Sarcopenia and Diabetes: A Detrimental Liaison of Advancing Age
Abstract
1. Introduction
2. Methods
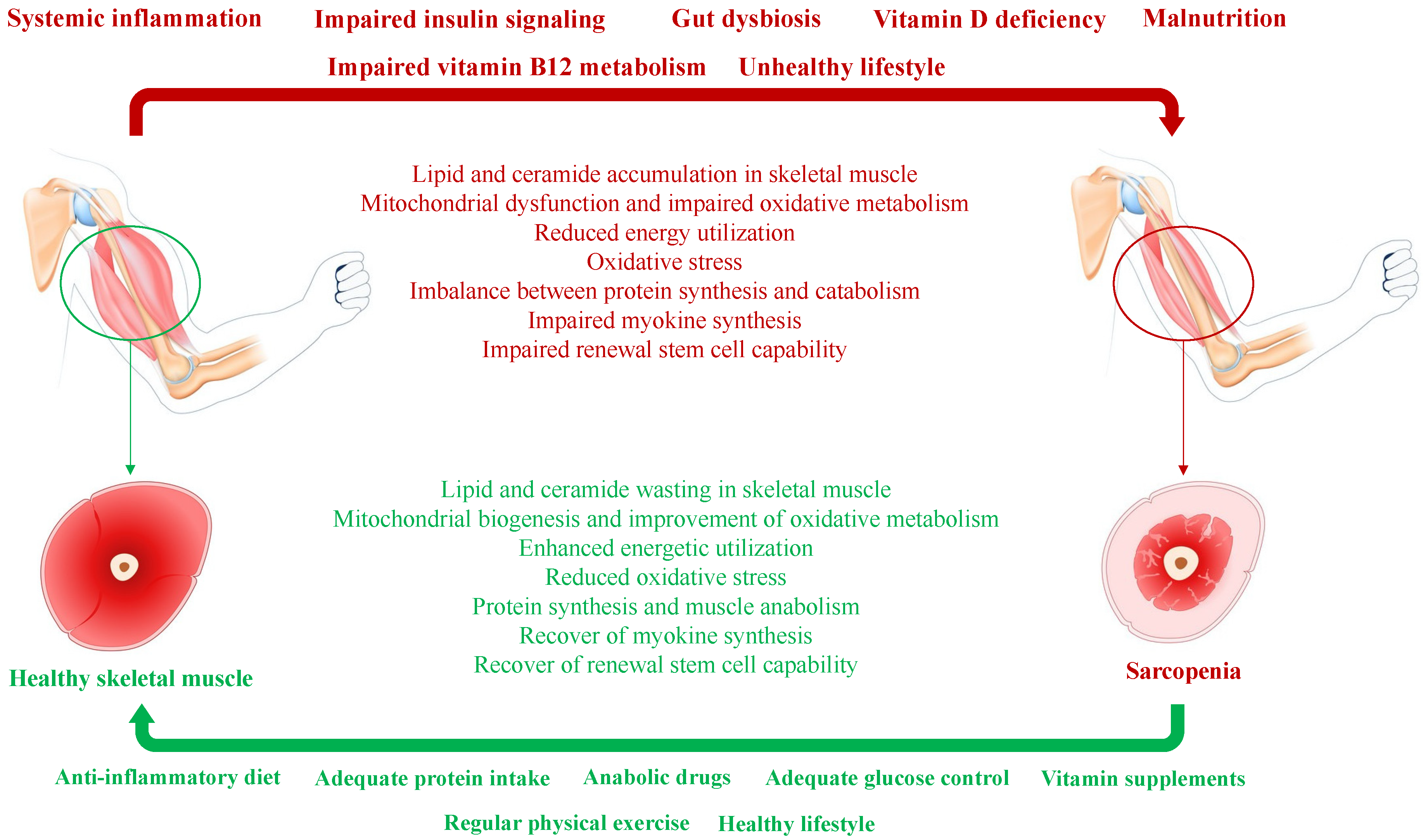
3. Mechanism of Diabetes-Induced Sarcopenia
4. Sarcopenic Obesity
5. Sarcopenia: A Determinant of Glucose Deterioration and Poor Outcomes
6. Modifiable Risk Factors
7. Preventing Sarcopenia: A Therapeutic Target in Primary and Secondary Prevention of T2D
7.1. The Physiological Role of Healthy Skeletal Muscle in Preventing Sarcopenia and Glucose Metabolism Deterioration
7.2. Non-Pharmacological Intervention: The Role of Lifestyle Changes and Supplements
7.3. Pharmacological Intervention
7.3.1. Biguanides
7.3.2. Secretagogues
7.3.3. Intestinal Glucosidase Inhibitor
7.3.4. Dipeptidyl Peptidase Type IV Inhibitors
7.3.5. Thiazolidinediones
7.3.6. Gliflozins
7.3.7. Glucagon-like Peptide 1 Receptor Agonists
7.3.8. Dual GLP-1/GIP Co-Agonists
7.3.9. Insulin Analogues
8. Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMPK | Adenosine monophosphate-activate protein kinase |
| DPPIVis | Dipeptidyl peptidase type IV inhibitors |
| FGF | Fibroblast growth factor |
| FGF19 | Fibroblast growth factor 19 |
| GLP-1 | Glucagon-like peptide 1 |
| GLP-1RAs | GLP-1 receptor agonists |
| GIP | Glucose-dependent insulinotropic polypeptide |
| GH | Growth hormone |
| IGF | Insulin-like growth factor |
| IL | Interleukin |
| mTOR | Mammalian target of rapamycin |
| PGC-1α | Peroxisome proliferator co-activator 1 alpha |
| SPARC | Secreted proteins acidic and rich in cysteine |
| Smad | Small mother against decapentaplegic |
| SGLT2is | Sodium-glucose (co) transporter type 2 inhibitors |
| TNFα | Tumor necrosis factor α |
| T2D | Type 2 diabetes |
| Vit-D | Vitamin D |
References
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical definition of sarcopenia. Clin. Cases Miner. Bone Metab. 2014, 11, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low Relative Skeletal Muscle Mass (Sarcopenia) in Older Persons Is Associated with Functional Impairment and Physical Disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, J.; Geerlings, M.A.; Reijnierse, E.M.; Phassouliotis, C.; Lim, W.K.; Maier, A.B. Prevalence of sarcopenia as a comorbid disease: A systematic review and meta-analysis. Exp. Gerontol. 2019, 131, 110801. [Google Scholar] [CrossRef] [PubMed]
- Anagnostis, P.; Gkekas, N.K.; Achilla, C.; Pananastasiou, G.; Taouxidou, P.; Mitsiou, M.; Kenanidis, E.; Potoupnis, M.; Tsiridis, E.; Goulis, D.G. Type 2 Diabetes Mellitus is Associated with Increased Risk of Sarcopenia: A Systematic Review and Meta-analysis. Calcif. Tissue Int. 2020, 107, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.-S.; Chai, Y.-H.; Gong, H.-J.; Zhuldyz, Z.; Stehouwer, C.D.A.; Zhou, J.-B.; Simó, R. The Association Between Diabetes Mellitus and Risk of Sarcopenia: Accumulated Evidences From Observational Studies. Front. Endocrinol. 2021, 12, 782391. [Google Scholar] [CrossRef] [PubMed]
- Ida, S.; Kaneko, R.; Imataka, K.; Murata, K. Association between Sarcopenia and Renal Function in Patients with Diabetes: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2019, 2019, 1365189. [Google Scholar] [CrossRef]
- Wannarong, T.; Sukpornchairak, P.; Naweera, W.; Geiger, C.D.; Ungprasert, P. Association between diabetic peripheral neuropathy and sarcopenia: A systematic review and meta-analysis. Geriatr. Gerontol. Int. 2022, 22, 785–789. [Google Scholar] [CrossRef]
- Feng, L.; Gao, Q.; Hu, K.; Wu, M.; Wang, Z.; Chen, F.; Mei, F.; Zhao, L.; Ma, B. Prevalence and risk factors of sarcopenia in patients with diabetes: A meta-analysis. J. Clin. Endocrinol. Metab. 2021, 107, 1470–1483. [Google Scholar] [CrossRef]
- Corcoran, M.P.; Lamon-Fava, S.; Fielding, R.A. Skeletal muscle lipid deposition and insulin resistance: Effect of dietary fatty acids and exercise. Am. J. Clin. Nutr. 2007, 85, 662–677. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, R.R.; Corriere, M.; Ferrucci, L. Age-related and disease-related muscle loss: The effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014, 2, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Buford, T.W.; Anton, S.D.; Judge, A.R.; Marzetti, E.; Wohlgemuth, S.E.; Carter, C.S.; Leeuwenburgh, C.; Pahor, M.; Manini, T.M. Models of accelerated sarcopenia: Critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res. Rev. 2010, 9, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Luevano-Contreras, C.; Chapman-Novakofski, K. Dietary Advanced Glycation End Products and Aging. Nutrients 2010, 2, 1247–1265. [Google Scholar] [CrossRef] [PubMed]
- Peroni, D.G.; Nuzzi, G.; Trambusti, I.; Di Cicco, M.E.; Comberiati, P. Microbiome Composition and Its Impact on the Development of Allergic Diseases. Front. Immunol. 2020, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Bonfrate, L.; Khalil, M.; Garruti, G.; Portincasa, P. Contribution of the microbiome for better phenotyping of people living with obesity. Rev. Endocr. Metab. Disord. 2023, 24, 839–870. [Google Scholar] [CrossRef]
- Sharma, S.; Tripathi, P. Gut microbiome and type 2 diabetes: Where we are and where to go? J. Nutr. Biochem. 2019, 63, 101–108. [Google Scholar] [CrossRef]
- Shimizu, Y. Gut microbiota in common elderly diseases affecting activities of daily living. World J. Gastroenterol. 2018, 24, 4750–4758. [Google Scholar] [CrossRef]
- Krok-Schoen, J.L.; Price, A.A.; Luo, M.; Kelly, O.J.; Taylor, C.A. Low Dietary Protein Intakes and Associated Dietary Patterns and Functional Limitations in an Aging Population: A NHANES Analysis. J. Nutr. Health Aging 2019, 23, 338–347. [Google Scholar] [CrossRef]
- McCarthy, K.; Laird, E.; O’Halloran, A.M.; Walsh, C.; Healy, M.; Fitzpatrick, A.L.; Walsh, J.B.; Hernández, B.; Fallon, P.; Molloy, A.M.; et al. Association between vitamin D deficiency and the risk of prevalent type 2 diabetes and incident prediabetes: A prospective cohort study using data from The Irish Longitudinal Study on Ageing (TILDA). EClinicalMedicine 2022, 53, 101654. [Google Scholar] [CrossRef] [PubMed]
- Kupisz-Urbańska, M.; Płudowski, P.; Marcinowska-Suchowierska, E. Vitamin D Deficiency in Older Patients—Problems of Sarcopenia, Drug Interactions, Management in Deficiency. Nutrients 2021, 13, 1247. [Google Scholar] [CrossRef]
- Cappola, A.R.; Auchus, R.J.; Fuleihan, G.E.-H.; Handelsman, D.J.; Kalyani, R.R.; McClung, M.; A Stuenkel, C.; O Thorner, M.; Verbalis, J.G. Hormones and Aging: An Endocrine Society Scientific Statement. J. Clin. Endocrinol. Metab. 2023, 108, 1835–1874. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska, K.; Adamska-Patruno, E.; Kretowski, A. The interplay between muscle mass decline, obesity, and type 2 diabetes. Pol. Arch. Intern. Med. 2019, 129, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Buckinx, F.; Aubertin-Leheudre, M. Sarcopenia in Menopausal Women: Current Perspectives. Int. J. Women’s Health 2022, 14, 805–819. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.; Morley, J.E.; Matsumoto, A.M.; Vinik, A. Sarcopenia: An Endocrine Disorder? Endocr. Pract. 2017, 23, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tan, Y.; Shi, Y.; Wang, X.; Liao, Z.; Wei, P. Diabetes and Sarcopenic Obesity: Pathogenesis, Diagnosis, and Treatments. Front. Endocrinol. 2020, 11, 568. [Google Scholar] [CrossRef] [PubMed]
- Remelli, F.; Maietti, E.; Abete, P.; Bellelli, G.; Bo, M.; Cherubini, A.; Corica, F.; Di Bari, M.; Maggio, M.; Rizzo, M.R.; et al. Prevalence of obesity and diabetes in older people with sarcopenia defined according to EWGSOP2 and FNHI criteria. Aging Clin. Exp. Res. 2021, 34, 113–120. [Google Scholar] [CrossRef]
- Baumgartner, R.N. Body Composition in Healthy Aging. Ann. N. Y. Acad. Sci. 2006, 904, 437–448. [Google Scholar] [CrossRef]
- Davison, K.K.; Ford, E.S.; Cogswell, M.E.; Dietz, W.H.; DrPH, M.E.C. Percentage of Body Fat and Body Mass Index Are Associated with Mobility Limitations in People Aged 70 and Older from NHANES III. J. Am. Geriatr. Soc. 2002, 50, 1802–1809. [Google Scholar] [CrossRef]
- Stenholm, S.; Harris, T.B.; Rantanen, T.; Visser, M.; Kritchevsky, S.B.; Ferrucci, L. Sarcopenic obesity: Definition, cause and consequences. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-H.; Choi, K.M. Sarcopenic Obesity, Insulin Resistance, and Their Implications in Cardiovascular and Metabolic Consequences. Int. J. Mol. Sci. 2020, 21, 494. [Google Scholar] [CrossRef] [PubMed]
- Mesinovic, J.; Zengin, A.; De Courten, B.; Ebeling, P.R.; Scott, D. Sarcopenia and type 2 diabetes mellitus: A bidirectional relationship. Diabetes Metab. Syndr. Obesity: Targets Ther. 2019, 12, 1057–1072. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hu, T.; Shen, Y.; Wang, Y.; Bao, Y.; Ma, X. Association of skeletal muscle mass and its change with diabetes occurrence: A population-based cohort study. Diabetol. Metab. Syndr. 2023, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Srikanthan, P.; Karlamangla, A.S. Relative Muscle Mass Is Inversely Associated with Insulin Resistance and Prediabetes. Findings from The Third National Health and Nutrition Examination Survey. J. Clin. Endocrinol. Metab. 2011, 96, 2898–2903. [Google Scholar] [CrossRef] [PubMed]
- Hulett, N.A.; Scalzo, R.L.; Reusch, J.E.B. Glucose Uptake by Skeletal Muscle within the Contexts of Type 2 Diabetes and Exercise: An Integrated Approach. Nutrients 2022, 14, 647. [Google Scholar] [CrossRef] [PubMed]
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr. Physiol. 2020, 10, 785–809. [Google Scholar] [CrossRef] [PubMed]
- Henningsen, J.; Rigbolt, K.T.G.; Blagoev, B.; Pedersen, B.K.; Kratchmarova, I. Dynamics of the Skeletal Muscle Secretome during Myoblast Differentiation. Mol. Cell. Proteom. 2010, 9, 2482–2496. [Google Scholar] [CrossRef]
- Raschke, S.; Eckel, J. Adipo-Myokines: Two Sides of the Same Coin—Mediators of Inflammation and Mediators of Exercise. Mediat. Inflamm. 2013, 2013, 320724. [Google Scholar] [CrossRef]
- Duggal, N.A.; Niemiro, G.; Harridge, S.D.R.; Simpson, R.J.; Lord, J.M. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat. Rev. Immunol. 2019, 19, 563–572. [Google Scholar] [CrossRef]
- Anand, A.C. Nutrition and Muscle in Cirrhosis. J. Clin. Exp. Hepatol. 2017, 7, 340–357. [Google Scholar] [CrossRef] [PubMed]
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of muscle atrophy and hypertrophy: Implications in health and disease. Nat. Commun. 2021, 12, 330. [Google Scholar] [CrossRef] [PubMed]
- Geladari, E.; Alexopoulos, T.; Kontogianni, M.D.; Vasilieva, L.; Mani, I.; Alexopoulou, A. Mechanisms of sarcopenia in liver cirrhosis and the role of myokines. Ann. Gastroenterol. 2023, 36, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Garneau, L.; Aguer, C. Role of myokines in the development of skeletal muscle insulin resistance and related metabolic defects in type 2 diabetes. Diabetes Metab. 2019, 45, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Eckel, J. Myokines in metabolic homeostasis and diabetes. Diabetologia 2019, 62, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Gianoudis, J.; Bailey, C.A.; Daly, R.M. Associations between sedentary behaviour and body composition, muscle function and sarcopenia in community-dwelling older adults. Osteoporos. Int. 2014, 26, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.T.; Hamilton, D.G.; Zderic, T.W. Sedentary behavior as a mediator of type 2 diabetes. Med. Sport Sci. 2014, 60, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, K.; Görgens, S.W.; Raschke, S.; Eckel, J. Myokines in insulin resistance and type 2 diabetes. Diabetologia 2014, 57, 1087–1099. [Google Scholar] [CrossRef]
- Buford, T.W.; Cooke, M.B.; Manini, T.M.; Leeuwenburgh, C.; Willoughby, D.S. Effects of Age and Sedentary Lifestyle on Skeletal Muscle NF- B Signaling in Men. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2010, 65, 532–537. [Google Scholar] [CrossRef]
- Joseph, J.J.; Echouffo-Tcheugui, J.B.; Golden, S.H.; Chen, H.; Jenny, N.S.; Carnethon, M.R.; Jacobs, D.; Burke, G.L.; Vaidya, D.; Ouyang, P.; et al. Physical activity, sedentary behaviors and the incidence of type 2 diabetes mellitus: The Multi-Ethnic Study of Atherosclerosis (MESA). BMJ Open Diabetes Res. Care 2016, 4, e000185. [Google Scholar] [CrossRef]
- Luo, C.; Liu, R.-Y.; Zhang, G.-W.; Hu, F.; Jin, Y.-H.; Liu, B.-Y. Possible sarcopenia and risk of new-onset type 2 diabetes mellitus in older adults in China: A 7-year longitudinal cohort study. BMC Geriatr. 2023, 23, 404. [Google Scholar] [CrossRef] [PubMed]
- Prokopidis, K.; Giannos, P.; Reginster, J.Y.; Bruyere, O.; Petrovic, M.; Cherubini, A.; Triantafyllidis, K.K.; Kechagias, K.S.; Dionyssiotis, Y.; Cesari, M.; et al. Sarcopenia is associated with a greater risk of polypharmacy and number of medications: A systematic review and meta-analysis. J. Cachex-Sarcopenia Muscle 2023, 14, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Sundar, V.V.; Ong, S.H.; Easaw, M.E.P.; Chee, W.S.S. Sarcopenia with co-existent type 2 diabetes mellitus is associated with worse clinical outcomes among hospitalised cardiac patients. Clin. Nutr. ESPEN 2021, 46, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Foong, Y.C.; Chherawala, N.; Aitken, D.; Scott, D.; Winzenberg, T.; Jones, G. Accelerometer-determined physical activity, muscle mass, and leg strength in community-dwelling older adults. J. Cachex-Sarcopenia Muscle 2015, 7, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Foong, Y.C.; Aitken, D.; Winzenberg, T.; Otahal, P.; Scott, D.; Jones, G. The association between physical activity and reduced body fat lessens with age — Results from a cross-sectional study in community-dwelling older adults. Exp. Gerontol. 2014, 55, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Yaegashi, A.; Shirahata, A.; Kudo, S.; Kozuka, M. Effects and contents of nutrition education relating to sarcopenia and frailty for Japanese older adults: A systematic review. Geriatr. Gerontol. Int. 2021, 21, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- The Look AHEAD Research Group; Earnest, C.P.; Church, T.S.; Lee, D.; Jacobs, D.R.; Lind, L.; Lind, P.M.; Normand, M.P.; Gibson, J.L.; Yeh, H.; et al. The Look AHEAD Study: A Description of the Lifestyle Intervention and the Evidence Supporting It. Obesity 2006, 14, 737–752. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Kim, J.; Kim, S.W.; Kong, H.-J. Effects of home-based tele-exercise on sarcopenia among community-dwelling elderly adults: Body composition and functional fitness. Exp. Gerontol. 2017, 87(Pt. A), 33–39. [Google Scholar] [CrossRef]
- Guo, M.; Yao, J.; Li, J.; Zhang, J.; Wang, D.; Zuo, H.; Zhang, Y.; Xu, B.; Zhong, Y.; Shen, F.; et al. Irisin ameliorates age-associated sarcopenia and metabolic dysfunction. J. Cachex-Sarcopenia Muscle 2022, 14, 391–405. [Google Scholar] [CrossRef]
- Kistner, T.M.; Pedersen, B.K.; Lieberman, D.E. Interleukin 6 as an energy allocator in muscle tissue. Nat. Metab. 2022, 4, 170–179. [Google Scholar] [CrossRef]
- Ozaki, Y.; Ohashi, K.; Otaka, N.; Kawanishi, H.; Takikawa, T.; Fang, L.; Takahara, K.; Tatsumi, M.; Ishihama, S.; Takefuji, M.; et al. Myonectin protects against skeletal muscle dysfunction in male mice through activation of AMPK/PGC1α pathway. Nat. Commun. 2023, 14, 4675. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Pescinini-Salzedas, L.M.; Minniti, G.A.d.S.; Laurindo, L.F.; Barbalho, S.M.; Sinatora, R.V.; Sloan, L.A.; Haber, R.S.d.A.; Araújo, A.C.; Quesada, K.; et al. Organokines, Sarcopenia, and Metabolic Repercussions: The Vicious Cycle and the Interplay with Exercise. Int. J. Mol. Sci. 2022, 23, 13452. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.S.; Rutti, S.; Arous, C.; Clemmesen, J.O.; Secher, N.H.; Drescher, A.; Gonelle-Gispert, C.; Halban, P.A.; Pedersen, B.K.; Weigert, C.; et al. Circulating Follistatin Is Liver-Derived and Regulated by the Glucagon-to-Insulin Ratio. J. Clin. Endocrinol. Metab. 2016, 101, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Lee, Y.-S.; Zimmers, T.A.; Soleimani, A.; Matzuk, M.M.; Tsuchida, K.; Cohn, R.D.; Barton, E.R. Regulation of Muscle Mass by Follistatin and Activins. Mol. Endocrinol. 2010, 24, 1998–2008. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; He, H.; Shen, X.; Tang, S.; Zhao, J.; Cao, X.; Han, S.; Cui, C.; Chen, Y.; Wei, Y.; et al. MicroRNA Profiling Reveals an Abundant miR-200a-3p Promotes Skeletal Muscle Satellite Cell Development by Targeting TGF-β2 and Regulating the TGF-β2/SMAD Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 3274. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.H.; Ahmad, K.; Moon, J.S.; Park, S.-Y.; Lim, J.H.; Chun, H.J.; Qadri, A.F.; Hwang, Y.C.; Jan, A.T.; Ahmad, S.S.; et al. Myostatin and its Regulation: A Comprehensive Review of Myostatin Inhibiting Strategies. Front. Physiol. 2022, 13, 876078. [Google Scholar] [CrossRef] [PubMed]
- Umezu, T.; Nakamura, S.; Sato, Y.; Kobayashi, T.; Ito, E.; Abe, T.; Kaneko, M.; Nomura, M.; Yoshimura, A.; Oya, A.; et al. Smad2 and Smad3 expressed in skeletal muscle promote immobilization-induced bone atrophy in mice. Biochem. Biophys. Res. Commun. 2021, 582, 111–117. [Google Scholar] [CrossRef]
- Rolland, Y.; Dray, C.; Vellas, B.; Barreto, P.D.S. Current and investigational medications for the treatment of sarcopenia. Metabolism 2023, 149, 155597. [Google Scholar] [CrossRef]
- Coelho-Júnior, H.J.; Calvani, R.; Tosato, M.; Landi, F.; Picca, A.; Marzetti, E. Protein intake and physical function in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2022, 81, 101731. [Google Scholar] [CrossRef]
- Ali, S.; Corbi, G.; Medoro, A.; Intrieri, M.; Scapagnini, G.; Davinelli, S. Relationship between monounsaturated fatty acids and sarcopenia: A systematic review and meta-analysis of observational studies. Aging Clin. Exp. Res. 2023, 35, 1823–1834. [Google Scholar] [CrossRef]
- Diao, H.; Yan, F.; He, Q.; Li, M.; Zheng, Q.; Zhu, Q.; Fang, F.; Cui, W. Association between Dietary Inflammatory Index and Sarcopenia: A Meta-Analysis. Nutrients 2023, 15, 219. [Google Scholar] [CrossRef] [PubMed]
- Jalili, C.; Talebi, S.; Bagheri, R.; Ghanavati, M.; Camera, D.M.; Amirian, P.; Zarpoosh, M.; Dizaji, M.K.; Kermani, M.A.H.; Moradi, S. The Association between Dietary Inflammatory Index and Aging Biomarkers/Conditions: A Systematic Review and Dose-response Meta-analysis. J. Nutr. Health Aging 2023, 27, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Júnior, H.J.; Trichopoulou, A.; Panza, F. Cross-sectional and longitudinal associations between adherence to Mediterranean diet with physical performance and cognitive function in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2021, 70, 101395. [Google Scholar] [CrossRef] [PubMed]
- Gielen, E.; Beckwée, D.; Delaere, A.; De Breucker, S.; Vandewoude, M.; Bautmans, I.; Sarcopenia Guidelines Development Group of the Belgian Society of Gerontology and Geriatrics (BSGG). Nutritional interventions to improve muscle mass, muscle strength, and physical performance in older people: An umbrella review of systematic reviews and meta-analyses. Nutr. Rev. 2020, 79, 121–147. [Google Scholar] [CrossRef]
- Beckwée, D.; Sarcopenia Guidelines Development Group of the Belgian Society of Gerontology and Geriatrics (BSGG); Delaere, A.; Aelbrecht, S.; Baert, V.; Beaudart, C.; Bruyere, O.; de Saint-Hubert, M.; Bautmans, I. Exercise Interventions for the Prevention and Treatment of Sarcopenia. A Systematic Umbrella Review. J. Nutr. Heal. Aging 2019, 23, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-Y.; Huang, K.-S.; Chen, K.-M.; Chou, C.-P.; Tu, Y.-K. Exercise, Nutrition, and Combined Exercise and Nutrition in Older Adults with Sarcopenia: A Systematic Review and Network Meta-analysis. Maturitas 2020, 145, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Shi, Q.; Nong, K.; Li, S.; Yue, J.; Huang, J.; Dong, B.; Beauchamp, M.; Hao, Q. Exercise for sarcopenia in older people: A systematic review and network meta-analysis. J. Cachex-Sarcopenia Muscle 2023, 14, 1199–1211. [Google Scholar] [CrossRef]
- Cienfuegos, S.; Corapi, S.; Gabel, K.; Ezpeleta, M.; Kalam, F.; Lin, S.; Pavlou, V.; Varady, K.A. Effect of Intermittent Fasting on Reproductive Hormone Levels in Females and Males: A Review of Human Trials. Nutrients 2022, 14, 2343. [Google Scholar] [CrossRef]
- Whittaker, J.; Wu, K. Low-fat diets and testosterone in men: Systematic review and meta-analysis of intervention studies. J. Steroid Biochem. Mol. Biol. 2021, 210, 105878. [Google Scholar] [CrossRef]
- Fantus, R.J.; Halpern, J.A.; Chang, C.; Keeter, M.K.; Bennett, N.E.; Helfand, B.; Brannigan, R.E. The Association between Popular Diets and Serum Testosterone among Men in the United States. J. Urol. 2020, 203, 398–404. [Google Scholar] [CrossRef]
- Whittaker, J.; Harris, M. Low-carbohydrate diets and men’s cortisol and testosterone: Systematic review and meta-analysis. Nutr. Health 2022, 28, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, J. High-protein diets and testosterone. Nutr. Health 2023, 29, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.d.S.; da Costa, C.M.; Souto, J.C.S.; Chiogna, L.M.; Santos, Z.E.d.A.; Rhoden, E.L.; Neto, B.S. The effects of a low carbohydrate diet on erectile function and serum testosterone levels in hypogonadal men with metabolic syndrome: A randomized clinical trial. BMC Endocr. Disord. 2023, 23, 30. [Google Scholar] [CrossRef]
- Furini, C.; Spaggiari, G.; Simoni, M.; Greco, C.; Santi, D. Ketogenic state improves testosterone serum levels—Results from a systematic review and meta-analysis. Endocrine 2022, 79, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Chidi-Ogbolu, N.; Baar, K. Effect of Estrogen on Musculoskeletal Performance and Injury Risk. Front. Physiol. 2019, 9, 1834. [Google Scholar] [CrossRef] [PubMed]
- Lisco, G.; Triggiani, D.; Giagulli, V.A.; De Pergola, G.; Guastamacchia, E.; Piazzolla, G.; Jirillo, E.; Triggiani, V. Endocrine, Metabolic, and Immune Pathogenesis of Postmenopausal Osteoporosis. Is there a Therapeutic Role in Natural Products? Endocrine Metab. Immune Disord. Drug Targets 2023, 23, 1278–1290. [Google Scholar] [CrossRef] [PubMed]
- Tiidus, P.M. Benefits of Estrogen Replacement for Skeletal Muscle Mass and Function in Post-Menopausal Females: Evidence from Human and Animal Studies. Eurasian J. Med. 2011, 43, 109–114. [Google Scholar] [CrossRef]
- Latham, C.M.; Brightwell, C.R.; Keeble, A.R.; Munson, B.D.; Thomas, N.T.; Zagzoog, A.M.; Fry, C.S.; Fry, J.L. Vitamin D Promotes Skeletal Muscle Regeneration and Mitochondrial Health. Front. Physiol. 2021, 12, 660498. [Google Scholar] [CrossRef]
- Luo, J.; Quan, Z.; Lin, S.; Cui, L. The association between blood concentration of 25-hydroxyvitamin D and sarcopenia: A meta-analysis. Asia Pac. J. Clin. Nutr. 2018, 27, 1258–1270. [Google Scholar] [CrossRef]
- Besora-Moreno, M.; Llauradó, E.; Valls, R.M.; Tarro, L.; Pedret, A.; Solà, R. Antioxidant-rich foods, antioxidant supplements, and sarcopenia in old-young adults ≥55 years old: A systematic review and meta-analysis of observational studies and randomized controlled trials. Clin. Nutr. 2022, 41, 2308–2324. [Google Scholar] [CrossRef]
- Gkekas, N.K.; Anagnostis, P.; Paraschou, V.; Stamiris, D.; Dellis, S.; Kenanidis, E.; Potoupnis, M.; Tsiridis, E.; Goulis, D.G. The effect of vitamin D plus protein supplementation on sarcopenia: A systematic review and meta-analysis of randomized controlled trials. Maturitas 2021, 145, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-H.; Chen, K.-H.; Chen, C.; Chu, W.-C.; Kang, Y.-N. The Optimal Strategy of Vitamin D for Sarcopenia: A Network Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 3589. [Google Scholar] [CrossRef] [PubMed]
- Nasimi, N.; Sohrabi, Z.; Nunes, E.A.; Sadeghi, E.; Jamshidi, S.; Gholami, Z.; Akbarzadeh, M.; Faghih, S.; Akhlaghi, M.; Phillips, S.M. Whey Protein Supplementation with or without Vitamin D on Sarcopenia-Related Measures: A Systematic Review and Meta-Analysis. Adv. Nutr. Int. Rev. J. 2023, 14, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Choo, Y.J. Effects of Whey Protein, Leucine, and Vitamin D Supplementation in Patients with Sarcopenia: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 521. [Google Scholar] [CrossRef] [PubMed]
- Otremski, I.; Lev-Ran, M.; Salama, R.; Edelstein, S. The metabolism of vitamin D3 in response to testosterone. Calcif. Tissue Int. 1997, 60, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.B. Vitamin D and male reproduction. Nat. Rev. Endocrinol. 2014, 10, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, H.; Wang, S.; Liu, H.; Guo, M.; Bai, H.; Zeng, W.; Zhang, T. Vitamin D Receptor affects male mouse fertility via regulation of lipid metabolism and testosterone biosynthesis in testis. Gene 2022, 834, 146589. [Google Scholar] [CrossRef]
- Holt, R.; Yahyavi, S.K.; Kooij, I.; Poulsen, N.N.; Juul, A.; Jørgensen, N.; Jensen, M.B. Effects of vitamin D on sex steroids, luteinizing hormone, and testosterone to luteinizing hormone ratio in 307 infertile men. Andrology 2023, in press. [Google Scholar] [CrossRef]
- D’andrea, S.; Martorella, A.; Coccia, F.; Castellini, C.; Minaldi, E.; Totaro, M.; Parisi, A.; Francavilla, F.; Francavilla, S.; Barbonetti, A. Relationship of Vitamin D status with testosterone levels: A systematic review and meta-analysis. Endocrine 2020, 72, 49–61. [Google Scholar] [CrossRef]
- Hagenfeldt, Y.; Linde, K.; Sjöberg, H.-E.; Zumkeller, W.; Arver, S. Testosterone increases serum 1,25–dihydroxyvitamin D and insulin-like growth factor-I in hypogonadal men. Int. J. Androl. 1992, 15, 93–102. [Google Scholar] [CrossRef]
- Saki, F.; Kasaee, S.R.; Sadeghian, F.; Koohpeyma, F.; Omrani, G.R. Investigating the effect of testosterone by itself and in combination with letrozole on 1,25-dihydroxy vitamin D and FGF23 in male rats. J. Endocrinol. Investig. 2018, 42, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Frisch, S.; Koertke, H.; Kuhn, J.; Dreier, J.; Obermayer-Pietsch, B.; Wehr, E.; Zittermann, A. Effect of Vitamin D Supplementation on Testosterone Levels in Men. Horm. Metab. Res. 2010, 43, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Lerchbaum, E.; Pilz, S.; Trummer, C.; Schwetz, V.; Pachernegg, O.; Heijboer, A.C.; Obermayer-Pietsch, B. Vitamin D and Testosterone in Healthy Men: A Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2017, 102, 4292–4302. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, Z.; Borochowitz, Z.; Benderli, A.; Vardi, P.; Oren, S.; Gougoux, A.; Spirer, Z.; Heyman, I.; Weisman, Y. Does 1,25-Dihydroxyvitamin D Participate in the Regulation of Hormone Release from Endocrine Glands? J. Clin. Endocrinol. Metab. 1985, 60, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Hofer, D.; Münzker, J.; Schwetz, V.; Ulbing, M.; Hutz, K.; Stiegler, P.; Zigeuner, R.; Pieber, T.R.; Müller, H.; Obermayer-Pietsch, B. Testicular Synthesis and Vitamin D Action. J. Clin. Endocrinol. Metab. 2014, 99, 3766–3773. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes—2023. Diabetes Care 2022, 46 (Suppl. 1), S140–S157. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liao, Z.; Xiao, Q. Metformin ameliorates skeletal muscle atrophy in Grx1 KO mice by regulating intramuscular lipid accumulation and glucose utilization. Biochem. Biophys. Res. Commun. 2020, 533, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Long, D.E.; Kosmac, K.; Dungan, C.M.; Bamman, M.M.; Peterson, C.A.; Kern, P.A. Potential Benefits of Combined Statin and Metformin Therapy on Resistance Training Response in Older Individuals. Front. Physiol. 2022, 13, 872745. [Google Scholar] [CrossRef]
- Song, Y.; Wu, Z.; Zhao, P. The Function of Metformin in Aging-Related Musculoskeletal Disorders. Front. Pharmacol. 2022, 13, 865524. [Google Scholar] [CrossRef]
- Toledo-Pérez, R.; Lopéz-Cervantes, S.P.; Hernández-Álvarez, D.; Mena-Montes, B.; Pedraza-Vázquez, G.; Sánchez-Garibay, C.; López-Diazguerrero, N.E.; Königsberg, M.; Luna-López, A. Metformin and tBHQ Treatment Combined with an Exercise Regime Prevents Osteosarcopenic Obesity in Middle-Aged Wistar Female Rats. Oxidative Med. Cell. Longev. 2021, 2021, 5294266. [Google Scholar] [CrossRef]
- Wahdan-Alaswad, R.; Harrell, J.C.; Fan, Z.; Edgerton, S.M.; Liu, B.; Thor, A.D. Metformin attenuates transforming growth factor beta (TGF-β) mediated oncogenesis in mesenchymal stem-like/claudin-low triple negative breast cancer. Cell Cycle 2016, 15, 1046–1059. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-M.; Lee, J.-H.; Yadav, H.; Kamaraju, A.K.; Liu, E.; Zhigang, D.; Vieira, A.; Kim, S.-J.; Collins, H.; Matschinsky, F.; et al. Transforming Growth Factor-β/Smad3 Signaling Regulates Insulin Gene Transcription and Pancreatic Islet β-Cell Function. J. Biol. Chem. 2009, 284, 12246–12257. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Makino, T.; Zhao, X.; Matsuto, M.; Sakurai, H.; Takahashi, Y.; Shimizu, M.; Sato, R.; Yamauchi, Y. Pathophysiological levels of GDF11 activate Smad2/Smad3 signaling and induce muscle atrophy in human iPSC-derived myocytes. Am. J. Physiol. Cell Physiol. 2022, 323, C1402–C1409. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.; Querfurth, H.W. Palmitate activates mTOR/p70S6K through AMPK inhibition and hypophosphorylation of raptor in skeletal muscle cells: Reversal by oleate is similar to metformin. Biochimie 2015, 118, 141–150. [Google Scholar] [CrossRef]
- Kang, M.J.; Moon, J.W.; Lee, J.O.; Kim, J.H.; Jung, E.J.; Kim, S.J.; Oh, J.Y.; Wu, S.W.; Lee, P.R.; Park, S.H.; et al. Metformin induces muscle atrophy by transcriptional regulation of myostatin via HDAC6 and FoxO3a. J. Cachex-Sarcopenia Muscle 2021, 13, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Gang, X.; Wang, G.; Xiao, X.; Li, Z.; Jiang, Z.; Wang, G. A cross-sectional study: Associations between sarcopenia and clinical characteristics of patients with type 2 diabetes. Medicine 2020, 99, e18708. [Google Scholar] [CrossRef]
- Ai, Y.; Xu, R.; Liu, L. The prevalence and risk factors of sarcopenia in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2021, 13, 93. [Google Scholar] [CrossRef]
- Witham, M.D.; Granic, A.; Pearson, E.; Robinson, S.M.; Sayer, A.A. Repurposing Drugs for Diabetes Mellitus as Potential Pharmacological Treatments for Sarcopenia—A Narrative Review. Drugs Aging 2023, 40, 703–719. [Google Scholar] [CrossRef]
- Ashcroft, F.M. Mechanisms of the Glycaemic Effects of Sulfonylureas. Horm. Metab. Res. 1996, 28, 456–463. [Google Scholar] [CrossRef]
- Bak, J.F.; Schmitz, O.; Sørensen, N.S.; Pedersen, O. Postreceptor effects of sulfonylurea on skeletal muscle glycogen synthase activity in type II diabetic patients. Diabetes 1989, 38, 1343–1350. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Chen, S.; Shao, H. Anti-diabetic drugs and sarcopenia: Emerging links, mechanistic insights, and clinical implications. J. Cachex-Sarcopenia Muscle 2021, 12, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, H. The mechanism of alpha-glucosidase inhibition in the management of diabetes. Clin. Investig. Med. 1995, 18, 303–311. [Google Scholar]
- Jiang, L.-L.; Xu, X.-H.; Luo, M.-H.; Wang, H.-Y.; Ding, B.; Yan, R.-N.; Hu, Y.; Ma, J.-H. Association of Acarbose with Decreased Muscle Mass and Function in Patients with Type 2 Diabetes: A Retrospective, Cross-Sectional Study. Diabetes Ther. 2021, 12, 2955–2969. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ni, Y.; Yang, S.; Li, H.; Li, X.; Feng, B. The Effects of Gliclazide, Metformin, and Acarbose on Body Composition in Patients with Newly Diagnosed Type 2 Diabetes Mellitus. Curr. Ther. Res. Clin Exp. 2013, 75, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Thornberry, N.A.; Gallwitz, B. Mechanism of action of inhibitors of dipeptidyl-peptidase-4 (DPP-4). Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 479–486. [Google Scholar] [CrossRef] [PubMed]
- A Nauck, M.; Meier, J.J. The incretin effect in healthy individuals and those with type 2 diabetes: Physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. 2016, 4, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Shyamaladevi, B.; Dash, I.; Badrachalam, R.; Krishnan, M.; Panneerselvam, A.; Undru, S. An update on diagnosis and therapeutics for type-2 diabetes mellitus. Bioinformation 2023, 19, 295–298. [Google Scholar] [CrossRef]
- Morieri, M.L.; Consoli, A.; Sesti, G.; Purrello, F.; Avogaro, A.; Fadini, G.P.; DARWIN-FUP Network. Comparative effectiveness of dapagliflozin vs. DPP-4 inhibitors on a composite endpoint of HbA1c, body weight and blood pressure reduction in the real world. Diabetes Metab. Res. Rev. 2020, 37, e3353. [Google Scholar] [CrossRef]
- Neidert, L.; Al-Tarhuni, M.; Goldman, D.; Kluess, H.A.; Jackson, D.N. Endogenous dipeptidyl peptidase IV modulates skeletal muscle arteriolar diameter in rats. Physiol. Rep. 2018, 6, e13564. [Google Scholar] [CrossRef]
- Giannocco, G.; Oliveira, K.C.; Crajoinas, R.O.; Venturini, G.; Salles, T.A.; Fonseca-Alaniz, M.H.; Maciel, R.M.; Girardi, A.C. Dipeptidyl peptidase IV inhibition upregulates GLUT4 translocation and expression in heart and skeletal muscle of spontaneously hypertensive rats. Eur. J. Pharmacol. 2013, 698, 74–86. [Google Scholar] [CrossRef]
- Lv, J.; Li, Y.; Shi, S.; Xu, X.; Wu, H.; Zhang, B.; Song, Q. Skeletal muscle mitochondrial remodeling in heart failure: An update on mechanisms and therapeutic opportunities. Biomed. Pharmacother. 2022, 155, 113833. [Google Scholar] [CrossRef] [PubMed]
- Boschmann, M.; Engeli, S.; Dobberstein, K.; Budziarek, P.; Strauss, A.; Boehnke, J.; Sweep, F.C.G.J.; Luft, F.C.; He, Y.; Foley, J.E.; et al. Dipeptidyl-Peptidase-IV Inhibition Augments Postprandial Lipid Mobilization and Oxidation in Type 2 Diabetic Patients. J. Clin. Endocrinol. Metab. 2009, 94, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Takada, S.; Masaki, Y.; Kinugawa, S.; Matsumoto, J.; Furihata, T.; Mizushima, W.; Kadoguchi, T.; Fukushima, A.; Homma, T.; Takahashi, M.; et al. Dipeptidyl peptidase-4 inhibitor improved exercise capacity and mitochondrial biogenesis in mice with heart failure via activation of glucagon-like peptide-1 receptor signalling. Cardiovasc. Res. 2016, 111, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Ogura, Y.; Inoue, K.; Tanabe, J.; Sugaya, T.; Ohata, K.; Nagai, Y.; Natsuki, Y.; Hoshino, S.; Watanabe, S.; et al. Effect of GLP-1 receptor agonist, liraglutide, on muscle in spontaneously diabetic torii fatty rats. Mol. Cell. Endocrinol. 2021, 539, 111472. [Google Scholar] [CrossRef] [PubMed]
- Vainshtein, A.; Desjardins, E.M.A.; Armani, A.; Sandri, M.; Hood, D.A. PGC-1α modulates denervation-induced mitophagy in skeletal muscle. Skelet. Muscle 2015, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, F.; Jiang, P. Effect of sitagliptin on expression of skeletal muscle peroxisome proliferator-activated receptor γ coactivator-1α and irisin in a rat model of type 2 diabetes mellitus. J. Int. Med Res. 2020, 48, 300060519885569. [Google Scholar] [CrossRef] [PubMed]
- Nahon, K.J.; Doornink, F.; Straat, M.E.; Botani, K.; Martinez-Tellez, B.; Abreu-Vieira, G.; van Klinken, J.B.; Voortman, G.J.; Friesema, E.C.H.; Ruiz, J.R.; et al. Effect of sitagliptin on energy metabolism and brown adipose tissue in overweight individuals with prediabetes: A randomised placebo-controlled trial. Diabetologia 2018, 61, 2386–2397. [Google Scholar] [CrossRef]
- Scalzo, R.L.; Rafferty, D.; Schauer, I.; Huebschmann, A.G.; Cree-Green, M.; Reusch, J.E.; Regensteiner, J.G. Sitagliptin improves diastolic cardiac function but not cardiorespiratory fitness in adults with type 2 diabetes. J. Diabetes Complicat. 2019, 33, 561–566. [Google Scholar] [CrossRef]
- Hällsten, K.; Virtanen, K.A.; Lönnqvist, F.; Sipilä, H.; Oksanen, A.; Viljanen, T.; Rönnemaa, T.; Viikari, J.; Knuuti, J.; Nuutila, P. Rosiglitazone but Not Metformin Enhances Insulin- and Exercise-Stimulated Skeletal Muscle Glucose Uptake in Patients with Newly Diagnosed Type 2 Diabetes. Diabetes 2002, 51, 3479–3485. [Google Scholar] [CrossRef]
- Meyer, M.M.; Levin, K.; Grimmsmann, T.; Perwitz, N.; Eirich, A.; Beck-Nielsen, H.; Klein, H.H. Troglitazone Treatment Increases Protein Kinase B Phosphorylation in Skeletal Muscle of Normoglycemic Subjects at Risk for the Development of Type 2 Diabetes. Diabetes 2002, 51, 2691–2697. [Google Scholar] [CrossRef][Green Version]
- Kim, Y.-B.; Ciaraldi, T.P.; Kong, A.; Kim, D.; Chu, N.; Mohideen, P.; Mudaliar, S.; Henry, R.R.; Kahn, B.B. Troglitazone but not Metformin Restores Insulin-Stimulated Phosphoinositide 3-Kinase Activity and Increases p110β Protein Levels in Skeletal Muscle of Type 2 Diabetic Subjects. Diabetes 2002, 51, 443–448. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tonelli, J.; Li, W.; Kishore, P.; Pajvani, U.B.; Kwon, E.; Weaver, C.; Scherer, P.E.; Hawkins, M. Mechanisms of Early Insulin-Sensitizing Effects of Thiazolidinediones in Type 2 Diabetes. Diabetes 2004, 53, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.D.; Debard, C.; Funahashi, T.; Humphreys, S.M.; Matsuzawa, Y.; Frayn, K.N.; Karpe, F.; Vidal, H. Changes in adiponectin receptor expression in muscle and adipose tissue of type 2 diabetic patients during rosiglitazone therapy. Diabetologia 2005, 48, 1585–1589. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Gupta, P.; Saini, A.S.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Dixon, E.D.; Nardo, A.D.; Claudel, T.; Trauner, M. The Role of Lipid Sensing Nuclear Receptors (PPARs and LXR) and Metabolic Lipases in Obesity, Diabetes and NAFLD. Genes 2021, 12, 645. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Vachliotis, I.D.; Mantzoros, C.S. Sarcopenia, sarcopenic obesity and nonalcoholic fatty liver disease. Metabolism 2023, 147, 155676. [Google Scholar] [CrossRef] [PubMed]
- Stuart, C.A.; Howell, M.E.; Yin, D. Overexpression of GLUT5 in Diabetic Muscle Is Reversed by Pioglitazone. Diabetes Care 2007, 30, 925–931. [Google Scholar] [CrossRef]
- McClelland, T.J.; Fowler, A.J.; Davies, T.W.; Pearse, R.; Prowle, J.; Puthucheary, Z. Can pioglitazone be used for optimization of nutrition in critical illness? A systematic review. J. Parenter. Enter. Nutr. 2023, 47, 459–475. [Google Scholar] [CrossRef]
- Fiorentino, T.V.; Monroy, A.; Kamath, S.; Sotero, R.; Cas, M.D.; Daniele, G.; Chavez, A.O.; Abdul-Ghani, M.; Hribal, M.L.; Sesti, G.; et al. Pioglitazone corrects dysregulation of skeletal muscle mitochondrial proteins involved in ATP synthesis in type 2 diabetes. Metabolism 2020, 114, 154416. [Google Scholar] [CrossRef]
- Warshauer, J.T.; Lopez, X.; Gordillo, R.; Hicks, J.; Holland, W.L.; Anuwe, E.; Blankfard, M.B.; Scherer, P.E.; Lingvay, I. Effect of pioglitazone on plasma ceramides in adults with metabolic syndrome. Diabetes Metab. Res. Rev. 2015, 31, 734–744. [Google Scholar] [CrossRef]
- Punthakee, Z.; Alméras, N.; Després, J.-P.; Dagenais, G.R.; Anand, S.S.; Hunt, D.L.; Sharma, A.M.; Jung, H.; Yusuf, S.; Gerstein, H.C. Impact of rosiglitazone on body composition, hepatic fat, fatty acids, adipokines and glucose in persons with impaired fasting glucose or impaired glucose tolerance: A sub-study of the DREAM trial. Diabet. Med. 2014, 31, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Kinugawa, S.; Hirabayashi, K.; Suga, T.; Takada, S.; Omokawa, M.; Kadoguchi, T.; Takahashi, M.; Fukushima, A.; Matsushima, S.; et al. Pioglitazone improves whole-body aerobic capacity and skeletal muscle energy metabolism in patients with metabolic syndrome. J. Diabetes Investig. 2016, 8, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.R.; de Jonge, L.; Volaufova, J.; Li, Y.; Xie, H.; Bray, G.A. Effect of pioglitazone on body composition and energy expenditure: A randomized controlled trial. Metabolism 2005, 54, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Zhang, B.; Xu, W.; Yang, H.; Feng, W.; Li, C.; Tong, G.; Li, M.; Wang, X.; Shen, S.; et al. Effects of exenatide, insulin, and pioglitazone on liver fat content and body fat distributions in drug-naive subjects with type 2 diabetes. Acta Diabetol. 2014, 51, 865–873. [Google Scholar] [CrossRef]
- Vettor, R.; Milan, G.; Franzin, C.; Sanna, M.; De Coppi, P.; Rizzuto, R.; Federspil, G. The origin of intermuscular adipose tissue and its pathophysiological implications. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E987–E998. [Google Scholar] [CrossRef]
- De Coppi, P.; Milan, G.; Scarda, A.; Boldrin, L.; Centobene, C.; Piccoli, M.; Pozzobon, M.; Pilon, C.; Pagano, C.; Gamba, P.; et al. Rosiglitazone modifies the adipogenic potential of human muscle satellite cells. Diabetologia 2006, 49, 1962–1973. [Google Scholar] [CrossRef]
- Balas, B.; Belfort, R.; Harrison, S.A.; Darland, C.; Finch, J.; Schenker, S.; Gastaldelli, A.; Cusi, K. Pioglitazone treatment increases whole body fat but not total body water in patients with non-alcoholic steatohepatitis. J. Hepatol. 2007, 47, 565–570. [Google Scholar] [CrossRef]
- Bray, G.A.; Smith, S.R.; Banerji, M.A.; Tripathy, D.; Clement, S.C.; Buchanan, T.A.; Henry, R.R.; Kitabchi, A.E.; Mudaliar, S.; Musi, N.; et al. Effect of pioglitazone on body composition and bone density in subjects with prediabetes in the ACT NOW trial. Diabetes, Obes. Metab. 2013, 15, 931–937. [Google Scholar] [CrossRef]
- Slim, R.; Ben Salem, C.; Zamy, M.; Biour, M. Pioglitazone-Induced Acute Rhabdomyolysis. Diabetes Care 2009, 32, e84. [Google Scholar] [CrossRef]
- Hsia, D.S.; Grove, O.; Cefalu, W.T. An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 73–79. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; DeFronzo, R.A.; Norton, L. Novel Hypothesis to Explain Why SGLT2 Inhibitors Inhibit Only 30–50% of Filtered Glucose Load in Humans. Diabetes 2013, 62, 3324–3328. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, G.; Hach, T.; Crowe, S.; Sanghvi, A.; Hall, K.D.; Ferrannini, E. Energy Balance After Sodium–Glucose Cotransporter 2 Inhibition. Diabetes Care 2015, 38, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Schork, A.; Saynisch, J.; Vosseler, A.; Jaghutriz, B.A.; Heyne, N.; Peter, A.; Häring, H.-U.; Stefan, N.; Fritsche, A.; Artunc, F. Effect of SGLT2 inhibitors on body composition, fluid status and renin–angiotensin–aldosterone system in type 2 diabetes: A prospective study using bioimpedance spectroscopy. Cardiovasc. Diabetol. 2019, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- Fathi, A.; Vickneson, K.; Singh, J.S. SGLT2-inhibitors; more than just glycosuria and diuresis. Heart Fail. Rev. 2020, 26, 623–642. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Nagata, N.; Nagashimada, M.; Zhuge, F.; Ni, Y.; Chen, G.; Mayoux, E.; Kaneko, S.; Ota, T. SGLT2 Inhibition by Empagliflozin Promotes Fat Utilization and Browning and Attenuates Inflammation and Insulin Resistance by Polarizing M2 Macrophages in Diet-induced Obese Mice. EBioMedicine 2017, 20, 137–149. [Google Scholar] [CrossRef]
- Monzo, L.; Ferrari, I.; Cicogna, F.; Tota, C.; Cice, G.; Girerd, N.; Calò, L. Sodium-glucose co-transporter 2 inhibitors in heart failure: An updated evidence-based practical guidance for clinicians. Eur. Heart J. Suppl. 2023, 25 (Suppl. C), C309–C315. [Google Scholar] [CrossRef]
- Sargeant, J.A.; Henson, J.; King, J.A.; Yates, T.; Khunti, K.; Davies, M.J. A Review of the Effects of Glucagon-Like Peptide-1 Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors on Lean Body Mass in Humans. Endocrinol. Metab. 2019, 34, 247–262. [Google Scholar] [CrossRef]
- Sugiyama, S.; Jinnouchi, H.; Kurinami, N.; Hieshima, K.; Yoshida, A.; Jinnouchi, K.; Nishimura, H.; Suzuki, T.; Miyamoto, F.; Kajiwara, K.; et al. Dapagliflozin Reduces Fat Mass without Affecting Muscle Mass in Type 2 Diabetes. J. Atheroscler. Thromb. 2018, 25, 467–476. [Google Scholar] [CrossRef]
- Pan, R.; Zhang, Y.; Wang, R.; Xu, Y.; Ji, H.; Zhao, Y. Effect of SGLT-2 inhibitors on body composition in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. PLoS ONE 2022, 17, e0279889. [Google Scholar] [CrossRef]
- Kalaitzoglou, E.; Fowlkes, J.L.; Popescu, I.; Thrailkill, K.M. Diabetes pharmacotherapy and effects on the musculoskeletal system. Diabetes Metab. Res. Rev. 2018, 35, e3100. [Google Scholar] [CrossRef]
- Zizola, C.; Schulze, P.C. Metabolic and structural impairment of skeletal muscle in heart failure. Heart Fail. Rev. 2012, 18, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Takada, S.; Sabe, H.; Kinugawa, S. Treatments for skeletal muscle abnormalities in heart failure: Sodium-glucose transporter 2 and ketone bodies. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H117–H128. [Google Scholar] [CrossRef] [PubMed]
- Voorrips, S.N.; Saucedo-Orozco, H.; Sánchez-Aguilera, P.I.; De Boer, R.A.; Van der Meer, P.; Westenbrink, B.D. Could SGLT2 Inhibitors Improve Exercise Intolerance in Chronic Heart Failure? Int. J. Mol. Sci. 2022, 23, 8631. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, J.M.; Nuffer, W.; Smith, B.A. GLP-1 receptor agonists: An updated review of head-to-head clinical studies. Ther. Adv. Endocrinol. Metab. 2021, 12, 2042018821997320. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.; Finlayson, G.; Axelsen, M.; Flint, A.; Gibbons, C.; Kvist, T.; Hjerpsted, J.B. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes. Metab. 2017, 19, 1242–1251. [Google Scholar] [CrossRef]
- Lisco, G.; De Tullio, A.; Guastamacchia, E.; Triggiani, V. Fixed-Ratio Combinations of Basal Insulin and GLP-1RA in the Management of Type 2 Diabetes Mellitus: Highlights from the Literature. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 626–646. [Google Scholar] [CrossRef]
- Liu, J.-S.; Su, S.-C.; Kuo, F.-C.; Li, P.-F.; Huang, C.-L.; Ho, L.-J.; Chen, K.-C.; Liu, Y.-C.; Lin, C.-P.; Cheng, A.-C.; et al. The efficacy and safety of combined GLP-1RA and basal insulin therapy among inadequately controlled T2D with premixed insulin therapy. Medicine 2023, 102, e33167. [Google Scholar] [CrossRef]
- Lisco, G.; De Tullio, A.; Disoteo, O.; De Geronimo, V.; Piazzolla, G.; De Pergola, G.; Giagulli, V.A.; Jirillo, E.; Guastamacchia, E.; Sabbà, C.; et al. Basal insulin intensification with GLP-1RA and dual GIP and GLP-1RA in patients with uncontrolled type 2 diabetes mellitus: A rapid review of randomized controlled trials and meta-analysis. Front. Endocrinol. 2022, 13, 920541. [Google Scholar] [CrossRef]
- Feng, W.; Bi, Y.; Li, P.; Yin, T.; Gao, C.; Shen, S.; Gao, L.; Yang, D.; Zhu, D. Effects of liraglutide, metformin and gliclazide on body composition in patients with both type 2 diabetes and non-alcoholic fatty liver disease: A randomized trial. J. Diabetes Investig. 2018, 10, 399–407. [Google Scholar] [CrossRef]
- Gibbons, C.; Blundell, J.; Hoff, S.T.; Dahl, K.; Bauer, R.; Bækdal, T. Effects of oral semaglutide on energy intake, food preference, appetite, control of eating and body weight in subjects with type 2 diabetes. Diabetes Obes. Metab. 2020, 23, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Bouchi, R.; Nakano, Y.; Fukuda, T.; Takeuchi, T.; Murakami, M.; Minami, I.; Izumiyama, H.; Hashimoto, K.; Yoshimoto, T.; Ogawa, Y. Reduction of visceral fat by liraglutide is associated with ameliorations of hepatic steatosis, albuminuria, and micro-inflammation in type 2 diabetic patients with insulin treatment: A randomized control trial. Endocr. J. 2017, 64, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Volpe, S.; Lisco, G.; Racaniello, D.; Fanelli, M.; Colaianni, V.; Vozza, A.; Triggiani, V.; Sabbà, C.; Tortorella, C.; De Pergola, G.; et al. Once-Weekly Semaglutide Induces an Early Improvement in Body Composition in Patients with Type 2 Diabetes: A 26-Week Prospective Real-Life Study. Nutrients 2022, 14, 2414. [Google Scholar] [CrossRef] [PubMed]
- Volpe, S.; Lisco, G.; Fanelli, M.; Racaniello, D.; Colaianni, V.; Lavarra, V.; Triggiani, D.; Crudele, L.; Triggiani, V.; Sabbà, C.; et al. Oral semaglutide improves body composition and preserves lean mass in patients with type 2 diabetes: A 26-week prospective real-life study. Front. Endocrinol. 2023, 14, 1240263. [Google Scholar] [CrossRef] [PubMed]
- Piazzolla, G.; Vozza, A.; Volpe, S.; Bergamasco, A.; Triggiani, V.; Lisco, G.; Falconieri, M.; Tortorella, C.; Solfrizzi, V.; Sabbà, C. Effectiveness and clinical benefits of new anti-diabetic drugs: A real life experience. Open Med. 2022, 17, 1203–1215. [Google Scholar] [CrossRef] [PubMed]
- Love, K.M.; Liu, J.; Regensteiner, J.G.; Reusch, J.E.; Liu, Z. GLP-1 and insulin regulation of skeletal and cardiac muscle microvascular perfusion in type 2 diabetes. J. Diabetes 2020, 12, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Iwai, S.; Kaji, K.; Nishimura, N.; Kubo, T.; Tomooka, F.; Shibamoto, A.; Suzuki, J.; Tsuji, Y.; Fujinaga, Y.; Kitagawa, K.; et al. Glucagon-like peptide-1 receptor agonist, semaglutide attenuates chronic liver disease-induced skeletal muscle atrophy in diabetic mice. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166770. [Google Scholar] [CrossRef]
- Xiang, J.; Qin, L.; Zhong, J.; Xia, N.; Liang, Y. GLP-1RA Liraglutide and Semaglutide Improves Obesity-Induced Muscle Atrophy via SIRT1 Pathway. Diabetes Metab. Syndr. Obes. Targets Ther. 2023, 16, 2433–2446. [Google Scholar] [CrossRef]
- Guarnotta, V.; Bianco, M.J.; Vigneri, E.; Panto’, F.; Sasso, B.L.; Ciaccio, M.; Pizzolanti, G.; Giordano, C. Effects of GLP-1 receptor agonists on myokine levels and pro-inflammatory cytokines in patients with type 2 diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 3193–3201. [Google Scholar] [CrossRef]
- Townsend, L.K.; Steinberg, G.R. AMPK and the Endocrine Control of Metabolism. Endocr. Rev. 2023, 44, 910–933. [Google Scholar] [CrossRef]
- Khin, P.P.; Hong, Y.; Yeon, M.; Lee, D.H.; Lee, J.H.; Jun, H.-S. Dulaglutide improves muscle function by attenuating inflammation through OPA-1-TLR-9 signaling in aged mice. Aging 2021, 13, 21962–21974. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Wu, W.; Fan, X.; Zhong, X.; Wang, N.; Wang, Y.; Pan, T.; Du, Y. Dulaglutide Protects Mice against Diabetic Sarcopenia-Mediated Muscle Injury by Inhibiting Inflammation and Regulating the Differentiation of Myoblasts. Int. J. Endocrinol. 2023, 2023, 9926462. [Google Scholar] [CrossRef]
- Giagulli, V.A.; Carbone, M.D.; Ramunni, M.I.; Licchelli, B.; De Pergola, G.; Sabbà, C.; Guastamacchia, E.; Triggiani, V. Adding liraglutide to lifestyle changes, metformin and testosterone therapy boosts erectile function in diabetic obese men with overt hypogonadism. Andrology 2015, 3, 1094–1103. [Google Scholar] [CrossRef]
- Lisco, G.; Bartolomeo, N.; De Tullio, A.; De Pergola, G.; Guastamacchia, E.; Jirillo, E.; Piazzolla, G.; Triggiani, V.; Giagulli, V.A. Long-acting glucagon-like peptide 1 receptor agonists boost erectile function in men with type 2 diabetes mellitus complaining of erectile dysfunction: A retrospective cohort study. Andrology 2023, in press. [Google Scholar] [CrossRef]
- Zhao, F.; Zhou, Q.; Cong, Z.; Hang, K.; Zou, X.; Zhang, C.; Chen, Y.; Dai, A.; Liang, A.; Ming, Q.; et al. Structural insights into multiplexed pharmacological actions of tirzepatide and peptide 20 at the GIP, GLP-1 or glucagon receptors. Nat. Commun. 2022, 13, 1057. [Google Scholar] [CrossRef]
- Coskun, T.; Urva, S.; Roell, W.C.; Qu, H.; Loghin, C.; Moyers, J.S.; O’farrell, L.S.; Briere, D.A.; Sloop, K.W.; Thomas, M.K.; et al. LY3437943, a novel triple glucagon, GIP, and GLP-1 receptor agonist for glycemic control and weight loss: From discovery to clinical proof of concept. Cell Metab. 2022, 34, 1234–1247.e9. [Google Scholar] [CrossRef]
- Urva, S.; Coskun, T.; Loh, M.T.; Du, Y.; Thomas, M.K.; Gurbuz, S.; Haupt, A.; Benson, C.T.; Hernandez-Illas, M.; A D’alessio, D.; et al. LY3437943, a novel triple GIP, GLP-1, and glucagon receptor agonist in people with type 2 diabetes: A phase 1b, multicentre, double-blind, placebo-controlled, randomised, multiple-ascending dose trial. Lancet 2022, 400, 1869–1881. [Google Scholar] [CrossRef]
- Heise, T.; DeVries, J.H.; Urva, S.; Li, J.; Pratt, E.J.; Thomas, M.K.; Mather, K.J.; Karanikas, C.A.; Dunn, J.; Haupt, A.; et al. Tirzepatide Reduces Appetite, Energy Intake, and Fat Mass in People With Type 2 Diabetes. Diabetes Care 2023, 46, 998–1004. [Google Scholar] [CrossRef]
- Rosenstock, J.; Wysham, C.; Frías, J.P.; Kaneko, S.; Lee, C.J.; Landó, L.F.; Mao, H.; Cui, X.; A Karanikas, C.A.; Thieu, V.T. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): A double-blind, randomised, phase 3 trial. Lancet 2021, 398, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Frías, J.P.; Davies, M.J.; Rosenstock, J.; Pérez Manghi, F.C.; Fernández Landó, L.; Bergman, B.K.; Liu, B.; Cui, X.; Brown, K. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Ludvik, B.; Giorgino, F.; Jódar, E.; Frias, J.P.; Landó, L.F.; Brown, K.; Bray, R.; Rodríguez, Á. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): A randomised, open-label, parallel-group, phase 3 trial. Lancet 2021, 398, 583–598. [Google Scholar] [CrossRef] [PubMed]
- Dahl, D.; Onishi, Y.; Norwood, P.; Huh, R.; Bray, R.; Patel, H.; Rodríguez, Á. Effect of Subcutaneous Tirzepatide vs. Placebo Added to Titrated Insulin Glargine on Glycemic Control in Patients With Type 2 Diabetes: The SURPASS-5 Randomized Clinical Trial. JAMA 2022, 327, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Frías, J.P.; Rodbard, H.W.; Tofé, S.; Sears, E.; Huh, R.; Landó, L.F.; Patel, H. Tirzepatide vs. Insulin Lispro Added to Basal Insulin in Type 2 Diabetes: The SURPASS-6 Randomized Clinical Trial. JAMA 2023, 330, 1631. [Google Scholar] [CrossRef] [PubMed]
- Del Prato, S.; E Kahn, S.; Pavo, I.; Weerakkody, G.J.; Yang, Z.; Doupis, J.; Aizenberg, D.; Wynne, A.G.; Riesmeyer, J.S.; Heine, R.J.; et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): A randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet 2021, 398, 1811–1824. [Google Scholar] [CrossRef] [PubMed]
- de Mesquita, Y.L.L.; Calvi, I.P.; Marques, I.R.; Cruz, S.A.; Padrao, E.M.H.; Carvalho, P.E.d.P.; da Silva, C.H.A.; Cardoso, R.; Moura, F.A.; Rafalskiy, V.V. Efficacy and safety of the dual GIP and GLP-1 receptor agonist tirzepatide for weight loss: A meta-analysis of randomized controlled trials. Int. J. Obes. 2023, 47, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Pan, X.-H.; Chew, H.S.J.; Goh, R.S.J.; Lin, C.; Anand, V.V.; Lee, E.C.Z.; Chan, K.E.; Kong, G.; Ong, C.E.Y.; et al. Efficacy and safety of tirzepatide for treatment of overweight or obesity. A systematic review and meta-analysis. Int. J. Obes. 2023, 47, 677–685. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Jastreboff, A.M.; Jastreboff, A.M.; Aronne, L.J.; Aronne, L.J.; Aronne, L.J.; Ahmad, N.N.; Ahmad, N.N.; Ahmad, N.N.; Wharton, S.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef]
- Frias, J.P.; Jastreboff, A.M.; le Roux, C.W.; Sattar, N.; Aizenberg, D.; Mao, H.; Zhang, S.; Ahmad, N.N.; Bunck, M.C.; Benabbad, I.; et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): A double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2023, 402, 613–626. [Google Scholar] [CrossRef]
- Wadden, T.A.; Chao, A.M.; Machineni, S.; Kushner, R.; Ard, J.; Srivastava, G.; Halpern, B.; Zhang, S.; Chen, J.; Bunck, M.C.; et al. Tirzepatide after intensive lifestyle intervention in adults with overweight or obesity: The SURMOUNT-3 phase 3 trial. Nat. Med. 2023, 29, 2909–2918. [Google Scholar] [CrossRef]
- O’brien, P.E.; Hindle, A.; Brennan, L.; Skinner, S.; Burton, P.; Smith, A.; Crosthwaite, G.; Brown, W. Long-Term Outcomes After Bariatric Surgery: A Systematic Review and Meta-analysis of Weight Loss at 10 or More Years for All Bariatric Procedures and a Single-Centre Review of 20-Year Outcomes After Adjustable Gastric Banding. Obes. Surg. 2018, 29, 3–14. [Google Scholar] [CrossRef]
- Buchwald, H.; Avidor, Y.; Braunwald, E.; Jensen, M.D.; Pories, W.; Fahrbach, K.; Schoelles, K. Bariatric Surgery: A systematic review and meta-analysis. JAMA 2004, 292, 1724–1737. [Google Scholar] [CrossRef]
- Sarma, S.; Palcu, P. Weight loss between glucagon-like peptide-1 receptor agonists and bariatric surgery in adults with obesity: A systematic review and meta-analysis. Obesity 2022, 30, 2111–2121. [Google Scholar] [CrossRef]
- Yabe, D.; Kawamori, D.; Seino, Y.; Oura, T.; Takeuchi, M. Change in pharmacodynamic variables following once-weekly tirzepatide treatment versus dulaglutide in Japanese patients with type 2 diabetes (SURPASS J-mono substudy). Diabetes Obes. Metab. 2022, 25, 398–406. [Google Scholar] [CrossRef]
- Lazzaroni, E.; Ben Nasr, M.; Loretelli, C.; Pastore, I.; Plebani, L.; Lunati, M.E.; Vallone, L.; Bolla, A.M.; Rossi, A.; Montefusco, L.; et al. Anti-diabetic drugs and weight loss in patients with type 2 diabetes. Pharmacol. Res. 2021, 171, 105782. [Google Scholar] [CrossRef]
- Hankosky, E.R.; Wang, H.; Neff, L.M.; Kan, H.; Wang, F.; Ahmad, N.N.; Stefanski, A.; Garvey, W.T. Tirzepatide reduces the predicted risk of developing type 2 diabetes in people with obesity or overweight: Post hoc analysis of the SURMOUNT-1 trial. Diabetes, Obes. Metab. 2023, 25, 3748–3756. [Google Scholar] [CrossRef]
- Fujita, S.; Rasmussen, B.B.; Cadenas, J.G.; Grady, J.J.; Volpi, E.; Rundqvist, H.C.; Esbjörnsson, M.; Rooyackers, O.; Österlund, T.; Moberg, M.; et al. Effect of insulin on human skeletal muscle protein synthesis is modulated by insulin-induced changes in muscle blood flow and amino acid availability. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E745–E754. [Google Scholar] [CrossRef]
- Rhoads, R.P.; Baumgard, L.H.; El-Kadi, S.W.; Zhao, L.D. PHYSIOLOGY AND ENDOCRINOLOGY SYMPOSIUM: Roles for insulin-supported skeletal muscle growth1,2. J. Anim. Sci. 2016, 94, 1791–1802. [Google Scholar] [CrossRef]
- Somwar, R.; Sweeney, G.; Ramlal, T.; Klip, A. Stimulation of glucose and amino acid transport and activation of the insulin signaling pathways by insulin lispro in L6 skeletal muscle cells. Clin. Ther. 1998, 20, 125–140. [Google Scholar] [CrossRef]
- Finocchietto, P.; Barreyro, F.; Holod, S.; Peralta, J.; Franco, M.C.; Méndez, C.; Converso, D.P.; Estévez, A.; Carreras, M.C.; Poderoso, J.J. Control of Muscle Mitochondria by Insulin Entails Activation of Akt2-mtNOS Pathway: Implications for the Metabolic Syndrome. PLoS ONE 2008, 3, e1749. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.L.; Chai, J.K.; Wang, X.T.; Han, S.F.; Liu, L.Y. Effects of insulin therapy on skeletal muscle wasting in severely scalded rats and its related mechanism. Zhonghua Shao Shang Za Zhi 2019, 35, 333–340. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Hong, O.; Choi, Y.; Kwon, H.; Jeong, H.; Son, J.; Lee, S.; Kim, S.; Yoon, K.; Yoo, S.J. Long-term insulin treatment leads to a change in myosin heavy chain fiber distribution in OLETF rat skeletal muscle. J. Cell. Biochem. 2019, 120, 2404–2412. [Google Scholar] [CrossRef]
- Poulsen, M.K.; Henriksen, J.E.; Hother-Nielsen, O.; Beck-Nielsen, H. The Combined Effect of Triple Therapy With Rosiglitazone, Metformin, and Insulin Aspart in Type 2 Diabetic Patients. Diabetes Care 2003, 26, 3273–3279. [Google Scholar] [CrossRef][Green Version]
- Osaka, T.; Hamaguchi, M.; Fukui, M. Favorable Appendicular Skeletal Muscle Mass Changes in Older Patients with Type 2 Diabetes Receiving GLP-1 Receptor Agonist and Basal Insulin Co-Therapy. Clin. Med. Insights Endocrinol. Diabetes 2023, 16, 11795514231161885. [Google Scholar] [CrossRef]
- Christoffersen, B.; Sanchez-Delgado, G.; John, L.M.; Ryan, D.H.; Raun, K.; Ravussin, E. Beyond appetite regulation: Targeting energy expenditure, fat oxidation, and lean mass preservation for sustainable weight loss. Obesity 2022, 30, 841–857. [Google Scholar] [CrossRef]
- Yang, X.; Wang, L.; Zhang, L.; Zhai, X.; Sheng, X.; Quan, H.; Lin, H. Exercise mitigates Dapagliflozin-induced skeletal muscle atrophy in STZ-induced diabetic rats. Diabetol. Metab. Syndr. 2023, 15, 171, Erratum in Diabetol. Metab. Syndr.2023, 15, 171. [Google Scholar] [CrossRef]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle–Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef]
- Massimino, E.; Izzo, A.; Riccardi, G.; Della Pepa, G. The Impact of Glucose-Lowering Drugs on Sarcopenia in Type 2 Diabetes: Current Evidence and Underlying Mechanisms. Cells 2021, 10, 1958. [Google Scholar] [CrossRef]
- Zheng, S.; Chen, N.; Kang, X.; Hu, Y.; Shi, S. Irisin alleviates FFA induced β-cell insulin resistance and inflammatory response through activating PI3K/AKT/FOXO1 signaling pathway. Endocrine 2022, M75, 740–751. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Kang, H.; Lin, C.-Y.; Fan, Y. Unlocking the Therapeutic Potential of Irisin: Harnessing Its Function in Degenerative Disorders and Tissue Regeneration. Int. J. Mol. Sci. 2023, 24, 6551. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.T.; Zhang, L.; Dubielecka, P.M.; Qin, G.; Chin, Y.E.; Gower, A.C.; Zhuang, S.; Liu, P.Y.; Zhao, T.C. Irisin deficiency exacerbates diet-induced insulin resistance and cardiac dysfunction in type II diabetes in mice. Am. J. Physiol. Cell Physiol. 2023, 325, C1085–C1096. [Google Scholar] [CrossRef] [PubMed]
- Colpitts, B.H.; Rioux, B.V.; Eadie, A.L.; Brunt, K.R.; Sénéchal, M. Irisin response to acute moderate intensity exercise and high intensity interval training in youth of different obesity statuses: A randomized crossover trial. Physiol. Rep. 2022, 10, e15198. [Google Scholar] [CrossRef]
- Rad, M.M.; Bijeh, N.; Hosseini, S.R.A.; Saeb, A.R. The effect of two concurrent exercise modalities on serum concentrations of FGF21, irisin, follistatin, and myostatin in men with type 2 diabetes mellitus. Arch. Physiol. Biochem. 2020, 129, 424–433. [Google Scholar] [CrossRef]
- Miazgowski, T.; Kaczmarkiewicz, A.; Miazgowski, B.; Kopeć, J. Cardiometabolic health, visceral fat and circulating irisin levels: Results from a real-world weight loss study. J. Endocrinol. Investig. 2021, 44, 1243–1252. [Google Scholar] [CrossRef]
- Nadimi, H.; Djazayery, A.; Javanbakht, M.H.; Dehpour, A.; Ghaedi, E.; Derakhshanian, H.; Mohammadi, H.; Zarei, M.; Djalali, M. The Effect of Vitamin D Supplementation on Serum and Muscle Irisin Levels, and FNDC5 Expression in Diabetic Rats. Rep. Biochem. Mol. Biol. 2019, 8, 236–243. [Google Scholar]
- Safarpour, P.; Daneshi-Maskooni, M.; Vafa, M.; Nourbakhsh, M.; Janani, L.; Maddah, M.; Amiri, F.-S.; Mohammadi, F.; Sadeghi, H. Vitamin D supplementation improves SIRT1, Irisin, and glucose indices in overweight or obese type 2 diabetic patients: A double-blind randomized placebo-controlled clinical trial. BMC Fam. Pract. 2020, 21, 26. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Y.; Huang, J.; Wu, H.; Meng, G.; Zhang, Q.; Liu, L.; Zhang, S.; Wang, X.; Zhang, J.; et al. Serum vitamin D status and circulating irisin levels in older adults with sarcopenia. Front. Nutr. 2022, 9, 1051870. [Google Scholar] [CrossRef]
- Kamenov, Z.; Assyov, Y.; Angelova, P.; Gateva, A.; Tsakova, A. Irisin and Testosterone in Men with Metabolic Syndrome. Horm. Metab. Res. 2017, 49, 755–759. [Google Scholar] [CrossRef]
- Ahmad, I.H.; Mostafa, E.R.M.; Mohammed, S.A.; Shipl, W.; Soliman, A.A.; Said, M. Correlations between serum testosterone and irisin levels in a sample of Egyptian men with metabolic syndrome; (case-control study). Arch. Physiol. Biochem. 2023, 129, 180–185. [Google Scholar] [CrossRef]
- Zügel, M.; Qiu, S.; Laszlo, R.; Bosnyák, E.; Weigt, C.; Müller, D.; Diel, P.; Steinacker, J.M.; Schumann, U. The role of sex, adiposity, and gonadectomy in the regulation of irisin secretion. Endocrine 2016, 54, 101–110. [Google Scholar] [CrossRef]
- Radellini, S.; Guarnotta, V.; Sciabica, V.; Pizzolanti, G.; Giordano, C. Metabolic Profile in a Cohort of Young Sicilian Patients with Klinefelter’s Syndrome: The Role of Irisin. Int. J. Endocrinol. 2022, 2022, 3780741. [Google Scholar] [CrossRef]
- Assyov, Y.; Gateva, A.; Karamfilova, V.; Gatev, T.; Nedeva, I.; Velikova, T.; Kamenov, Z.A. Impact of testosterone treatment on circulating irisin in men with late-onset hypogonadism and metabolic syndrome. Aging Male 2020, 23, 1381–1387. [Google Scholar] [CrossRef]
- Yardimci, A.; Ulker, N.; Bulmus, O.; Sahin, E.; Alver, A.; Gungor, I.H.; Turk, G.; Artas, G.; Tektemur, N.K.; Ozcan, M.; et al. Irisin Improves High-Fat Diet-Induced Sexual Dysfunction in Obese Male Rats. Neuroendocrinology 2022, 112, 1087–1103. [Google Scholar] [CrossRef]
- Mu, Y.; Dai, H.-G.; Luo, L.-B.; Yang, J. Irisin alleviates obesity-related spermatogenesis dysfunction via the regulation of the AMPKα signalling pathway. Reprod. Biol. Endocrinol. 2021, 19, 135. [Google Scholar] [CrossRef]
- Tekin, S.; Beytur, A.; Erden, Y.; Beytur, A.; Cigremis, Y.; Vardi, N.; Turkoz, Y.; Tekedereli, I.; Sandal, S. Effects of intracerebroventricular administration of irisin on the hypothalamus–pituitary–gonadal axis in male rats. J. Cell. Physiol. 2018, 234, 8815–8824. [Google Scholar] [CrossRef]
- Pereira, S.d.C.; Benoit, B.; Junior, F.C.A.d.A.; Chanon, S.; Vieille-Marchiset, A.; Pesenti, S.; Ruzzin, J.; Vidal, H.; Toscano, A.E. Fibroblast growth factor 19 as a countermeasure to muscle and locomotion dysfunctions in experimental cerebral palsy. J. Cachex-Sarcopenia Muscle 2021, 12, 2122–2133. [Google Scholar] [CrossRef]
- Guo, A.; Li, K.; Xiao, Q. Fibroblast growth factor 19 alleviates palmitic acid-induced mitochondrial dysfunction and oxidative stress via the AMPK/PGC-1α pathway in skeletal muscle. Biochem. Biophys. Res. Commun. 2020, 526, 1069–1076. [Google Scholar] [CrossRef]
- Benoit, B.; Meugnier, E.; Castelli, M.; Chanon, S.; Vieille-Marchiset, A.; Durand, C.; Bendridi, N.; Pesenti, S.; Monternier, P.-A.; Durieux, A.-C.; et al. Fibroblast growth factor 19 regulates skeletal muscle mass and ameliorates muscle wasting in mice. Nat. Med. 2017, 23, 990–996. [Google Scholar] [CrossRef]
- Soytas, R.B.; Suzan, V.; Arman, P.; Gedik, T.E.; Unal, D.; Cengiz, M.; Bolayirli, I.M.; Erdincler, D.S.; Doventas, A.; Yavuzer, H. Association of FGF-19 and FGF-21 levels with primary sarcopenia. Geriatr. Gerontol. Int. 2021, 21, 959–962. [Google Scholar] [CrossRef]
- Guo, A.; Li, K.; Tian, H.; Fan, Z.; Chen, Q.; Yang, Y.; Yu, J.; Wu, Y.; Xiao, Q. FGF19 protects skeletal muscle against obesity-induced muscle atrophy, metabolic derangement and abnormal irisin levels via the AMPK/SIRT-1/PGC-α pathway. J. Cell. Mol. Med. 2021, 25, 3585–3600. [Google Scholar] [CrossRef]
- Benoit, B.; Beau, A.; Bres, É.; Chanon, S.; Pinteur, C.; Vieille-Marchiset, A.; Jalabert, A.; Zhang, H.; Garg, P.; Strigini, M.; et al. Treatment with fibroblast growth factor 19 increases skeletal muscle fiber size, ameliorates metabolic perturbations and hepatic inflammation in 5/6 nephrectomized mice. Sci. Rep. 2023, 13, 5520. [Google Scholar] [CrossRef]
- Bailey, C.J.; Flatt, P.R.; Conlon, J.M. An update on peptide-based therapies for type 2 diabetes and obesity. Peptides 2023, 161, 170939. [Google Scholar] [CrossRef]
- Morvan, F.; Rondeau, J.-M.; Zou, C.; Minetti, G.; Scheufler, C.; Scharenberg, M.; Jacobi, C.; Brebbia, P.; Ritter, V.; Toussaint, G.; et al. Blockade of activin type II receptors with a dual anti-ActRIIA/IIB antibody is critical to promote maximal skeletal muscle hypertrophy. Proc. Natl. Acad. Sci. USA 2017, 114, 12448–12453. [Google Scholar] [CrossRef]
- Ghanim, H.; Dhindsa, S.; Batra, M.; Green, K.; Abuaysheh, S.; Kuhadiya, N.D.; Makdissi, A.; Chaudhuri, A.; Dandona, P. Effect of Testosterone on FGF2, MRF4, and Myostatin in Hypogonadotropic Hypogonadism: Relevance to Muscle Growth. J. Clin. Endocrinol. Metab. 2019, 104, 2094–2102. [Google Scholar] [CrossRef]
- Liu, W.; Thomas, S.G.; Asa, S.L.; Gonzalez-Cadavid, N.; Bhasin, S.; Ezzat, S. Myostatin Is a Skeletal Muscle Target of Growth Hormone Anabolic Action. J. Clin. Endocrinol. Metab. 2003, 88, 5490–5496. [Google Scholar] [CrossRef]
- Campbell, C.; McMillan, H.J.; Mah, J.K.; Tarnopolsky, M.; Selby, K.; McClure, T.; Wilson, D.M.; Sherman, M.L.; Escolar, D.; Attie, K.M. Myostatin inhibitor ACE-031 treatment of ambulatory boys with Duchenne muscular dystrophy: Results of a randomized, placebo-controlled clinical trial. Muscle Nerve 2016, 55, 458–464. [Google Scholar] [CrossRef]
- Barrett, D.; Bilic, S.; Chyung, Y.; Cote, S.M.; Iarrobino, R.; Kacena, K.; Kalra, A.; Long, K.; Nomikos, G.; Place, A.; et al. A Randomized Phase 1 Safety, Pharmacokinetic and Pharmacodynamic Study of the Novel Myostatin Inhibitor Apitegromab (SRK-015): A Potential Treatment for Spinal Muscular Atrophy. Adv. Ther. 2021, 38, 3203–3222. [Google Scholar] [CrossRef]
- Becker, C.; Lord, S.R.; A Studenski, S.; Warden, S.J.; A Fielding, R.; Recknor, C.P.; Hochberg, M.C.; Ferrari, S.L.; Blain, H.; Binder, E.F.; et al. Myostatin antibody (LY2495655) in older weak fallers: A proof-of-concept, randomised, phase 2 trial. Lancet Diabetes Endocrinol. 2015, 3, 948–957. [Google Scholar] [CrossRef]
| Type of Intervention | Possible Positive Effects on Skeletal Muscle | Possible Detrimental Effects on Skeletal Muscle | Overall Effect |
|---|---|---|---|
| Protein supplementation | Attenuates myofibrillar catabolism | - | Prevent sarcopenia |
| Vitamin D supplementation | Improve insulin sensitivity | - | Prevent sarcopenia |
| Boost testosterone synthesis | |||
| Boost myokine synthesis (e.g., irisin) | |||
| Diets | Insulin-sensitizing effect | Impairment of testosterone synthesis (low-fat diets, intermittent fasting protocols, vegetable-based diets) | Prevent sarcopenia |
| Improve glucose utilization | |||
| Reduce systemic inflammation | |||
| Prevent muscle steatosis | |||
| Induce weight loss | |||
| (Facilitate adherence and resistance to physical exercise) | |||
| Physical exercise (high-intensity more than low-to-moderate intensity) | Insulin-sensitizing effect | - | Improve muscle mass and strength |
| Improve glucose utilization | |||
| Prevent muscle steatosis | |||
| Induce weight loss | |||
| Boost testosterone synthesis | |||
| Boost myokine synthesis | |||
| Increase myofibrillar synthesis | |||
| Reduce myofibrillar catabolism |
| Classes of Antihyperglycemic Agents | Possible Positive Effects on Skeletal Muscle | Possible Detrimental Effects on Skeletal Muscle | Overall Effect |
|---|---|---|---|
| Biguanides (e.g., metformin) | Insulin-like sensitizing effect | Proteolytic effect (Inhibition of AMPK/mTORc1 pathway) Stimulate myostatin synthesis (AMPK/FoxO3a transcription factor) | Neutral or favors sarcopenia |
| Improve glucose metabolism | |||
| Ameliorate energy utilization | |||
| Anti-inflammatory/antioxidative properties | |||
| Improve satellite cell viability/regenerative effects | |||
| Antiproteolytic effect | |||
| (Inhibition of TGF-β/Smad signaling) | |||
| Secretagogues (e.g., sulfonylureas, glinides) | Unclear | Inhibit ATP-sensitive potassium channels | Favors sarcopenia |
| (Muscle atrophy) | |||
| Enhance caspase-3 activity | |||
| (Apoptosis) | |||
| Thiazolidinediones (e.g., pioglitazone) | Insulin-sensitizing effect | Direct muscle toxicity? (Rhabdomyolysis, rare adverse event) | Neutral |
| Improve glucose utilization | |||
| Prevent muscle steatosis | |||
| Intestinal glucosidase inhibitors (e.g., acarbose) | Unclear | Unclear | Unclear |
| DPPIVis | Potentiate microvascular supply | Unclear | Neutral |
| Insulin-sensitizing effect | |||
| Improve glucose utilization | |||
| Antioxidative/anti-inflammatory effects | |||
| Enhance the synthesis of PGC-1α | |||
| (Mitochondrial biogenesis) | |||
| SGLT2is | Metabolic shift toward fatty acids and ketones | Clinical evidence of fat-free mass loss | Favors sarcopenia. Prevent sarcopenia in heart failure |
| Improve tissue oxygenation | |||
| Antioxidative/anti-inflammatory effects | |||
| Improve cardiac pump efficiency | |||
| Improve exercise tolerance | |||
| Boost myokine secretion | |||
| GLP-1RAs | Improve glucose utilization | Excessive weight loss Reduce appetite (might hamper sufficient caloric and protein intake) | Prevent sarcopenia or improve skeletal muscle |
| Antioxidative/anti-inflammatory effects | |||
| Stimulate hepatic synthesis of IGF1 (myogenesis) | |||
| Boost myokine secretion | |||
| Improve satellite cell viability | |||
| Improve satellite cell viability/regenerative effects | |||
| Promote myofiber repair | |||
| Boost testosterone synthesis | |||
| Insulin analogues | Improve glucose utilization | Long-term, dose-dependent impairment of insulin sensitivity Muscle steatosis Weight gain and hypoglycemia (Facilitate discontinuation of physical exercise and sedentarism) | Unclear |
| Antioxidative/anti-inflammatory effects | |||
| Potentiate microvascular supply | |||
| Direct stimulation of myofibrillar synthesis | |||
| Direct inhibition of myofiber proteolysis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisco, G.; Disoteo, O.E.; De Tullio, A.; De Geronimo, V.; Giagulli, V.A.; Monzani, F.; Jirillo, E.; Cozzi, R.; Guastamacchia, E.; De Pergola, G.; et al. Sarcopenia and Diabetes: A Detrimental Liaison of Advancing Age. Nutrients 2024, 16, 63. https://doi.org/10.3390/nu16010063
Lisco G, Disoteo OE, De Tullio A, De Geronimo V, Giagulli VA, Monzani F, Jirillo E, Cozzi R, Guastamacchia E, De Pergola G, et al. Sarcopenia and Diabetes: A Detrimental Liaison of Advancing Age. Nutrients. 2024; 16(1):63. https://doi.org/10.3390/nu16010063
Chicago/Turabian StyleLisco, Giuseppe, Olga Eugenia Disoteo, Anna De Tullio, Vincenzo De Geronimo, Vito Angelo Giagulli, Fabio Monzani, Emilio Jirillo, Renato Cozzi, Edoardo Guastamacchia, Giovanni De Pergola, and et al. 2024. "Sarcopenia and Diabetes: A Detrimental Liaison of Advancing Age" Nutrients 16, no. 1: 63. https://doi.org/10.3390/nu16010063
APA StyleLisco, G., Disoteo, O. E., De Tullio, A., De Geronimo, V., Giagulli, V. A., Monzani, F., Jirillo, E., Cozzi, R., Guastamacchia, E., De Pergola, G., & Triggiani, V. (2024). Sarcopenia and Diabetes: A Detrimental Liaison of Advancing Age. Nutrients, 16(1), 63. https://doi.org/10.3390/nu16010063









