013, 36, 529–536. [Google Scholar] [CrossRef]
- Jin, W.-Y.; Lin, S.-L.; Hou, R.-L.; Chen, X.-Y.; Han, T.; Jin, Y.; Tang, L.; Zhu, Z.-W.; Zhao, Z.-Y. Associations between maternal lipid profile and pregnancy complications and perinatal outcomes: A population-based study from China. BMC Pregnancy Childbirth 2016, 16, 60. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.; Ortega-Senovilla, H. Disturbances in lipid metabolism in diabetic pregnancy—Are these the cause of the problem? Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, D.R.; Grundy, S.M. Pregnancy-associated Hypertriglyceridemia in Normal and Diabetic Women: Differences in Insulin-dependent, Non-insulin-dependent, and Gestational Diabetes. Diabetes 1982, 31, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Knopp, R.H.; Chapman, M.; Bergelin, R.; Wahl, P.W.; Warth, M.R.; Irvine, S. Relationships of Lipoprotein Lipids to Mild Fasting Hyperglycemia and Diabetes in Pregnancy. Diabetes Care 1980, 3, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Shelley-Jones, D.C.; Wein, P.; Nolan, C.; Beischer, N.A. Why do Asian-born Women Have a Higher Incidence of Gestational Diabetes? An Analysis of Racial Differences in Body Habitus, Lipid Metabolism and the Serum Insulin Response to an Oral Glucose Load. Aust. N. Z. J. Obstet. Gynaecol. 1993, 33, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Radaelli, T.; Lepercq, J.; Varastehpour, A.; Basu, S.; Catalano, P.M.; Hauguel-De Mouzon, S. Differential regulation of genes for fetoplacental lipid pathways in pregnancy with gestational and type 1 diabetes mellitus. Am. J. Obstet. Gynecol. 2009, 201, e1–e209. [Google Scholar] [CrossRef]
- Vrijkotte, T.G.; Krukziener, N.; Hutten, B.A.; Vollebregt, K.C.; van Eijsden, M.; Twickler, M.B. Maternal lipid profile during early pregnancy and pregnancy complications and outcomes: The ABCD study. J. Clin. Endocrinol. Metab. 2012, 97, 3917–3925. [Google Scholar] [CrossRef] [PubMed]
- Szabo, A.J.; Szabo, O. Placental free-fatty-acid transfer and fetal adipose-tissue development: An explanation of fetal adiposity in infants of diabetic mothers. Lancet 1974, 304, 498–499. [Google Scholar] [CrossRef]
- Shafrir, E.; Khassis, S. Maternal-fetal fat transport versus new fat synthesis in the pregnant diabetic rat. Diabetologia 1982, 22, 111–117. [Google Scholar] [CrossRef]
- Barrett, H.L.; Dekker Nitert, M.; D’Emden, M.; Lingwood, B.; de Jersey, S.; McIntyre, H.D.; Callaway, L.K. Capillary Triglycerides in Late Pregnancy-Challenging to Measure, Hard to Interpret: A Cohort Study of Practicality. Nutrients 2021, 13, 1266. [Google Scholar] [CrossRef]
- McAdams, M.A.; Van Dam, R.M.; Hu, F.B. Comparison of Self-reported and Measured BMI as Correlates of Disease Markers in U.S. Adults. Obesity 2007, 15, 188. [Google Scholar] [CrossRef] [PubMed]
- Paradis, A.M.; Pérusse, L.; Godin, G.; Vohl, M.C. Validity of a self-reported measure of familial history of obesity. Nutr. J. 2008, 7, 27. [Google Scholar] [CrossRef] [PubMed]
| N = 100 | |
|---|---|
| Age (years) | 35.0 ± 5.3 |
| Family history of diabetes | 27% (27) |
| Pre-pregnancy weight (kg) * | 70 (62–83) |
| Pre-pregnancy BMI (kg/m2) * | 25.8 (23.7–30.9) |
| Multiparity | 50.0% (50) |
| GDM diagnosis | |
| First trimester | 49.0% (49) |
| Second trimester | 51.0% (51) |
| Metformin therapy | 22.0% (22) |
| Insulin therapy | 23.0% (23) |
| Percentage of gestational weight gain (%) | 16.0 ± 10.8 |
| Gestational age at delivery (weeks) * | 39 (38–39) |
| Prematurity | 8.0% (8) |
| Neonatal birth weight (g) | 3250.2 ± 496.7 |
| Small for gestational age (SGA) | 4.0% (4) |
| Large for gestational age (LGA) | 18.0% (18) |
| Macrosomia | 7.0% (7) |
| Adverse neonatal outcome 1 | 35.0% (35) |
| Congenital malformations | 3.0% (3) |
| Third-trimester fasting glucose (mg/dL) * | 75 (71–82) |
| Third-trimester HbA1c (%) | 5.2 ± 0.4 |
| Third-trimester TG (mg/dL) * | 213 (179–268) |
| Third-trimester HDL cholesterol (mg/dL) | 67.4 ± 13.0 |
| Third-trimester LDL cholesterol (mg/dL) * | 147 (117–176) |
| Hypertriglyceridemia | 25.0% (25) |
| Women with LGA Newborns | Women without LGA Newborns | p | |
|---|---|---|---|
| Age (years) | 35.1 ± 5.9 | 34.9 ± 5.5 | 0.892 |
| Multiparity | 61.1% (11/18) | 47.6% (39/82) | 0.436 |
| Pre-pregnancy BMI (kg/m2) * | 31.6 (25.6–38.1) | 25.0 (22.9–29.7) | 0.002 |
| Percentage of gestational weight gain (%) | 14.7 ± 13.1 | 16.3 ± 10.2 | 0.579 |
| Metformin therapy + | 50.0% (9/18) | 15.9% (13/82) | 0.004 |
| Insulin therapy + | 50.0% (9/18) | 17.1% (14/82) | 0.005 |
| Adverse neonatal outcome 1 | 61.1% (11/18) | 30.8% (24/78) | 0.028 |
| Third-trimester fasting glucose (mg/dL) * | 83 (72–92) | 74 (70–79) | 0.011 |
| Third-trimester HbA1c (%) | 5.4 ± 0.4 | 5.2 ± 0.4 | 0.030 |
| Third-trimester TG (mg/dL) * | 296 (187–354) | 208 (171–253) | 0.027 |
| Third-trimester HDL cholesterol (mg/dL) | 64.8 ± 14.4 | 67.9 ± 12.7 | 0.362 |
| Third-trimester LDL cholesterol (mg/dL) * | 149 (118–154) | 146 (115–180) | 0.507 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Crude OR (CI 95%) | p | Adjusted OR (CI 95%) | p | |
| Age (years) | 1.007 (0.917–1.105) | 0.891 | 0.964 (0.856–1.085) | 0.540 |
| Multiparity | 1.733 (0.611–4.912) | 0.301 | 1.139 (0.283–4.578) | 0.855 |
| Pre-pregnancy BMI (kg/m2) | 1.127 (1.044–1.215) | 0.002 | 1.190 (1.026–1.380) | 0.022 |
| Percentage of gestational weight gain (%) | 0.986 (0.939–1.036) | 0.575 | 1.054 (0.970–1.145) | 0.213 |
| Metformin therapy | 5.308 (1.771–15.908) | 0.003 | 4.209 (0.856–20.058) | 0.077 |
| Insulin therapy | 4.857 (1.636–14.423) | 0.004 | 0.790 (0.121–5.138) | 0.805 |
| Third-trimester fasting glucose (mg/dL) | 1.077 (1.025–1.132) | 0.003 | 1.073 (0.999–1.150) | 0.054 |
| Third-trimester HbA1c (%) | 4.042 (1.107–14.759) | 0.035 | 1.153 (0.197–6.741) | 0.874 |
| Hypertriglyceridemia | 5.583 (1.886–16.528) | 0.002 | 7.603 (1.695–34.097) | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques Puga, F.; Borges Duarte, D.; Benido Silva, V.; Pereira, M.T.; Garrido, S.; Vilaverde, J.; Sales Moreira, M.; Pichel, F.; Pinto, C.; Dores, J. Maternal Hypertriglyceridemia in Gestational Diabetes: A New Risk Factor? Nutrients 2024, 16, 1577. https://doi.org/10.3390/nu16111577
Marques Puga F, Borges Duarte D, Benido Silva V, Pereira MT, Garrido S, Vilaverde J, Sales Moreira M, Pichel F, Pinto C, Dores J. Maternal Hypertriglyceridemia in Gestational Diabetes: A New Risk Factor? Nutrients. 2024; 16(11):1577. https://doi.org/10.3390/nu16111577
Chicago/Turabian StyleMarques Puga, Francisca, Diana Borges Duarte, Vânia Benido Silva, Maria Teresa Pereira, Susana Garrido, Joana Vilaverde, Marta Sales Moreira, Fernando Pichel, Clara Pinto, and Jorge Dores. 2024. "Maternal Hypertriglyceridemia in Gestational Diabetes: A New Risk Factor?" Nutrients 16, no. 11: 1577. https://doi.org/10.3390/nu16111577
APA StyleMarques Puga, F., Borges Duarte, D., Benido Silva, V., Pereira, M. T., Garrido, S., Vilaverde, J., Sales Moreira, M., Pichel, F., Pinto, C., & Dores, J. (2024). Maternal Hypertriglyceridemia in Gestational Diabetes: A New Risk Factor? Nutrients, 16(11), 1577. https://doi.org/10.3390/nu16111577






