All That Glitters Is Not Gold: Assessment of Bee Pollen Supplementation Effects on Gastric Mucosa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Studies
2.2. Laboratory Phase
2.3. Data Analysis
3. Results
3.1. Histological Evaluation of Gastric Mucosa in Haematoxylin and Eosin Staining
3.2. Immunohistochemical Evaluation of COX-1, COX-2, iNOS, and ADMA Levels of Concentration
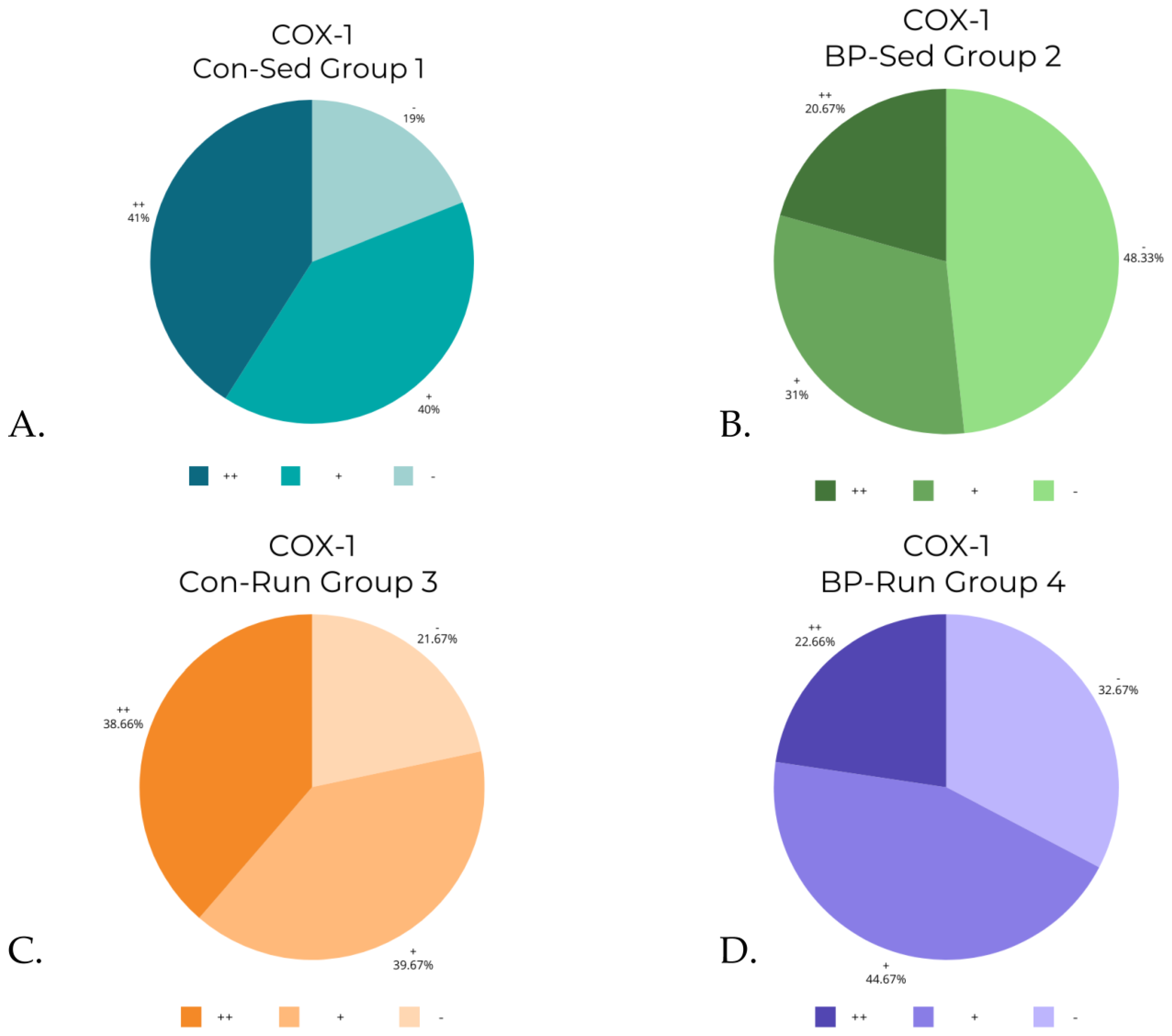
3.3. COX-1 Levels
3.4. COX-2 Levels
3.5. iNOS Levels
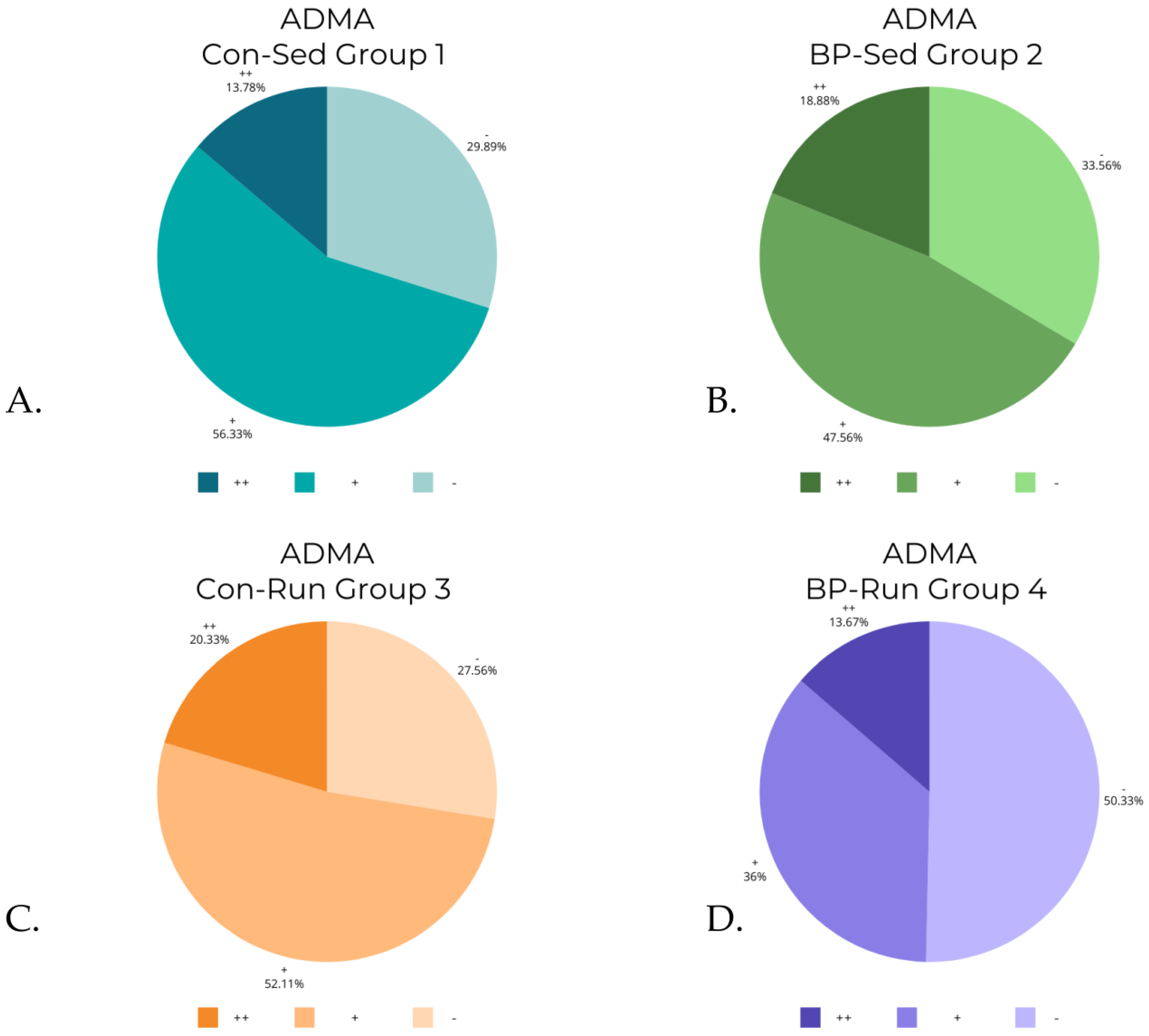
3.6. ADMA Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thakur, M.; Nanda, V. Composition and Functionality of Bee Pollen: A Review. Trends Food Sci. Technol. 2020, 98, 82–106. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elashal, M.H.; Yosri, N.; Du, M.; Musharraf, S.G.; Nahar, L.; Sarker, S.D.; Guo, Z.; Cao, W.; Zou, X.; et al. Bee Pollen: Current Status and Therapeutic Potential. Nutrients 2021, 13, 1876. [Google Scholar] [CrossRef] [PubMed]
- El Ghouizi, A.; Bakour, M.; Laaroussi, H.; Ousaaid, D.; El Menyiy, N.; Hano, C.; Lyoussi, B. Bee Pollen as Functional Food: Insights into Its Composition and Therapeutic Properties. Antioxidants 2023, 12, 557. [Google Scholar] [CrossRef] [PubMed]
- Jannesar, M.; Shoushtari, M.S.; Majd, A.; Pourpak, Z. Bee Pollen Flavonoids as a Therapeutic Agent in Allergic and Immunological Disorders. Iran. J. Allergy Asthma Immunol. 2017, 16, 171–182. [Google Scholar] [PubMed]
- Higuchi, K.; Watanabe, T.; Tanigawa, T.; Tominaga, K.; Fujiwara, Y.; Arakawa, T. Sofalcone, a Gastroprotective Drug, Promotes Gastric Ulcer Healing Following Eradication Therapy for Helicobacter Pylori: A Randomized Controlled Comparative Trial with Cimetidine, an H2-Receptor Antagonist. J. Gastroenterol. Hepatol. 2010, 25 (Suppl. S1), S155–S160. [Google Scholar] [CrossRef] [PubMed]
- De Lira Mota, K.S.; Dias, G.E.N.; Pinto, M.E.F.; Luiz-Ferreira, Â.; Souza-Brito, A.R.M.; Hiruma-Lima, C.A.; Barbosa-Filho, J.M.; Batista, L.M. Flavonoids with Gastroprotective Activity. Molecules 2009, 14, 979. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Salam, O.M.E.; Czimmer, J.; Debreceni, A.; Szolcsányi, J.; Mózsik, G. Gastric Mucosal Integrity: Gastric Mucosal Blood Flow and Microcirculation. An Overview. J. Physiol. Paris 2001, 95, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Sunairi, M.; Watanabe, K.; Suzuki, T.; Tanaka, N.; Kuwayama, H.; Nakajima, M. Effects of Anti-Ulcer Agents on Antibiotic Activity against Helicobacter Pylori. Eur. J. Gastroenterol. Hepatol. 1994, 6 (Suppl. S1), S121–S124. [Google Scholar]
- Zhang, H.; Liu, R.; Lu, Q. Separation and Characterization of Phenolamines and Flavonoids from Rape Bee Pollen, and Comparison of Their Antioxidant Activities and Protective Effects against Oxidative Stress. Molecules 2020, 25, 1264. [Google Scholar] [CrossRef]
- Šarić, A.; Balog, T.; Sobočanec, S.; Kušić, B.; Šverko, V.; Rusak, G.; Likić, S.; Bubalo, D.; Pinto, B.; Reali, D.; et al. Antioxidant Effects of Flavonoid from Croatian Cystus Incanus L. Rich Bee Pollen. Food Chem. Toxicol. 2009, 47, 547–554. [Google Scholar] [CrossRef]
- Belina-Aldemita, M.D.; Schreiner, M.; D’Amico, S. Characterization of Phenolic Compounds and Antioxidative Potential of Pot-Pollen Produced by Stingless Bees (Tetragonula Biroi Friese) from the Philippines. J. Food Biochem. 2020, 44, e13102. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Ishihara, M.; Segami, T.; Ito, M. Anti-Ulcer Effects of Antioxidants, Quercetin, Alpha-Tocopherol, Nifedipine and Tetracycline in Rats. Jpn. J. Pharmacol. 1998, 78, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lian, Y.; Li, Q.; Sun, L.; Chen, R.; Lai, X.; Lai, Z.; Yuan, E.; Sun, S. Preventative and Therapeutic Potential of Flavonoids in Peptic Ulcers. Molecules 2020, 25, 4626. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.P.; Pollock, S.J.; Kaur, K.; Felgar, R.E.; Bernstein, S.H.; Chiorazzi, N.; Phipps, R.P. Constitutive and Activation-Inducible Cyclooxygenase-2 Expression Enhances Survival of Chronic Lymphocytic Leukemia B Cells. Clin. Immunol. 2006, 120, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sun, R.; Zenga, J.; Himburg, H.; Wang, L.; Duan, S.; Liu, J.; Bui, D.; Xie, Z.; Du, T.; et al. Comparison of Absolute Expression and Turnover Number of COX-1 and COX-2 in Human and Rodent Cells and Tissues. J. Inflamm. Res. 2022, 15, 4435–4447. [Google Scholar] [CrossRef] [PubMed]
- Čalija, B. Microsized and Nanosized Carriers for Nonsteroidal Anti-Inflammatory Drugs: Formulation Challenges and Potential Benefits; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780128040805. [Google Scholar]
- Stiller, C.O.; Hjemdahl, P. Lessons from 20 Years with COX-2 Inhibitors: Importance of Dose–Response Considerations and Fair Play in Comparative Trials. J. Intern. Med. 2022, 292, 557–574. [Google Scholar] [CrossRef] [PubMed]
- Zarghi, A.; Arfaei, S. Selective COX-2 Inhibitors: A Review of Their Structure-Activity Relationships. Iran. J. Pharm. Res. IJPR 2011, 10, 655. [Google Scholar] [PubMed]
- Desai, S.J.; Prickril, B.; Rasooly, A. Mechanisms of Phytonutrient Modulation of Cyclooxygenase-2 (COX-2) and Inflammation Related to Cancer. Nutr. Cancer 2018, 70, 350. [Google Scholar] [CrossRef]
- Mahboubi-Rabbani, M.; Abbasi, M.; Zarghi, A. Natural-Derived COX-2 Inhibitors as Anticancer Drugs: A Review of Their Structural Diversity and Mechanism of Action. Anticancer. Agents Med. Chem. 2022, 23, 15–36. [Google Scholar] [CrossRef]
- Dannenberg, A.J.; Altorki, N.K.; Boyle, J.O.; Dang, C.; Howe, L.R.; Weksler, B.B.; Subbaramaiah, K. Cyclo-Oxygenase 2: A Pharmacological Target for the Prevention of Cancer. Lancet Oncol. 2001, 2, 544–551. [Google Scholar] [CrossRef]
- Jackson, L.M.; Wu, K.C.; Mahida, Y.R.; Jenkins, D.; Hawkey, C.J. Cyclooxygenase (COX) 1 and 2 in Normal, Inflamed, and Ulcerated Human Gastric Mucosa. Gut 2000, 47, 762. [Google Scholar] [CrossRef] [PubMed]
- Emery, P.; Zeidler, H.; Kvien, T.K.; Guslandi, M.; Naudin, R.; Stead, H.; Verburg, K.M.; Isakson, P.C.; Hubbard, R.C.; Geis, G.S. Celecoxib versus Diclofenac in Long-Term Management of Rheumatoid Arthritis: Randomised Double-Blind Comparison. Lancet 1999, 354, 2106–2111. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.S.; Weaver, A.L.; Graham, D.Y.; Kavitz, A.J.; Lipsky, P.E.; Hubbard, R.C.; Isakson, P.C.; Verburg, K.M.; Yu, S.S.; Zhao, W.W.; et al. Anti-Inflammatory and Upper Gastrointestinal Effects of Celecoxib in Rheumatoid Arthritis: A Randomized Controlled Trial. JAMA 1999, 282, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Keklikoglu, N.; Koray, M.; Kocaelli, H.; Akinci, S. INOS Expression in Oral and Gastrointestinal Tract Mucosa. Dig. Dis. Sci. 2008, 53, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Lee, Y.; Kellum, J.A. A New Perspective on NO Pathway in Sepsis and ADMA Lowering as a Potential Therapeutic Approach. Crit. Care 2022, 26. [Google Scholar] [CrossRef] [PubMed]
- Sibal, L.; Agarwal, S.C.; Home, P.D.; Boger, R.H. The Role of Asymmetric Dimethylarginine (ADMA) in Endothelial Dysfunction and Cardiovascular Disease. Curr. Cardiol. Rev. 2010, 6, 82. [Google Scholar] [CrossRef]
- Nair, N.; Gongora, E. Oxidative Stress and Cardiovascular Aging: Interaction between NRF-2 and ADMA. Curr. Cardiol. Rev. 2017, 13, 183. [Google Scholar] [CrossRef]
- Mohamed, Y.T.; Naguib, I.A.; Abo-Saif, A.A.; Elkomy, M.H.; Alghamdi, B.S.; Mohamed, W.R. Role of ADMA/DDAH-1 and INOS/ENOS Signaling in the Gastroprotective Effect of Tadalafil against Indomethacin-Induced Gastric Injury. Biomed. Pharmacother. 2022, 150, 113026. [Google Scholar] [CrossRef]
- Moita, E.; Gil-Izquierdo, A.; Sousa, C.; Ferreres, F.; Silva, L.R.; Valentão, P.; Domínguez-Perles, R.; Baenas, N.; Andrade, P.B. Integrated Analysis of COX-2 and INOS Derived Inflammatory Mediators in LPS-Stimulated RAW Macrophages Pre-Exposed to Echium Plantagineum L. Bee Pollen Extract. PLoS ONE 2013, 8, e59131. [Google Scholar] [CrossRef]
- Kwon, D.J.; Ju, S.M.; Youn, G.S.; Choi, S.Y.; Park, J. Suppression of INOS and COX-2 Expression by Flavokawain A via Blockade of NF-ΚB and AP-1 Activation in RAW 264.7 Macrophages. Food Chem. Toxicol. 2013, 58, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Rzepecka-Stojko, A.; Kabała-Dzik, A.; Kubina, R.; Jasik, K.; Kajor, M.; Wrze’śniok, D.; Stojko, J. Protective Effect of Polyphenol-Rich Extract from Bee Pollen in a High-Fat Diet. Molecules 2018, 23, 805. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, P.M.; Jasielski, P.P.; Zarobkiewicz, M.K.; Sławiński, M.A.; Wawryk-Gawda, E.; Jodłowska-Jędrych, B. Changes in Histological Structure, Interleukin 12, Smooth Muscle Actin and Nitric Oxide Synthase 1. and 3. Expression in the Liver of Running and Non-Running Wistar Rats Supplemented with Bee Pollen or Whey Protein. Foods 2022, 11, 1131. [Google Scholar] [CrossRef] [PubMed]
- Zarobkiewicz, M.K.; Sławiński, M.A.; Wawryk-Gawda, E.; Woźniakowski, M.M.; Kulak-Janczy, E.; Korzeniowska, S.; Jodłowska-Jędrych, B. Changes in Histological Structure and Nitric Oxide Synthase Expression in Aorta of Rats Supplemented with Bee Pollen or Whey Protein. Appl. Physiol. Nutr. Metab. 2019, 44, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. Apitherapy for Age-Related Skeletal Muscle Dysfunction (Sarcopenia): A Review on the Effects of Royal Jelly, Propolis, and Bee Pollen. Foods 2020, 9, 1362. [Google Scholar] [CrossRef] [PubMed]
- Komosinska-Vassev, K.; Olczyk, P.; Kaźmierczak, J.; Mencner, L.; Olczyk, K. Bee Pollen: Chemical Composition and Therapeutic Application. Evid. Based. Complement. Alternat. Med. 2015, 2015, 297425. [Google Scholar] [CrossRef] [PubMed]
- Végh, R.; Csóka, M.; Sörös, C.; Sipos, L. Food Safety Hazards of Bee Pollen—A Review. Trends Food Sci. Technol. 2021, 114, 490–509. [Google Scholar] [CrossRef]
- Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011.
- Feldman, A.T.; Wolfe, D. Tissue Processing and Hematoxylin and Eosin Staining. Methods Mol. Biol. 2014, 1180, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Lis-Sochocka, M.; Chylińska-Wrzos, P.; Wawryk-Gawda, E.; Jodłowska-Jędrych, B. Expression of Caspase 1 and Histomorphology of Lung after Cladribine Treatment. Adv. Clin. Exp. Med. 2019, 28, 59–65. [Google Scholar] [CrossRef]
- Rosaneli, C.F.; Bighetti, A.E.; Antonio, M.A.; Carvalho, J.E.; Sgarbieri, V.C. Efficacy of a Whey Protein Concentrate on the Inhibition of Stomach Ulcerative Lesions Caused by Ethanol Ingestion. J. Med. Food 2004, 5, 221–228. [Google Scholar] [CrossRef]
- Wang, D.; Cabalag, C.S.; Clemons, N.J.; DuBois, R.N. Cyclooxygenases and Prostaglandins in Tumor Immunology and Microenvironment of Gastrointestinal Cancer. Gastroenterology 2021, 161, 1813–1829. [Google Scholar] [CrossRef]
- Nagaraju, G.P.; El-Rayes, B.F. Cyclooxygenase-2 in Gastrointestinal Malignancies. Cancer 2019, 125, 1221–1227. [Google Scholar] [CrossRef]
- Jaiswal, M.; Larusso, N.F.; Gores, G.J. Nitric Oxide in Gastrointestinal Epithelial Cell Carcinogenesis: Linking Inflammation to Oncogenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G626–G634. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, G.A.; Cheng, R.Y.S.; Ridnour, L.A.; Basudhar, D.; Somasundaram, V.; McVicar, D.W.; Monteiro, H.P.; Wink, D.A. Inducible Nitric Oxide Synthase in the Carcinogenesis of Gastrointestinal Cancers. Antioxid. Redox Signal. 2017, 26, 1059–1077. [Google Scholar] [CrossRef] [PubMed]
- Riccioni, G.; Scotti, L.; Guagnano, M.T.; Bosco, G.; Bucciarelli, V.; Di Ilio, E.; Speranza, L.; Martini, F.; Bucciarelli, T. Physical Exercise Reduces Synthesis of ADMA, SDMA, and L-Arg. Front. Biosci. Elit. 2015, 7E, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.J.O.; Vasconcelos, C.C.; Pereira, F.A.N.; Silva, R.H.M.; Queiroz, P.F.D.S.Q.; Fernandes, C.V.; Garcia, J.B.S.; Ramos, R.M.; Da Rocha, C.Q.; Lima, S.T.D.J.R.M.; et al. Anti-Inflammatory and Antinociceptive Activity of Pollen Extract Collected by Stingless Bee Melipona Fasciculata. Int. J. Mol. Sci. 2019, 20, 4512. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Sakamoto, T.; Araki, Y.; Hara, H. Anti-Inflammatory Effect of Bee Pollen Ethanol Extract from Cistus Sp. of Spanish on Carrageenan-Induced Rat Hind Paw Edema. BMC Complement. Altern. Med. 2010, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.C.; Huang, Y.T.; Tsai, S.H.; Lin-Shiau, S.Y.; Chen, C.F.; Lin, J.K. Suppression of Inducible Cyclooxygenase and Inducible Nitric Oxide Synthase by Apigenin and Related Flavonoids in Mouse Macrophages. Carcinogenesis 1999, 20, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Rzepecka-Stojko, A.; Stojko, J.; Jasik, K.; Buszman, E. Anti-Atherogenic Activity of Polyphenol-Rich Extract from Bee Pollen. Nutrients 2017, 9, 1369. [Google Scholar] [CrossRef] [PubMed]
- Tomić, M.; Micov, A.; Pecikoza, U.; Stepanović-Petrović, R. Clinical Uses of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) and Potential Benefits of NSAIDs Modified-Release Preparations. In Microsized and Nanosized Carriers for Nonsteroidal Anti-Inflammatory Drugs, Formulation Challenges and Potential Benefits; Academic Press: Cambridge, MA, USA, 2017; pp. 1–29. [Google Scholar] [CrossRef]
- Zhang, Z.; Zou, Y.Y.; Li, F.J.; Hu, C.P. Asymmetric Dimethylarginine: A Novel Biomarker of Gastric Mucosal Injury? World J. Gastroenterol. 2011, 17, 2178–2180. [Google Scholar] [CrossRef]
- Chelucci, E.; Chiellini, C.; Cavallero, A.; Gabriele, M. Bio-Functional Activities of Tuscan Bee Pollen. Antioxidants 2023, 12, 115. [Google Scholar] [CrossRef]
- Campos, M.G.R.; Bogdanov, S.; Bicudo De Almeida-Muradian, L.; Szczesna, T.; Mancebo, Y.; Frigerio, C.; Ferreira, F. Pollen Composition and Standardisation of Analytical Methods. J. Apic. Res. 2008, 47, 154–161. [Google Scholar] [CrossRef]
- Ketkar, S.; Rathore, A.; Kandhare, A.; Lohidasan, S.; Bodhankar, S.; Paradkar, A.; Mahadik, K. Alleviating Exercise-Induced Muscular Stress Using Neat and Processed Bee Pollen: Oxidative Markers, Mitochondrial Enzymes, and Myostatin Expression in Rats. Integr. Med. Res. 2015, 4, 147. [Google Scholar] [CrossRef]
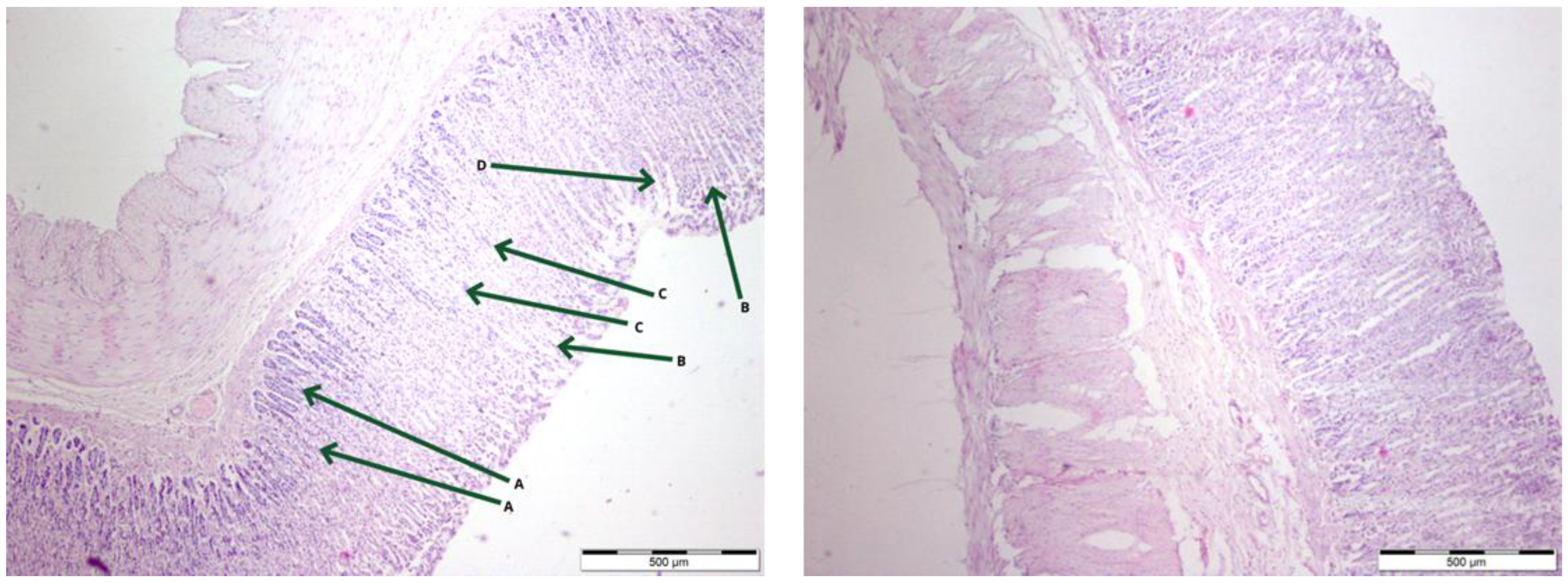
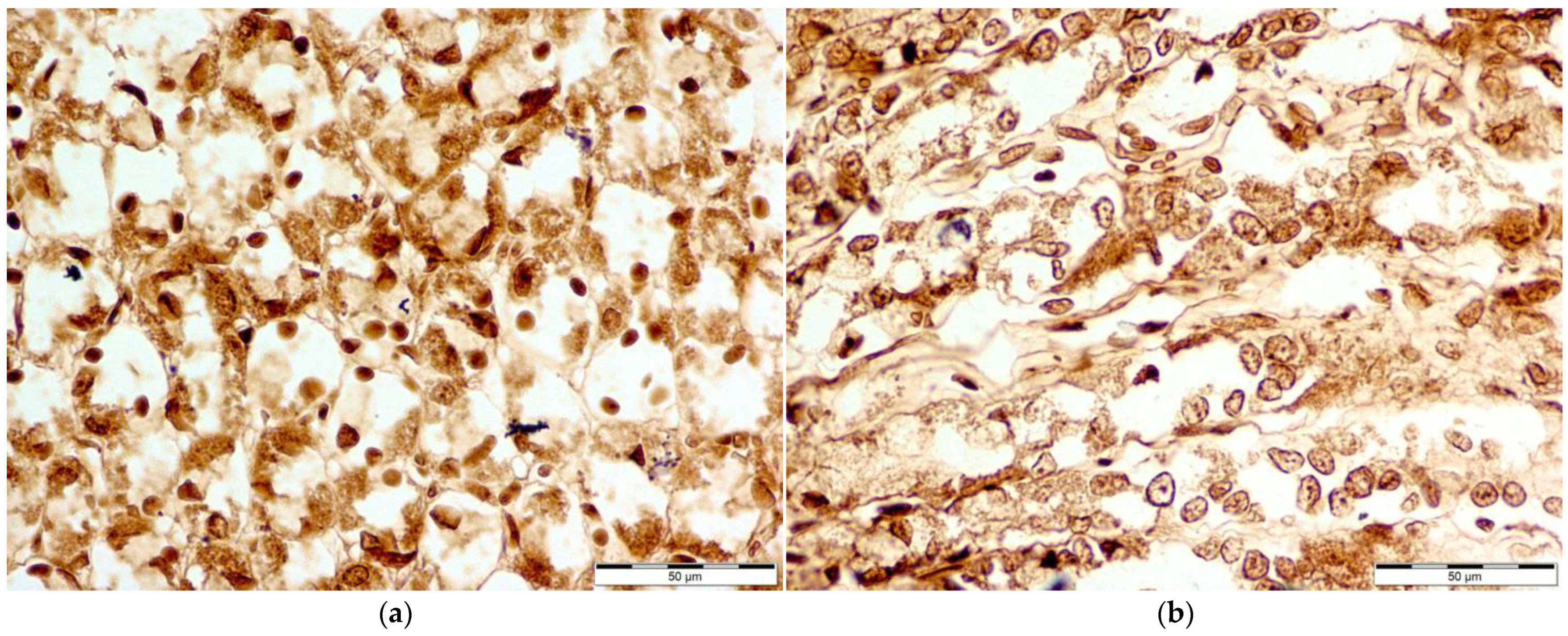
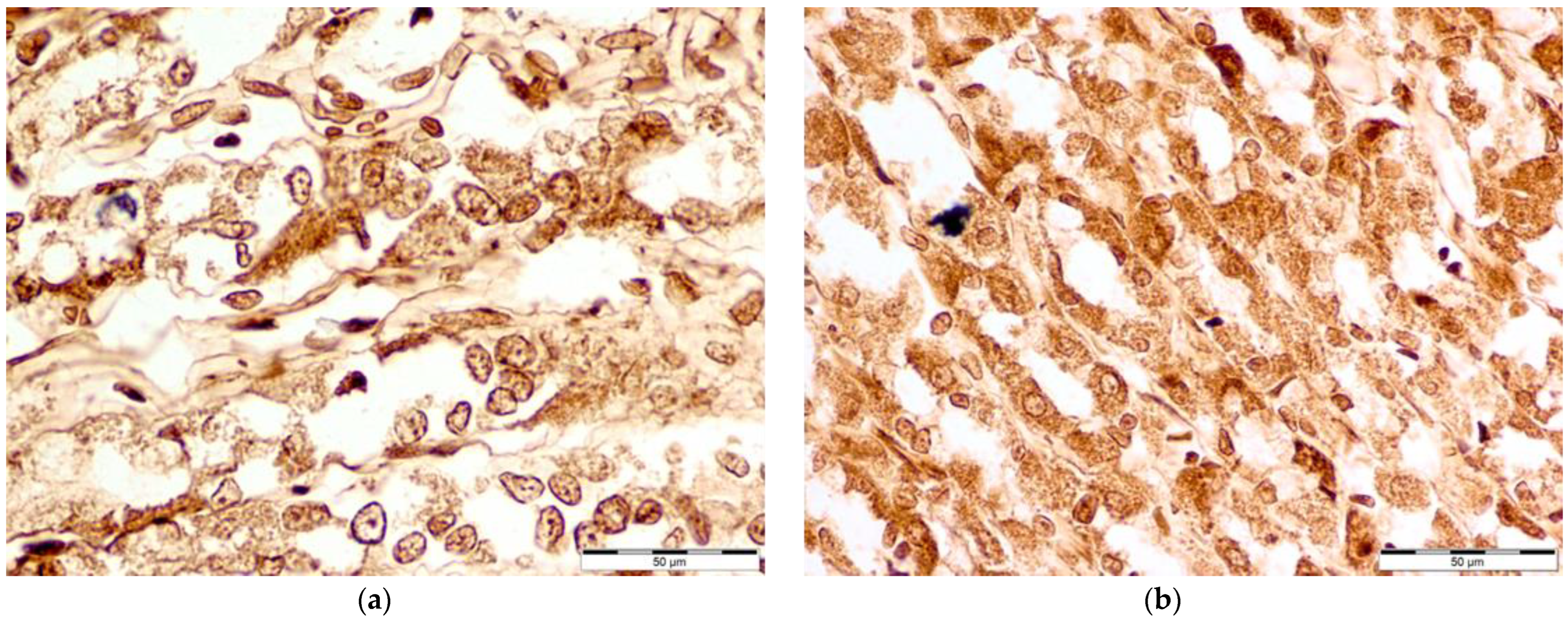
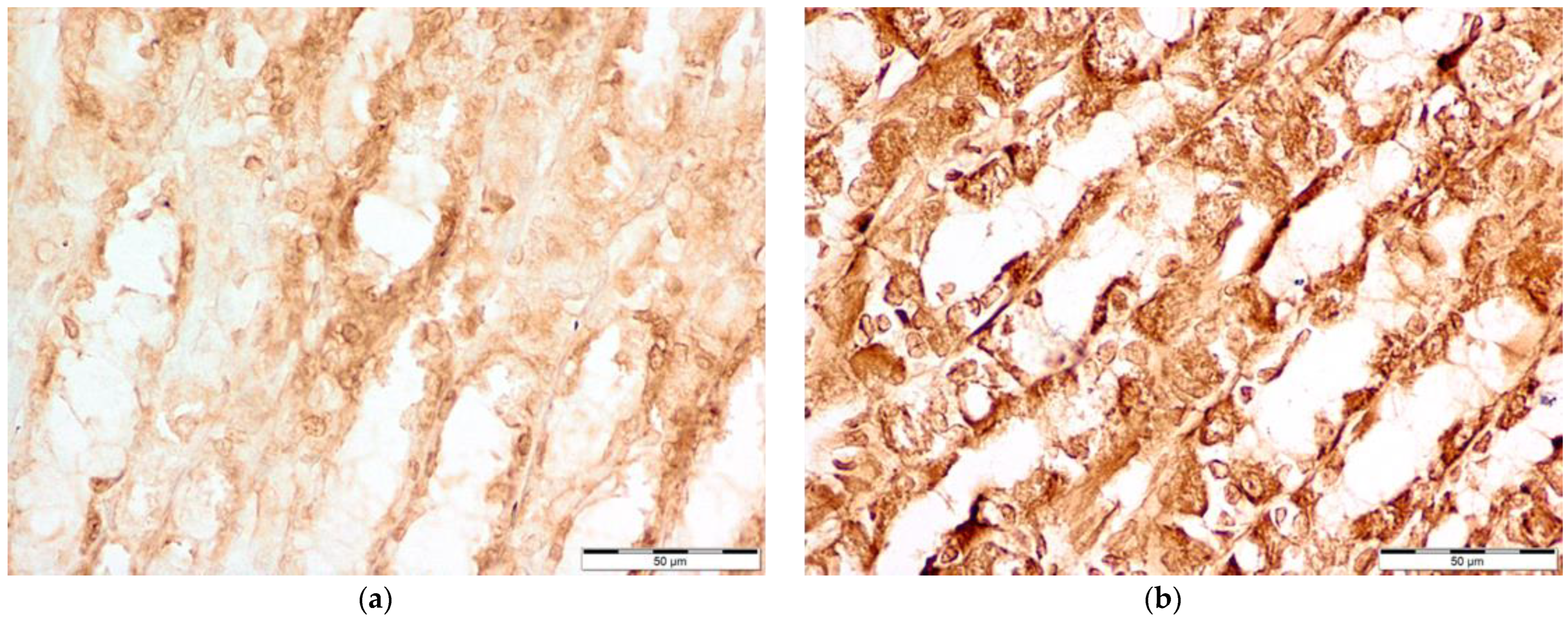



| Group | Running | Supplementation | Group | Running | Supplementation |
|---|---|---|---|---|---|
| Con-Sed (1) | No | No | Con-Run (3) | Yes | No |
| BP-Sed (2) | No | Bee pollen | BP-Run (4) | Yes | Bee pollen |
| Targeted Molecule | Group | − % | − (n) | + % | + (n) | ++ % | ++ (n) | Total (n) Evaluated |
|---|---|---|---|---|---|---|---|---|
| COX-1 | 1 (Con-Sed) | 19.00% | 57 | 40.00% | 120 | 41.00% | 123 | 300 |
| 2 (BP-Sed) | 48.33% | 145 | 31.00% | 93 | 20.67% | 62 | 300 | |
| 3 (Con-Run) | 21.67% | 65 | 39.67% | 119 | 38.66% | 116 | 300 | |
| 4 (BP-Run) | 32.67% | 98 | 44.67% | 134 | 22.66% | 68 | 300 | |
| COX-2 | 1 (Con-Sed) | 26.25% | 105 | 34.50% | 138 | 39.25% | 157 | 400 |
| 2 (BP-Sed) | 27.25% | 109 | 37.00% | 148 | 35.75% | 143 | 400 | |
| 3 (Con-Run) | 16.50% | 66 | 43.00% | 172 | 40.50% | 162 | 400 | |
| 4 (BP-Run) | 22.75% | 91 | 36.50% | 146 | 40.75% | 163 | 400 | |
| iNOS | 1 (Con-Sed) | 27.25% | 109 | 64.00% | 256 | 8.75% | 35 | 400 |
| 2 (BP-Sed) | 16.25% | 65 | 56.75% | 227 | 27.00% | 108 | 400 | |
| 3 (Con-Run) | 27.75% | 111 | 53.00% | 212 | 19.25% | 77 | 400 | |
| 4 (BP-Run) | 29.50% | 118 | 39.75% | 159 | 30.75% | 123 | 400 | |
| ADMA | 1 (Con-Sed) | 29.89% | 269 | 56.33% | 507 | 13.78% | 124 | 900 |
| 2 (BP-Sed) | 33.56% | 302 | 47.56% | 428 | 18.88% | 170 | 900 | |
| 3 (Con-Run) | 27.56% | 248 | 52.11% | 469 | 20.33% | 183 | 900 | |
| 4 (BP-Run) | 50.33% | 453 | 36.00% | 324 | 13.67% | 123 | 900 |
| Groups Compared | Con-Sed (1) | Con-Run (3) | BP-Sed (2) | BP-Run (4) |
| Con-Sed (1) | 1.000000 | <0.000001 1 | 0.000009 | |
| Con-Run (3) | 1.000000 | <0.000001 | 0.000272 | |
| BP-Sed (2) | <0.000001 | <0.000001 | 0.073937 | |
| BP-Run (4) | 0.000009 | 0.000272 | 0.073937 |
| Groups Compared | Con-Sed (1) | Con-Run (3) | BP-Sed (2) | BP-Run (4) |
| Con-Sed (1) | 0.405873 | <0.000001 1 | 0.001928 | |
| Con-Run (3) | 0.405873 | 0.001687 | 0.460279 | |
| BP-Sed (2) | <0.000001 | 0.001687 | 0.375597 | |
| BP-Run (4) | 0.001928 | 0.460279 | 0.375597 |
| Groups Compared | Con-Sed (1) | Con-Run (3) | BP-Sed (2) | BP-Run (4) |
| Con-Sed (1) | 0.129412 | 1.000000 | <0.000001 1 | |
| Con-Run (3) | 0.129412 | 0.164388 | <0.000001 | |
| BP-Sed (2) | 1.000000 | 0.164388 | <0.000001 | |
| BP-Run (4) | <0.000001 | <0.000001 | <0.000001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oszczędłowski, P.; Górecki, K.; Greluk, A.; Krawczyk, M.; Pacyna, K.; Kędzierawski, J.A.; Ziółko, A.K.; Chromiak, K.; Sławiński, M.A.; Raczkiewicz, P.; et al. All That Glitters Is Not Gold: Assessment of Bee Pollen Supplementation Effects on Gastric Mucosa. Nutrients 2024, 16, 37. https://doi.org/10.3390/nu16010037
Oszczędłowski P, Górecki K, Greluk A, Krawczyk M, Pacyna K, Kędzierawski JA, Ziółko AK, Chromiak K, Sławiński MA, Raczkiewicz P, et al. All That Glitters Is Not Gold: Assessment of Bee Pollen Supplementation Effects on Gastric Mucosa. Nutrients. 2024; 16(1):37. https://doi.org/10.3390/nu16010037
Chicago/Turabian StyleOszczędłowski, Paweł, Kamil Górecki, Aleksandra Greluk, Milena Krawczyk, Katarzyna Pacyna, Jan Andrzej Kędzierawski, Artur Kacper Ziółko, Karol Chromiak, Mirosław A. Sławiński, Przemysław Raczkiewicz, and et al. 2024. "All That Glitters Is Not Gold: Assessment of Bee Pollen Supplementation Effects on Gastric Mucosa" Nutrients 16, no. 1: 37. https://doi.org/10.3390/nu16010037
APA StyleOszczędłowski, P., Górecki, K., Greluk, A., Krawczyk, M., Pacyna, K., Kędzierawski, J. A., Ziółko, A. K., Chromiak, K., Sławiński, M. A., Raczkiewicz, P., Chylińska-Wrzos, P., Jodłowska-Jędrych, B., & Pedrycz-Wieczorska, A. (2024). All That Glitters Is Not Gold: Assessment of Bee Pollen Supplementation Effects on Gastric Mucosa. Nutrients, 16(1), 37. https://doi.org/10.3390/nu16010037









