Amurensin G Sensitized Cholangiocarcinoma to the Anti-Cancer Effect of Gemcitabine via the Downregulation of Cancer Stem-like Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Cell Viability Assay
2.3. Colony Formation Assay
2.4. Cell Apoptosis Assay
2.5. Wound Healing Assay
2.6. Transwell Migration Assay
2.7. Flow Cytometry Analysis
2.8. Mitochondrial Superoxide (MitoSOX) Assay
2.9. Western Blot Assay
2.10. Statistical Analysis
3. Results
3.1. AMG Decreases Cell Growth in SNU-478 Cells
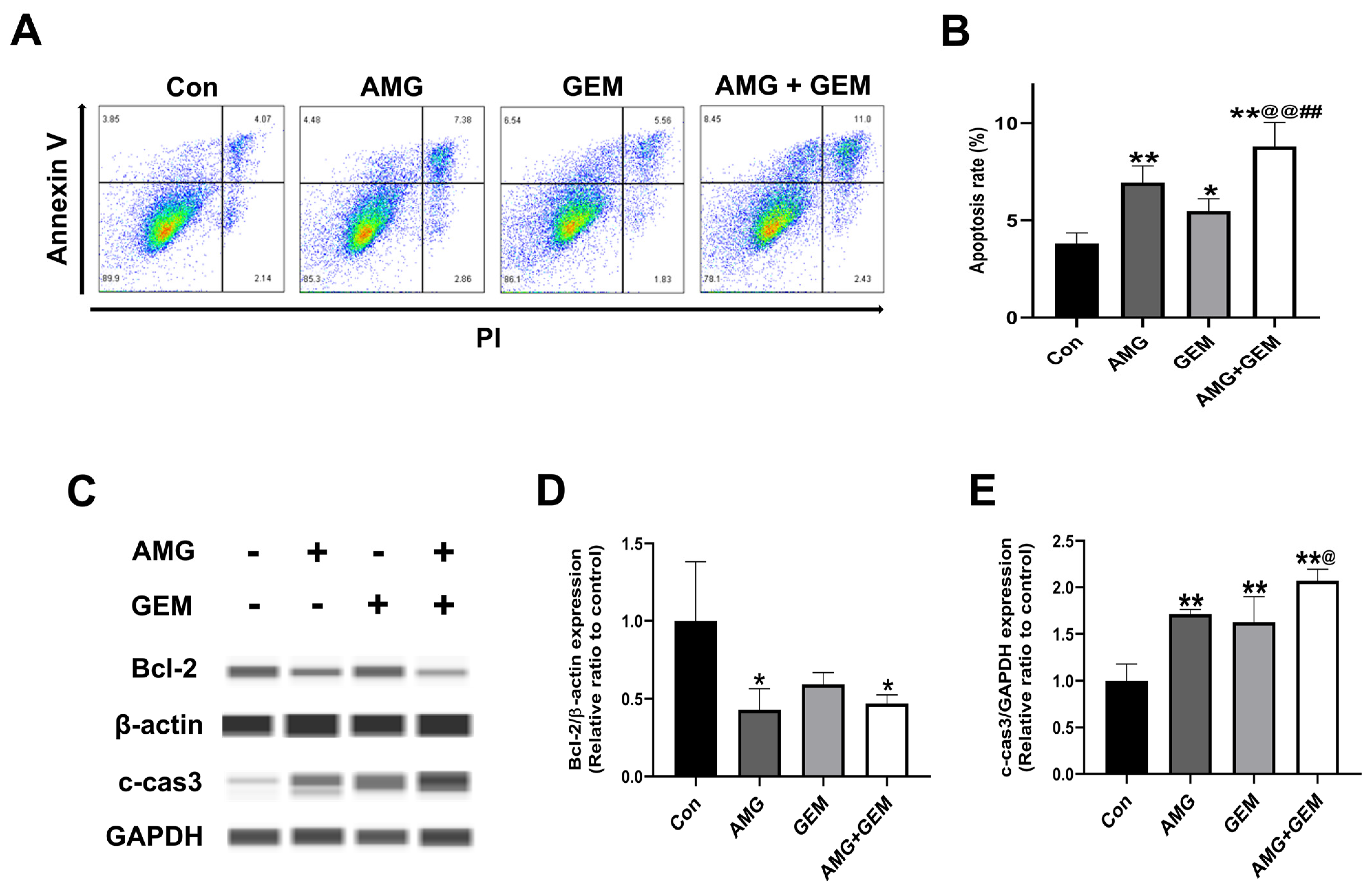
3.2. Combination Treatment of AMG with Gemcitabine Induces Apoptosis in SNU-478 Cells
3.3. AMG Suppresses the Migratory Ability in SNU-478 Cells
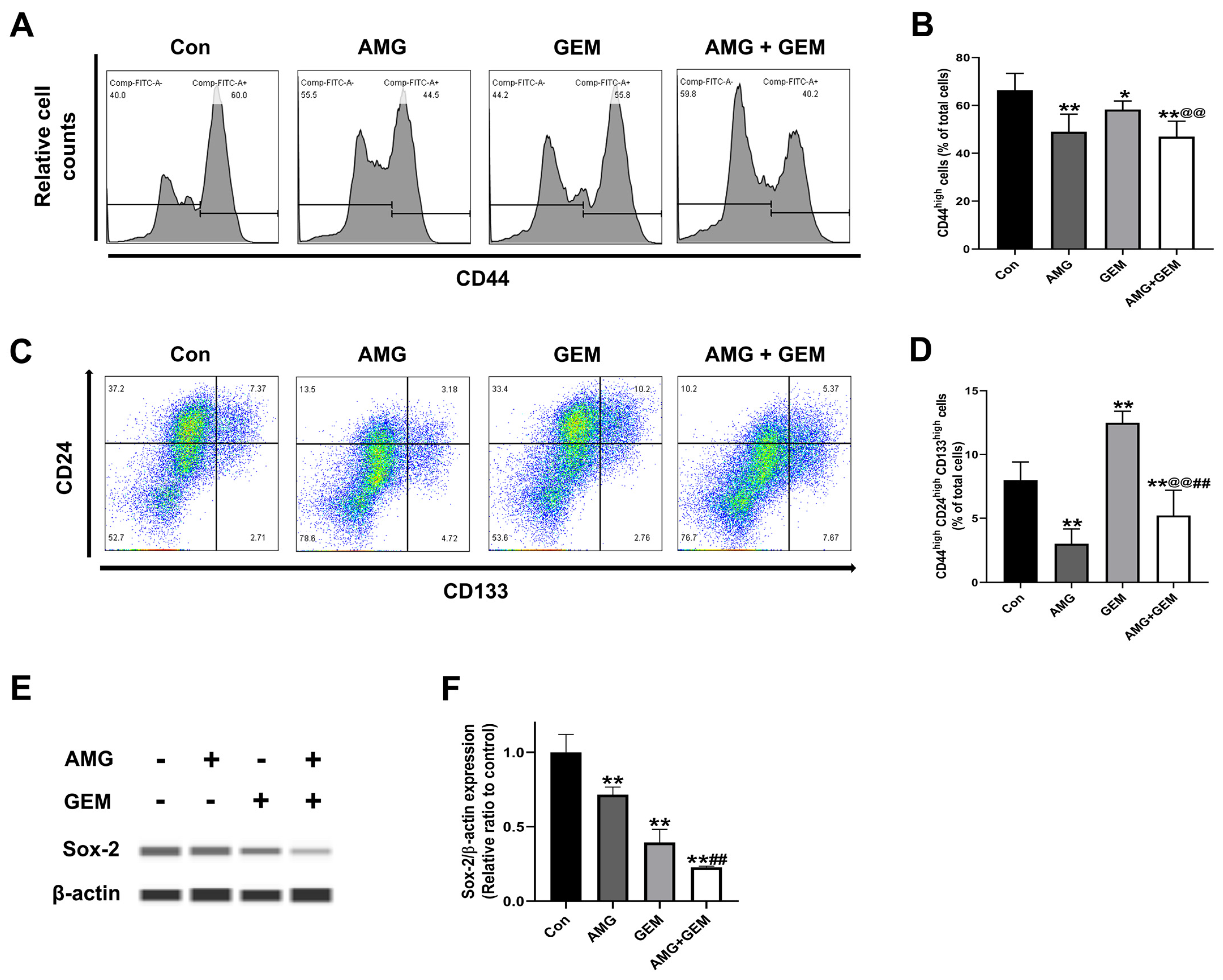
3.4. AMG Inhibits Cancer Stem-like Cell Properties in SNU-478 Cells
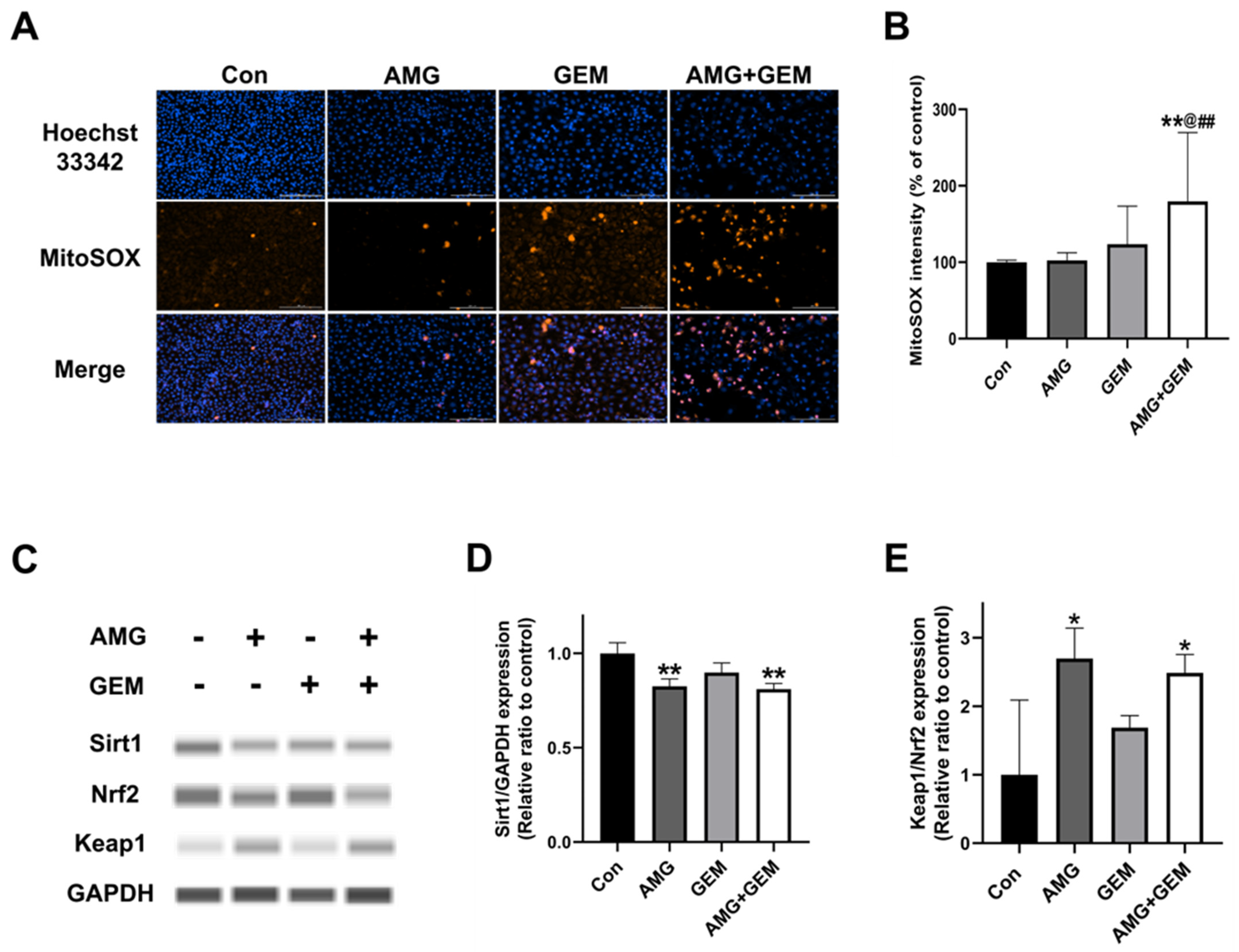
3.5. Combination Treatment of AMG with Gemcitabine Increases Mitochondrial ROS Production in SNU-478 Cells
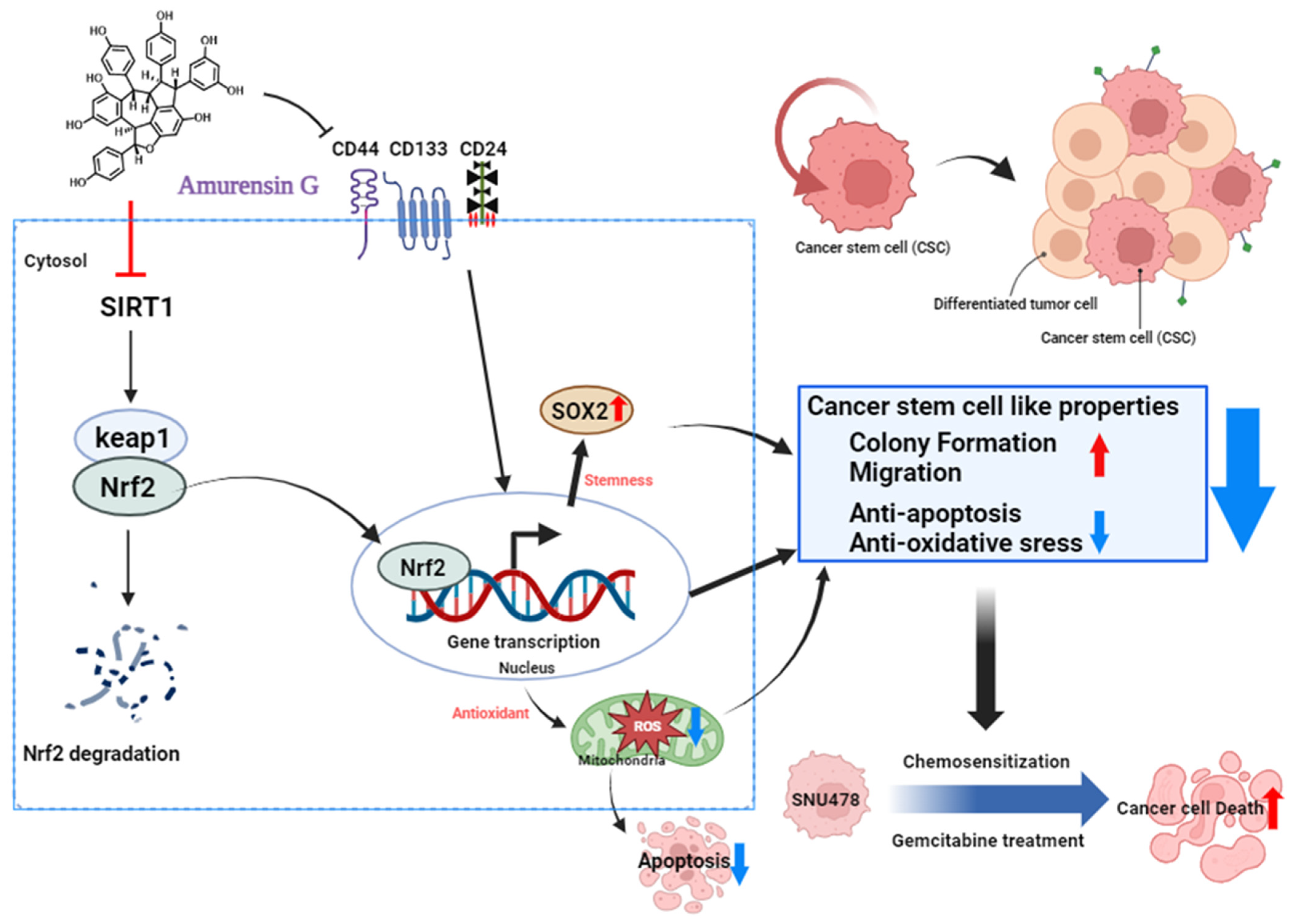
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Chu, P.Y. Role of cancer stem cells in cholangiocarcinoma and therapeutic implications. Int. J. Mol. Sci. 2019, 20, 4154. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Elvevi, A.; Laffusa, A.; Scaravaglio, M.; Rossi, R.E.; Longarini, R.; Stagno, A.M.; Cristoferi, L.; Ciaccio, A.; Cortinovis, D.L.; Invernizzi, P.; et al. Clinical treatment of cholangiocarcinoma: An updated comprehensive review. Ann. Hepatol. 2022, 27, 100737. [Google Scholar] [CrossRef] [PubMed]
- Pandit, B.; Royzen, M. Recent development of prodrugs of gemcitabine. Genes 2022, 13, 466. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Chen, X.; Luan, Y. Small molecular gemcitabine prodrugs for cancer therapy. Curr. Med. Chem. 2020, 27, 5562–5582. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Xie, J. Promising molecular mechanisms responsible for gemcitabine resistance in cancer. Genes. Dis. 2015, 2, 299–306. [Google Scholar] [CrossRef]
- Lei, M.M.L.; Lee, T.K.W. Cancer stem cells: Emerging key players in immune evasion of cancers. Front. Cell Dev. Biol. 2021, 9, 692940. [Google Scholar] [CrossRef]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target. Ther. 2020, 5, 8. [Google Scholar] [CrossRef]
- Ryu, H.W.; Oh, W.K.; Jang, I.S.; Park, J. Amurensin g induces autophagy and attenuates cellular toxicities in a rotenone model of parkinson’s disease. Biochem. Biophys. Res. Commun. 2013, 433, 121–126. [Google Scholar] [CrossRef]
- Butnariu, M.; Quispe, C.; Herrera-Bravo, J.; Pentea, M.; Sarac, I.; Kusumler, A.S.; Ozcelik, B.; Painuli, S.; Semwal, P.; Imran, M.; et al. Papaver plants: Current insights on phytochemical and nutritional composition along with biotechnological applications. Oxid. Med. Cell Longev. 2022, 2022, 2041769. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.S.; Park, J.I.; Kim, M.J.; Kim, H.B.; Lee, J.W.; Dao, T.T.; Oh, W.K.; Kang, C.D.; Kim, S.H. Involvement of sirt1 in hypoxic down-regulation of c-myc and beta-catenin and hypoxic preconditioning effect of polyphenols. Toxicol. Appl. Pharmacol. 2012, 259, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.Y.; Kim, J.Y.; Lee, H.K.; Ha do, T.; Song, K.S.; Bae, K.; Seong, Y.H. Leaf and stem of vitis amurensis and its active components protect against amyloid beta protein (25-35)-induced neurotoxicity. Arch. Pharm. Res. 2010, 33, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Kim, M.R.; Kim, O.; Phuong, N.T.; Yun, J.; Oh, W.K.; Bae, K.; Kang, K.W. Amurensin g inhibits angiogenesis and tumor growth of tamoxifen-resistant breast cancer via pin1 inhibition. Food Chem. Toxicol. 2012, 50, 3625–3634. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, M.J.; Kim, D.W.; Kang, C.D.; Kim, S.H. Amurensin g enhances the susceptibility to tumor necrosis factor-related apoptosis-inducing ligand-mediated cytotoxicity of cancer stem-like cells of hct-15 cells. Cancer Sci. 2013, 104, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.K.; Cho, K.B.; Hien, T.T.; Kim, T.H.; Kim, H.S.; Dao, T.T.; Han, H.K.; Kwon, S.M.; Ahn, S.G.; Yoon, J.H.; et al. Amurensin g, a potent natural sirt1 inhibitor, rescues doxorubicin responsiveness via down-regulation of multidrug resistance 1. Mol. Pharmacol. 2010, 78, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Lee, H.K.; Nam, M.W.; Lee, G.; Choi, K.C. The role of kiss1 gene on the growth and migration of prostate cancer and the underlying molecular mechanisms. Life Sci. 2022, 310, 121009. [Google Scholar] [CrossRef]
- Nam, M.W.; Lee, H.K.; Kim, C.W.; Choi, Y.; Ahn, D.; Go, R.E.; Choi, K.C. Effects of ccn6 overexpression on the cell motility and activation of p38/bone morphogenetic protein signaling pathways in pancreatic cancer cells. Biomed. Pharmacother. 2023, 163, 114780. [Google Scholar] [CrossRef]
- Kim, C.W.; Lee, H.K.; Nam, M.W.; Choi, Y.; Choi, K.C. Overexpression of kiss1 induces the proliferation of hepatocarcinoma and increases metastatic potential by increasing migratory ability and angiogenic capacity. Mol. Cells 2022, 45, 935–949. [Google Scholar] [CrossRef]
- Kokuryo, T.; Yokoyama, Y.; Nagino, M. Recent advances in cancer stem cell research for cholangiocarcinoma. J. Hepatobiliary Pancreat. Sci. 2012, 19, 606–613. [Google Scholar] [CrossRef]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the european network for the study of cholangiocarcinoma (ens-cca). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Duan, Q.; Zhao, H.; Liu, T.; Wu, H.; Shen, Q.; Wang, C.; Yin, T. Gemcitabine treatment promotes pancreatic cancer stemness through the nox/ros/nf-kappab/stat3 signaling cascade. Cancer Lett. 2016, 382, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Ji, H.; Gan, X.; Cao, S.; Li, Z.; Jin, Y. Knockdown of cd44 inhibits proliferation, migration and invasion of osteosarcoma cells accompanied by downregulation of cathepsin s. J. Orthop. Surg. Res. 2022, 17, 154. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Huang, S.; Chen, S.; Chen, J.; Wang, Z.; Wang, Y.; Zheng, H. Sox2 promotes chemoresistance, cancer stem cells properties, and epithelial-mesenchymal transition by beta-catenin and beclin1/autophagy signaling in colorectal cancer. Cell Death Dis. 2021, 12, 449. [Google Scholar] [CrossRef] [PubMed]
- Thanee, M.; Dokduang, H.; Kittirat, Y.; Phetcharaburanin, J.; Klanrit, P.; Titapun, A.; Namwat, N.; Khuntikeo, N.; Wangwiwatsin, A.; Saya, H.; et al. Cd44 modulates metabolic pathways and altered ros-mediated akt signal promoting cholangiocarcinoma progression. PLoS ONE 2021, 16, e0245871. [Google Scholar] [CrossRef] [PubMed]
- Moloney, J.N.; Cotter, T.G. Ros signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, T.; Nagano, O.; Yae, T.; Tamada, M.; Motohara, T.; Oshima, H.; Oshima, M.; Ikeda, T.; Asaba, R.; Yagi, H.; et al. Cd44 variant regulates redox status in cancer cells by stabilizing the xct subunit of system xc(−) and thereby promotes tumor growth. Cancer Cell 2011, 19, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, S.; Li, H.; Duan, Q.; Zhang, Z.; Shen, Q.; Wang, C.; Yin, T. Ros/kras/ampk signaling contributes to gemcitabine-induced stem-like cell properties in pancreatic cancer. Mol. Ther. Oncolytics 2019, 14, 299–312. [Google Scholar] [CrossRef]
- Li, Z. Cd133: A stem cell biomarker and beyond. Exp. Hematol. Oncol. 2013, 2, 17. [Google Scholar] [CrossRef]
- Nomura, A.; Dauer, P.; Gupta, V.; McGinn, O.; Arora, N.; Majumdar, K.; Uhlrich, C., 3rd; Dalluge, J.; Dudeja, V.; Saluja, A.; et al. Microenvironment mediated alterations to metabolic pathways confer increased chemo-resistance in cd133+ tumor initiating cells. Oncotarget 2016, 7, 56324–56337. [Google Scholar] [CrossRef]
- Kim, H.B.; Kim, M.J.; Lee, S.H.; Lee, J.W.; Bae, J.H.; Kim, D.W.; Dao, T.T.; Oh, W.K.; Kang, C.D.; Kim, S.H. Amurensin g, a novel sirt1 inhibitor, sensitizes trail-resistant human leukemic k562 cells to trail-induced apoptosis. Biochem. Pharmacol. 2012, 84, 402–410. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Wang, B.; Wang, X.; Dong, G.; Jia, J.; Yang, Q. Sirt1 inhibits chemoresistance and cancer stemness of gastric cancer by initiating an ampk/foxo3 positive feedback loop. Cell Death Dis. 2020, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, W. Emerging roles of sirt1 in cancer drug resistance. Genes Cancer 2013, 4, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Alves-Fernandes, D.K.; Jasiulionis, M.G. The role of sirt1 on DNA damage response and epigenetic alterations in cancer. Int. J. Mol. Sci. 2019, 20, 3153. [Google Scholar] [CrossRef]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The role of sirtuins in antioxidant and redox signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, H.Y.; Gao, L.; Bai, M.Z.; Fu, W.K.; Huang, C.F.; Mi, N.N.; Ma, H.D.; Lu, Y.W.; Jiang, N.Z.; et al. Lanatoside c decelerates proliferation and induces apoptosis through inhibition of stat3 and ros-mediated mitochondrial membrane potential transformation in cholangiocarcinoma. Front. Pharmacol. 2023, 14, 1098915. [Google Scholar] [CrossRef]


| Protein | Manufacturer | Molecular Weight (kDa) | Source | Clone | Dilution Ratio |
|---|---|---|---|---|---|
| Bcl-2 | BioLegend | 22, 26 | Mouse | 100 | 1:100 |
| c-cas3 | Cell Signaling Technology | 17, 19 | Rabbit | Polyclonal | 1:100 |
| Sox-2 | Cell Signaling Technology | 35 | Rabbit | D6D9 | 1:100 |
| Sirt1 | Cell Signaling Technology | 120 | Mouse | 1F3 | 1:100 |
| Nrf2 | Cell Signaling Technology | 97–100 | Rabbit | D1Z9C | 1:100 |
| Keap1 | Cell Signaling Technology | 60–64 | Rabbit | D6B12 | 1:100 |
| GAPDH | Abcam | 40.2 | Mouse | 6C5 | 1:200 |
| β-actin | Cell Signaling Technology | 45 | Rabbit | 13E5 | 1:200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, Y.-J.; Lee, H.K.; Choi, K.-C. Amurensin G Sensitized Cholangiocarcinoma to the Anti-Cancer Effect of Gemcitabine via the Downregulation of Cancer Stem-like Properties. Nutrients 2024, 16, 73. https://doi.org/10.3390/nu16010073
Na Y-J, Lee HK, Choi K-C. Amurensin G Sensitized Cholangiocarcinoma to the Anti-Cancer Effect of Gemcitabine via the Downregulation of Cancer Stem-like Properties. Nutrients. 2024; 16(1):73. https://doi.org/10.3390/nu16010073
Chicago/Turabian StyleNa, Yun-Jung, Hong Kyu Lee, and Kyung-Chul Choi. 2024. "Amurensin G Sensitized Cholangiocarcinoma to the Anti-Cancer Effect of Gemcitabine via the Downregulation of Cancer Stem-like Properties" Nutrients 16, no. 1: 73. https://doi.org/10.3390/nu16010073
APA StyleNa, Y.-J., Lee, H. K., & Choi, K.-C. (2024). Amurensin G Sensitized Cholangiocarcinoma to the Anti-Cancer Effect of Gemcitabine via the Downregulation of Cancer Stem-like Properties. Nutrients, 16(1), 73. https://doi.org/10.3390/nu16010073








