Prevalence of T-2 Toxin in the Food and Beverages of Residents Living in a Kashin–Beck-Disease Area of Qamdo, Tibet
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Investigation Regions, Sites and Population
2.2. Survey Objects and Blood Sample Collection
2.3. Collection of Food Sample
2.4. Determination of Selenium Content in Blood
2.5. Determination of T-2 Toxin Contamination Levels in Food and Water Samples
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Population
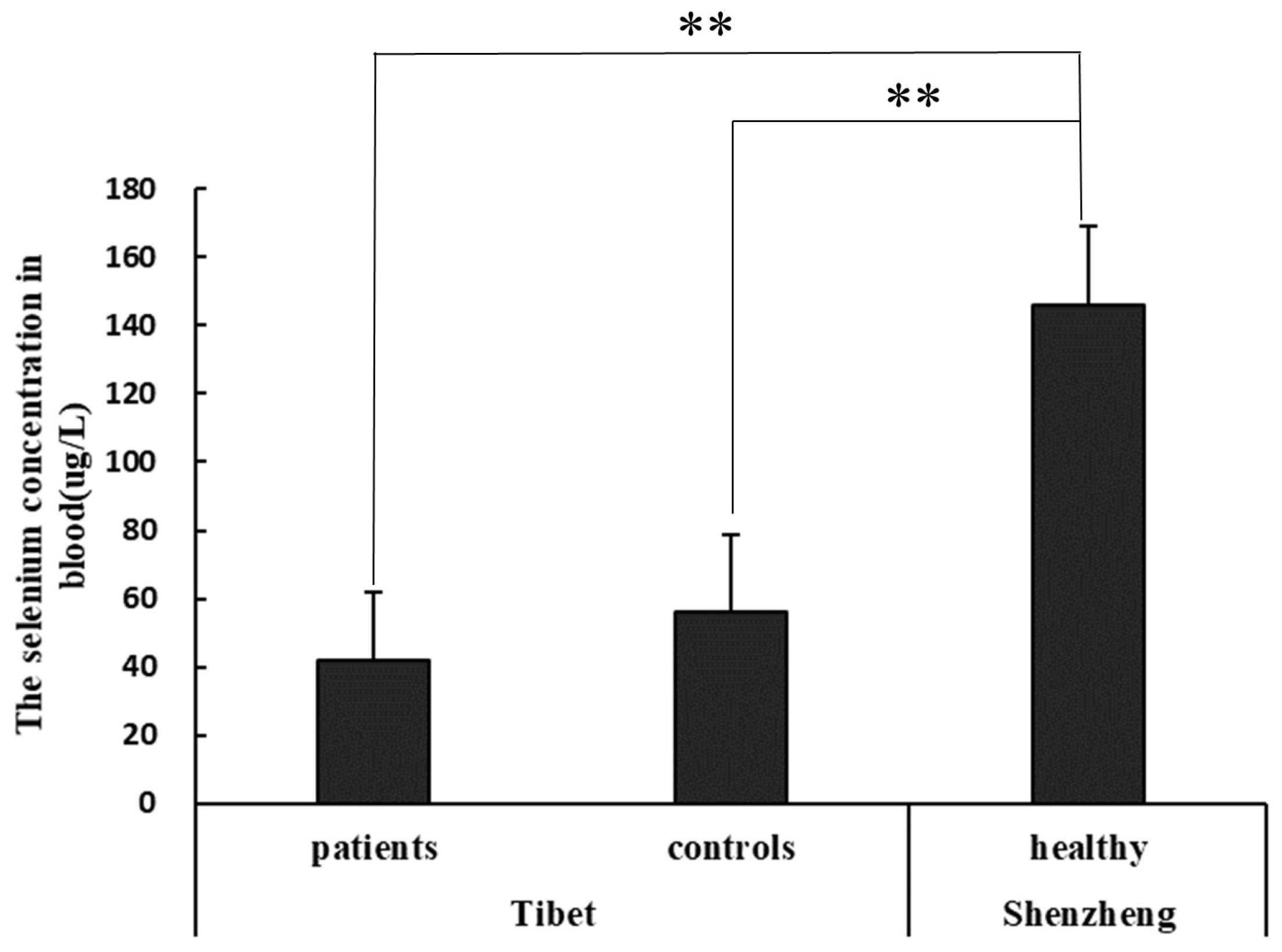
3.2. Selenium Concentration in Blood of KBD Area and Non-KBD Area
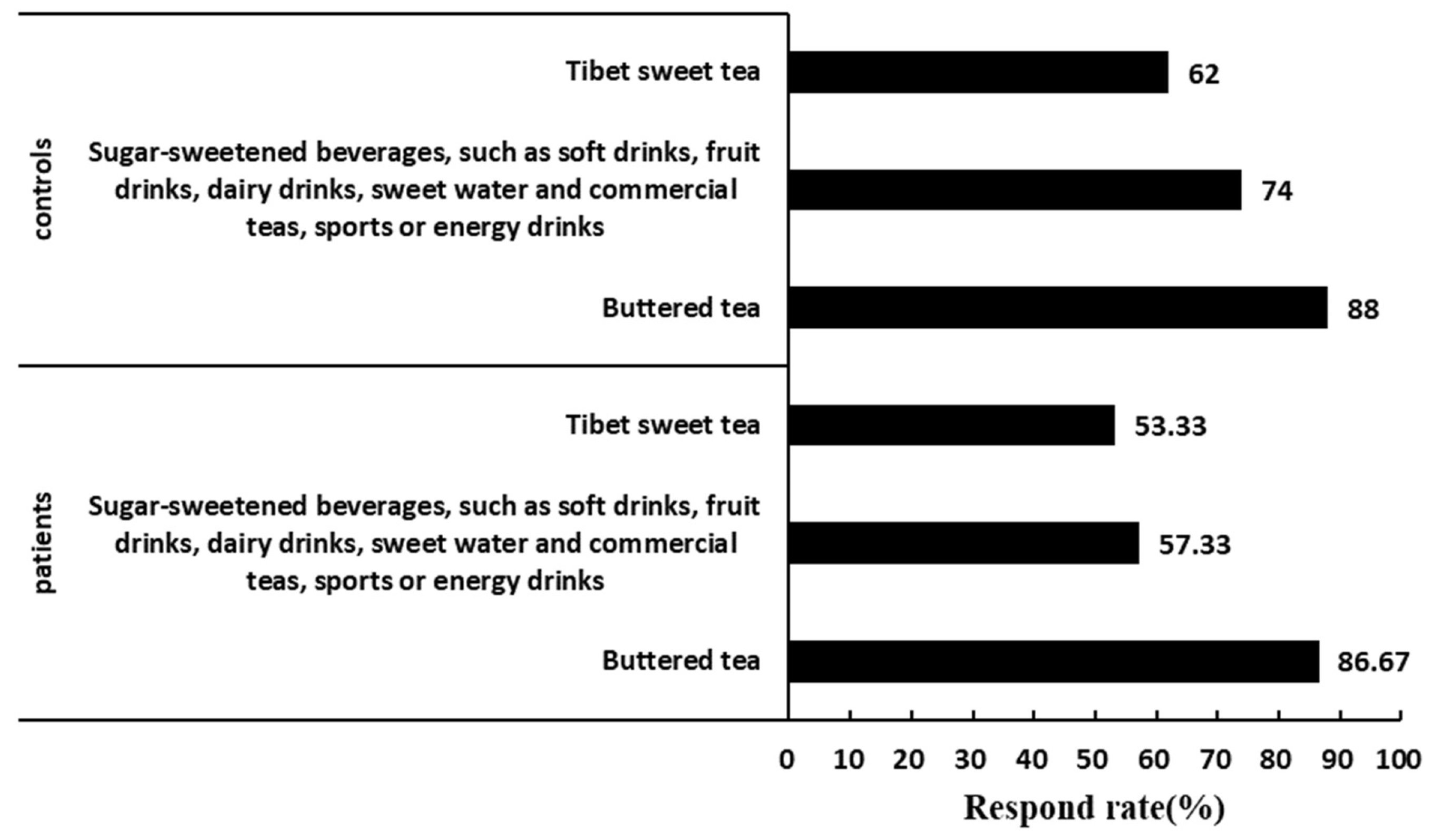
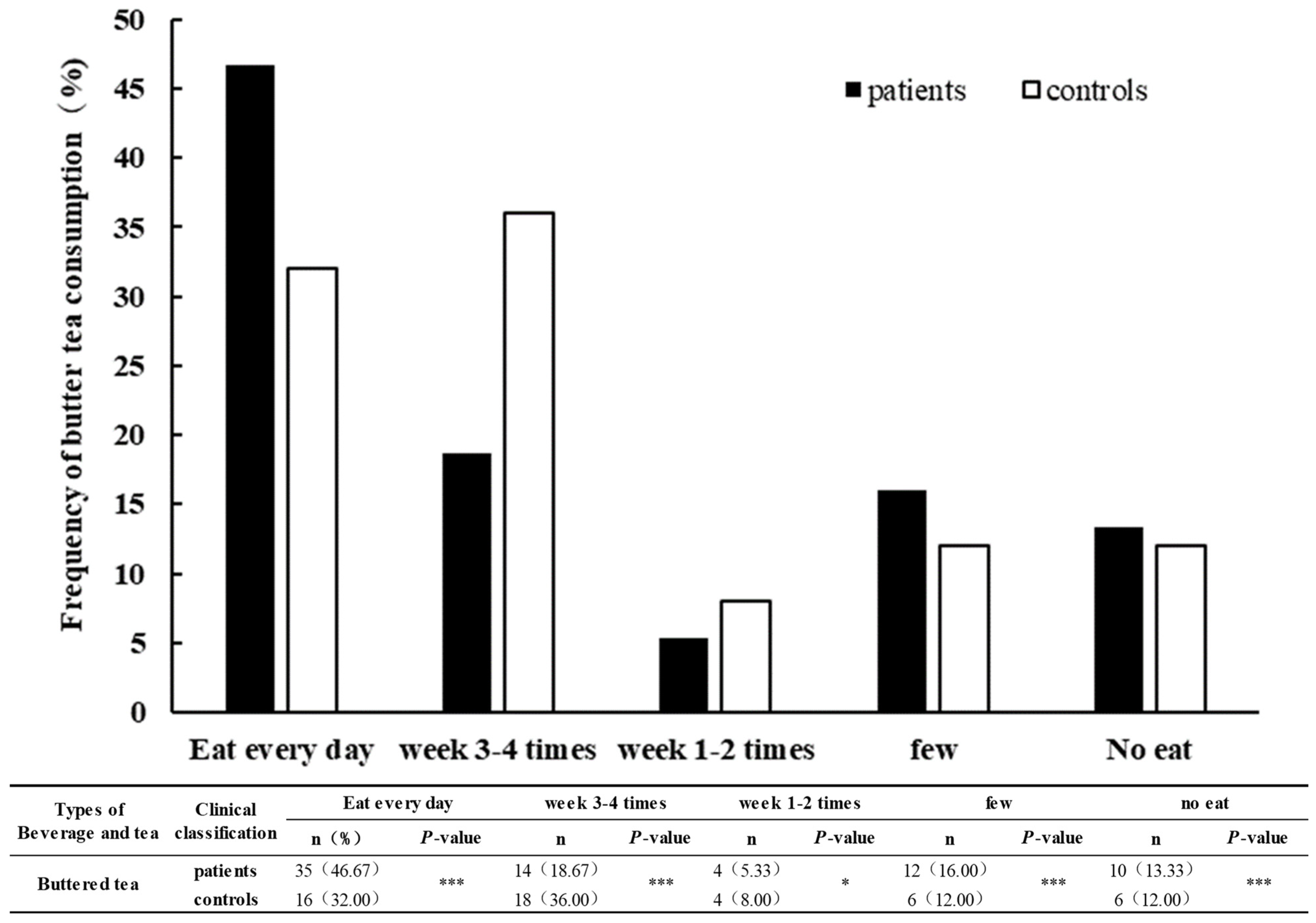
3.3. Comparison of Drink and Beverage Dietary Intake and Frequency in KBD Area between Patients and Controls
3.4. T-2 Toxin Contamination Level in Food and Drinking Water Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, K.; Yu, J.; Liu, H.; Liu, Y.; Liu, N.; Cao, Y.; Zhang, X.; Sun, D. Endemic Kashin-Beck disease: A food-sourced osteoarthropathy. Semin. Arthritis Rheum. 2020, 50, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Allander, E. Kashin-Beck disease. An analysis of research and public health activities based on a bibliography 1849–1992. Scand. J. Rheumatol. Suppl. 1994, 99, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Reyes, R.; Suetens, C.; Mathieu, F.; Begaux, F.; Zhu, D.; Rivera, M.T.; Boelaert, M.; Nève, J.; Perlmutter, N.; Vanderpas, J. KashinBeck osteoarthropathy in rural Tibet in relation to selenium and iodine status. N. Engl. J. Med. 1998, 339, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Q.; Li, Q.; Zhao, S.; Guo, M.; Zhang, S.; Cheng, X.; Li, Z.; Yang, Z.; Qiu, Y.; et al. Prevalence of pediatric Kashin-Beck disease in Tibet. Clin. Rheumatol. 2021, 40, 3717–3722. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, J.; Zhao, H. The Prevalence of Kashin-Beck Disease in China: A Systematic Review and Meta-analysis. Biol. Trace Elem. Res. 2022, 201, 3175–3184. [Google Scholar] [CrossRef] [PubMed]
- Qun, W. The disease and pathogenesis of KBD in Tibet and population intervention of KBD epidemic control by food exchange. China Sci. Technol. Achiev. 2019, 20, 56–58. [Google Scholar]
- Li, D.; Han, J.; Guo, X.; Qu, C.; Yu, F.; Wu, X. The effects of T-2 toxin on the prevalence and development of Kashin-Beck disease in China: A meta-analysis and systematic review. Toxicol. Res. 2016, 5, 731–751. [Google Scholar] [CrossRef] [PubMed]
- Gou, X. Progression and prospect of etiology and pathogenesis of Kashin-Beck disease. J. Xi’an Jiaotong Univ. (Med. Sci.) 2008, 29, 481–488. [Google Scholar]
- Li, X.; Chen, Q. The progress and prospect of the etiology and pathogenesis of Kashin-Beck disease. Foreign Med. Sci. 2018, 39, 361–364. [Google Scholar]
- T-2 toxin and Kashin-Beck disease. Chin. J. Control. Endem. Dis. 2000, 15, 35–39.
- Zhang, Y.; Li, Z.; He, Y.; Liu, Y.; Mi, G.; Chen, J. T-2 toxin induces articular cartilage damage by increasing the expression of MMP-13 via the TGF-β receptor pathway. Hum. Exp. Toxicol. 2022, 41, 9603271221075555. [Google Scholar] [CrossRef] [PubMed]
- Polak-Śliwińska, M.; Paszczyk, B. Trichothecenes in food and feed, relevance to human and animal health and methods of detection: A systematic review. Molecules 2021, 26, 454. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Z.; Beier, R.C.; Shen, J.; Smet, D.D.; De Saeger, S.; Zhang, S. T-2 toxin, a trichothecene mycotoxin: Review of toxicity, metabolism, and analytical methods. J. Agric. Food Chem. 2011, 59, 3441–3453. [Google Scholar] [CrossRef] [PubMed]
- GB 16395-2011; Criteria for Delimitation and Classification of Kaschin-Beck Disease Endemic Area. State General Administration of the People’s Republic of China for Quality Supervision and Inspection and Quarantine: Beijing, China, 2011.
- WS/T207-2010; Diagnosis of Kaschin-Beck Disease. The Ministry of Health of the People’s Republic of China: Beijing, China, 2010.
- Lian, Q.; Jia, P.P.; Xiao, M.; Cui, M.; Sun, X. Test and analysis on mycotoxin level of hulless barley. Sci. Technol. Cereals Oils Foods 2022, 30, 157–161. [Google Scholar]
- Wei, N.; Zhang, F.L.; Zhang, Y. Distribution of fungal toxin contamination in main grain and oil crops on the Tibetan plateau. Qual. Saf. Agro-Prod. 2018, 3, 66–70. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific opinion on the risks for animal and public health related to the presence of T-2 and HT-2 toxin in food and feed. EFSA J. 2011, 9, 2481. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, Q.; Xie, F.; Jiang, J. Brick tea consumption and its relationship with fluorosis in Tibetan areas. Front. Nutr. 2022, 9, 1030344. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.Y.; Li, Q.; Meng, F.G.; Fu, Y.; Zhao, Z.J.; Wang, L.H. T-2 toxin contamination in grains and selenium concentration in drinking water and grains in Kaschin-Beck disease endemic areas of Qinghai Province. Biol. Trace Elem. Res. 2012, 150, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Wang, Z.L.; Yang, H.J.; Xue, S.H.; Song, D.Q.; Dong, L.; Ma, T.Y. Histopathology of ehondroneerosis in knee articular cartilage of rat at T-2 toxin and selenium deficiency conditions. Chin. J. Control Endem. Dis. 2010, 25, 98–101. [Google Scholar]
- Schwake-Anduschus, C.; Langenkämper, G.; Unbehend, G.; Dietrich, R.; Märtlbauer, E.; Münzing, K. Occurrence of Fusarium T-2 and HT-2 toxins in oats from eultivar studies in Germany and degradation of the toxins during grain cleaning treatment and food processing. Food Addit. Contam. 2010, 27, 1253–1260. [Google Scholar] [CrossRef]
- WHO; FAO. Safety evaluation of certain mycotoxins in food. In Proceedings of the Fifty-Sixth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), Geneva, Switzerland, 6–15 February 2001; WHO Food Additives Series 47, FAO Food and Nutrition Paper 74; International Programme on Chemical Safety. World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Zhao, S.; Degyi, Y.; Nimakura, D.; Tu, D.; Gong, H.; Guo, M. Investigation report on the influencing factors of internal and external environmental conditions of historical serious disease areas of kashin-beck in Tibet. J. Med. Pest Control 2020, 36, 1002–1004, 1009. [Google Scholar]
| Drinks and Beverage Category | Patient (n = 75) | Controls (n = 50) | X2 | p-Value 1 |
|---|---|---|---|---|
| Liquor (including all types) | 1 (1.33) | 0 (0.00) | 0.672 | 0.412 |
| Wine/rice wine/rice wine | 1 (1.33) | 0 (0.00) | 0.672 | 0.412 |
| Beer | 1 (1.33) | 0 (0.00) | 0.672 | 0.412 |
| Hulless barley wine | 2 (2.67) | 0 (0.00) | 1.355 | 0.244 |
| No added water or soda | 38 (50.67) | 20 (40.00) | 1.372 | 0.241 |
| Artificial sugary drinks include carbonated drinks and commercial teas | 35 (46.67) | 30 (60.00) | 2.137 | 0.144 |
| Sugar-sweetened beverages, such as soft drinks, fruit drinks, dairy drinks, sweet water and commercial teas, sports or energy drinks | 43 (57.33) | 37 (74.00) | 3.617 | 0.057 |
| Buttered tea | 65 (86.67) | 44 (88.00) | 0.048 | 0.827 |
| Tibet sweet tea | 40 (53.33) | 31 (62.00) | 0.918 | 0.338 |
| Other teas (no added natural green/black/oolong tea, etc.) | 38 (50.67) | 28 (56.00) | 0.342 | 0.558 |
| Coffee (with or without sugar/milk) | 17 (22.67) | 21 (42.00) * | 5.300 | 0.021 |
| 100% pure juice | 17 (22.67) | 15 (30.00) | 0.847 | 0.357 |
| Food Type | Survey Sites | n | AVE. (μg/kg) |
|---|---|---|---|
| Brick tea | Detong village | 5 | 424 ± 56 |
| Langcuo village | 3 | 396 ± 24 | |
| Zanba powder | Detong village | 5 | Tr |
| Langcuo village | 3 | Tr | |
| Hulless barley | Detong village | 5 | Tr |
| Langcuo village | 3 | Tr | |
| Milk residue | Detong village | 5 | Tr |
| Langcuo village | 3 | Tr | |
| Drinking water | Detong village | 6 | Tr |
| Langcuo village | 4 | Tr |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, T.; Yan, J.; Tan, H.; Pu, Z.; Wang, O.; Liu, T.; Chen, Z.; Gao, J.; Wang, J.; Lin, J.; et al. Prevalence of T-2 Toxin in the Food and Beverages of Residents Living in a Kashin–Beck-Disease Area of Qamdo, Tibet. Nutrients 2024, 16, 1449. https://doi.org/10.3390/nu16101449
Jiang T, Yan J, Tan H, Pu Z, Wang O, Liu T, Chen Z, Gao J, Wang J, Lin J, et al. Prevalence of T-2 Toxin in the Food and Beverages of Residents Living in a Kashin–Beck-Disease Area of Qamdo, Tibet. Nutrients. 2024; 16(10):1449. https://doi.org/10.3390/nu16101449
Chicago/Turabian StyleJiang, Tong, Junan Yan, Hongxing Tan, Zhu Pu, Ou Wang, Tao Liu, Zhaoyu Chen, Jiaxiang Gao, Jun Wang, Jianhao Lin, and et al. 2024. "Prevalence of T-2 Toxin in the Food and Beverages of Residents Living in a Kashin–Beck-Disease Area of Qamdo, Tibet" Nutrients 16, no. 10: 1449. https://doi.org/10.3390/nu16101449
APA StyleJiang, T., Yan, J., Tan, H., Pu, Z., Wang, O., Liu, T., Chen, Z., Gao, J., Wang, J., Lin, J., Huo, J., & Huang, J. (2024). Prevalence of T-2 Toxin in the Food and Beverages of Residents Living in a Kashin–Beck-Disease Area of Qamdo, Tibet. Nutrients, 16(10), 1449. https://doi.org/10.3390/nu16101449






