Potential Defence Mechanisms Triggered by Monosodium Glutamate Sub-Chronic Consumption in Two-Year-Old Wistar Rats
Abstract
1. Introduction
Positive and Negative Aspects of the Biological Behaviour of MSG
2. Materials and Methods
2.1. Approvals
2.2. The Used Chemical Nutrient
2.3. Experimental Design
2.3.1. Animals and the Experimental Conditions
2.3.2. Self-Administration of MSG in Rats
2.3.3. The Doses Used in the Animal Model Experiment
2.3.4. Measurements during the Study
2.3.5. Biological Tests
2.4. Statistical Analysis of the Results
3. Results
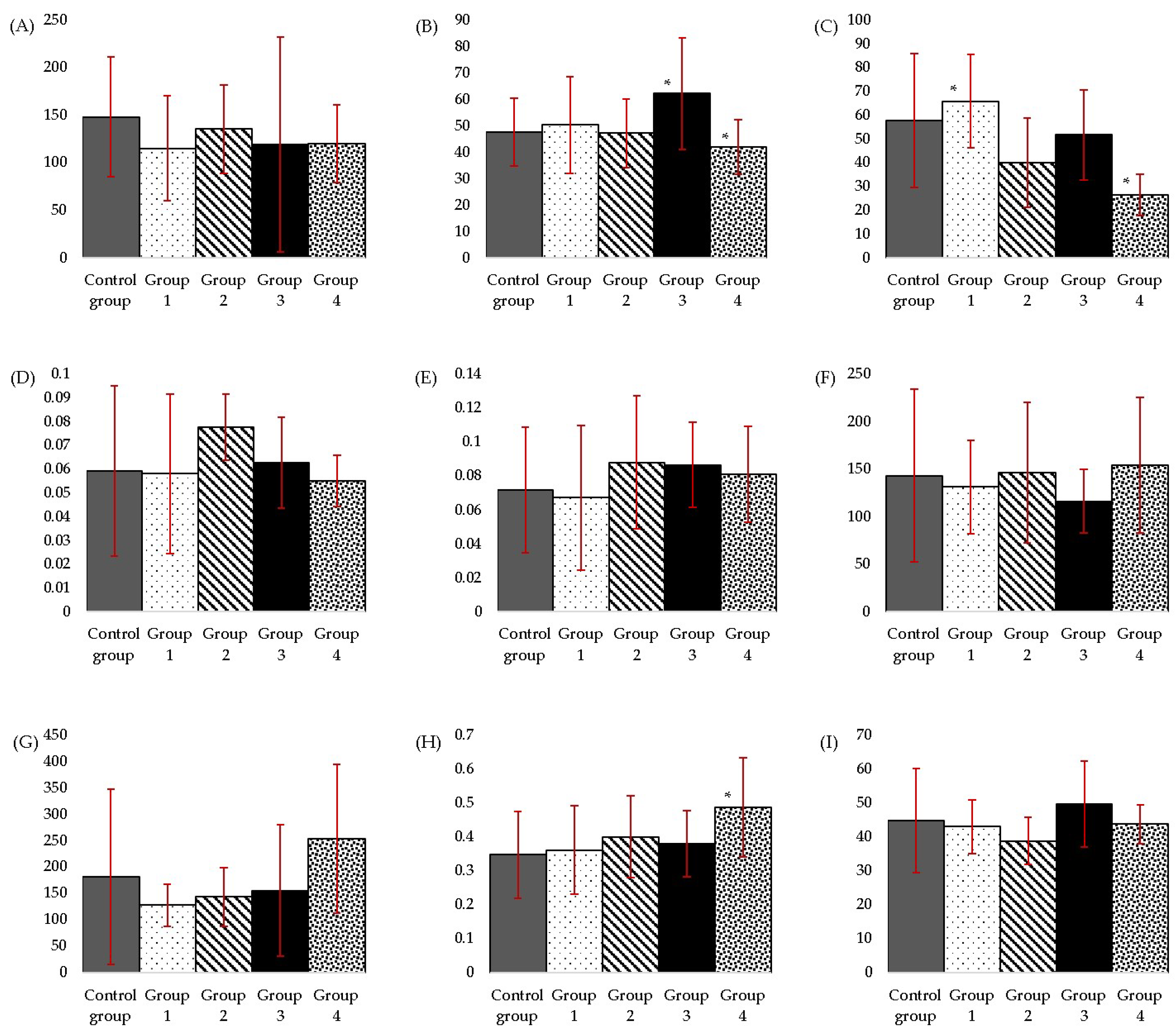
3.1. Biochemical and Metabolic Parameters
3.1.1. Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT)
3.1.2. Alkaline Phosphatase (ALP)
3.1.3. Direct Bilirubin (DB) and Total Bilirubin (TB)
3.1.4. Total Cholesterol (CHOL)
3.1.5. Triglycerides (TG)
3.1.6. Creatinine (CR)
3.1.7. Urea (UR)
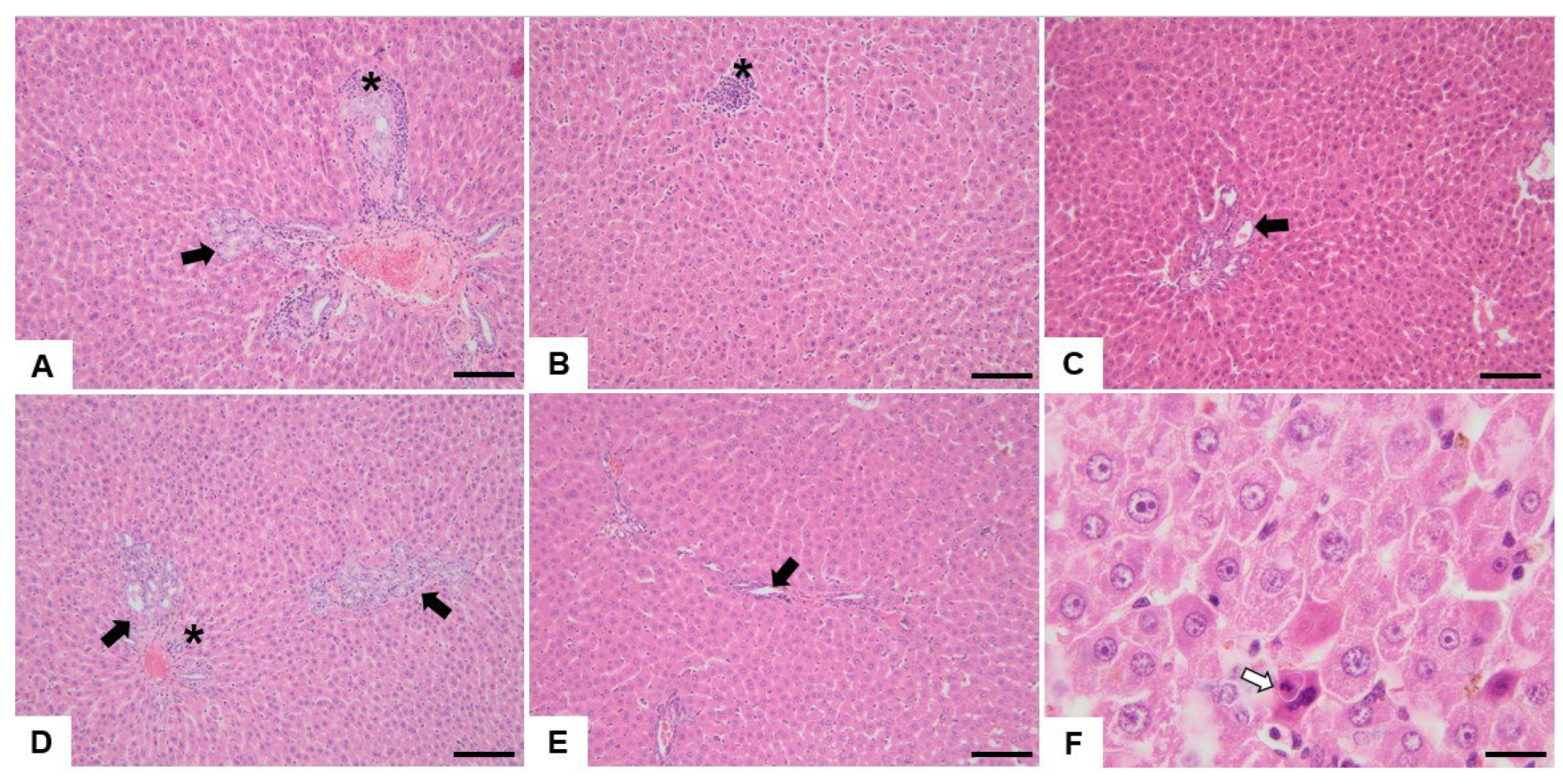
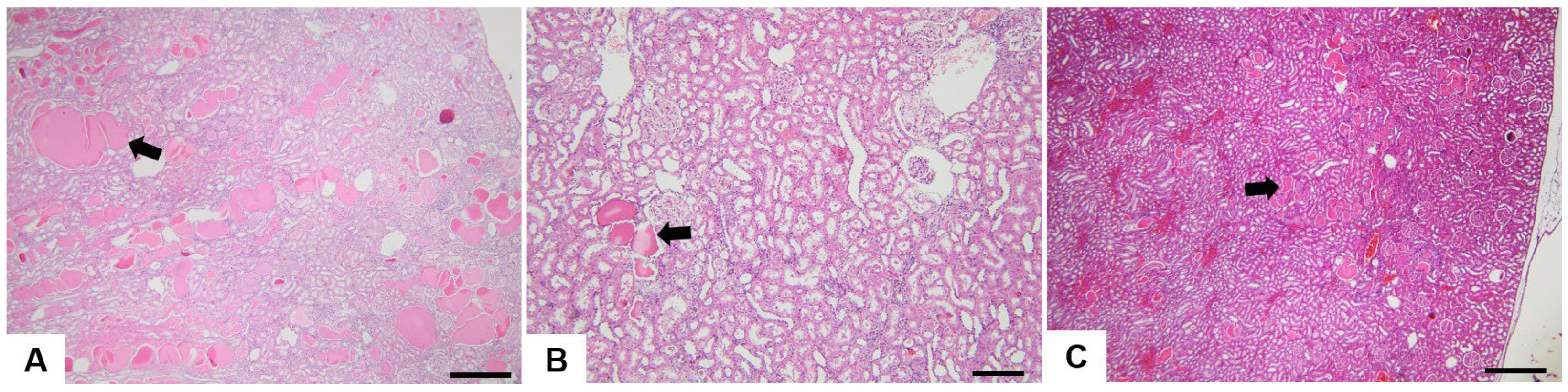
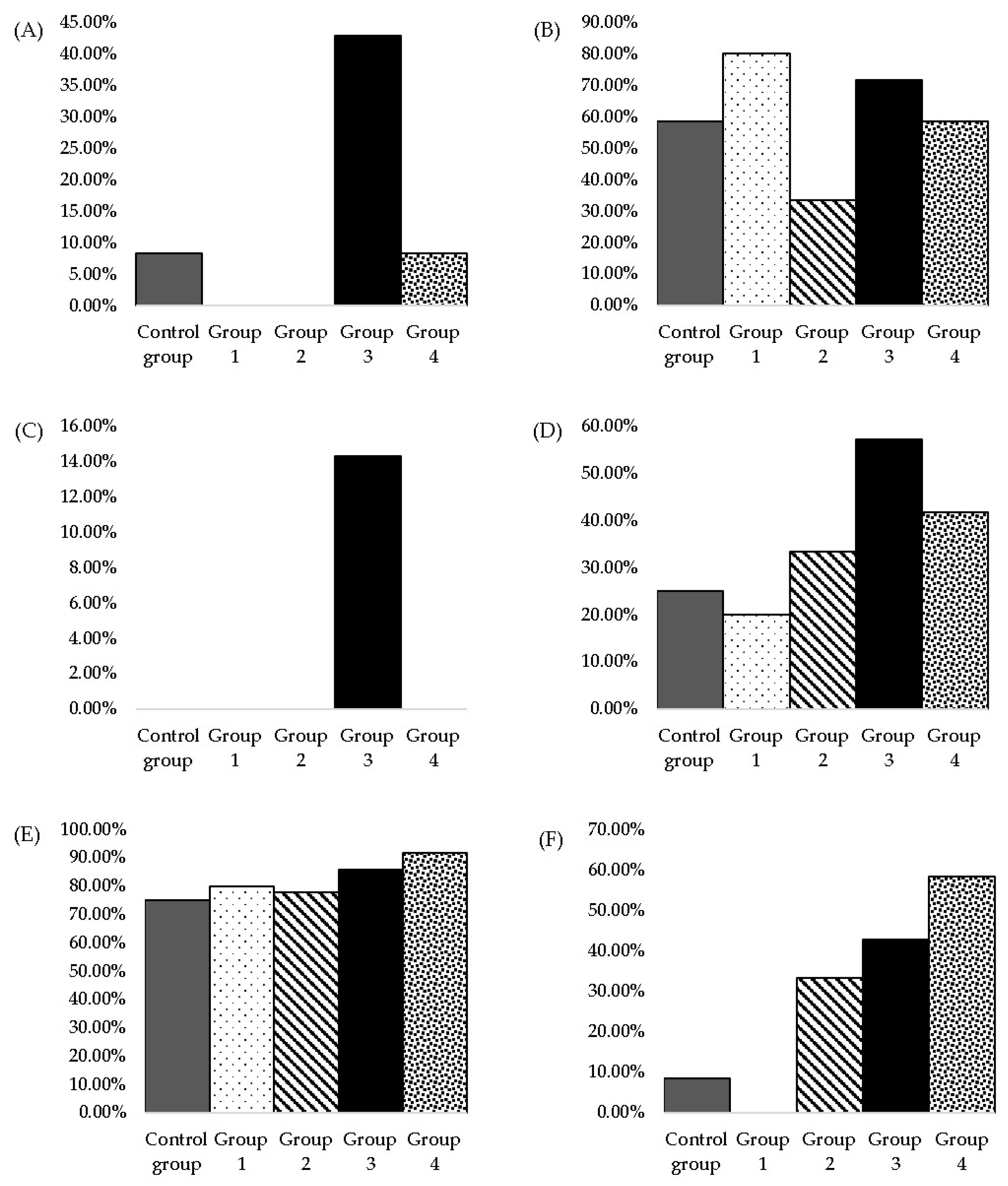
3.2. Histopathological Analysis
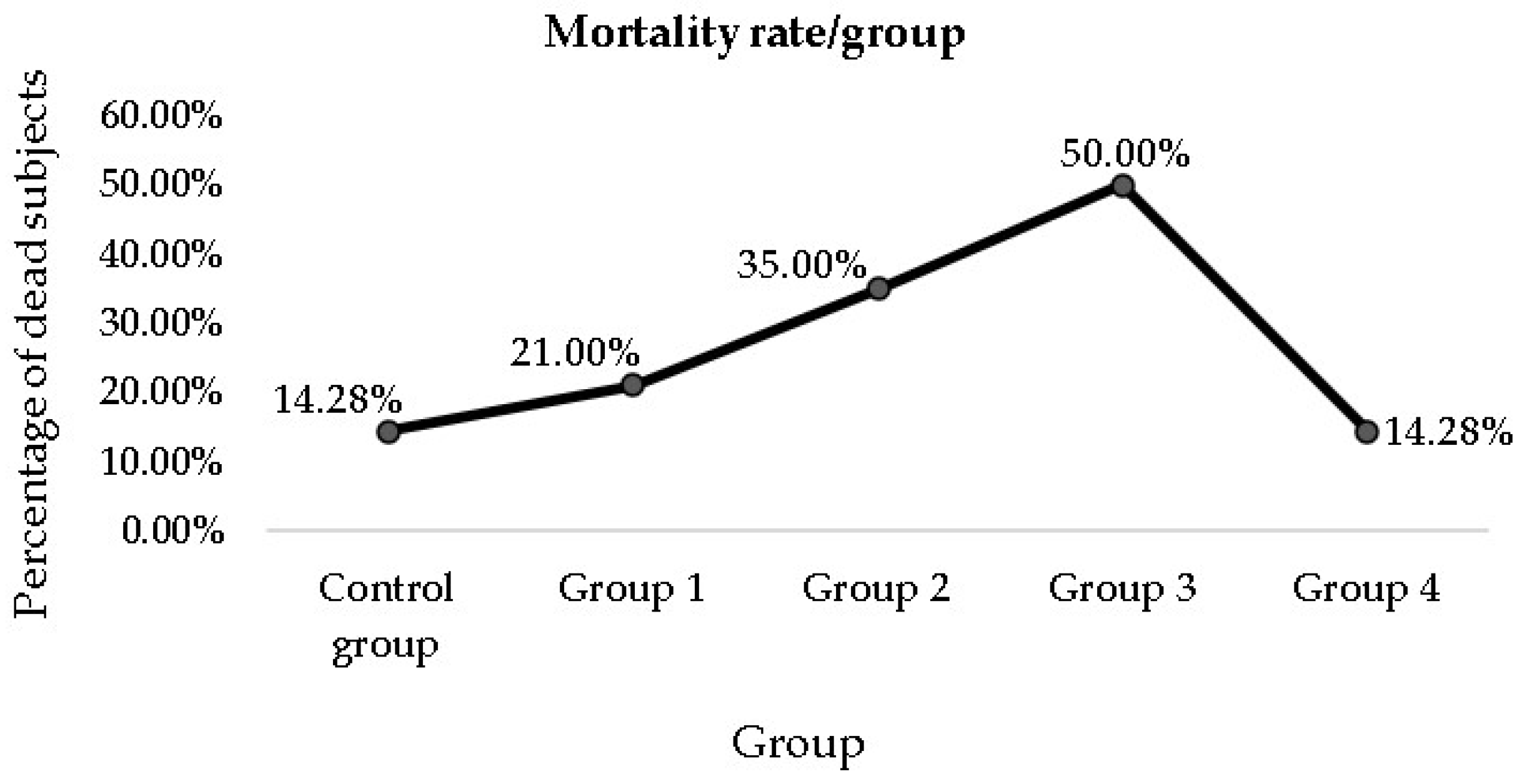
3.3. Mortality Rate among the Groups
4. Discussion
4.1. Analysis of Changes in Biochemical Parameters
4.1.1. Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT) Changes
4.1.2. Alkaline Phosphatase (ALP)
4.1.3. Direct Bilirubin (DB) and Total Bilirubin (TB)
4.1.4. Total Cholesterol (CHOL)
4.1.5. Triglycerides (TG)
4.1.6. Urea (UR) and Creatinine (CR)
4.2. Histopathological Analysis
4.2.1. Bile Duct Hyperplasia
4.2.2. Oval Cell Hyperplasia
4.2.3. Mononuclear Cell Infiltrate
4.2.4. Hepatic Macrovesicular and Microvesicular Steatosis
4.2.5. Other Hepatic Histological Alterations
4.2.6. Hepatic Histological Modifications Produced by MSG in Rats Reported in the Scientific Literature
4.2.7. Hyaline Droplets
4.2.8. Mineralisation
4.2.9. Cytoplasmic Vacuolisation
4.2.10. Chronic Progressive Nephropathy
4.2.11. Renal Histological Modifications Produced by MSG in Rats Reported in the Scientific Literature
4.2.12. Other Observations
5. The Hypothesis of the Development of a Defence Mechanism Triggered by MSG Sub-Chronic Consumption in Ageing Rats
6. Limitations of the Study
- Logistical difficulties prevented us from conducting a parallel study on younger adult Wistar rats to compare MSG’s effect on different age groups. We could obtain valuable results through this approach, which would be a future research direction we propose. Also, a higher number of subjects in each group would have been indicated for better precision of the results.
- Also, due to logistical difficulties, it was not possible to examine the subjects who died before the end of the administration of the entire quantity of MSG. Therefore, some possible toxic effects of MSG that could have caused death were omitted.
- Extrapolating the results obtained on experimental animals to the human species is quite challenging. Achieving highly relevant data in this regard would require clinical trials.
- The self-administration method of MSG used in the study has advantages and disadvantages. Its limitations include the lower dosage accuracy than oral gavage or parenteral methods. Also, it relies on the natural circadian timing of alimentation and depends on individual preferences for flavours, palatability issues and changes in behaviour over time.
- Selecting the MSG doses administered within the groups to be relevant for the human species was difficult. The heterogeneity of doses administered in similar scientific studies contributed to this aspect.
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gottardo, F.M.; da Silva, A.P.A.; dos Santos, L.R.; Colla, L.M.; Reinehr, C.O. Use of Monosodium Glutamate in Foods: The Good, the Bad, and the Controversial Side. ABCS Health Sci. 2022, 47, e022305. [Google Scholar] [CrossRef]
- Itez, P.; Aloh, G.S.; Osuocha, K.U.; Iwueke, A.V.; Chukwu, E.C. Ameliorative Potentials of Methanol Leaf Extract of Newbouldia Laevis on Monosodium Glutamate Induced Toxicity in Female Albino Rats. Afr. J. Bio. Sci. 2020, 2, 37–50. [Google Scholar]
- Rizk, F.H.; Soliman, N.A.; Abo-Elnasr, S.E.; Mahmoud, H.A.; Abdel Ghafar, M.T.; Elkholy, R.A.; ELshora, O.A.; Mariah, R.A.; Amin Mashal, S.S.; El Saadany, A.A. Fisetin Ameliorates Oxidative Glutamate Testicular Toxicity in Rats via Central and Peripheral Mechanisms Involving SIRT1 Activation. Redox Rep. 2022, 27, 177–185. [Google Scholar] [CrossRef]
- Bayram, H.M.; Akgoz, H.F.; Kizildemir, O.; Ozturkan, A. Monosodium Glutamate: Review on Preclinical and Clinical Reports. Biointerface Res. Appl. Chem. 2022, 13, 149. [Google Scholar] [CrossRef]
- Abdulghani, M.A.M.; Alshehade, S.A.; Kamran, S.; Alshawsh, M.A. Effect of Monosodium Glutamate on Serum Sex Hormones and Uterine Histology in Female Rats along with Its Molecular Docking and In-Silico Toxicity. Heliyon 2022, 8, e10967. [Google Scholar] [CrossRef] [PubMed]
- Zanfirescu, A.; Ungurianu, A.; Tsatsakis, A.M.; Nițulescu, G.M.; Kouretas, D.; Veskoukis, A.; Tsoukalas, D.; Engin, A.B.; Aschner, M.; Margină, D. A Review of the Alleged Health Hazards of Monosodium Glutamate. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1111–1134. [Google Scholar] [CrossRef] [PubMed]
- Reeds, P.J.; Burrin, D.G.; Stoll, B.; Jahoor, F. Intestinal Glutamate Metabolism. J. Nutr. 2000, 130, 978S–982S. [Google Scholar] [CrossRef]
- Moldovan, O.-L.; Rusu, A.; Tanase, C.; Vari, C.-E. Glutamate—A Multifaceted Molecule: Endogenous Neurotransmitter, Controversial Food Additive, Design Compound for Anti-Cancer Drugs. A Critical Appraisal. Food Chem. Toxicol. 2021, 153, 112290. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Re-evaluation of Glutamic Acid (E 620), Sodium Glutamate (E 621), Potassium Glutamate (E 622), Calcium Glutamate (E 623), Ammonium Glutamate (E 624) and Magnesium Glutamate (E 625) as Food Additives. EFS2 2017, 15, e04910. [Google Scholar] [CrossRef]
- Tennant, D.R. Review of Glutamate Intake from Both Food Additive and Non-Additive Sources in the European Union. Ann. Nutr. Metab. 2018, 73, 21–28. [Google Scholar] [CrossRef]
- Roberts, A.; Lynch, B.; Rietjens, I.M.C.M. Risk Assessment Paradigm for Glutamate. Ann. Nutr. Metab. 2018, 73, 53–64. [Google Scholar] [CrossRef]
- Beyreuther, K.; Biesalski, H.K.; Fernstrom, J.D.; Grimm, P.; Hammes, W.P.; Heinemann, U.; Kempski, O.; Stehle, P.; Steinhart, H.; Walker, R. Consensus Meeting: Monosodium Glutamate—An Update. Eur. J. Clin. Nutr. 2007, 61, 304–313. [Google Scholar] [CrossRef]
- Abd-Elkareem, M.; Soliman, M.; Abd El-Rahman, M.A.M.; Abou Khalil, N.S. Effect of Nigella sativa L. Seed on the Kidney of Monosodium Glutamate Challenged Rats. Front. Pharmacol. 2022, 13, 789988. [Google Scholar] [CrossRef] [PubMed]
- Lemberg, A.; Alejandra Fernández, M. Hepatic Encephalopathy, Ammonia, Glutamate, Glutamine and Oxidative Stress. Ann. Hepatol. 2009, 8, 95–102. [Google Scholar] [CrossRef]
- Mohiuddin, S.S.; Khattar, D. Biochemistry, Ammonia; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Galland, F.; Negri, E.; Da Ré, C.; Fróes, F.; Strapazzon, L.; Guerra, M.C.; Tortorelli, L.S.; Gonçalves, C.-A.; Leite, M.C. Hyperammonemia Compromises Glutamate Metabolism and Reduces BDNF in the Rat Hippocampus. NeuroToxicology 2017, 62, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, J.; Zielińska, M.; Norenberg, M.D. Glutamine as a Mediator of Ammonia Neurotoxicity: A Critical Appraisal. Biochem. Pharmacol. 2010, 80, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Raizel, R.; Tirapegui, J. Role of Glutamine, as Free or Dipeptide Form, on Muscle Recovery from Resistance Training: A Review Study. Nutrire 2018, 43, 28. [Google Scholar] [CrossRef]
- Hakimi, M.; Mohamadi, M.A.; Ghaderi, Z. The Effects of Glutamine Supplementation on Performance and Hormonal Responses in Non-Athlete Male Students during Eight Week Resistance Training. JHSE 2012, 7, 770–782. [Google Scholar] [CrossRef]
- Coqueiro, A.Y.; Rogero, M.M.; Tirapegui, J. Glutamine as an Anti-Fatigue Amino Acid in Sports Nutrition. Nutrients 2019, 11, 863. [Google Scholar] [CrossRef]
- Wismanadi, H.; Kafrawi, F.R.; Sulistyarto, S.; Hakim, A.A.; Rusdiawan, A.; Khuddus, L.A. Acceleration of Sports Recovery with Glutamine Supplementation in Vertical Jump and Badminton Smash Velocity. In Proceedings of the International Joint Conference on Arts and Humanities, Surabaya, Indonesia, 2 October 2021. [Google Scholar]
- Curthoys, N.P.; Watford, M. Regulation of Glutaminase Activity and Glutamine Metabolism. Annu. Rev. Nutr. 1995, 15, 133–159. [Google Scholar] [CrossRef]
- Riedel, G. Glutamate Receptor Function in Learning and Memory. Behav. Brain Res. 2003, 140, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Gasbarri, A.; Pompili, A. Involvement of Glutamate in Learning and Memory. In Identification of Neural Markers Accompanying Memory; Elsevier: Amsterdam, The Netherlands, 2014; pp. 63–77. ISBN 978-0-12-408139-0. [Google Scholar]
- Mohamed, P.; Radwan, R.; Mohamed, S.; Mohamed, S. Toxicity of Monosodium Glutamate on Liver and Body Weight with the Protective Effect of Tannic Acid in Adult Male Rats. Mansoura J. Forensic Med. Clin. Toxicol. 2021, 29, 23–32. [Google Scholar] [CrossRef]
- Adam, S.; Alsanousi, N.; Abdalla, S.; Shareef, A. The Toxic Effect of Monosodium Glutamate on Liver and Kidney Functions in Wister Rats. NJST 2019, 3, 7–14. [Google Scholar]
- Hussin, A.M.; Tala’a, A.A.; Fadhil, S.A.N.; Salman, H.A. The Adverse Effect of Long Term Intake of Monosodium Glutamate on Kidney Performance. IOP Conf. Ser. Earth Environ. Sci. 2021, 880, 012056. [Google Scholar] [CrossRef]
- Oyebode, O.T.; Obiekwe, M.E.; Olorunsogo, O.O. Protective Effects of Alpha Stone on Monosodium Glutamate-Induced Uterine Hyperplasia in Female Wistar Rats. J. Ayurveda Integr. Med. 2020, 11, 217–223. [Google Scholar] [CrossRef] [PubMed]
- El-Sawy, H.B.I.; Soliman, M.M.; El-Shazly, S.A.; Abdel-Maksoud Ali, H. Protective Effects of Camel Milk and Vitamin E against Monosodium Glutamate Induced Biochemical and Testicular Dysfunctions. Prog. Nutr. 2018, 20, 76–85. [Google Scholar] [CrossRef]
- Banerjee, A.; Mukherjee, S.; Maji, B.K. Monosodium Glutamate Causes Hepato-Cardiac Derangement in Male Rats. Hum. Exp. Toxicol. 2021, 40, S359–S369. [Google Scholar] [CrossRef]
- Desoky, S.; Abdel-Fattah, E.A.-R.; Mazen, N. Study of the Toxic Effects of Monosodium Glutamate on the Central Nervous System. Clin. Med. 2021, 8, 9. [Google Scholar]
- Nnadozie, J.O.; Chijioke, U.O.; Okafor, O.C.; Olusina, D.B.; Oli, A.N.; Nwonu, P.C.; Mbagwu, H.O.; Chijioke, C.P. Chronic Toxicity of Low Dose Monosodium Glutamate in Albino Wistar Rats. BMC Res. Notes 2019, 12, 593. [Google Scholar] [CrossRef]
- Shukry, M.; El-Shehawi, A.M.; El-Kholy, W.M.; Elsisy, R.A.; Hamoda, H.S.; Tohamy, H.G.; Abumandour, M.M.; Farrag, F.A. Ameliorative Effect of Graviola (Annona Muricata) on Mono Sodium Glutamate-Induced Hepatic Injury in Rats: Antioxidant, Apoptotic, Anti-Inflammatory, Lipogenesis Markers, and Histopathological Studies. Animals 2020, 10, 1996. [Google Scholar] [CrossRef]
- Longodor, A.; Coroian, A.; Balta, I.; Taulescu, M.; Toma, C.; Sevastre, B.; Marchis, Z.; Andronie, L.; Ioana, P.; Matei, F.; et al. Protective Effects of Dietary Supplement Spirulina (Spirulina Platensis) against Toxically Impacts of Monosodium Glutamate in Blood and Behavior of Swiss Mouse. Separations 2021, 8, 218. [Google Scholar] [CrossRef]
- Al-Mousawi, N.H. Study on Effect of Glutamate Monosodium Exposure on Some Blood and Biochemical Parameters in Adult Albino Rats. J. Entomol. Zool. Stud. 2017, 5, 1029–1031. [Google Scholar]
- Mohamed, M.; El-Nahrawy, W.; Zaher, A.; Amer, A. Therapeutic Role of Nanocurcumin Versus Monosodium Glutamate Toxicity. Egypt. Acad. J. Biol. Sci. B. Zool. 2022, 14, 55–65. [Google Scholar] [CrossRef]
- Albrahim, T.; Binobead, M.A. Roles of Moringa Oleifera Leaf Extract in Improving the Impact of High Dietary Intake of Monosodium Glutamate-Induced Liver Toxicity, Oxidative Stress, Genotoxicity, DNA Damage, and PCNA Alterations in Male Rats. Oxidative Med. Cell. Longev. 2018, 2018, 4501097. [Google Scholar] [CrossRef] [PubMed]
- Onobrudu, D.; Nwiloh, B. Monosodium Glutamate Alter Hepatic Functions, Redox Potential and Lipid Metabolism: Omega 3 Fatty Acids Ameliorative Intervention. GSC Biol. Pharm. Sci. 2020, 3, 101–110. [Google Scholar] [CrossRef]
- Farombi, E.O.; Onyema, O.O. Monosodium Glutamate-Induced Oxidative Damage and Genotoxicity in the Rat: Modulatory Role of Vitamin C, Vitamin E and Quercetin. Hum. Exp. Toxicol. 2006, 25, 251–259. [Google Scholar] [CrossRef]
- Philemon, A.; Anthony, C.; Cemaluk, E.; Egu, E. Terminalia Catappa Flour Extract Mitigated Monosodium Glutamate Intoxicated Rats’ Kidney Biofunction and Histology. J. Phytopharm. 2020, 9, 164–168. [Google Scholar] [CrossRef]
- El-Alfy, N.; Mahmoud, M.; Eissa, M.; Abdelmohsen, A. Role of Propolis Against Toxic Effects of Monosodium Glutamate on Histology and Expression Lipid Metabolism Genes of Mice Liver. Indian J. Public Health Res. Dev. 2020, 11, 867–873. [Google Scholar] [CrossRef]
- Kassab, R.B.; Theyab, A.; Al-Ghamdy, A.O.; Algahtani, M.; Mufti, A.H.; Alsharif, K.F.; Abdella, E.M.; Habotta, O.A.; Omran, M.M.; Lokman, M.S.; et al. Protocatechuic Acid Abrogates Oxidative Insults, Inflammation, and Apoptosis in Liver and Kidney Associated with Monosodium Glutamate Intoxication in Rats. Environ. Sci. Pollut. Res. Int. 2022, 29, 12208–12221. [Google Scholar] [CrossRef]
- Ibrahim, M.; Khalifa, A.; Saleh, A.; Tammam, H. Histopathological and Histochemical Assessment of Monosodium Glutamate-Induced Hepatic Toxicity and the Amelioration with Propolis. Ain Shams J. Forensic Med. Clin. Toxicol. 2019, 33, 24–36. [Google Scholar] [CrossRef]
- Mohammed, M.; Gomaa, A.; Mohammed, M.; Hosny, G.; Ahmed, M. Effects of Mono-Sodium Glutamate Administration On Metabolic Parameters, Hepatic and Renal Functions in Adult and Neonate Male Rats. BESPS 2022, 42, 74–89. [Google Scholar] [CrossRef]
- Mohammedsaleh, Z.M. Histological Studies of the Effects of Monosodium Glutamate on the Stomach in Adult Rats. J. Cytol. Histol. 2014, 5, 1000i102. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose Translation from Animal to Human Studies Revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Thoolen, B.; Maronpot, R.R.; Harada, T.; Nyska, A.; Rousseaux, C.; Nolte, T.; Malarkey, D.E.; Kaufmann, W.; Küttler, K.; Deschl, U.; et al. Proliferative and Nonproliferative Lesions of the Rat and Mouse Hepatobiliary System. Toxicol. Pathol. 2010, 38, 5S–81S. [Google Scholar] [CrossRef]
- Nolte, T.; Brander-Weber, P.; Dangler, C.; Deschl, U.; Elwell, M.R.; Greaves, P.; Hailey, R.; Leach, M.W.; Pandiri, A.R.; Rogers, A.; et al. Nonproliferative and Proliferative Lesions of the Gastrointestinal Tract, Pancreas and Salivary Glands of the Rat and Mouse. J. Toxicol. Pathol. 2016, 29, 1S–125S. [Google Scholar] [CrossRef]
- Frazier, K.S.; Seely, J.C.; Hard, G.C.; Betton, G.; Burnett, R.; Nakatsuji, S.; Nishikawa, A.; Durchfeld-Meyer, B.; Bube, A. Proliferative and Nonproliferative Lesions of the Rat and Mouse Urinary System. Toxicol. Pathol. 2012, 40, 14S–86S. [Google Scholar] [CrossRef]
- Keenan, C.M.; Baker, J.; Bradley, A.; Goodman, D.G.; Harada, T.; Herbert, R.; Kaufmann, W.; Kellner, R.; Mahler, B.; Meseck, E.; et al. International Harmonization of Nomenclature and Diagnostic Criteria (INHAND): Progress to Date and Future Plans. Toxicol. Pathol. 2015, 43, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Schafer, K.A.; Eighmy, J.; Fikes, J.D.; Halpern, W.G.; Hukkanen, R.R.; Long, G.G.; Meseck, E.K.; Patrick, D.J.; Thibodeau, M.S.; Wood, C.E.; et al. Use of Severity Grades to Characterize Histopathologic Changes. Toxicol. Pathol. 2018, 46, 256–265. [Google Scholar] [CrossRef]
- Mann, P.C.; Vahle, J.; Keenan, C.M.; Baker, J.F.; Bradley, A.E.; Goodman, D.G.; Harada, T.; Herbert, R.; Kaufmann, W.; Kellner, R.; et al. International Harmonization of Toxicologic Pathology Nomenclature: An Overview and Review of Basic Principles. Toxicol. Pathol. 2012, 40, 7S–13S. [Google Scholar] [CrossRef]
- The Society of Toxicologic Pathology (STP). Available online: https://www.toxpath.org/inhand.asp (accessed on 4 July 2023).
- Girling, S.J.; Campbell-Palmer, R.; Pizzi, R.; Fraser, M.A.; Cracknell, J.; Arnemo, J.; Rosell, F. Haematology and Serum Biochemistry Parameters and Variations in the Eurasian Beaver (Castor Fiber). PLoS ONE 2015, 10, e0128775. [Google Scholar] [CrossRef]
- Kazmi, Z.; Fatima, I.; Perveen, S.; Malik, S.S. Monosodium Glutamate: Review on Clinical Reports. Int. J. Food Prop. 2017, 20, 1807–1815. [Google Scholar] [CrossRef]
- Tousson, E.; El-Atrash, A.; Karson, Y. Protective Role of Rockect Seed (Eruca Sativa) Extract against Monosodium Glutamate-Induced Hepato-Renal Toxicity in Male Rats. Asian J. Res. Med. Pharm. Sci. 2020, 8, 1–10. [Google Scholar] [CrossRef]
- Okediran, B.; Olurotimi, A.; Rahman, S.; Michael, O.; Olukunle, J. Alterations in the Lipid Profile and Liver Enzymes of Rats Treated with Monosodium Glutamate. Sokoto J. Vet. Sci. 2015, 12, 42. [Google Scholar] [CrossRef]
- ALhamed, T.A.; Al-marzook, F.A.; Al-Asady, A.M. The Harmful Effects of Monosodium Glutamate on Blood Parameters Liver and Kidney Functions in Adult White Rats and the Protective Role of Omega-3. Indian J. Forensic Med. Toxicol. 2021, 15, 5245–5250. [Google Scholar] [CrossRef]
- Pal, L.C.; Kumar, A.; Pande, V.; Rao, C.V. Hepatoprotective Effect of Bioactive Fraction of Lagerstroemia Speciosa (L.) Pers. Bark Against Monosodium Glutamate-Induced Liver Toxicity. Pharmacogn. J. 2020, 12, 1630–1640. [Google Scholar] [CrossRef]
- Banerjee, A.; Das, D.; Paul, R.; Roy, S.; Das, U.; Saha, S.; Dey, S.; Adhikary, A.; Mukherjee, S.; Maji, B.K. Mechanistic Study of Attenuation of Monosodium Glutamate Mixed High Lipid Diet Induced Systemic Damage in Rats by Coccinia Grandis. Sci. Rep. 2020, 10, 15443. [Google Scholar] [CrossRef] [PubMed]
- Mirzakhani, N.; Farshid, A.A.; Tamaddonfard, E.; Tehrani, A.; Imani, M. Comparison of the Effects of Hydroalcoholic Extract of Capparis Spinosa Fruit, Quercetin and Vitamin E on Monosodium Glutamate-Induced Toxicity in Rats. Vet. Res. Forum 2020, 11, 127–134. [Google Scholar] [CrossRef]
- Johnlouis, I.; Cemaluk, A. Correlations of Biomechanical Characteristics with Ball Speed in Penalty Corner Push-In Effects of Ethanol Extract of Cocoa (Theobroma Cacao) Pod on Normal and Monosodium Glutamate-Intoxicated Rats’ Hepatic Histo-Morphology, Serum Bio-Functional Parameters and Serum Antioxidant Activities. Int. J. Recent Res. Appl. Stud. 2019, 6, 14. [Google Scholar]
- Jayasri Krupaa, R.; Hariharan, R.; Aravindha Babu, N.; Masthan, K.M.K. Alkaline Phosphatase and Its Clinical Importance—A Review. Eur. J. Mol. Clin. Med. 2020, 7, 1409–1413. [Google Scholar]
- Arise, R. Ivermectin Protects Against Monosodium Glutamate-Induced Excitotoxicity in the Rat. Adv. Food Sci. 2013, 36, 38–47. [Google Scholar] [CrossRef]
- Kayode, O.T.; Rotimi, D.E.; Olaolu, T.D.; Adeyemi, O.S. Ketogenic Diet Improves and Restores Redox Status and Biochemical Indices in Monosodium Glutamate-Induced Rat Testicular Toxicity. Biomed. Pharmacother. 2020, 127, 110227. [Google Scholar] [CrossRef] [PubMed]
- Manal Said, T.; Nawal, A.-B. Adverse Effects of Monosodium Glutamate on Liver and Kidney Functions in Adult Rats and Potential Protective Effect of Vitamins C and E. Food Nutr. Sci. 2012, 3, 651–659. [Google Scholar] [CrossRef]
- Bartoš, V.; Dastych, M.; Dastych, M., Jr.; Franěk, T.; Jirsa, M.; Kalousová, M.; Karlík, T.; Kocna, P. Clinical Biochemistry; Karolinum Press: Praga, Czech Republic, 2016. [Google Scholar]
- Crook, M.A. Clinical Biochemistry and Metabolic Medicine; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Reed, R. Clinical Chemistry; Abbott: Chicago, IL, USA, 2022. [Google Scholar]
- Chikezie, P.C.; Ezeocha, B.C.; Nwankpa, P. Changes in Hepato-Renal Tissues Biomarkers of Alloxan-Induced Diabetic Wistar Rats Co-Administered Monosodium Glutamate and Ascorbic Acid. MOJ Food Process Technol. 2018, 6, 312–317. [Google Scholar] [CrossRef][Green Version]
- Airaodion, A.; Ngwogu, K.; Ngwogu, A.; Megwas, A.; Ekenjoku, J. Nephrotoxicity of Monosodium Glutamate (MSG) in Wistar Rats. Int. J. Adv. Nephrol. Res. 2020, 31, 1–10. [Google Scholar]
- Omogbiya, A.I.; Ben-Azu, B.; Eduviere, A.T.; Eneni, A.-E.O.; Nwokoye, P.O.; Ajayi, A.M.; Umukoro, S. Monosodium Glutamate Induces Memory and Hepatic Dysfunctions in Mice: Ameliorative Role of Jobelyn® through the Augmentation of Cellular Antioxidant Defense Machineries. Toxicol. Res. 2021, 37, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Insawang, T.; Selmi, C.; Cha’on, U.; Pethlert, S.; Yongvanit, P.; Areejitranusorn, P.; Boonsiri, P.; Khampitak, T.; Tangrassameeprasert, R.; Pinitsoontorn, C.; et al. Monosodium Glutamate (MSG) Intake Is Associated with the Prevalence of Metabolic Syndrome in a Rural Thai Population. Nutr. Metab. 2012, 9, 50. [Google Scholar] [CrossRef]
- Hirata, A.E.; Andrade, I.S.; Vaskevicius, P.; Dolnikoff, M.S. Monosodium Glutamate (MSG)-Obese Rats Develop Glucose Intolerance and Insulin Resistance to Peripheral Glucose Uptake. Braz. J. Med. Biol. Res. 1997, 30, 671–674. [Google Scholar] [CrossRef]
- Frayn, K.N. Insulin Resistance, Impaired Postprandial Lipid Metabolism and Abdominal Obesity. Med. Princ. Pract. 2002, 11, 31–40. [Google Scholar] [CrossRef]
- Helal, E.G.E.; Barayan, A.W.; Abdelaziz, M.A.; EL-Shenawe, N.S.A. Adverse Effects of Mono Sodium Glutamate, Sodium Benzoate and Chlorophyllins on Some Physiological Parameters in Male Albino Rats. Egypt. J. Hosp. Med. 2019, 8, 1857–1864. [Google Scholar] [CrossRef]
- AlDeri, A. Hematological Study of Silymarin on Monosodium Glutamate Toxicity in Rabbits. Plant Arch. 2020, 20, 1–6. [Google Scholar]
- Ogbuagu, E.O.; Airaodion, A.I.; Okoroukwu, V.N.; Ogbuagu, U. Hyperglycemic and Hypocholesterolemic Effect of Monosodium Glutamate in Wistar Rats. Int. J. Res. Rep. Hematol. 2019, 2, 1–7. [Google Scholar]
- Lîsîi, L. Biochimie Medicală; Nicolae Testemițanu State University of Medicine and Pharmacy: Chișinău, Moldova, 2007. [Google Scholar]
- Aguilera, A.; Álvarez-Delgado, C.; Hernández-Godinez, D.; Fernandez-Mejia, C. Hepatic Diseases Related to Triglyceride Metabolism. Mini Rev. Med. Chem. 2013, 13, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Saliu, J.; Oboh, G.; Schetinger, M.; Stefanello, N.; Rocha, J. Antidiabetic Potentials of Jute Leaf (Corchorus Olitorius) on Type-2 Diabetic Rats. J. Emerg. Trends Eng. Appl. Sci. 2015, 6, 223–230. [Google Scholar]
- Koohpeyma, F.; Siri, M.; Allahyari, S.; Mahmoodi, M.; Saki, F.; Dastghaib, S. The Effects of L-Carnitine on Renal Function and Gene Expression of Caspase-9 and Bcl-2 in Monosodium Glutamate-induced Rats. BMC Nephrol. 2021, 22, 162. [Google Scholar] [CrossRef] [PubMed]
- MN, C.; Rana, S. Textbook of Medical Biochemistry; Jaypee Brothers Medical Publishers, Ltd.: New Delhi, India, 2012. [Google Scholar]
- Bile Duct—Hyperplasia. Available online: https://ntp.niehs.nih.gov/atlas/nnl/hepatobiliary-system/liver/BileDuct-Hyperplasia (accessed on 4 July 2023).
- Hailey, J.R.; Nold, J.B.; Brown, R.H.; Cullen, J.M.; Holder, J.C.; Jordan, H.L.; Ennulat, D.; Miller, R.T. Biliary Proliferative Lesions in the Sprague-Dawley Rat: Adverse/Non-Adverse. Toxicol. Pathol. 2014, 42, 844–854. [Google Scholar] [CrossRef]
- Oval Cell—Hyperplasia. Available online: https://ntp.niehs.nih.gov/atlas/nnl/hepatobiliary-system/liver/OvalCell-Hyperplasia (accessed on 4 July 2023).
- Senaldi, G.; Portmann, B.; Mowat, A.P.; Mieli-Vergani, G.; Vergani, D. Immunohistochemical Features of the Portal Tract Mononuclear Cell Infiltrate in Chronic Aggressive Hepatitis. Arch. Dis. Child. 1992, 67, 1447–1453. [Google Scholar] [CrossRef]
- Satapathy, S.K.; Kuwajima, V.; Nadelson, J.; Atiq, O.; Sanyal, A.J. Drug-Induced Fatty Liver Disease: An Overview of Pathogenesis and Management. Ann. Hepatol. 2015, 14, 789–806. [Google Scholar] [CrossRef]
- Garikipati, S.C.; Roy, P. Biliary Tract Cholangiocarcinoma; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hepatocyte—Karyomegaly. Available online: https://ntp.niehs.nih.gov/atlas/nnl/hepatobiliary-system/liver/Hepatocyte-Karyomegaly (accessed on 25 July 2023).
- Cebada Chaparro, E.; Lloret Del Hoyo, J.; Méndez Fernández, R. Chronic Cholangitis: Differential Diagnosis and Role of MRI. Radiología 2020, 62, 452–463. [Google Scholar] [CrossRef]
- Renal Tubule—Accumulation, Hyaline Droplet. Available online: https://ntp.niehs.nih.gov/atlas/nnl/urinary-system/kidney/RenalTubule-AccumulationHyalineDroplet (accessed on 26 July 2023).
- Hard, G.C.; Snowden, R.T. Hyaline Droplet Accumulation in Rodent Kidney Proximal Tubules: An Association with Histiocytic Sarcoma. Toxicol. Pathol. 1991, 19, 88–97. [Google Scholar] [CrossRef]
- Seely, J.C.; Hard, G.C. Chronic Progressive Nephropathy (CPN) in the Rat: Review of Pathology and Relationship to Renal Tumorigenesis. J. Toxicol. Pathol. 2008, 21, 199–205. [Google Scholar] [CrossRef][Green Version]
- Hard, G.C.; Banton, M.I.; Bretzlaff, R.S.; Dekant, W.; Fowles, J.R.; Mallett, A.K.; McGregor, D.B.; Roberts, K.M.; Sielken, R.L.; Valdez-Flores, C.; et al. Consideration of Rat Chronic Progressive Nephropathy in Regulatory Evaluations for Carcinogenicity. Toxicol. Sci. 2013, 132, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Abrass, C.K. The Nature of Chronic Progressive Nephropathy in Aging Rats. Adv. Ren. Replace. Ther. 2000, 7, 4–10. [Google Scholar] [CrossRef]
- Schirrmacher, V. Less Can Be More: The Hormesis Theory of Stress Adaptation in the Global Biosphere and Its Implications. Biomedicines 2021, 9, 293. [Google Scholar] [CrossRef]
- Hernández Bautista, R.J.; Mahmoud, A.M.; Königsberg, M.; López Díaz Guerrero, N.E. Obesity: Pathophysiology, Monosodium Glutamate-Induced Model and Anti-Obesity Medicinal Plants. Biomed. Pharmacother. 2019, 111, 503–516. [Google Scholar] [CrossRef]
- Kendig, E.L.; Le, H.H.; Belcher, S.M. Defining Hormesis: Evaluation of a Complex Concentration Response Phenomenon. Int. J. Toxicol. 2010, 29, 235–246. [Google Scholar] [CrossRef]
- Luna–López, A.; González-Puertos, V.Y.; López-Diazguerrero, N.E.; Königsberg, M. New Considerations on Hormetic Response against Oxidative Stress. J. Cell Commun. Signal. 2014, 8, 323–331. [Google Scholar] [CrossRef]
- Hernández-Bautista, R.; Alarcón-Aguilar, F.; Del, C.; Escobar-Villanueva, M.; Almanza-Pérez, J.; Merino-Aguilar, H.; Fainstein, M.; López-Diazguerrero, N. Biochemical Alterations during the Obese-Aging Process in Female and Male Monosodium Glutamate (MSG)-Treated Mice. Int. J. Mol. Sci. 2014, 15, 11473–11494. [Google Scholar] [CrossRef]
- Walker, M.C.; van der Donk, W.A. The Many Roles of Glutamate in Metabolism. J. Ind. Microbiol. Biotechnol. 2016, 43, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Ragonnaud, E.; Biragyn, A. Gut Microbiota as the Key Controllers of “Healthy” Aging of Elderly People. Immun. Ageing 2021, 18, 2. [Google Scholar] [CrossRef]
- Karaca, M.; Martin-Levilain, J.; Grimaldi, M.; Li, L.; Dizin, E.; Emre, Y.; Maechler, P. Liver Glutamate Dehydrogenase Controls Whole-Body Energy Partitioning Through Amino Acid–Derived Gluconeogenesis and Ammonia Homeostasis. Diabetes 2018, 67, 1949–1961. [Google Scholar] [CrossRef] [PubMed]
- Soniya, K.; Awasthi, S.; Nair, N.N.; Chandra, A. Transimination Reaction at the Active Site of Aspartate Aminotransferase: A Proton Hopping Mechanism through Pyridoxal 5′-Phosphate. ACS Catal. 2019, 9, 6276–6283. [Google Scholar] [CrossRef]
- Thakur, S.; Gupta, S.K.; Ali, V.; Singh, P.; Verma, M. Aldose Reductase: A Cause and a Potential Target for the Treatment of Diabetic Complications. Arch. Pharm. Res. 2021, 44, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Escrivá, F.; Gavete, M.L.; Fermín, Y.; Pérez, C.; Gallardo, N.; Alvarez, C.; Andrés, A.; Ros, M.; Carrascosa, J.M. Effect of Age and Moderate Food Restriction on Insulin Sensitivity in Wistar Rats: Role of Adiposity. J. Endocrinol. 2007, 194, 131–141. [Google Scholar] [CrossRef] [PubMed]


| Year | Tested Animals | Animals/Group | Daily MSG Dose/kg bw | Study Duration | Tested Parameters | Results | Ref. |
|---|---|---|---|---|---|---|---|
| 2021 | Male rats | 10/group | 15 mg | 30 days and 60 days | Kidney tissue | Proliferated and enlarged mesangial cells | [27] |
| Serum CR | Increased levels | ||||||
| 2020 | Male Wistar rats | 10/group | 2.4 g | 8 weeks | Liver tissue | Periportal hepatic necrosis; mononuclear cell infiltration | [33] |
| GSH, GST, SOD, CAT * | Decreased levels | ||||||
| AST, ALT, ALP, TB, GGT, CHOL, TG ** | Increased levels | ||||||
| 2019 | Male Albino Rats | 12/group | 97 mg | 6 weeks | Liver tissue | Hydropic degeneration; congested sinusoidal vessels; binucleation | [43] |
| SOD, CAT, GSH, GPx *** | Decreased levels | ||||||
| ALT, AST, ALP, Bilirubin | Increased levels | ||||||
| 2021 | Male rats | 10/group | 120 mg | 3 months | Liver and kidney tissues | Dilatation of portal veins; vacuolar cytoplasmic degeneration of hepatocytes; hyaline and cellular casts in the renal tubules | [44] |
| Total antioxidant capacity | Decreased levels | ||||||
| AST, ALT, UR, CR | Increased levels | ||||||
| 2022 | Male rats | 10/group | 10 mg | 4 weeks | CHOL, TG, LDL **, AST, ALT | Increased levels | [36] |
| HDL ** | Decreased levels | ||||||
| 8 weeks | CHOL, TG, LDL, AST, ALT | Increased levels | |||||
| HDL | Decreased levels | ||||||
| 2020 | Virgin female Wistar rats | 7/group | 200 mg | 14 days (acute toxicity) | Progesterone, Oestrogen | Increased levels | [28] |
| TG, CHOL | Increased levels | ||||||
| Ovarian tissue | Moderate to severe deposits of collagen tissue with fibrosis were observed in the ovarian stroma | ||||||
| 2017 | Male rats | 10/group | 5 g | 30 days | AST, ALT, ALP, UR, CR | Increased levels | [35] |
| 2014 | Wistar rats | 6/group | 3 g | 7 days (acute toxicity) | Stomach tissue | Multiple congested blood vessels in the gastric submucosa | [45] |
| 6 g | Increase of connective tissues lamina propria and around the basal parts of the gastric gland |
| Group | Dose | Observations |
|---|---|---|
| 1 | 185 mg MSG/kg bw/day | Approximately equal to the maximum daily dose of MSG in the human species—30 mg/kg bw per day according to EFSA [9] |
| 2 | 1500 mg MSG/kg bw/day | Half of the third group dose |
| 3 | 3000 mg MSG/kg bw/day | A similar value to the NOAEL dose for MSG −3200 mg/kg bw according to EFSA [9] |
| 4 | 6000 mg MSG/kg bw/day | Twice the approximate NOAEL dose |
| Control | MSG non-consumer group | - |
| Biochemical/ Metabolic Parameter | Analysis Method | Reagent |
|---|---|---|
| Aspartate Aminotransferase (AST) | IFCC (International Federation for Clinical Chemistry) standardised kinetics with the pyridoxal phosphate method | Aspartate aminotransferase, Roche Diagnostics, GmbH, Mannheim, Germany (ASTL) |
| Alanine Aminotransferase (ALT) | IFCC (International Federation for Clinical Chemistry) standardised kinetics with the pyridoxal phosphate method | Alanine aminotransferase, Roche Diagnostics, GmbH, Mannheim, Germany (ALTL) |
| Alkaline phosphatase (ALP) | Spectrophotometric method (colourimetric test) | Alkaline phosphatase IFCC, 2nd generation, Roche Diagnostics, GmbH, Mannheim, Germany (ALP2) |
| Total bilirubin (TB) | Spectrophotometric (colourimetric) method | Total bilirubin DPD, 2nd generation, Roche Diagnostics, GmbH, Mannheim, Germany (BILT2) |
| Direct bilirubin (DB) | Spectrophotometric (colourimetric) method | Direct bilirubin, 2nd generation, Roche Diagnostics, GmbH, Mannheim, Germany (BILD2) |
| Total cholesterol (CHOL) | Spectrophotometric (enzymatic-colourimetric) method | Cholesterol Roche Diagnostics, GmbH, Mannheim, Germany (CHOL2) |
| Triglycerides (TG) | Spectrophotometric (enzymatic-colourimetric) method | Triglycerides Roche Diagnostics, GmbH, Mannheim, Germany (TRIGL) |
| Creatinine (CR) | Kinetic (enzymatic-colourimetric) Jaffé method | Creatinine Jaffé, 2nd generation, Roche Diagnostics, GmbH, Mannheim, Germany (CREJ2) |
| Urea (UR) | Spectrophotometric (kinetic) method | Urea, Roche Diagnostics, GmbH, Mannheim, Germany (Ureal) |
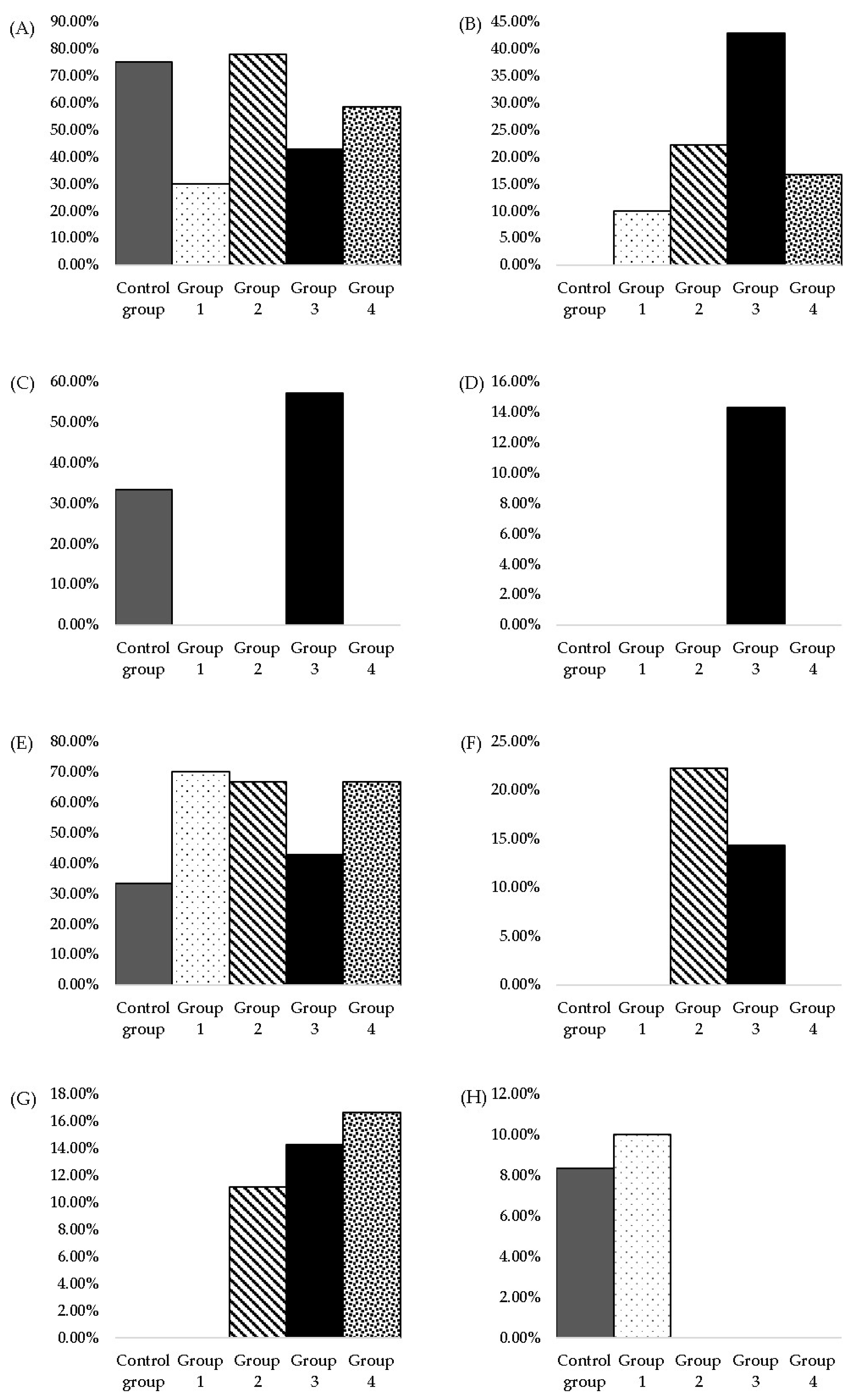
| Histopathological Modification | Occurrence Frequency of Modification/Group * | ||||
|---|---|---|---|---|---|
| Control Group (n † = 12) | Group 1 (n = 10) | Group 2 (n = 9) | Group 3 (n = 7) | Group 4 (n = 12) | |
| Bile duct hyperplasia (minimal) | 75% (9/12) | 30% (3/10) | 77.77% (7/9) | 42.86% (3/7) | 58.33% (7/12) |
| Bile duct hyperplasia (moderate) | - | 10% (1/10) | 22.22% (2/9) | 42.86% (3/7) | 16.66% (2/12) |
| Oval cell hyperplasia (minimal) | 33.33% (4/12) | - | - | 57.14% (4/7) | - |
| Oval cell hyperplasia (moderate) | - | - | - | 14.29% (1/7) | - |
| Focal necrotic hepatitis (minimal) | 8.33% (1/12) | - | - | - | - |
| Multifocal mononuclear cell infiltrate (mainly portal—minimal) | 33.33% (4/12) | 70% (7/10) | 66.66% (6/9) | 42.86% (3/7) | 66.66% (8/12) |
| Multifocal mononuclear cell infiltrate (mainly portal—moderate) | - | - | 22.22% (2/9) | 14.29% (1/7) | - |
| Focal chronic necro haemorrhagic cholangitis | 8.33% (1/12) | - | - | - | - |
| Hepatic macro- and micro-vesicular steatosis (mainly centrilobular) | 8.33% (1/12) | - | - | - | - |
| Hepatic focal microvesicular steatosis (minimal) | - | - | 11.11% (1/9) | 14.29% (1/7) | 16.66% (2/12) |
| Focal hydropic change (minimal) | 8.33% (1/12) | - | - | - | - |
| Glycogenosis (glycogen storage—minimal) | 8.33% (1/12) | 10% (1/10) | - | - | - |
| Eosinophlic focal cell alteration | 8.33% (1/12) | - | - | - | - |
| Diffuse lymphohistiocytic hepatitis (moderate) and portal fibrosis (minimal) | - | - | - | 14.29% (1/7) | - |
| Individual hepatocytic death (apoptosis/necrosis) | - | - | - | 14.29% (1/7) | - |
| Cholangiocarcinoma | - | 10% (1/10) | - | - | - |
| Karyomegaly | - | - | - | - | 16.66% (2/12) |
| Histopathological Modifications | Occurrence Frequency of Modification/Group * | ||||
|---|---|---|---|---|---|
| Control Group (n † = 12) | Group 1 (n = 10) | Group 2 (n = 9) | Group 3 (n = 7) | Group 4 (n = 12) | |
| Hyaline droplet accumulation | 8.33% (1/12) | - | - | 42.86% (3/7) | 8.33% (1/12) |
| Tubular pigment | 58.33% (7/12) | 80.00% (8/10) | 33.33% (3/9) | 71.43% (5/7) | 58.33% (7/12) |
| Mineralisation | - | - | - | 14.29% (1/7) | - |
| Cytoplasmic vacuolisation | 8.33% (1/12) | - | 33.33% (3/9) | 42.86% (3/7) | 58.33% (7/12) |
| Chronic progressive nephropathy | 75.00% (9/12) | 80.00% (8/10) | 77.77% (7/9) | 85.71% (6/7) | 91.66% (11/12) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moldovan, O.-L.; Vari, C.-E.; Tero-Vescan, A.; Cotoi, O.S.; Cocuz, I.G.; Tabaran, F.A.; Pop, R.; Fülöp, I.; Chis, R.F.; Lungu, I.-A.; et al. Potential Defence Mechanisms Triggered by Monosodium Glutamate Sub-Chronic Consumption in Two-Year-Old Wistar Rats. Nutrients 2023, 15, 4436. https://doi.org/10.3390/nu15204436
Moldovan O-L, Vari C-E, Tero-Vescan A, Cotoi OS, Cocuz IG, Tabaran FA, Pop R, Fülöp I, Chis RF, Lungu I-A, et al. Potential Defence Mechanisms Triggered by Monosodium Glutamate Sub-Chronic Consumption in Two-Year-Old Wistar Rats. Nutrients. 2023; 15(20):4436. https://doi.org/10.3390/nu15204436
Chicago/Turabian StyleMoldovan, Octavia-Laura, Camil-Eugen Vari, Amelia Tero-Vescan, Ovidiu Simion Cotoi, Iuliu Gabriel Cocuz, Flaviu Alexandru Tabaran, Romelia Pop, Ibolya Fülöp, Rafael Florin Chis, Ioana-Andreea Lungu, and et al. 2023. "Potential Defence Mechanisms Triggered by Monosodium Glutamate Sub-Chronic Consumption in Two-Year-Old Wistar Rats" Nutrients 15, no. 20: 4436. https://doi.org/10.3390/nu15204436
APA StyleMoldovan, O.-L., Vari, C.-E., Tero-Vescan, A., Cotoi, O. S., Cocuz, I. G., Tabaran, F. A., Pop, R., Fülöp, I., Chis, R. F., Lungu, I.-A., & Rusu, A. (2023). Potential Defence Mechanisms Triggered by Monosodium Glutamate Sub-Chronic Consumption in Two-Year-Old Wistar Rats. Nutrients, 15(20), 4436. https://doi.org/10.3390/nu15204436