Vegetarian Nutrition in Chronic Kidney Disease
Abstract
:1. Introduction
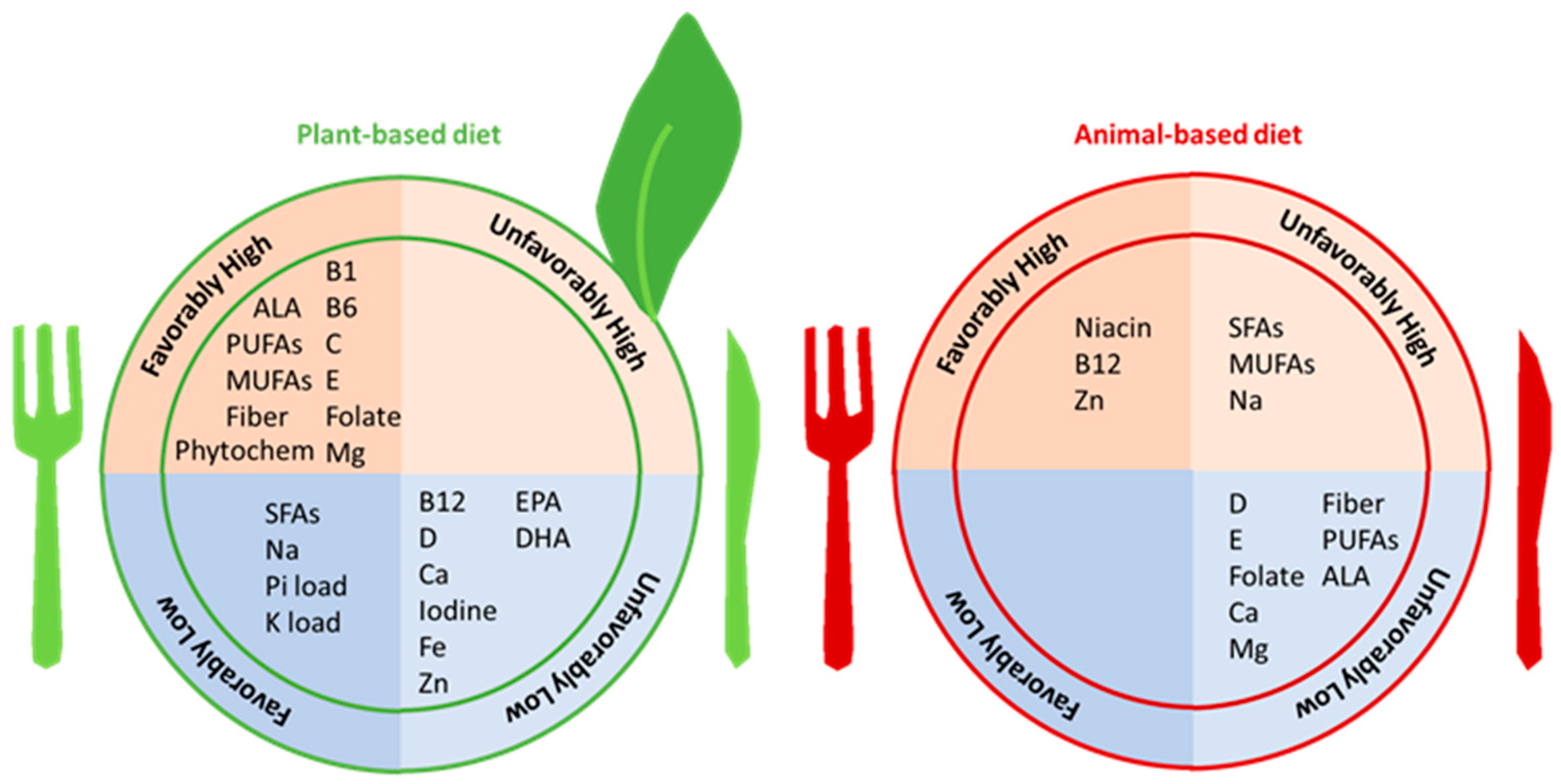
2. Overview of Vegetarian and Plant-Based Diets
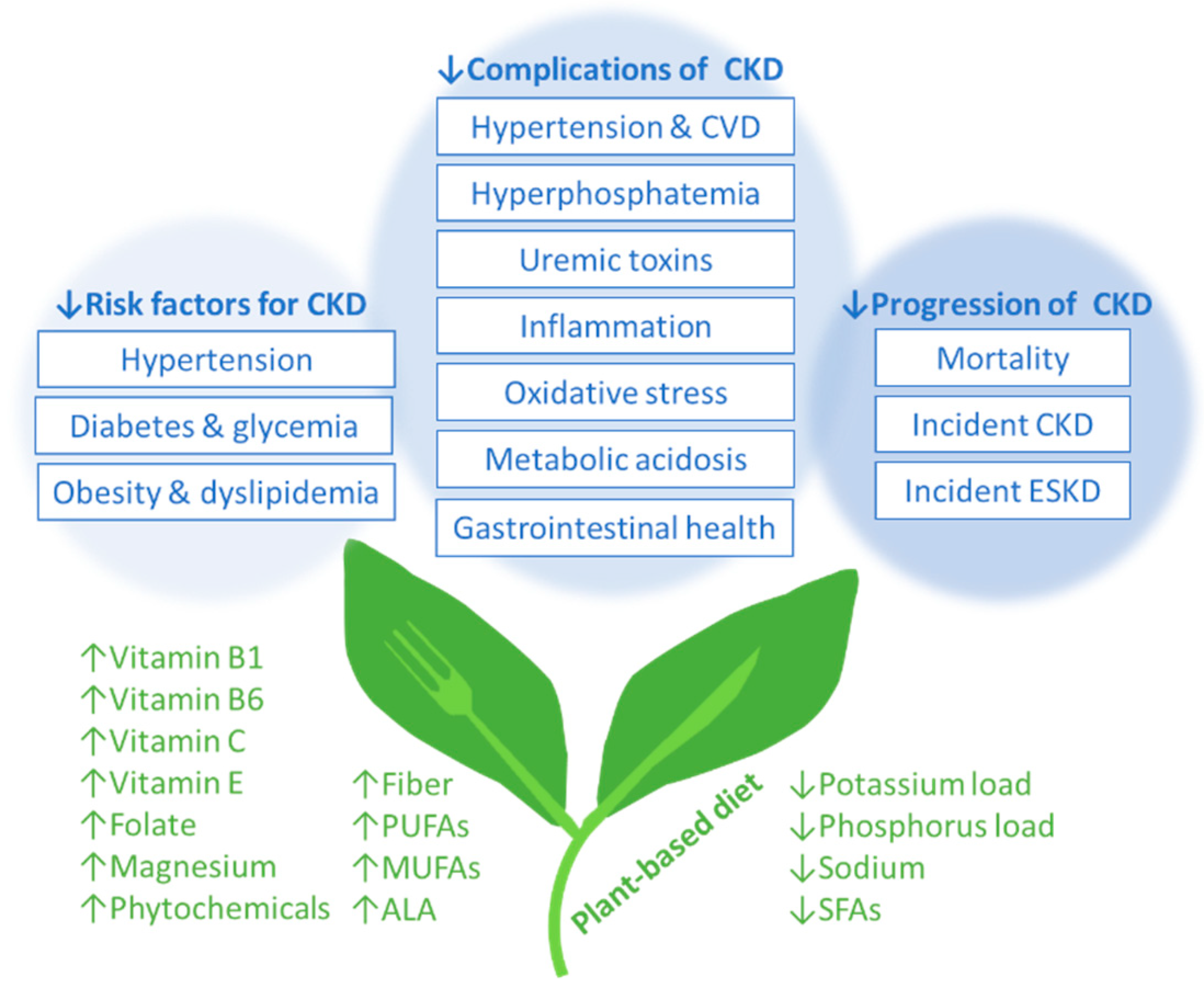
3. Vegetarian Diets and Risk Factors for Incident CKD
3.1. Hypertension in Non-CKD Populations
3.2. Diabetes Mellitus in Non-CKD Populations
4. Vegetarian Diets and CKD Complications
4.1. Hypertension in CKD Populations
4.2. Hyperphosphatemia in CKD Populations
4.3. Uremic Toxins, Inflammation, and Oxidative Stress in CKD
4.4. Metabolic Acidosis
5. Vegetarian Diets, Incident CKD, and CKD Progression
5.1. Incident CKD and CKD Progression
5.2. Progression of ESKD
6. Practical Application of Vegetarian Diets in CKD
6.1. Protein-Energy Wasting
6.2. Overall Nutritional Adequacy
6.3. Protein Adequacy Overall and with Physical Activity
6.4. Soy Protein and Isoflavones
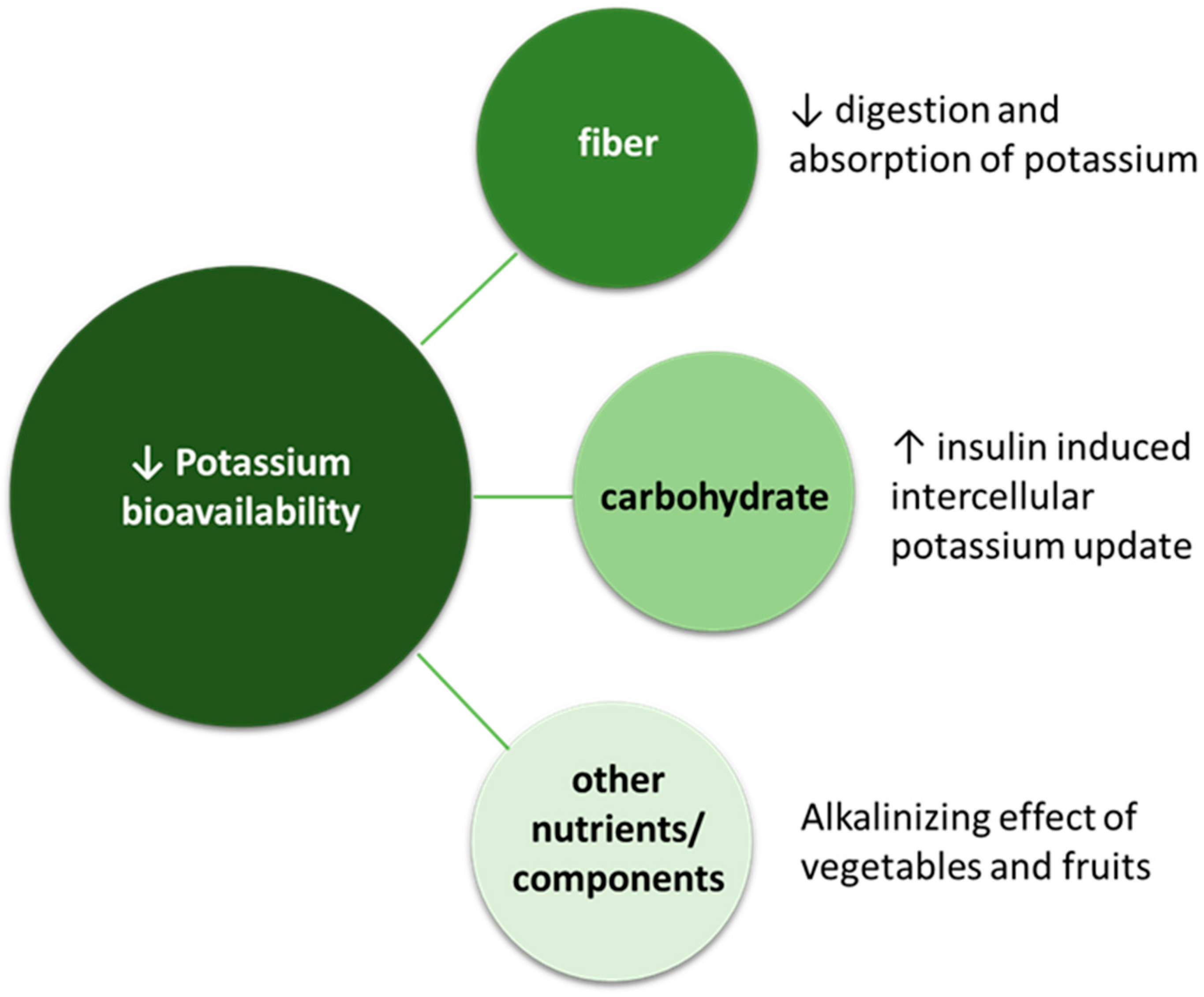
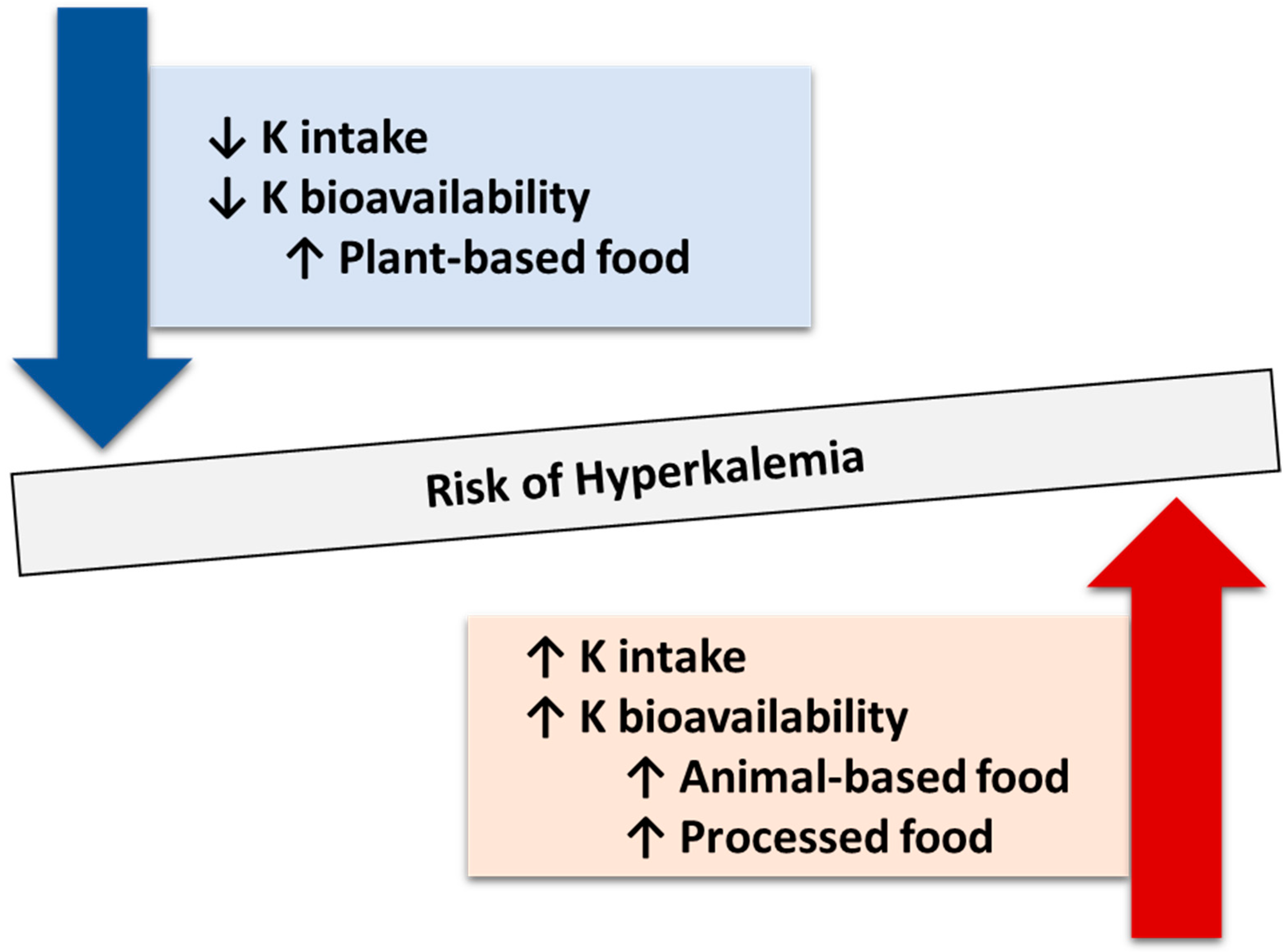
6.5. Hyperkalemia
6.6. All-Cause Mortality
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medawar, E.; Huhn, S.; Villringer, A.; Veronica Witte, A. The effects of plant-based diets on the body and the brain: A systematic review. Transl. Psychiatry 2019, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Satija, A.; Bhupathiraju, S.N.; Rimm, E.B.; Spiegelman, D.; Chiuve, S.E.; Borgi, L.; Willett, W.C.; Manson, J.E.; Sun, Q.; Hu, F.B. Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS Med. 2016, 13, e1002039. [Google Scholar] [CrossRef] [PubMed]
- Satija, A.; Bhupathiraju, S.N.; Spiegelman, D.; Chiuve, S.E.; Manson, J.E.; Willett, W.; Rexrode, K.M.; Rimm, E.B.; Hu, F.B. Healthful and Unhealthful Plant-Based Diets and the Risk of Coronary Heart Disease in U.S. Adults. J. Am. Coll. Cardiol. 2017, 70, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Baden, M.Y.; Liu, G.; Satija, A.; Li, Y.; Sun, Q.; Fung, T.T.; Rimm, E.B.; Willett, W.C.; Hu, F.B.; Bhupathiraju, S.N. Changes in Plant-Based Diet Quality and Total and Cause-Specific Mortality. Circulation 2019, 140, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Bellizzi, V.; Cupisti, A.; Locatelli, F.; Bolasco, P.; Brunori, G.; Cancarini, G.; Caria, S.; De Nicola, L.; Di Iorio, B.R.; Di Micco, L.; et al. Low-protein diets for chronic kidney disease patients: The Italian experience. BMC Nephrol. 2016, 17, 77. [Google Scholar] [CrossRef] [PubMed]
- Garneata, L.; Stancu, A.; Dragomir, D.; Stefan, G.; Mircescu, G. Ketoanalogue-Supplemented Vegetarian Very Low-Protein Diet and CKD Progression. J. Am. Soc. Nephrol. 2016, 27, 2164–2176. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Joshi, S.; Schlueter, R.; Cooke, J.; Brown-Tortorici, A.; Donnelly, M.; Schulman, S.; Lau, W.L.; Rhee, C.M.; Streja, E.; et al. Plant-Dominant Low-Protein Diet for Conservative Management of Chronic Kidney Disease. Nutrients 2020, 12, 1931. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Rhee, C.M.; Joshi, S.; Brown-Tortorici, A.; Kramer, H.M. Medical nutrition therapy using plant-focused low-protein meal plans for management of chronic kidney disease in diabetes. Curr. Opin. Nephrol. Hypertens. 2022, 31, 26–35. [Google Scholar] [CrossRef]
- Reyes-López, M.A.; Piccoli, G.B.; Leone, F.; Orozco-Guillén, A.; Perichart-Perera, O. Nutrition care for chronic kidney disease during pregnancy: An updated review. Eur. J. Clin. Nutr. 2020, 74, 983–990. [Google Scholar] [CrossRef]
- Piccoli, G.B.; Vigotti, F.N.; Leone, F.; Capizzi, I.; Daidola, G.; Cabiddu, G.; Avagnina, P. Low-protein diets in CKD: How can we achieve them? A narrative, pragmatic review. Clin. Kidney J. 2015, 8, 61–70. [Google Scholar] [CrossRef]
- Gluba-Brzózka, A.; Franczyk, B.; Rysz, J. Vegetarian Diet in Chronic Kidney Disease-A Friend or Foe. Nutrients 2017, 9, 374. [Google Scholar] [CrossRef]
- Foundation, N.K. Plant-Based Diet or Vegetarian Diet—What is the Difference? Available online: https://www.kidney.org/atoz/content/plant-based-diet-or-vegetarian-diet-difference (accessed on 13 December 2023).
- Ku, E.; Lee, B.J.; Wei, J.; Weir, M.R. Hypertension in CKD: Core Curriculum 2019. Am. J. Kidney Dis. 2019, 74, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Rouse, I.L.; Beilin, L.J.; Armstrong, B.K.; Vandongen, R. Blood-pressure-lowering effect of a vegetarian diet: Controlled trial in normotensive subjects. Lancet 1983, 1, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Margetts, B.M.; Beilin, L.J.; Vandongen, R.; Armstrong, B.K. Vegetarian diet in mild hypertension: A randomised controlled trial. Br. Med. J. (Clin. Res. Ed.) 1986, 293, 1468–1471. [Google Scholar] [CrossRef] [PubMed]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Nishimura, K.; Barnard, N.D.; Takegami, M.; Watanabe, M.; Sekikawa, A.; Okamura, T.; Miyamoto, Y. Vegetarian diets and blood pressure: A meta-analysis. JAMA Intern. Med. 2014, 174, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Tonstad, S.; Butler, T.; Yan, R.; Fraser, G.E. Type of vegetarian diet, body weight, and prevalence of type 2 diabetes. Diabetes Care 2009, 32, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Liu, G.; Hu, F.B.; Bhupathiraju, S.N.; Sun, Q. Association Between Plant-Based Dietary Patterns and Risk of Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2019, 179, 1335–1344. [Google Scholar] [CrossRef]
- Kahleova, H.; Tura, A.; Hill, M.; Holubkov, R.; Barnard, N.D. A Plant-Based Dietary Intervention Improves Beta-Cell Function and Insulin Resistance in Overweight Adults: A 16-Week Randomized Clinical Trial. Nutrients 2018, 10, 189. [Google Scholar] [CrossRef]
- Kahleova, H.; Matoulek, M.; Malinska, H.; Oliyarnik, O.; Kazdova, L.; Neskudla, T.; Skoch, A.; Hajek, M.; Hill, M.; Kahle, M.; et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with Type 2 diabetes. Diabet. Med. A J. Br. Diabet. Assoc. 2011, 28, 549–559. [Google Scholar] [CrossRef]
- Huang, R.Y.; Huang, C.C.; Hu, F.B.; Chavarro, J.E. Vegetarian Diets and Weight Reduction: A Meta-Analysis of Randomized Controlled Trials. J. Gen. Intern. Med. 2016, 31, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Hao, T.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med. 2011, 364, 2392–2404. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D.; Hou, T.; Ludwig, D.S.; Rimm, E.B.; Willett, W.; Hu, F.B.; Mozaffarian, D. Changes in intake of protein foods, carbohydrate amount and quality, and long-term weight change: Results from 3 prospective cohorts. Am. J. Clin. Nutr. 2015, 101, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Baden, M.Y.; Satija, A.; Hu, F.B.; Huang, T. Change in Plant-Based Diet Quality Is Associated with Changes in Plasma Adiposity-Associated Biomarker Concentrations in Women. J. Nutr. 2019, 149, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Eichelmann, F.; Schwingshackl, L.; Fedirko, V.; Aleksandrova, K. Effect of plant-based diets on obesity-related inflammatory profiles: A systematic review and meta-analysis of intervention trials. Obes. Rev. 2016, 17, 1067–1079. [Google Scholar] [CrossRef]
- Bowman, S.A. A Vegetarian-Style Dietary Pattern Is Associated with Lower Energy, Saturated Fat, and Sodium Intakes; and Higher Whole Grains, Legumes, Nuts, and Soy Intakes by Adults: National Health and Nutrition Examination Surveys 2013-2016. Nutrients 2020, 12, 2668. [Google Scholar] [CrossRef]
- McMahon, E.J.; Campbell, K.L.; Bauer, J.D.; Mudge, D.W.; Kelly, J.T. Altered dietary salt intake for people with chronic kidney disease. Cochrane Database Syst. Rev. 2021, 6, Cd010070. [Google Scholar] [CrossRef]
- Garofalo, C.; Borrelli, S.; Provenzano, M.; De Stefano, T.; Vita, C.; Chiodini, P.; Minutolo, R.; De Nicola, L.; Conte, G. Dietary Salt Restriction in Chronic Kidney Disease: A Meta-Analysis of Randomized Clinical Trials. Nutrients 2018, 10, 732. [Google Scholar] [CrossRef]
- Shi, H.; Su, X.; Li, C.; Guo, W.; Wang, L. Effect of a low-salt diet on chronic kidney disease outcomes: A systematic review and meta-analysis. BMJ Open 2022, 12, e050843. [Google Scholar] [CrossRef]
- Wang, W.; Soltero, L.; Zhang, P.; Huang, X.R.; Lan, H.Y.; Adrogue, H.J. Renal inflammation is modulated by potassium in chronic kidney disease: Possible role of Smad7. Am. J. Physiol. Ren. Physiol. 2007, 293, F1123–F1130. [Google Scholar] [CrossRef]
- Tyson, C.C.; Lin, P.H.; Corsino, L.; Batch, B.C.; Allen, J.; Sapp, S.; Barnhart, H.; Nwankwo, C.; Burroughs, J.; Svetkey, L.P. Short-term effects of the DASH diet in adults with moderate chronic kidney disease: A pilot feeding study. Clin. Kidney J. 2016, 9, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Goraya, N.; Simoni, J.; Jo, C.H.; Wesson, D.E. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin. J. Am. Soc. Nephrol. 2013, 8, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Goraya, N.; Simoni, J.; Jo, C.H.; Wesson, D.E. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014, 86, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Turban, S.; Juraschek, S.P.; Miller, E.R., 3rd; Anderson, C.A.M.; White, K.; Charleston, J.; Appel, L.J. Randomized Trial on the Effects of Dietary Potassium on Blood Pressure and Serum Potassium Levels in Adults with Chronic Kidney Disease. Nutrients 2021, 13, 2678. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Muniz, F.J. Dietary fibre and cardiovascular health. Nutr. Hosp. 2012, 27, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, K.R.; Bakris, G.L.; Bilous, R.W.; Chiang, J.L.; de Boer, I.H.; Goldstein-Fuchs, J.; Hirsch, I.B.; Kalantar-Zadeh, K.; Narva, A.S.; Navaneethan, S.D.; et al. Diabetic kidney disease: A report from an ADA Consensus Conference. Diabetes Care 2014, 37, 2864–2883. [Google Scholar] [CrossRef]
- Trautwein, E.A.; McKay, S. The Role of Specific Components of a Plant-Based Diet in Management of Dyslipidemia and the Impact on Cardiovascular Risk. Nutrients 2020, 12, 2671. [Google Scholar] [CrossRef]
- Montemurno, E.; Cosola, C.; Dalfino, G.; Daidone, G.; De Angelis, M.; Gobbetti, M.; Gesualdo, L. What would you like to eat, Mr CKD Microbiota? A Mediterranean Diet, please! Kidney Blood Press. Res. 2014, 39, 114–123. [Google Scholar] [CrossRef]
- Altorf-van der Kuil, W.; Engberink, M.F.; Brink, E.J.; van Baak, M.A.; Bakker, S.J.; Navis, G.; van’t Veer, P.; Geleijnse, J.M. Dietary protein and blood pressure: A systematic review. PLoS ONE 2010, 5, e12102. [Google Scholar] [CrossRef]
- Tielemans, S.M.; Altorf-van der Kuil, W.; Engberink, M.F.; Brink, E.J.; van Baak, M.A.; Bakker, S.J.; Geleijnse, J.M. Intake of total protein, plant protein and animal protein in relation to blood pressure: A meta-analysis of observational and intervention studies. J. Hum. Hypertens. 2013, 27, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Montalcini, T.; De Bonis, D.; Ferro, Y.; Carè, I.; Mazza, E.; Accattato, F.; Greco, M.; Foti, D.; Romeo, S.; Gulletta, E.; et al. High Vegetable Fats Intake Is Associated with High Resting Energy Expenditure in Vegetarians. Nutrients 2015, 7, 5933–5947. [Google Scholar] [CrossRef] [PubMed]
- Narasaki, Y.; Rhee, C.M. Dietary Therapy for Managing Hyperphosphatemia. Clin. J. Am. Soc. Nephrol. 2020, 16, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Gutekunst, L.; Mehrotra, R.; Kovesdy, C.P.; Bross, R.; Shinaberger, C.S.; Noori, N.; Hirschberg, R.; Benner, D.; Nissenson, A.R.; et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Narasaki, Y.; Yamasaki, M.; Matsuura, S.; Morinishi, M.; Nakagawa, T.; Matsuno, M.; Katsumoto, M.; Nii, S.; Fushitani, Y.; Sugihara, K.; et al. Phosphatemic Index Is a Novel Evaluation Tool for Dietary Phosphorus Load: A Whole-Foods Approach. J. Ren. Nutr. 2020, 30, 493–502. [Google Scholar] [CrossRef]
- Moe, S.M.; Chen, N.X.; Seifert, M.F.; Sinders, R.M.; Duan, D.; Chen, X.; Liang, Y.; Radcliff, J.S.; White, K.E.; Gattone, V.H., 2nd. A rat model of chronic kidney disease-mineral bone disorder. Kidney Int. 2009, 75, 176–184. [Google Scholar] [CrossRef]
- Moe, S.M.; Zidehsarai, M.P.; Chambers, M.A.; Jackman, L.A.; Radcliffe, J.S.; Trevino, L.L.; Donahue, S.E.; Asplin, J.R. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 257–264. [Google Scholar] [CrossRef]
- Azadbakht, L.; Esmaillzadeh, A. Soy-protein consumption and kidney-related biomarkers among type 2 diabetics: A crossover, randomized clinical trial. J. Ren. Nutr. 2009, 19, 479–486. [Google Scholar] [CrossRef]
- Cases, A.; Cigarrán-Guldrís, S.; Mas, S.; Gonzalez-Parra, E. Vegetable-Based Diets for Chronic Kidney Disease? It Is Time to Reconsider. Nutrients 2019, 11, 1263. [Google Scholar] [CrossRef]
- Koppe, L.; Fouque, D.; Soulage, C.O. The Role of Gut Microbiota and Diet on Uremic Retention Solutes Production in the Context of Chronic Kidney Disease. Toxins 2018, 10, 155. [Google Scholar] [CrossRef]
- Marzocco, S.; Dal Piaz, F.; Di Micco, L.; Torraca, S.; Sirico, M.L.; Tartaglia, D.; Autore, G.; Di Iorio, B. Very low protein diet reduces indoxyl sulfate levels in chronic kidney disease. Blood Purif. 2013, 35, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Sirich, T.L.; Plummer, N.S.; Gardner, C.D.; Hostetter, T.H.; Meyer, T.W. Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, V.M.R.; Wei, G.; Baird, B.C.; Murtaugh, M.; Chonchol, M.B.; Raphael, K.L.; Greene, T.; Beddhu, S. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int. 2012, 81, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Liu, S.M.; Lau, W.L.; Khazaeli, M.; Nazertehrani, S.; Farzaneh, S.H.; Kieffer, D.A.; Adams, S.H.; Martin, R.J. High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS ONE 2014, 9, e114881. [Google Scholar] [CrossRef] [PubMed]
- Dahl, W.J.; Stewart, M.L. Position of the Academy of Nutrition and Dietetics: Health Implications of Dietary Fiber. J. Acad. Nutr. Diet. 2015, 115, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Solmi, M.; Caruso, M.G.; Giannelli, G.; Osella, A.R.; Evangelou, E.; Maggi, S.; Fontana, L.; Stubbs, B.; Tzoulaki, I. Dietary fiber and health outcomes: An umbrella review of systematic reviews and meta-analyses. Am. J. Clin. Nutr. 2018, 107, 436–444. [Google Scholar] [CrossRef]
- Xiong, R.G.; Zhou, D.D.; Wu, S.X.; Huang, S.Y.; Saimaiti, A.; Yang, Z.J.; Shang, A.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Health Benefits and Side Effects of Short-Chain Fatty Acids. Foods 2022, 11, 2863. [Google Scholar] [CrossRef]
- de Brito-Ashurst, I.; Varagunam, M.; Raftery, M.J.; Yaqoob, M.M. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J. Am. Soc. Nephrol. 2009, 20, 2075–2084. [Google Scholar] [CrossRef]
- Yuzbashian, E.; Asghari, G.; Mirmiran, P.; Hosseini, F.S.; Azizi, F. Associations of dietary macronutrients with glomerular filtration rate and kidney dysfunction: Tehran lipid and glucose study. J. Nephrol. 2015, 28, 173–180. [Google Scholar] [CrossRef]
- Nettleton, J.A.; Steffen, L.M.; Palmas, W.; Burke, G.L.; Jacobs, D.R., Jr. Associations between microalbuminuria and animal foods, plant foods, and dietary patterns in the Multiethnic Study of Atherosclerosis. Am. J. Clin. Nutr. 2008, 87, 1825–1836. [Google Scholar] [CrossRef] [PubMed]
- Haring, B.; Selvin, E.; Liang, M.; Coresh, J.; Grams, M.E.; Petruski-Ivleva, N.; Steffen, L.M.; Rebholz, C.M. Dietary Protein Sources and Risk for Incident Chronic Kidney Disease: Results From the Atherosclerosis Risk in Communities (ARIC) Study. J. Ren. Nutr. 2017, 27, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Knight, E.L.; Stampfer, M.J.; Hankinson, S.E.; Spiegelman, D.; Curhan, G.C. The Impact of Protein Intake on Renal Function Decline in Women with Normal Renal Function or Mild Renal Insufficiency. Ann. Intern. Med. 2003, 138, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.R.; Tappenden, K.A.; Carson, L.; Jones, R.; Prabhudesai, M.; Marshall, W.P.; Erdman, J.W., Jr. Isolated soy protein consumption reduces urinary albumin excretion and improves the serum lipid profile in men with type 2 diabetes mellitus and nephropathy. J. Nutr. 2004, 134, 1874–1880. [Google Scholar] [CrossRef] [PubMed]
- Azadbakht, L.; Atabak, S.; Esmaillzadeh, A. Soy protein intake, cardiorenal indices, and C-reactive protein in type 2 diabetes with nephropathy: A longitudinal randomized clinical trial. Diabetes Care 2008, 31, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Świątek, Ł.; Jeske, J.; Miedziaszczyk, M.; Idasiak-Piechocka, I. The impact of a vegetarian diet on chronic kidney disease (CKD) progression—A systematic review. BMC Nephrol. 2023, 24, 168. [Google Scholar] [CrossRef]
- Kelly, J.T.; Carrero, J.J. Dietary Sources of Protein and Chronic Kidney Disease Progression: The Proof May Be in the Pattern. J. Ren. Nutr. 2017, 27, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Lew, Q.-L.J.; Jafar, T.H.; Koh, H.W.L.; Jin, A.; Chow, K.Y.; Yuan, J.-M.; Koh, W.-P. Red meat intake and risk of ESRD. J. Am. Soc. Nephrol. 2017, 28, 304–312. [Google Scholar] [CrossRef]
- Kelly, J.T.; Palmer, S.C.; Wai, S.N.; Ruospo, M.; Carrero, J.J.; Campbell, K.L.; Strippoli, G.F. Healthy Dietary Patterns and Risk of Mortality and ESRD in CKD: A Meta-Analysis of Cohort Studies. Clin. J. Am. Soc. Nephrol. 2017, 12, 272–279. [Google Scholar] [CrossRef]
- Gutiérrez, O.M.; Muntner, P.; Rizk, D.V.; McClellan, W.M.; Warnock, D.G.; Newby, P.K.; Judd, S.E. Dietary patterns and risk of death and progression to ESRD in individuals with CKD: A cohort study. Am. J. Kidney Dis. 2014, 64, 204–213. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Ikizler, T.A.; Block, G.; Avram, M.M.; Kopple, J.D. Malnutrition-inflammation complex syndrome in dialysis patients: Causes and consequences. Am. J. Kidney Dis. 2003, 42, 864–881. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J.; Bolton, K.; Culleton, B.; Harvey, K.S.; Ikizler, T.A.; Johnson, C.A.; Kausz, A.; Kimmel, P.L.; Kusek, J. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002, 39, S1–S266. [Google Scholar]
- Ogborn, M.R.; Bankovic-Calic, N.; Shoesmith, C.; Buist, R.; Peeling, J. Soy protein modification of rat polycystic kidney disease. Am. J. Physiol. 1998, 274, F541–F549. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, J.; Ramírez, V.; Pérez, J.; Torre-Villalvazo, I.; Torres, N.; Tovar, A.R.; Muñoz, R.M.; Uribe, N.; Gamba, G.; Bobadilla, N.A. Renal protection by a soy diet in obese Zucker rats is associated with restoration of nitric oxide generation. Am. J. Physiol. Ren. Physiol. 2005, 288, F108–F116. [Google Scholar] [CrossRef] [PubMed]
- Mitch, W.E.; Remuzzi, G. Diets for patients with chronic kidney disease, should we reconsider? BMC Nephrol. 2016, 17, 80. [Google Scholar] [CrossRef] [PubMed]
- Jibani, M.M.; Bloodworth, L.L.; Foden, E.; Griffiths, K.D.; Galpin, O.P. Predominantly vegetarian diet in patients with incipient and early clinical diabetic nephropathy: Effects on albumin excretion rate and nutritional status. Diabet. Med. A J. Br. Diabet. Assoc. 1991, 8, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Barsotti, G.; Morelli, E.; Cupisti, A.; Meola, M.; Dani, L.; Giovannetti, S. A low-nitrogen low-phosphorus Vegan diet for patients with chronic renal failure. Nephron 1996, 74, 390–394. [Google Scholar] [CrossRef]
- Milovanova, L.Y.; Volkov, A.V.; Milovanova, S.Y.; Taranova, M.V.; Nezhdanov, K.S. Soy Protein as a Part of a Low-protein Diet is a New Direction in Cardio- and Nephroprotection in Patients With 3b-4 Stages of Chronic kidney Disease. J. Ren. Nutr. 2023, 33, 435–442. [Google Scholar] [CrossRef]
- Wu, T.T.; Chang, C.Y.; Hsu, W.M.; Wang, I.K.; Hsu, C.H.; Cheng, S.H.; Liang, C.C.; Chang, C.T.; Huang, C.C. Nutritional status of vegetarians on maintenance haemodialysis. Nephrology 2011, 16, 582–587. [Google Scholar] [CrossRef]
- Neufingerl, N.; Eilander, A. Nutrient Intake and Status in Adults Consuming Plant-Based Diets Compared to Meat-Eaters: A Systematic Review. Nutrients 2021, 14, 29. [Google Scholar] [CrossRef]
- Rizzo, N.S.; Jaceldo-Siegl, K.; Sabate, J.; Fraser, G.E. Nutrient profiles of vegetarian and nonvegetarian dietary patterns. J. Acad. Nutr. Diet. 2013, 113, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Young, V.R.; Pellett, P.L. Plant proteins in relation to human protein and amino acid nutrition. Am. J. Clin. Nutr. 1994, 59, 1203s–1212s. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.; Lynch, H.; Dickinson, J.M.; Reed, K.E. No Difference Between the Effects of Supplementing With Soy Protein Versus Animal Protein on Gains in Muscle Mass and Strength in Response to Resistance Exercise. Int. J. Sport. Nutr. Exerc. Metab. 2018, 28, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Additives, E.P.o.F.; Food, N.S.a.t. Risk assessment for peri-and post-menopausal women taking food supplements containing isolated isoflavones. EFSA J. 2015, 13, 4246. [Google Scholar]
- Joshi, S.; McMacken, M.; Kalantar-Zadeh, K. Plant-Based Diets for Kidney Disease: A Guide for Clinicians. Am. J. Kidney Dis. 2021, 77, 287–296. [Google Scholar] [CrossRef]
- Te Dorsthorst, R.P.M.; Hendrikse, J.; Vervoorn, M.T.; van Weperen, V.Y.H.; van der Heyden, M.A.G. Review of case reports on hyperkalemia induced by dietary intake: Not restricted to chronic kidney disease patients. Eur. J. Clin. Nutr. 2019, 73, 38–45. [Google Scholar] [CrossRef]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet. 2016, 116, 1970–1980. [Google Scholar] [CrossRef]
- Zarantonello, D.; Brunori, G. The Role of Plant-Based Diets in Preventing and Mitigating Chronic Kidney Disease: More Light than Shadows. J. Clin. Med. 2023, 12, 6137. [Google Scholar] [CrossRef]
- Zong, G.; Li, Y.; Sampson, L.; Dougherty, L.W.; Willett, W.C.; Wanders, A.J.; Alssema, M.; Zock, P.L.; Hu, F.B.; Sun, Q. Monounsaturated fats from plant and animal sources in relation to risk of coronary heart disease among US men and women. Am. J. Clin. Nutr. 2018, 107, 445–453. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Zong, G.; Willett, W.C.; Zock, P.L.; Wanders, A.J.; Hu, F.B.; Sun, Q. Associations of Monounsaturated Fatty Acids From Plant and Animal Sources With Total and Cause-Specific Mortality in Two US Prospective Cohort Studies. Circ. Res. 2019, 124, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Hertzler, S.R.; Lieblein-Boff, J.C.; Weiler, M.; Allgeier, C. Plant Proteins: Assessing Their Nutritional Quality and Effects on Health and Physical Function. Nutrients 2020, 12, 3704. [Google Scholar] [CrossRef] [PubMed]
- Hartman, J.W.; Tang, J.E.; Wilkinson, S.B.; Tarnopolsky, M.A.; Lawrence, R.L.; Fullerton, A.V.; Phillips, S.M. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am. J. Clin. Nutr. 2007, 86, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Volk, B.M.; Gómez, A.L.; Kunces, L.J.; Kupchak, B.R.; Freidenreich, D.J.; Aristizabal, J.C.; Saenz, C.; Dunn-Lewis, C.; Ballard, K.D.; et al. Whey protein supplementation during resistance training augments lean body mass. J. Am. Coll. Nutr. 2013, 32, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, Y.; Kye, S.; Chung, Y.S.; Kim, K.M. Association of vegetables and fruits consumption with sarcopenia in older adults: The Fourth Korea National Health and Nutrition Examination Survey. Age Ageing 2015, 44, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Inoshita, H.; Asaoka, D.; Matsuno, K.; Yanagisawa, N.; Suzuki, Y.; Miyauchi, K. Cross-Sectional Study on the Association between Dietary Patterns and Sarcopenia in Elderly Patients with Chronic Kidney Disease Receiving Conservative Treatment. Nutrients 2023, 15, 4994. [Google Scholar] [CrossRef] [PubMed]
- Naismith, D.J.; Braschi, A. An investigation into the bioaccessibility of potassium in unprocessed fruits and vegetables. Int. J. Food Sci. Nutr. 2008, 59, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Braschi, A.; Gill, L.; Naismith, D.J. Partial substitution of sodium with potassium in white bread: Feasibility and bioavailability. Int. J. Food Sci. Nutr. 2009, 60, 507–521. [Google Scholar] [CrossRef]
- Narasaki, Y.; You, A.S.; Malik, S.; Moore, L.W.; Bross, R.; Cervantes, M.K.; Daza, A.; Kovesdy, C.P.; Nguyen, D.V.; Kalantar-Zadeh, K.; et al. Dietary potassium intake, kidney function, and survival in a nationally representative cohort. Am. J. Clin. Nutr. 2022, 116, 1123–1134. [Google Scholar] [CrossRef]
- Chen, X.; Wei, G.; Jalili, T.; Metos, J.; Giri, A.; Cho, M.E.; Boucher, R.; Greene, T.; Beddhu, S. The Associations of Plant Protein Intake With All-Cause Mortality in CKD. Am. J. Kidney Dis. 2016, 67, 423–430. [Google Scholar] [CrossRef]
- Narasaki, Y.; Okuda, Y.; Moore, L.W.; You, A.S.; Tantisattamo, E.; Inrig, J.K.; Miyagi, T.; Nakata, T.; Kovesdy, C.P.; Nguyen, D.V.; et al. Dietary protein intake, kidney function, and survival in a nationally representative cohort. Am. J. Clin. Nutr. 2021, 114, 303–313. [Google Scholar] [CrossRef] [PubMed]
| Diet | CKD Stage | Protein | Carbohydrates |
|---|---|---|---|
| LPD vegan | 3–4 | 0.7 g/kg/day (100% from grain and legumes) | From cereals |
| LPDs vegan | 3–4 Indicated in pregnant women with advanced CKD [9], in people at high risk of malnutrition, or in people who do not tolerate legumes [10] | 0.6 g/kg/day (100% from cereals and legumes) + EAAs/KAs (1 tablet every 10 kg of body weight) | From cereals |
| PLADO diet | 3–5 | 0.6 g/kg/day (with >50% plant-based sources) | From whole cereals |
| PLAFOND diet | 3–5 Diabetic nephropathy | 0.6 to <0.8 g/kg/day (with >50% plant-based sources) | From whole cereals |
| VLPDs | 4–5 | 0.3–0.4 g/kg/day + EAAs/KAs (1 tablet every 5 kg of body weight) | Especially from low-protein substitutes |
| Property | Function | Health Benefits |
|---|---|---|
| Bulk |
|
|
| Viscosity |
|
|
| Fermentability |
|
|
| Topic | Concern/Myth | Evidence |
|---|---|---|
| Nutritional adequacy | Plant-based diets lack adequate contents of nutrients largely found in animal-based foods |
|
| Protein adequacy | Plant-based diets provide inferior protein quantity compared to animal-based diets |
|
| Plant-based diets provide inferior protein quality compared to animal-based diets |
| |
| Plant proteins are inferior to animal proteins in terms of lean body mass and strength |
| |
| Hormonal abnormalities | Isoflavones from soy have potential adverse effects (e.g., thyroid dysfunction, breast cancer) |
|
| Hyperkalemia | Plant-based diets cause hyperkalemia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narasaki, Y.; Kalantar-Zadeh, K.; Rhee, C.M.; Brunori, G.; Zarantonello, D. Vegetarian Nutrition in Chronic Kidney Disease. Nutrients 2024, 16, 66. https://doi.org/10.3390/nu16010066
Narasaki Y, Kalantar-Zadeh K, Rhee CM, Brunori G, Zarantonello D. Vegetarian Nutrition in Chronic Kidney Disease. Nutrients. 2024; 16(1):66. https://doi.org/10.3390/nu16010066
Chicago/Turabian StyleNarasaki, Yoko, Kamyar Kalantar-Zadeh, Connie M. Rhee, Giuliano Brunori, and Diana Zarantonello. 2024. "Vegetarian Nutrition in Chronic Kidney Disease" Nutrients 16, no. 1: 66. https://doi.org/10.3390/nu16010066








