The Next-Generation Probiotic E. coli 1917-pSK18a-MT Ameliorates Cadmium-Induced Liver Injury by Surface Display of Metallothionein and Modulation of Gut Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Construction and Evaluation of Next-Generation Probiotics In Vitro
2.2. Design of Animal Experiments and Collection of Samples
2.3. Liver Index
2.4. Histology and Histopathology
2.5. Real-Time Fluorescence Quantitative PCR
2.6. Measurement of Cd in Feces and Liver Tissue
2.7. Western Blotting
2.8. Determination of Oxidation-Related Factors in Liver and Serum
2.9. 16S rRNA Gene Sequencing
2.10. Statistical Analysis
3. Results
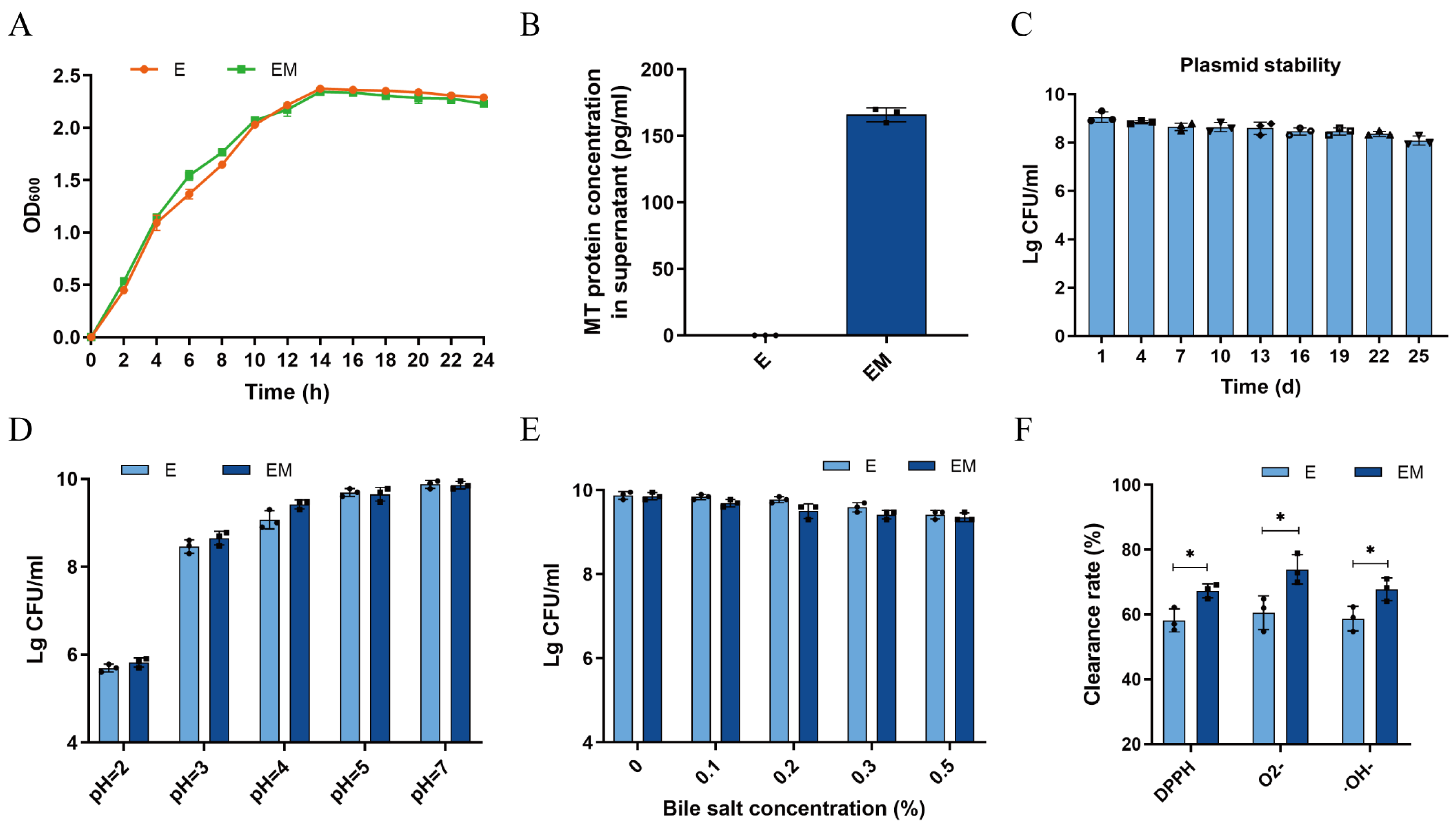
3.1. Evaluation of the Probiotic Properties of EM In Vitro
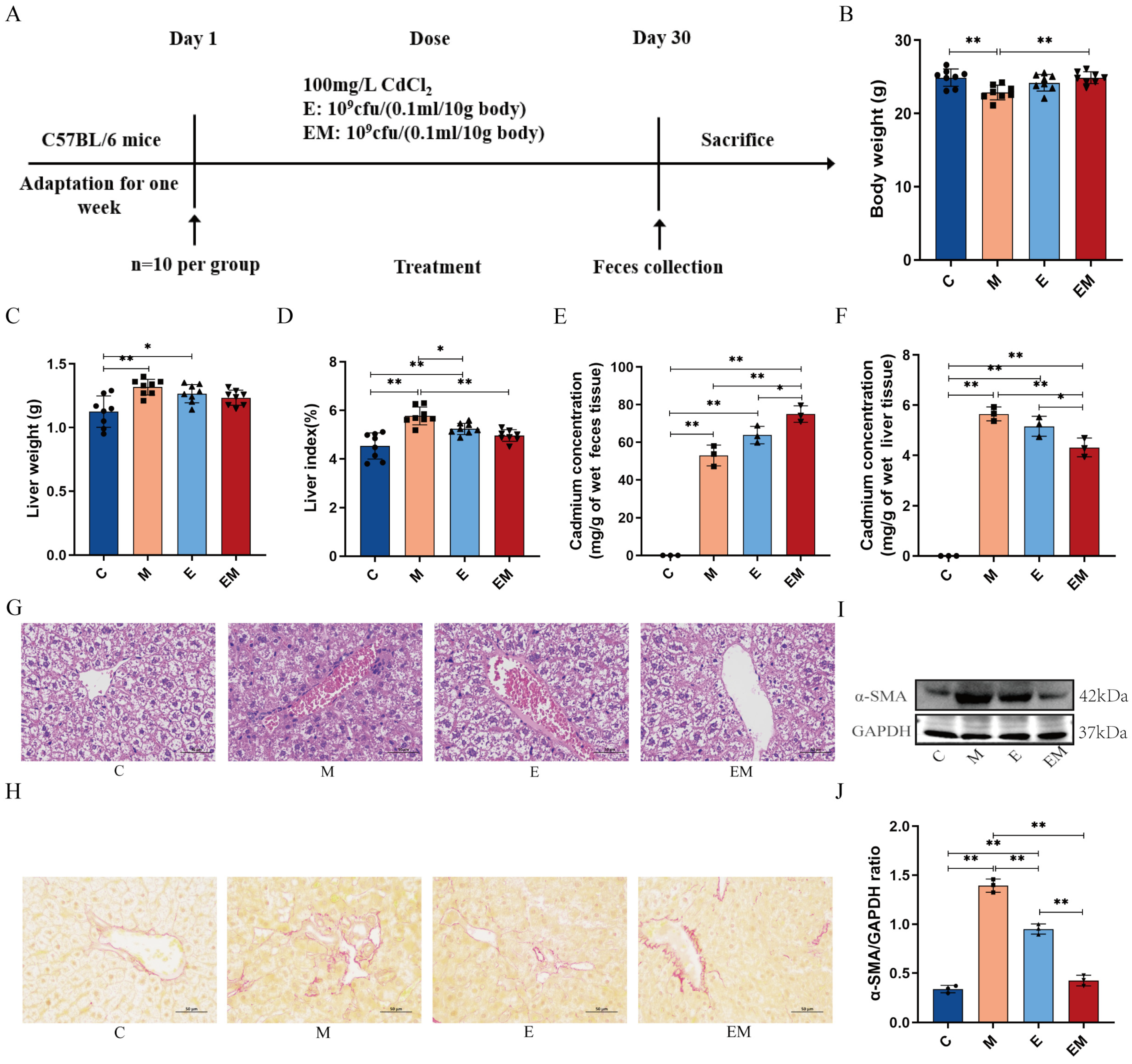
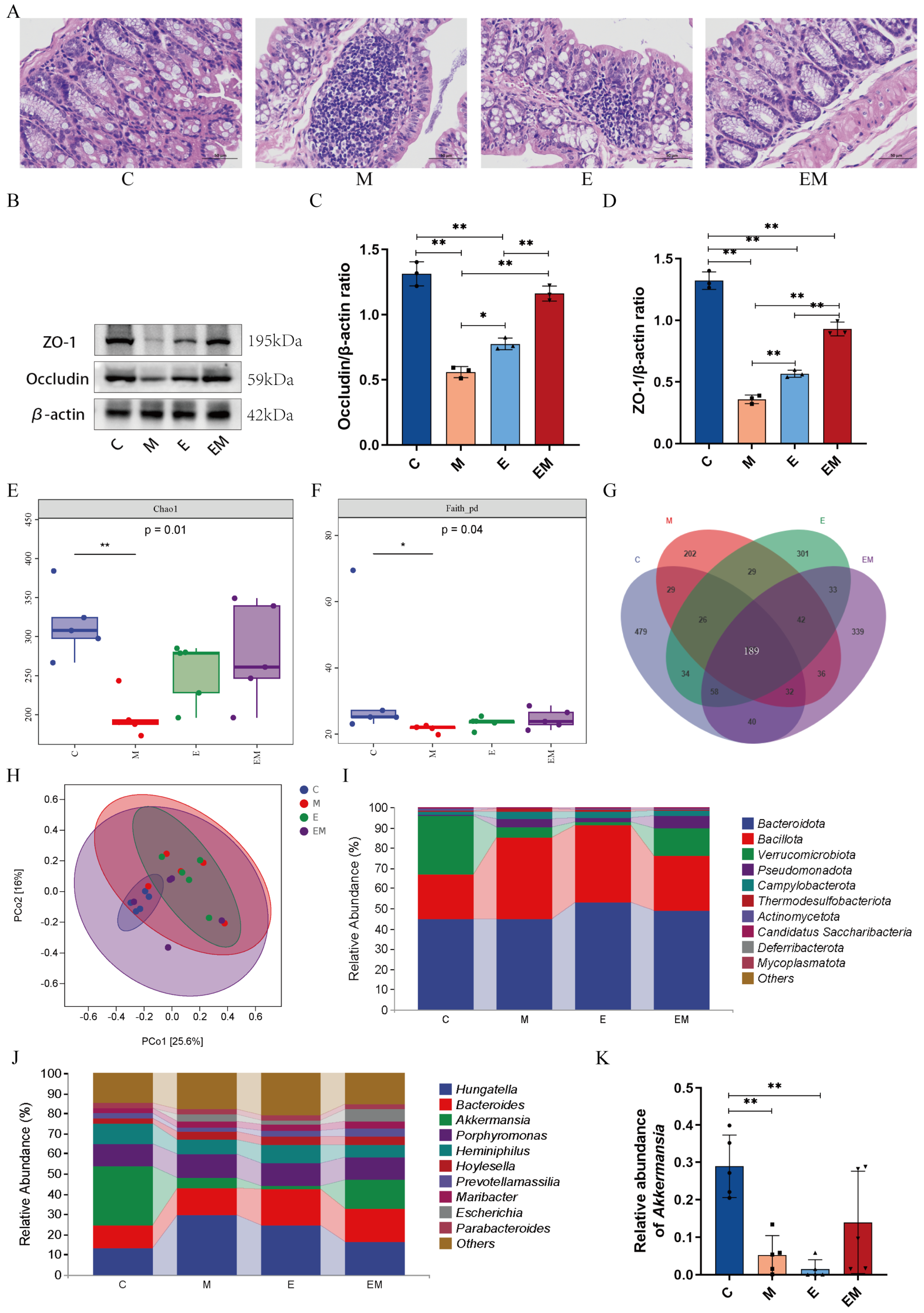
3.2. EM Treatment Reversed the Pathologic Changes in Cd-Intoxicated Mice
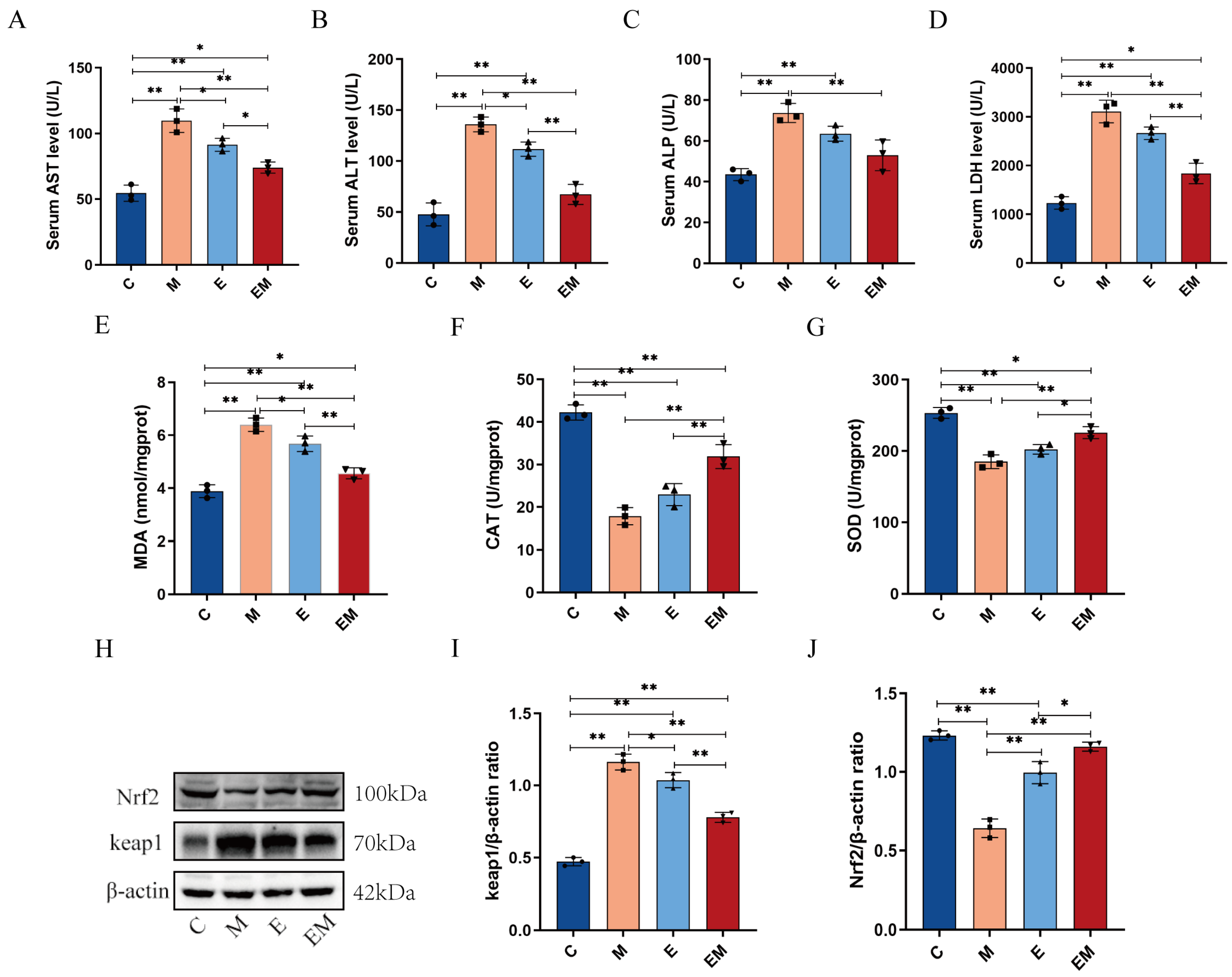
3.3. Enhancement of Hepatic Dysfunction and Oxidative Stress Induced by Cd Intoxication by EM Administration
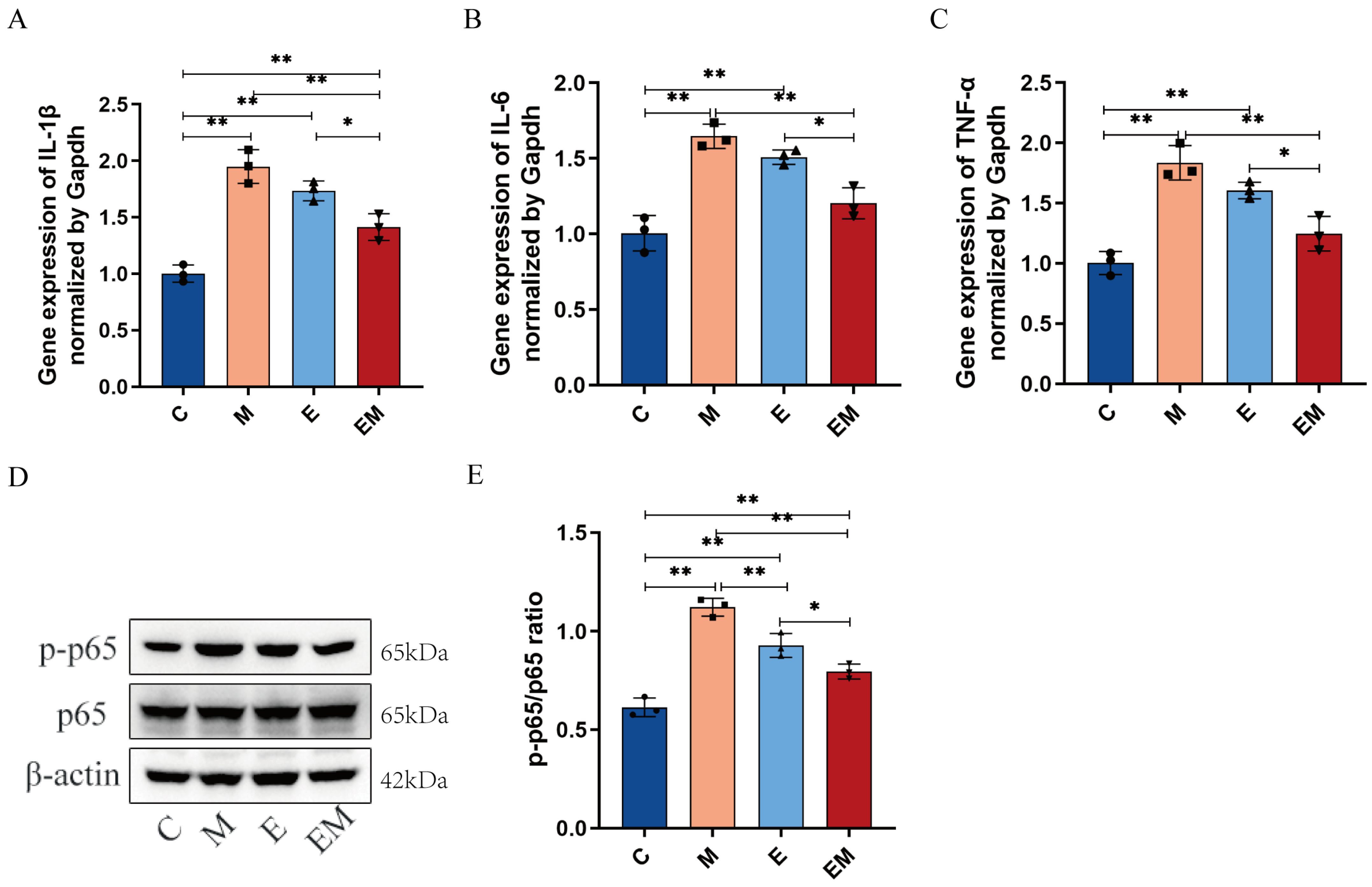
3.4. EM Treatment Reduces Inflammation Levels in the Liver Tissues of Cd-Exposed Mice
3.5. EM Maintained the Intestinal Barrier Function and Reversed the Dysbiosis of Gut Microbiota in Cd-Intoxicated Mice
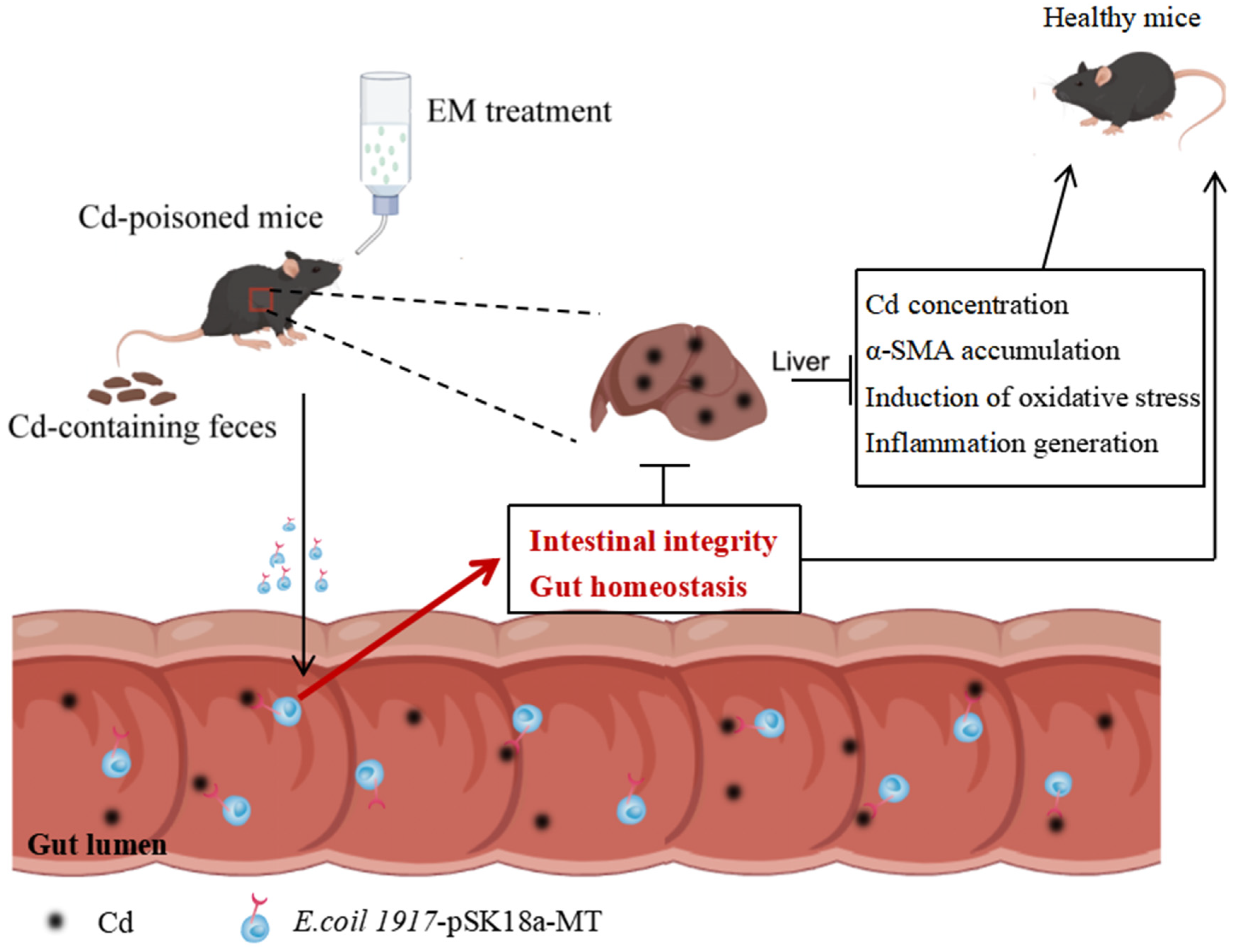
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernal, W.; Auzinger, G.; Dhawan, A.; Wendon, J. Acute Liver Failure. Lancet 2010, 376, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Brenner, D.A. Liver Inflammation and Fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Pi, H.; Xu, S.; Reiter, R.J.; Guo, P.; Zhang, L.; Li, Y.; Li, M.; Cao, Z.; Tian, L.; Xie, J.; et al. SIRT3-SOD2-mROS-Dependent Autophagy in Cadmium-Induced Hepatotoxicity and Salvage by Melatonin. Autophagy 2015, 11, 1037–1051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, X.; Elsabagh, M.; Zhang, Y.; Ma, Y.; Jin, Y.; Wang, M.; Wang, H.; Jiang, H. Effects of the Gut Microbiota and Barrier Function on Melatonin Efficacy in Alleviating Liver Injury. Antioxidants 2022, 11, 1727. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Che, L.; Qi, C.; Meng, Z. Protective Effects of Polysaccharides on Hepatic Injury: A Review. Int. J. Biol. Macromol. 2019, 141, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Rafati-Rahimzadeh, M.; Rafati-Rahimzadeh, M.; Kazemi, S.; Moghadamnia, A. Cadmium Toxicity and Treatment: An Update. Casp. J. Intern. Med. 2017, 8, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Yin, H.; Yang, Z.; Tan, M.; Wang, F.; Chen, K.; Zuo, Z.; Shu, G.; Cui, H.; Ouyang, P.; et al. Vitamin E Protects ahainst Cadmium-Induced Sub-Chronic Liver Injury Associated with the Inhibition of Oxidative Stress and Activation of Nrf2 Pathway. Ecotoxicol. Environ. Saf. 2021, 208, 111610. [Google Scholar] [CrossRef] [PubMed]
- García-Niño, W.R.; Pedraza-Chaverrí, J. Protective Effect of Curcumin against Heavy Metals-Induced Liver Damage. Food Chem. Toxicol. 2014, 69, 182–201. [Google Scholar] [CrossRef]
- Włostowski, T.; Krasowska, A.; Bonda, E. Joint Effects of Dietary Cadmium and Polychlorinated Biphenyls on Metallothionein Induction, Lipid Peroxidation and Histopathology in the Kidneys and Liver of Bank Voles. Ecotoxicol. Environ. Saf. 2008, 69, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Sánchez, C.; Egido, J.; Sánchez-González, P.D.; Pérez-Barriocanal, F.; López-Novoa, J.M.; Morales, A.I. Effect of the Flavonoid Quercetin on Cadmium-Induced Hepatotoxicity. Food Chem. Toxicol. 2008, 46, 2279–2287. [Google Scholar] [CrossRef]
- Cénit, M.C.; Matzaraki, V.; Tigchelaar, E.F.; Zhernakova, A. Rapidly Expanding Knowledge on the Role of the Gut Microbiome in Health and Disease. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2014, 1842, 1981–1992. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Madsen, K.; Spiller, R.; Van Meerveld, B.G.; Verne, G.N. Intestinal Barrier Function in Health and Gastrointestinal Disease. Neurogastroenterol. Motil. 2012, 24, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Anderson, J.M.; Bharti, R.; Raes, J.; Rosenstiel, P. The Resilience of the Intestinal Microbiota Influences Health and Disease. Nat. Rev. Microbiol. 2017, 15, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Zhang, H.; Jiang, X.; Zou, J.; Li, Q.; Mai, H.; Su, D.; Ling, W.; Feng, X. Bisphenol A Exposure Induces Gut Microbiota Dysbiosis and Consequent Activation of Gut-Liver Axis Leading to Hepatic Steatosis in CD-1 Mice. Environ. Pollut. 2020, 265, 114880. [Google Scholar] [CrossRef]
- Khanian, M.; Karimi-Torshizi, M.-A.; Allameh, A. Alleviation of Aflatoxin-Related Oxidative Damage to Liver and Improvement of Growth Performance in Broiler Chickens Consumed Lactobacillus plantarum 299v for Entire Growth Period. Toxicon 2019, 158, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, S.; Zhao, Y.; Wang, M.; Hu, L.; Li, W.; Xu, H. Combination of Houttuynia Cordata Polysaccharide and Lactiplantibacillus Plantarum P101 Alleviates Acute Liver Injury by Regulating Gut Microbiota in Mice. J. Sci. Food Agric. 2022, 102, 6848–6857. [Google Scholar] [CrossRef] [PubMed]
- Hancock, V.; Dahl, M.; Klemm, P. Probiotic Escherichia coli Strain Nissle 1917 Outcompetes Intestinalpathogens during Biofilm Formation. J. Med. Microbiol. 2010, 59, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhao, Z.; Zhao, Z.; Chen, Y.; Zhang, L. Native and Engineered Probiotics: Promising Agents against Related Systemic and Intestinal Diseases. Int. J. Mol. Sci. 2022, 23, 594. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Qi, X.; Han, J.; Ni, H.; Zhao, S. Reducing Cadmium Accumulation in Shrimp Using Escherichia coli with Surface-Displayed Peptide. Ecotoxicol. Environ. Saf. 2023, 256, 114858. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Chen, Y.; Li, H.; Li, W.; Wei, J.; Li, Z.; Wang, X.; Chen, T.; Huang, H. Engineered Bacteria EcN-MT Alleviate Liver Injury in Cadmium-Exposed Mice via Its Probiotics Characteristics and Expressing of Metallothionein. Front. Pharmacol. 2022, 13, 857869. [Google Scholar] [CrossRef]
- Wu, H.; Wei, J.; Zhao, X.; Liu, Y.; Chen, Z.; Wei, K.; Lu, J.; Chen, W.; Jiang, M.; Li, S.; et al. Neuroprotective Effects of an Engineered Escherichia coli Nissle 1917 on Parkinson’s Disease in Mice by Delivering GLP-1 and Modulating Gut Microbiota. Bioeng. Transla Med 2023, 8, e10351. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.-X.; Wang, X.-H.; Xu, X.; Chen, W.-J.; Wei, J.; Chen, T.-T.; Wei, H. Anti-Tumor Effects of Engineered VNP20009-Abvec-Igκ-mPD-1 Strain in Melanoma Mice via Combining the Oncolytic Therapy and Immunotherapy. Pharmaceutics 2022, 14, 2789. [Google Scholar] [CrossRef] [PubMed]
- Elazab, S.T.; Hsu, W.H. Antagonism of Cadmium-Induced Liver Injury in Ducks by α-Bisabolol. Front. Vet. Sci. 2022, 9, 1024549. [Google Scholar] [CrossRef] [PubMed]
- Schinner, S.A.C.; Mokszycki, M.E.; Adediran, J.; Leatham-Jensen, M.; Conway, T.; Cohen, P.S. Escherichia coli EDL933 Requires Gluconeogenic Nutrients To Successfully Colonize the Intestines of Streptomycin-Treated Mice Precolonized with E. coli Nissle 1917. Infect. Immun. 2015, 83, 1983–1991. [Google Scholar] [CrossRef] [PubMed]
- Reister, M.; Hoffmeier, K.; Krezdorn, N.; Rotter, B.; Liang, C.; Rund, S.; Dandekar, T.; Sonnenborn, U.; Oelschlaeger, T.A. Complete Genome Sequence of the Gram-Negative Probiotic Escherichia coli Strain Nissle 1917. J. Biotechnol. 2014, 187, 106–107. [Google Scholar] [CrossRef] [PubMed]
- Toloza, L.; Giménez, R.; Fábrega, M.J.; Alvarez, C.S.; Aguilera, L.; Cañas, M.A.; Martín-Venegas, R.; Badia, J.; Baldomà, L. The Secreted Autotransporter Toxin (Sat) Does Not Act as a Virulence Factor in the Probiotic Escherichia coli Strain Nissle 1917. BMC Microbiol. 2015, 15, 250. [Google Scholar] [CrossRef] [PubMed]
- Sanjeev, S.; Bidanchi, R.M.; Murthy, M.K.; Gurusubramanian, G.; Roy, V.K. Influence of Ferulic Acid Consumption in Ameliorating the Cadmium-Induced Liver and Renal Oxidative Damage in Rats. Environ. Sci. Pollut. Res. 2019, 26, 20631–20653. [Google Scholar] [CrossRef]
- Kaur, G.; Shivanandappa, T.B.; Kumar, M.; Kushwah, A.S. Fumaric Acid Protect the Cadmium-Induced Hepatotoxicity in Rats: Owing to Its Antioxidant, Anti-Inflammatory Action and Aid in Recast the Liver Function. Naunyn-Schmiedeberg’s Arch Pharm. 2020, 393, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Abu-El-Zahab, H.S.H.; Hamza, R.Z.; Montaser, M.M.; El-Mahdi, M.M.; Al-Harthi, W.A. Antioxidant, Antiapoptotic, Antigenotoxic, and Hepatic Ameliorative Effects of L-Carnitine and Selenium on Cadmium-Induced Hepatotoxicity and Alterations in Liver Cell Structure in Male Mice. Ecotoxicol. Environ. Saf. 2019, 173, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, Z.; Li, Y.; Sun, L.; Liu, Y.; Deng, Q.; Zhong, S. Ameliorative Effects of Oyster Protein Hydrolysates on Cadmium-Induced Hepatic Injury in Mice. Mar. Drugs 2022, 20, 758. [Google Scholar] [CrossRef] [PubMed]
- Kuester, R.K.; Waalkes, M.P.; Goering, P.L.; Fisher, B.L.; McCuskey, R.S.; Sipes, I.G. Differential Hepatotoxicity Induced by Cadmium in Fischer 344 and Sprague-Dawley Rats. Toxicol. Sci. 2002, 65, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.U.; Aziz, S.; Hamza, A.; Batool, M.; Alkahtani, S.; Riaz, M.N.; Ashraf, A. Curative Effects of Kaempferide on Cadmium-Instigated Hepatotoxicity in Male Albino Rats. J. King Saud Univ. Sci. 2023, 35, 102885. [Google Scholar] [CrossRef]
- Refaie, M.M.M.; El-Hussieny, M.; Zenhom, N.M. Protective Role of Nebivolol in Cadmium-Induced Hepatotoxicity via Downregulation of Oxidative Stress, Apoptosis and Inflammatory Pathways. Environ. Toxicol. Pharmacol. 2018, 58, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Qi, K.; Zhang, L.; Bai, Z.; Ren, C.; Xu, X.; Zhang, Z.; Li, X. Glutathione Might Attenuate Cadmium-Induced Liver Oxidative Stress and Hepatic Stellate Cell Activation. Biol. Trace Elem. Res. 2019, 191, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yan, J.; Xie, Y.; Chang, X.; Li, J.; Ren, C.; Zhu, J.; Ren, L.; Qi, K.; Bai, Z.; et al. Dual Role of Cadmium in Rat Liver: Inducing Liver Injury and Inhibiting the Progression of Early Liver Cancer. Toxicol. Lett. 2022, 355, 62–81. [Google Scholar] [CrossRef] [PubMed]
- Souza-Arroyo, V.; Fabián, J.J.; Bucio-Ortiz, L.; Miranda-Labra, R.U.; Gomez-Quiroz, L.E.; Gutiérrez-Ruiz, M.C. The Mechanism of the Cadmium-Induced Toxicity and Cellular Response in the Liver. Toxicology 2022, 480, 153339. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Ge, J.; Ren, Y.; Song, X.; Jiang, Y.; Sun-Waterhouse, D.; Li, F.; Li, D. Caffeic Acid Phenethyl Ester Mitigates Cadmium-Induced Hepatotoxicity in Mice: Role of miR-182-5p/TLR4 Axis. Ecotoxicol. Environ. Saf. 2021, 207, 111578. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; He, Y.; Wang, H.; Zhang, Q. Protective Effect of Melatonin against Chronic Cadmium-Induced Hepatotoxicity by Suppressing Oxidative Stress, Inflammation, and Apoptosis in Mice. Ecotoxicol. Environ. Saf. 2021, 228, 112947. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Weng, Z.; Liang, J.; Liu, Q.; Zhang, X.; Xu, J.; Xu, C.; Gu, A. Association between Urinary Cadmium Concentrations and Liver Function in Adolescents. Environ. Sci. Pollut. Res. 2022, 29, 39768–39776. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Imafidon, C.E.; Olukiran, O.S.; Ogundipe, D.J.; Eluwole, A.O.; Adekunle, I.A.; Oke, G.O. Acetonic Extract of Vernonia amygdalina (Del.) Attenuates Cd-Induced Liver Injury: Potential Application in Adjuvant Heavy Metal Therapy. Toxicol. Rep. 2018, 5, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-M.; Wan, X.-M.; Xiao, M.; Zheng, C.; Zhou, X.-L. Puerarin Induces Nrf2 as a Cytoprotective Mechanism to Prevent Cadmium-Induced Autophagy Inhibition and NLRP3 Inflammasome Activation in AML12 Hepatic Cells. J. Inorg. Biochem. 2021, 217, 111389. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhu, Y.; Lu, Z.; Guo, W.; Tumen, B.; He, Y.; Chen, C.; Hu, S.; Xu, K.; Wang, Y.; et al. Cadmium Induces Acute Liver Injury by Inhibiting Nrf2 and the Role of NF-κB, NLRP3, and MAPKs Signaling Pathway. Int. J. Environ. Res. Public Health 2020, 17, 138. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.K.; Li, H.Y.; Bai, L.H.; Wang, L.S.; Zou, X.T. Histological Changes, Lipid Metabolism, and Oxidative and Endoplasmic Reticulum Stress in the Liver of Laying Hens Exposed to Cadmium Concentrations. Poult. Sci. 2020, 99, 3215–3228. [Google Scholar] [CrossRef] [PubMed]
- Noor, K.K.; Ijaz, M.U.; Ehsan, N.; Tahir, A.; Yeni, D.K.; Neamul Kabir Zihad, S.M.; Uddin, S.J.; Ashraf, A.; Simal-Gandara, J. Hepatoprotective Role of Vitexin against Cadmium-Induced Liver Damage in Male Rats: A Biochemical, Inflammatory, Apoptotic and Histopathological Investigation. Biomed. Pharmacother. 2022, 150, 112934. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Luo, Y.; Xie, Z. Sulforaphane Ameliorates Cadmium Induced Hepatotoxicity through the Up-Regulation of /Nrf2/ARE Pathway and the Inactivation of NF-κB. J. Funct. Foods 2021, 77, 104297. [Google Scholar] [CrossRef]
- Kong, Y.; Li, M.; Wu, X.; Xia, C.; Liu, X.; Wang, G. Protective Mechanism of Homologous Lactic Acid Bacteria against Cholestatic Liver Injury in Snakehead Fish. Aquaculture 2022, 550, 737845. [Google Scholar] [CrossRef]
- Brdarić, E.; Soković Bajić, S.; Đokić, J.; Đurđić, S.; Ruas-Madiedo, P.; Stevanović, M.; Tolinački, M.; Dinić, M.; Mutić, J.; Golić, N.; et al. Protective Effect of an Exopolysaccharide Produced by Lactiplantibacillus plantarum BGAN8 Against Cadmium-Induced Toxicity in Caco-2 Cells. Front. Microbiol. 2021, 12, 759378. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Yin, R.; Yu, L.; Wang, G.; Tian, F.; Yu, R.; Zhao, J.; Liu, X.; Chen, Y.Q.; Zhang, H.; et al. Screening of Lactic Acid Bacteria with Potential Protective Effects against Cadmium Toxicity. Food Control 2015, 54, 23–30. [Google Scholar] [CrossRef]
- Liu, Y.; Kang, W.; Liu, S.; Li, J.; Liu, J.; Chen, X.; Gan, F.; Huang, K. Gut Microbiota–Bile Acid–Intestinal Farnesoid X Receptor Signaling Axis Orchestrates Cadmium-Induced Liver Injury. Sci. Total Environ. 2022, 849, 157861. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Huang, H.; Luo, C.; Zhang, X.; Chen, Y.; Yue, F.; Xie, B.; Chen, T.; Zou, C. The Next-Generation Probiotic E. coli 1917-pSK18a-MT Ameliorates Cadmium-Induced Liver Injury by Surface Display of Metallothionein and Modulation of Gut Microbiota. Nutrients 2024, 16, 1468. https://doi.org/10.3390/nu16101468
Zhang Y, Huang H, Luo C, Zhang X, Chen Y, Yue F, Xie B, Chen T, Zou C. The Next-Generation Probiotic E. coli 1917-pSK18a-MT Ameliorates Cadmium-Induced Liver Injury by Surface Display of Metallothionein and Modulation of Gut Microbiota. Nutrients. 2024; 16(10):1468. https://doi.org/10.3390/nu16101468
Chicago/Turabian StyleZhang, Yan, Hong Huang, Chuanlin Luo, Xinfeng Zhang, Yanjing Chen, Fenfang Yue, Bingqing Xie, Tingtao Chen, and Changwei Zou. 2024. "The Next-Generation Probiotic E. coli 1917-pSK18a-MT Ameliorates Cadmium-Induced Liver Injury by Surface Display of Metallothionein and Modulation of Gut Microbiota" Nutrients 16, no. 10: 1468. https://doi.org/10.3390/nu16101468
APA StyleZhang, Y., Huang, H., Luo, C., Zhang, X., Chen, Y., Yue, F., Xie, B., Chen, T., & Zou, C. (2024). The Next-Generation Probiotic E. coli 1917-pSK18a-MT Ameliorates Cadmium-Induced Liver Injury by Surface Display of Metallothionein and Modulation of Gut Microbiota. Nutrients, 16(10), 1468. https://doi.org/10.3390/nu16101468







