1. Introduction
Esophageal cancer presents high mortality rates (about 500,000 cancer deaths each year), ranking sixth among all cancer-related deaths [
1]. As reported in a recent review [
2], the worldwide five-year overall survival rate ranges from 15% to 25% [
3]. The esophageal cancer incidence increases with age, is more prevalent in men, and is associated with risk factors such as smoking habit, obesity, and alcoholism [
4,
5].
The main treatment for esophageal cancer is still esophagectomy (EPG), with about one in five patients requiring surgical resection. EPG is a major surgical intervention involving total or partial esophageal resection and restoration of gastrointestinal continuity [
4]. EPG is associated with high rates of morbidity and mortality [
4,
6] and frequently presents postoperative complications such as anastomosis dehiscence, chylothorax, or even pneumonia [
4,
6,
7]. In addition to these surgical complications, trauma from major surgeries like EPG activates the cytokine cascade, resulting in an alteration of the immune system and the development of a systemic inflammatory response. Different studies demonstrated an association between postoperative complications or mortality and postoperative inflammatory markers such as C-reactive protein (CRP), interleukin-6 (IL-6), or tumor necrosis factor-α (TNF-α) [
8]. So, it is reasonable to hypothesize that postoperative complications could be minimized if inflammatory markers are reduced through anti-inflammatory mechanisms.
There are, however, controversial results in the literature, as some enteral pharmaconutrients showed effectiveness in the reduction of postoperative complications [
6], and early enteral nutrition (EN) can be beneficial in improving immunocompetence, reducing infection rates, and maintaining intestinal functionality. Omega-3 fatty acids (ω-3FA) are one of the pharmaconutrients with beneficial effects on postoperative morbidity [
9,
10]. ω-3FA are composed of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which are essential polyunsaturated fatty acids (PUFAs) derived from fish oil, and their administration can reduce proinflammatory cytokine production and regulate eicosanoid synthesis derived from arachidonic acid (AA), which are mediators of inflammation [
8,
9,
10]. Also, DHA and EPA are associated with reduced proinflammatory transcription factor NF-κB activation. Furthermore, it is known that these PUFAs lead to the production of resolvins E and D, that have anti-inflammatory properties as they inhibit the migration of neutrophils and their infiltration into areas of inflammation and inhibit interleukin-1β (IL-1β) production [
8].
One previous study evaluating the perioperative parenteral administration of ω-3FA in major abdominal surgery showed beneficial effects on patient outcomes [
11]. Also, a previous review of 16 clinical trials assessing the effect of enteral immunonutrition with ω-3FA in gastrointestinal surgery patients demonstrated their benefits in reducing infectious postoperative complications and length of stay, particularly among individuals with preoperative malnutrition [
12]. However, recent reviews evaluating the effect on the postoperative inflammatory response after perioperative ω-3FA administration (mainly enteral) in patients undergoing gastrointestinal surgery or EPG could not demonstrate any significant effect, although reduced inflammatory markers were observed [
2,
8]. In addition, focusing on patients who underwent EPG, they could not demonstrate any significant reduction in infectious complications or anastomotic leakage; however, it did not increase in-hospital mortality [
2].
Studies on parenterally administered ω-3FA in esophagectomized patients are scarcely reported in the literature. A recent meta-analysis assessed the effect of ω-3FA-supplemented parenteral nutrition on inflammatory and immune function in postoperative patients with gastrointestinal malignancy. The analysis showed that early intervention with ω-3FA (maximum dose received was 0.2 g/kg/day) reduces the inflammatory reaction and improves the postoperative curative effect and the immune suppression induced by conventional parenteral nutrition (PN) or tumor [
13].
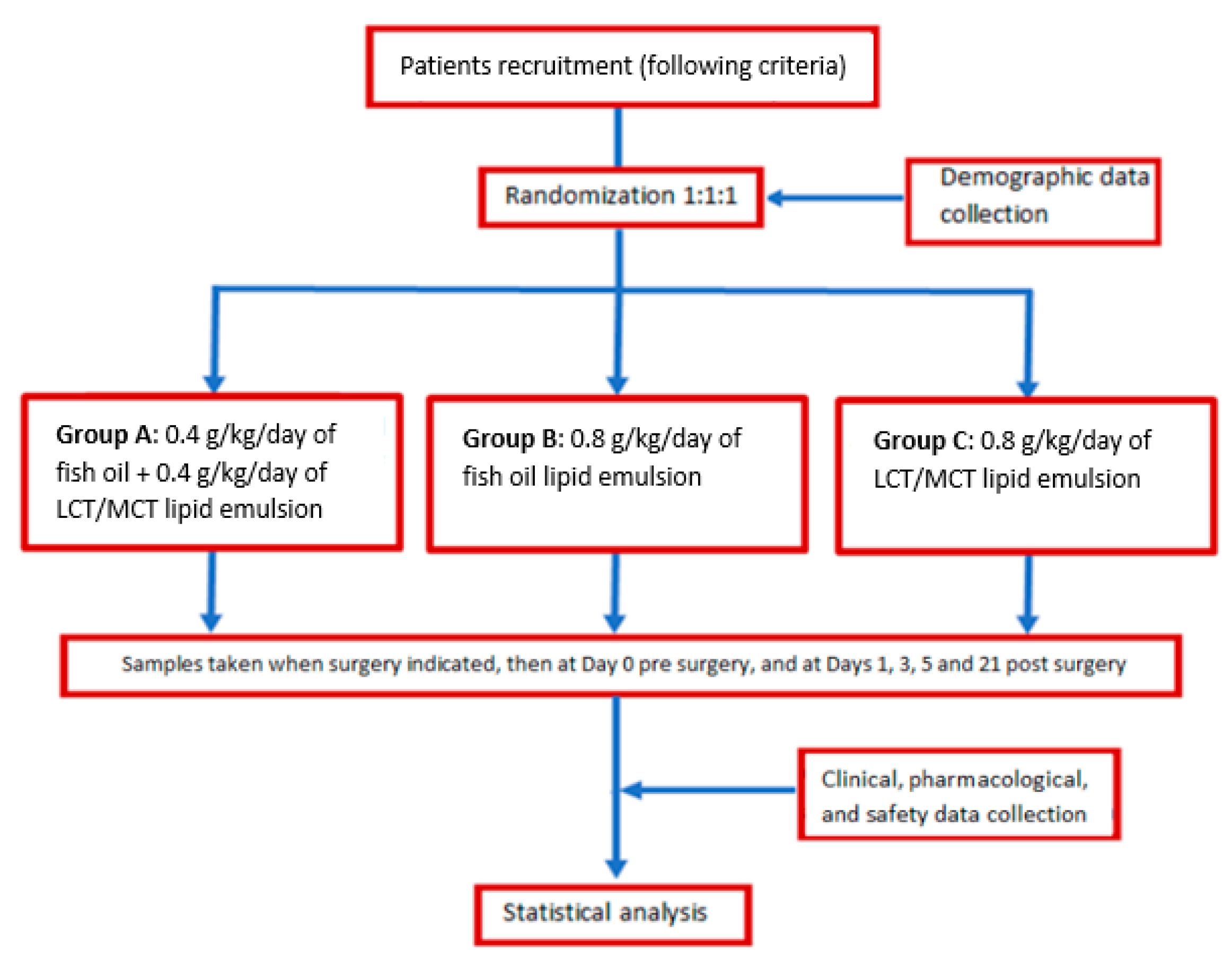
This study proposes to investigate the effect of combining fish oil lipid emulsions (LE) administered parenterally with EN support within the framework of a randomized clinical trial. The main objective of this study is to establish whether the intravenous administration of ω-3FA fish oil LE for five days in esophagectomized patients is effective in reducing inflammation, measured as the serum concentration of IL-6, and to determine if 0.8 g/kg/day doses are more effective than 0.4 g/kg/day doses in the normalization of IL-6. Secondary objectives are to determine any differences regarding other inflammatory, hepatic, nutritional, and safety parameters, as well as differences in postoperative complications and mortality during hospitalization.
2. Materials & Methods
This work contains the results of a prospective, single-center, randomized, double-blind study in patients diagnosed with esophageal cancer who underwent EPG by the Ivor-Lewis or McKeown techniques (EudraCT number 2016-004978-18; REEC protocol code FAR-NP-2017-01) [
14]. Subsequently, patients received an LE via continuous infusion of ω-3FA or a 50% mixture of ω-6 long-chain triglycerides (LCT)/short-chain triglycerides (MCT), along with standard EN support for five days post-surgery [
14] (Impact Neutre® 500 mL containing ω-3, ω-6, and arginine, excluding glutamine). As per usual clinical practice in our hospital, every patient with an indication for esophagectomy will be visited by a nutritionist who will make a first nutritional assessment and follow up on the tolerance of this enteral support. Impact Neutre is initiated at 21 mL/h and titrated to 1500 mL daily for 7 days. Impact Neutre has a caloric input of 144 kcal/100 mL.
Patients who meet the following inclusion criteria were enrolled: 18 years old or older, any gender and any race; those able to give their written informed consent (IC) for the study and having access to the digestive tract. The exclusion criteria were the following [
14]: hypersensitivity type 1 or idiosyncratic reactions to any of the LE components; pregnant or breastfeeding women; plasmatic triglyceride (TRG) concentration > 3 mmol/L; chronic treatment with corticosteroids or immunosuppressants in the last month; HIV diagnosis; transplanted; hepatic impairment classified as Child-Pugh grade B or C. Every patient could voluntarily refuse to continue in the study and the following situations were also considered treatment withdrawal criteria: presentation of any adverse effect related to the treatment; any patient who presents a Clavien-Dindo grade IV complication (i.e., life-threatening complication requiring intensive care unit (ICU) management).
The patients recruited were randomized 1:1:1 into three groups:
Group A received an LE mixture of 0.4 g/kg/day of fish oil and 0.4 g/kg/day of LCT/MCT 50:50,
Group B received 0.8 g/kg/day of fish oil LE and,
Group C received an LE mixture of 0.8 g/kg/day of LCT/MCT 50:50.
This allocation was blind for all patients and health personnel. The allocation sequence (computer-generated random numbers) was generated by the pharmaceutical researcher, and it was be performed by randomized blocks permutated with the block size defined at random. In the first step, blocks of size 3 with possible sequences ABC, ACB, CAB, CBA, and blocks of size 6 were defined. In a second step, the clusters were randomized, and in a third step, the sequences were randomized within the blocks. This method ensures that the groups are balanced throughout the study and that the researcher cannot predict the sequence. The pharmaceutical researcher was responsible for patient assignment to the corresponding intervention group and also assigned a numbered patient identification code for each patient in the trial. The composition of the emulsion administered to each patient was known by the pharmaceutical researcher responsible for clinical trials, the pharmaceutical researcher who validated its conduct, and the personnel who conducted it.
Each subject was followed up for a year post-surgical intervention.
Figure 1 illustrates the diagram flowchart of this study [
14].
- ✓
Serum concentrations of inflammatory parameters: IL-6, CRP, TNF-α, interleukin-10 (IL-10), interleukin-8 (IL-8) and soluble interleukin-2 receptor (sIL-2R/CD25s).
- ✓
Postoperative complications: measured as suture dehiscence, chylothorax, pneumonia, and other respiratory tract infections.
- ✓
Safety: measured as hepatic impairment, TRG, and alterations in coagulation parameters.
- ✓
Nutritional parameters: albumin, prealbumin, and lymphocytes.
- ✓
Mortality during hospitalization.
Variables were recorded pre-operatively at the time of recruitment and at days 0, +1, +3, +5 after randomization. Variables at day 0 inform about the basal inflammatory status of patients prior to esophagectomy and parenteral administration of LE.
The demographics and clinical and analytical data generated were collected and recorded in the Data Collection Logbook following good clinical practices.
Cytokine values were determined by an Enzyme-Linked Immunosorbent Assay (ELISA). Samples were centrifuged at 700× g one hour post-extraction and were then aliquoted and stored frozen at −80 °C until analysis.
Relative to statistical analyses, descriptive statistics were employed for all variable data, such as baseline values for inflammatory, nutritional, hepatic, and safety parameters, using frequency tables. For continuous variables, statistical descriptions were used (n, average, standard deviation, value range, and median), while grouped percentages were used for categorical variables.
The statistical analysis was carried out according to the following protocol and intention-to-treat, which included patients who required ICU care.
For the univariate approach, analyses of variance (ANOVA) using the Scheffe post hoc multiple-comparison test were performed to determine differences in the variables studied at randomization time and on days 0, 1, 3, and 5 post-intervention. For categorical variables, the chi-square test was used when necessary.
For the multivariate approach, a general linear model of repeated measures was used: MANCOVA models were performed for the different dependent variables (inflammatory and safety variables) with temporal measurements at days 0, 1, 3, and 5, and were adjusted for the different LEs administered. To establish temporal differences between dependent variables, the F statistic was used (the assumed sphericity when variances between different levels were equal and the Greenhouse-Geisser F statistic when variances were unequal). The Bonferroni test was used to compare temporal effects within subjects. Significance was established at p < 0.05. For all MANCOVA models, inter-subject contrast tests were performed to study the association of variable values measured at different times and with respect to the group variable (following the protocol). The coefficient B of determination was established at a significance level of p < 0.05. When no significance is observed between the groups, the variables studied will be transformed into natural (Napierian) logarithms for all times (at days 0, +1, +3, and +5).
Data were processed with SPPS v 22.
This clinical trial and its posterior analysis were carried out following good clinical practices and with the approval of the ethics committee of the University Hospital of Bellvitge.
3. Results
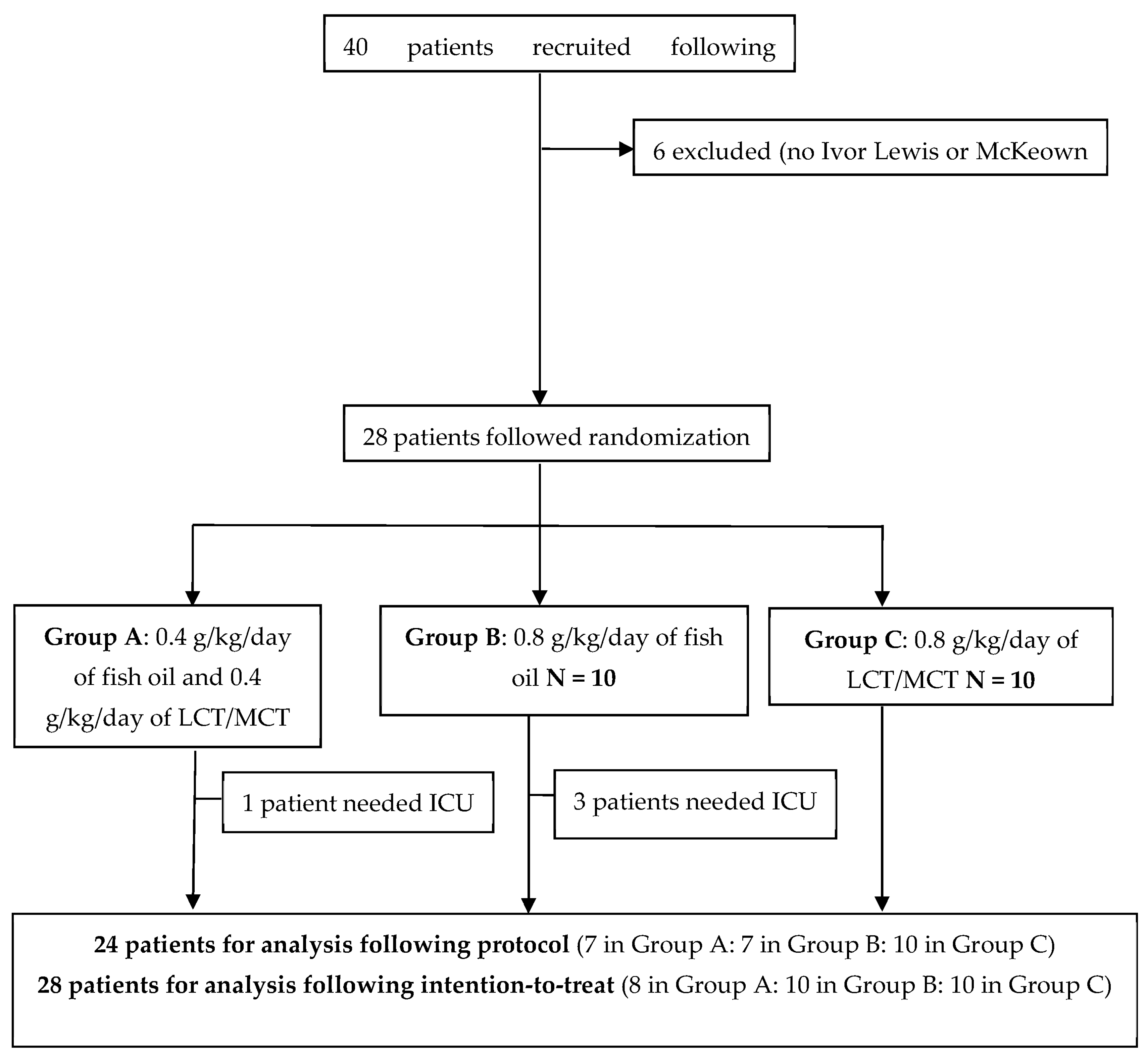
During the recruitment period, forty patients fulfilling the inclusion criteria were enrolled. Six patients were excluded because they did not undergo Ivor Lewis or McKeown surgery (exclusion criteria). In addition, three patients were lost after recruitment due to the SARS-CoV-2 pandemic and did not follow randomization, and another three were removed due to withdrawal of the lipid infusion following medical criteria.
Thus, after inclusion, 28 patients were randomized 1:1:1 into three groups. Four patients needed assistance in the ICU. Finally, 24 patients were analyzed following the protocol. See these results in
Figure 2.
Data collected at baseline did not present any significant differences between groups (
Table 1). Related to the characteristics and staging of the tumor, 22 patients presented adenocarcinoma and six presented squamous carcinoma. None of them had metastasis diagnosed when surgery was indicated. Only one patient was staged based on tumor size as T1, five were stratified as T2, fourteen as T3, and eight as T4.
In the univariate analysis, no significant differences were observed for any variable analyzed taking into account the type of lipid administered and the different times studied. The results for the main variables are depicted in
Table 2.
We performed a multivariant analysis (MANCOVA). As shown in
Table 3, only IL6, CRP, and TRG showed significant differences in global temporal evolution. Maximum values were reached on day +1 for IL-6, day +3 for CRP, and day +5 for TRG. The results for IL-6, CRP, and TRG can be seen in
Table 3. No differences were found in the temporal evolution of any variable when studying each group depending on the type of LE administered.
To compare differences in inflammatory parameters according to group at different times (0, +1, +3, and +5 days), an inter-subject contrast test based on coefficient B was performed. Results are shown in
Table 4. Significance could be observed only in TRG variation on day +5 and a tendency on day +3 in favor of the group receiving 0.8 g/kg/day of ω-3.
In the intention-to-treat multivariant analysis (MANCOVA), the inclusion of critically ill patients showed statistically significant differences in the global evolution of inflammatory parameters, regardless of the lipids administered. Statistically significant differences were found in the temporal evolution of CRP values when critical patients were studied. CRP presents the highest value at day +3.
Concerning the other inflammatory parameters evaluated (TNF-α, IL-10, IL-8, and sl2R/CD25s), no statistically significant differences were observed, neither in global evolution nor according to groups.
MANCOVA analysis for the variables IL6 and CRP transformed into natural logarithms was repeated. The statistical difference in global temporal evolution is maintained, with no significant differences being observed between the groups studied.
Regarding safety, measured by liver test function, TRGs, and alterations in coagulation and nutritional parameters, groups receiving ω-3 LE demonstrated it to be safe and to achieve nutrition goals. In fact, TRG levels from LE administration were reduced quicker in groups receiving 0.8 and 0.4 g/kg/day of ω-3, and especially lower levels were observed in the group receiving 0.8 g/kg/day of ω-3 at days 3 and 5 post-intervention, as shown in
Table 4.
Complications were analyzed immediately post-surgery and during one year of follow-up, with the findings presented in
Table 5. According to the group, no significant difference was observed in mechanical complications derived from surgery, like leakage or fistula and chylothorax, even when they presented mainly in the control group. No significant difference was observed in the incidence of respiratory tract infections between groups. Only two patients presented surgery site infections (SSI) (8.3%), which were resolved after short-term antibiotic therapy. The number of patients without complications was similar between groups: five patients in the group receiving 0.4 g/kg/day of ω-3 LE; six in the group receiving 0.8 g/kg/day of ω-3 LE; and seven in the control group, with no statistical differences between them (
p = 0.091). No patient died during their hospitalization.
4. Discussion
EPG is an aggressive surgery that involves a rapid initial elevation of inflammatory parameters that quickly return to normal, as seen in this study. The administration of an LE with an immunomodulatory effect in the immediate postoperative period did not modulate this earlier inflammatory activity. Thus, we found statistically significant differences in the overall temporal evolution of IL-6 and CRP that were not found when studying the type of LE administered. This situation is confirmed even when critically ill patients are included. The administration of ω-3 is not only safe at all-time points administered but, in such a short period, it demonstrates its ability to improve hypertriglyceridemia depending on the administered dose, showing a greater improvement with the highest ω-3 dose.
It is important to highlight that, in this study, ω-3 was administered intravenously, affording some advantages compared with enteral administration. First of all, due to its iso-osmolarity, intravenous LE administration does not require a central line, thereby avoiding any extra-invasive treatment for the patient; in fact, LEs may have protective properties for the vascular wall. Moreover, in the patients considered for the study, the LE could be administered simultaneously with EN, so gastrointestinal tract stimulation was not interrupted and the caloric goal was achieved. Also, the use of only one parenteral pharmaconutrient (ω-3FA) and at a higher dose than in previous studies facilitates the caloric goal, achieves faster incorporation of ω-3FA into the cellular membranes and, consequently, into the inflammatory cascade [
22,
23], and enables the study of the immunomodulatory effect of one nutrient without any interference from other nutrients. When immunonutrition is administered enterally, the commercialized products available are combinations of different immunonutrients
Regarding inflammatory parameters, IL6 and CRP presented early high levels but returned to levels similar to baseline at day +5. Similar results were shown in a recent meta-analysis [
13] that evaluated the evolution of inflammatory and immune function in postoperative patients with gastrointestinal malignancy after administration of enriched PN with ω3 LE. They also demonstrated that CRP levels show a rapid change in acute trauma and could reflect a change in the inflammatory response. Patients presented increased CRP levels within the first 4–12 h post-surgery, reaching the peak at 24–72 h, and returned to baseline on day +14. There were significant differences between groups on day +6, in favor of the ω3 group that presented lower levels than the control group, concluding that PUFAS can reduce the post-surgical inflammatory response in patients with gastrointestinal tumors. However, it is important to highlight that one of the limitations reported by the authors of this meta-analysis was that all studies included were carried out in Chinese populations and were also seeking a nutritional goal, so these results might not be extrapolatable to all populations.
Previous studies evaluating the effect of PN enriched with ω3 LE on the immune response of patients with gastric or colorectal tumors found significant differences between groups in reducing the inflammatory response, measured by parameters like IL-6, CRP, and TNF-α [
18,
21]. In our previous study [
24], we also observed that for longer periods of PN, inflammation in the presence of plasmatic phytosterols (PS) exhibits a synergistic effect on impairing hepatic function, mainly altering GGT but also ALT.
In this study, no significant differences were observed between groups for the other inflammatory markers: IL8, IL10, TNF-α, and CD25s. In a previous study that evaluated the effect of PN enriched with ω3 LE on reducing the levels of CD25s and procalcitonin (PCT) and improving cellular immunity, they demonstrated significant differences in PCT and cellular immunity; however, there were no differences between groups for CD25s [
20]. Similar results were obtained in another study that assessed the influence of PN enriched with glutamine and/or ω3 on the inflammatory response of colorectal cancer patients measured as IL8 and cellular immunity. Cancer-induced alterations in cellular immunity could be corrected in the ω3-supplemented group, but there were no significant differences between groups in IL8 levels [
19]. Furthermore, a recent meta-analysis [
25] evaluated the influence of ω3 on humoral and cellular immunity and nutritional status in colorectal cancer patients. It demonstrated the effectiveness of ω3 administered postoperatively as enriched PN or EN in improving humoral and cellular immunity. They concluded that ω3 PUFAS can be a potential immunonutrition therapy in the post-operative care of patients with colorectal cancer. These studies suggest the need for further research to evaluate this anti-inflammatory effect. Taking this into account along with our study, IL8 and CD25s might not be suitable markers to evaluate inflammation in critical patients, which might be better evaluated using cellular immunity.
Concerning morbimortality, there were more respiratory tract infections in the groups receiving 0.4 than in the control group and in the 0.8 g/kg/day of the ω-3 group (n = 2 vs. n = 1 and n = 1, respectively). Therefore, we could not demonstrate that ω3 LE could prevent infections as suggested in the hypothesis; however, these infections were not complicated and were easily resolved. A recent review
2 analyzed the administration of ω3 as enriched EN in the perioperative period, demonstrating that enriched EN could reduce overall infectious complications. However, they also recognized that respiratory tract infections after EPG, which is one of the commonest complications, can be caused by other factors independent of the immunonutrition administered, e.g., surgical trauma, postoperative immunosuppression, or sputum accumulation. Even so, there were no significant differences in the incidence of pulmonary infection between the enteral immunonutrition group (EIN) and the EN group (Relative Risk = 0.96, Confidence Interval: 0.73–1.27,
p = 0.79). They also suggested that it could be related to the surgical technique, it is well known that the pain of the surgical incision in EPG inhibits the patient’s voluntary cough, and impaired expectoration could have an impact on pulmonary infection. Related to SSI, only two patients presented this complication, which was resolved with antibiotic therapy. Every patient undergoing esophagectomy in our hospital receives a single dose of metronidazole 1 g intravenously and two single doses of cefuroxime 1.5 g intravenously. The incidence of SSI in this study was similar to that of a recent review [
26], where patients who received a single dose of first-generation cephalosporins and nitroimidazole had in a 9.7% SSI rate. They concluded that prophylaxis with antibiotics can reduce SSI in upper gastrointestinal surgery, like esophagectomy, and also that a single-dose regimen is non-inferior to multiple doses, which is preferred to reduce microbiologic resistance.
Finally, the intravenous administration of ω3 LE for five days after surgery was safe, as well as being associated with lower TRG levels compared with the control group, especially in the 0.8 g/kg/day group. This suggests that administration of ω3 LE for a short period can control lipid metabolism and also does not have an impact on liver test functions (GGT, FA, ALT, and AST). Safety is warranted as ω-3 LE administration has demonstrated no liver damage compared with LCT LE, as ω-3 LEs do not contain phytosterols, which are involved in hepatotoxicity.
One limitation of this study is the small number of patients included; however, they are uniformly distributed between groups, and the results obtained remain consistent across the different statistical tests carried out. Further studies with a greater number of patients could provide more results; they could also analyze the influence of tumor staging on the inflammatory response and the influence of cellular immunity as a possible marker of inflammation.
5. Conclusions
In this study, we found that ω-3 FA has no impact on the control of the early inflammatory postoperative response assessed over a short period, so it cannot be ruled out that the administration of fish oil LE could improve this inflammatory response in complex situations, like those characterized by major metabolic stress and prolonged in time. Administration of fish oil proved to be safe and, even for a short period, could normalize TRG levels, proving to be dose-dependent and able to control lipid metabolism. Further studies and longer administration periods are needed to evaluate the utility of fish oil as a pharmaconutrient in controlling inflammatory processes and the influence of nutritional assessment and enteral immunonutrition on this response. Moreover, further studies are needed to evaluate the inflammatory response measured by cellular immunity.
Author Contributions
A.S.-L.G., J.M.L.T. and E.L.B. equally contributed to the conception and design of the research. A.S.-L.G., J.M.L.T., E.L.B. and M.B.B.T. contributed equally to the writing of the manuscript. L.F.T., M.M.M., J.B.M., S.N.V., G.C.C., N.V.C. and M.F.Á. contributed equally to the final review of this article. All authors critically revised the manuscript, agreed to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript. Conceptualization, A.S.-L.G., J.M.L.T., E.L.B. and M.B.B.T.; Data curation, A.S.-L.G., J.M.L.T., E.L.B. and M.B.B.T.; Formal analysis, A.S.-L.G., J.M.L.T., E.L.B. and M.B.B.T.; Investigation, A.S.-L.G., J.M.L.T., E.L.B. and M.B.B.T.; Methodology, A.S.-L.G., J.M.L.T., E.L.B. and M.B.B.T.; Project administration, A.S.-L.G., J.M.L.T., E.L.B. and M.B.B.T.; Resources, L.F.T., M.M.M., J.B.M., S.N.V., G.C.C., N.V.C. and M.F.Á.; Supervision, M.B.B.T.; Validation, L.F.T., M.M.M., J.B.M., S.N.V., G.C.C., N.V.C. and M.F.Á.; Writing—original draft, A.S.-L.G., J.M.L.T., E.L.B. and M.B.B.T.; Writing—review and editing, A.S.-L.G., J.M.L.T., E.L.B., L.F.T., M.M.M., J.B.M., S.N.V., G.C.C., N.V.C., M.F.Á. and M.B.B.T. All authors have read and agreed to the published version of the manuscript.
Funding
There are no institutions or grants that support this study.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Ethics Committee for investigation with medicinal products of Bellvitge University Hospital (protocol code AC012/17, approval date: 8 June 2017).
Informed Consent Statement
All the patients that participated in the study have given their written informed consent.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy.
Acknowledgments
We want to thank all the health personnel involved in the care of these patients and the development of this study. We thank Helena Kruyer for editorial support and English revision in the preparation of this manuscript. We thank the CERCA Programme/Generalitat de Catalunya for institutional support.
Conflicts of Interest
There are no potential conflicts of interests by any of the authors.
Correction Statement
This article has been republished with a minor correction to the readability of tables 3 and 4.
This change does not affect the scientific content of the article.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, T.-T.; Yang, Z.-L.; Tan, Z.-M.; Yang, C.-F.; Wang, Z. The effect of perioperative immunonutrition on patients undergoing esophagectomy: A systematic review and updated meta-analysis. Nutr. Hosp. 2023, 40, 839–847. [Google Scholar] [PubMed]
- Pennathur, A.; Gibson, M.K.; Jobe, B.A.; Luketich, J.D. Oesophageal carcinoma. Lancet 2013, 381, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Park, D.P.; Welch, C.A.; Harrison, D.A.; Palser, T.R.; Cromwell, D.A.; Gao, F.; Alderson, D.; Rowan, K.M.; Perkins, G.D. Outcomes following oesophagectomy in patients with oesophageal cancer: A secondary analysis of the ICNARC Case Mix Programme Database. Crit. Care 2009, 13 (Suppl. S2), S1. [Google Scholar] [CrossRef] [PubMed]
- Uhlenhopp, D.J.; Then, E.O.; Sunkara, T.; Gaduputi, V. Epidemiology of esophageal cancer: Update in global trends, etiology and risk factors. Clin. J. Gastroenterol. 2020, 13, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Iwahashi, M.; Takifuji, K.; Nakamori, M.; Naka, T.; Ishida, K.; Ojima, T.; Iida, T.; Katsuda, M.; Hayata, K.; et al. Optimal dose of preoperative enteral immunonutrition for patients with esophageal cancer. Surg. Today 2009, 39, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.A.; Docherty, N.G.; Eckhardt, H.G.; Doyle, S.L.; Guinan, E.M.; Ravi, N.; Reynolds, J.V.; le Roux, C.W. Weight loss, satiety, and the postprandial gut hormone response after esophagectomy: A prospective study. Ann. Surg. 2017, 266, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, G.; Stroemer, A.; Mayr, A.; Kunsorg, A.; Stoppe, C.; Wittmann, M.; Velten, M. Effects of Omega-3 Fatty Acids on Postoperative Inflammatory Response: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 3414. [Google Scholar] [CrossRef]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef]
- Xu, J.; Yunshi, Z.; Li, R. Immunonutrition in surgical patients. Curr. Drug Targets 2009, 10, 771–777. [Google Scholar] [CrossRef]
- Tsekos, E.; Reuter, C.; Stehle, P.; Boeden, G. Perioperative administration of parenteral fish oil supplements in a routine clinical setting improves patient outcome after major abdominal surgery. Clin. Nutr. Edinb. Scotl. 2004, 23, 325–330. [Google Scholar] [CrossRef]
- Akbarshahi, H.; Andersson, B.; Nordén, M.; Andersson, R. Perioperative nutrition in elective gastrointestinal surgery–potential for improvement? Dig. Surg. 2008, 25, 165–174. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C. Effect of ω-3 polyunsaturated fatty acid-supplemented parenteral nutrition on inflammatory and immune function in postoperative patients with gastrointestinal malignancy A meta-analysis of randomized control trials in China. Medicine 2018, 97, 16. [Google Scholar] [CrossRef]
- Suárez-Lledó, A.; Leiva-Badosa, E.; Llop-Talaveron, J.M.; Fernández-Alvarez, M.; Farran-Teixidor, L.; Miró-Martín, M.; Virgili-Casas, N.; Creus-Costas, G.; Bas-Minguet, J.; Poyatos-Canton, E.; et al. Clinical, randomized, double blind clinical trial to study the effect of parenteral supplementation with fish oil emulsion in the nutritional support in esophagectomized patients. Medicine 2021, 100, e26426. [Google Scholar] [CrossRef]
- Furukawa, K.; Yamamori, H.; Takagi, K.; Hayashi, N.; Suzuki, R.; Nakajima, N.; Tashiro, T. Influences of soybean oil emulsion on stress response and cell-mediated immune function in moderately or severely stressed patients. Nutrition 2002, 18, 235–240. [Google Scholar] [CrossRef]
- Baena-Gómez, M.A.; de la Torre-Aguilar, M.J.; Aguilera-García, C.M.; Olza, J.; Pérez-Navero, J.L.; Gil-Campos, M. Inflammatory response using different lipid parenteral nutrition formulas in children after hematopoietic stem cell transplantation. Nutr. Cancer 2016, 68, 804–810. [Google Scholar] [CrossRef]
- Betiati Dda, S.; de Oliveira, P.F.; Camargo, C.D.Q.; Nunes, E.A.; Trinda, D.E.B. Effects of omega-3 fatty acids on regulatory T cells in hematologic neoplasm. Rev. Bras. Hematol. Hemoter. 2013, 35, 119–125. [Google Scholar] [CrossRef][Green Version]
- Wei, Z.; Wang, W.; Chen, J.; Yang, D.; Yan, R.; Cai, Q. A prospective, randomized, controlled study of omega-3 fish oil fat emulsion-based parenteral nutrition for patients following surgical resection of gastric tumor. Nutr. J. 2014, 13, 25. [Google Scholar] [CrossRef]
- Aliyazicioglu, T.; Cantürk, N.Z.; Simsek, T.; Kolayli, F.; Çekmen, M. Effects of standard and/or glutamine dipeptide and/or omega-3 fatty acid-supplemented parenteral nutrition on neutrophil functions, interleukin-8 level and length of stay- A double blind, controlled, randomized study. East. Afr. Med. J. 2013, 90, 59–66. [Google Scholar]
- Long, H.; Yang, H.; Lin, Y.; Situ, D.; Liu, W. Fish oil- supplemented parenteral nutrition in patients following esophageal cancer surgery: Effect on inflammation and immune function. Nutr. Cancer 2013, 65, 71–75. [Google Scholar] [CrossRef]
- Ma, C.J.; Wu, J.M.; Tsai, H.L.; Huang, C.W.; Lu, C.Y.; Sun, L.C.; Shih, Y.L.; Chen, C.W.; Chuang, J.F.; Wu, M.H.; et al. Prospective double-blind randomized study on the efficacy of an n-3 fatty acid enriched intravenous fat emulsion in postsurgical gastric and colorectal cancer patients. Nutr. J. 2015, 21, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Hagi, A.; Nakayama, M.; Shinzaki, W.; Haji, S.; Ohyanagi, H. Effects of the omega-6: Omega-3 fatty acid ratio of fat emulsions on the fatty acid composition in cell membranes and the anti-inflammatory action. J. Parenter. Enter. Nutr. 2010, 34, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Waitzberg, D.L.; Torrinhas, R.S.; Jacintho, T.M. New parenteral lipid emulsions for clinical use. J. Parenter. Enter. Nutr. 2006, 30, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Llop Talaveron, J.M.; Suárez-Lledó Grande, A.; Leiva Badosa, E.; Bas Minguet, J.; Climent Martí, J.; Poyatos Cantón, E.; Badia Tahull, M.B. Inflammatory processes involved in the alteration of liver function biomarkers in adult hospitalized patients treated with parenteral nutrition. Front. Nutr. 2023, 10, 1034481. [Google Scholar] [CrossRef]
- Yue, T.; Xiong, K.; Deng, J.; Hu, W.; Tan, T.; Li, S.; Yang, T.; Xiao, T. Meta-analysis of omega-3 polyunsaturated fatty acids on immune functions and nutritional status of patients with colorectal cancer. Front. Nutr. 2022, 9, 945590. [Google Scholar] [CrossRef]
- Marano, L.; Carbone, L.; Poto, G.E.; Calomino, N.; Neri, A.; Piagnerelli, R.; Fontani, A.; Verre, L.; Savelli, V.; Roviello, F.; et al. Antimicrobial Prophylaxis Reduces the Rate of Surgical Site Infection in Upper Gastrointestinal Surgery: A Systematic Review. Antibiotics 2022, 11, 230. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).