Association between Western Dietary Patterns, Typical Food Groups, and Behavioral Health Disorders: An Updated Systematic Review and Meta-Analysis of Observational Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Extraction and Methodological Quality Assessment
2.5. Sensitivity Analysis and Subgroup Analysis
2.6. Statistical Analysis
3. Results
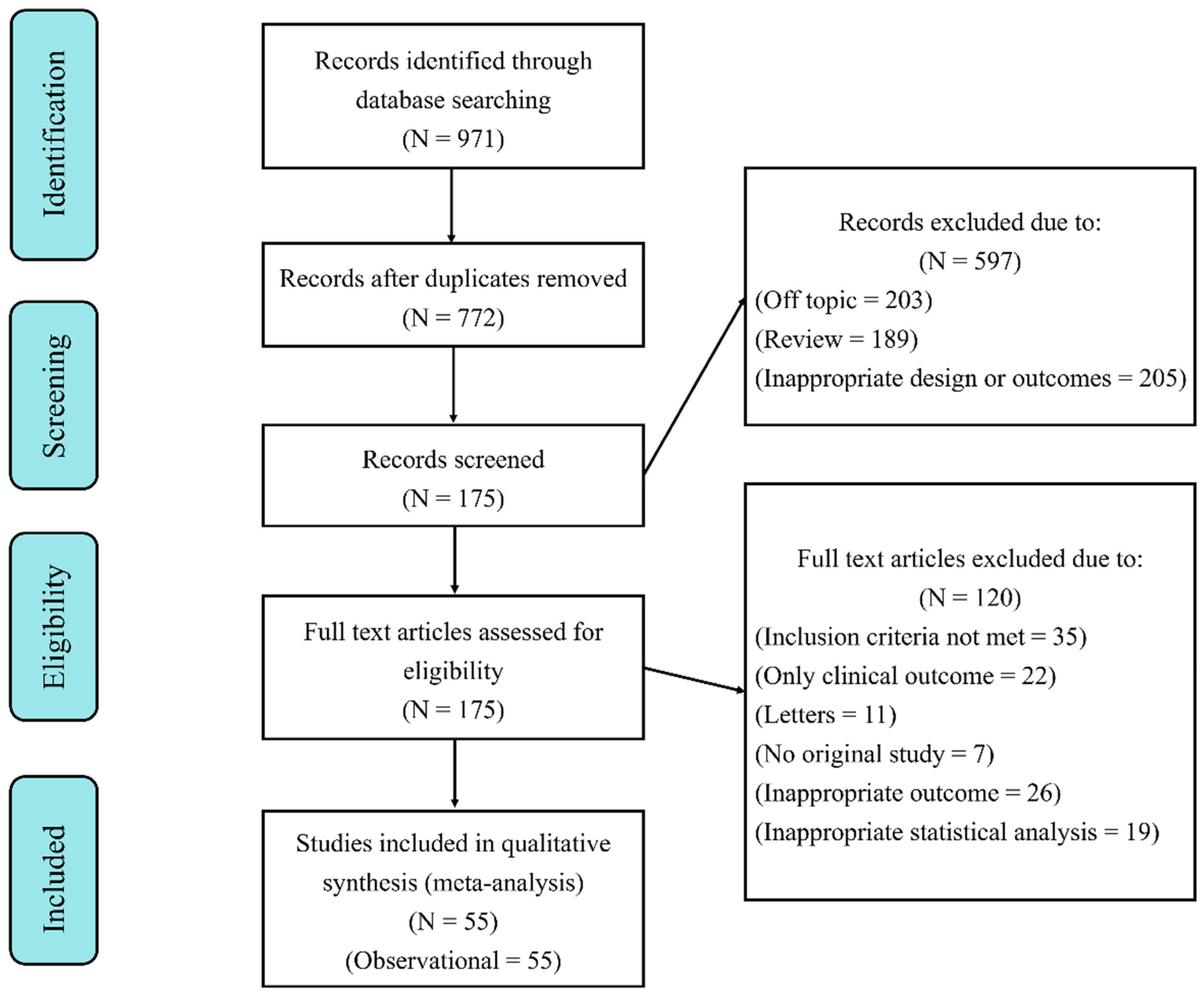
3.1. Search Results and Study Characteristics
3.2. Characteristics of Included Studies
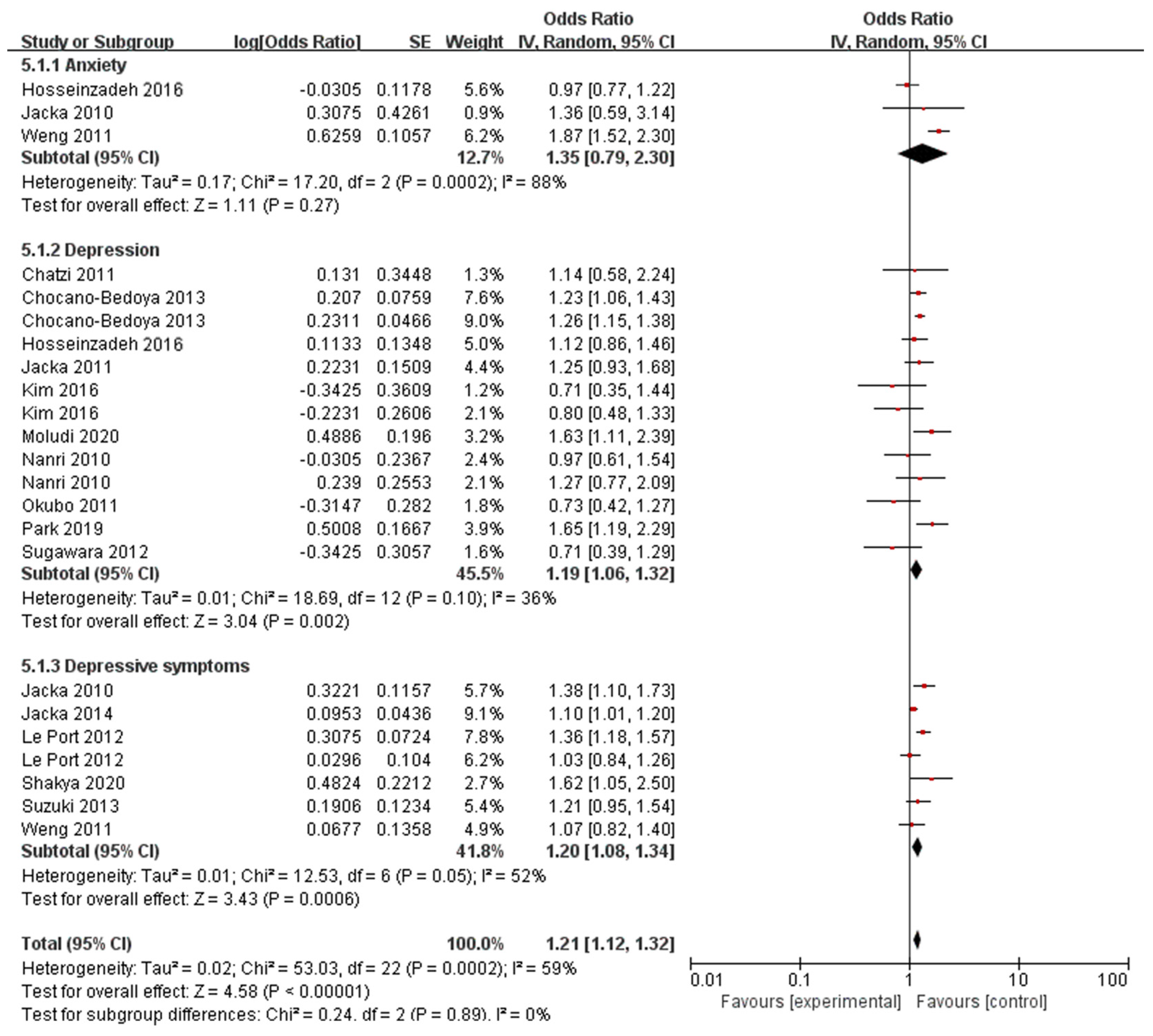
3.3. Western Dietary Pattern and Behavioral Health Disorders
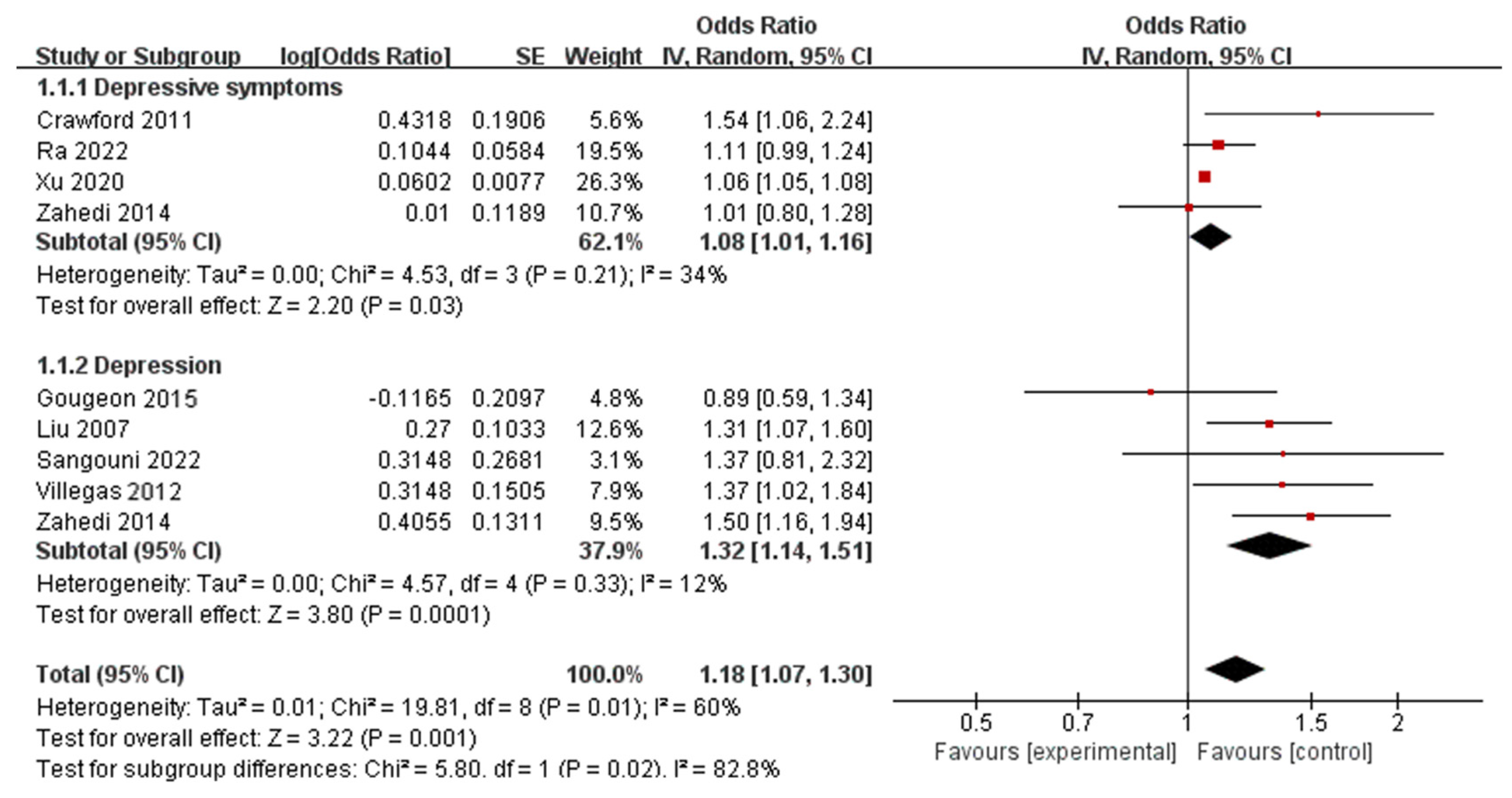
3.4. Fast Foods and Behavioral Health Disorders
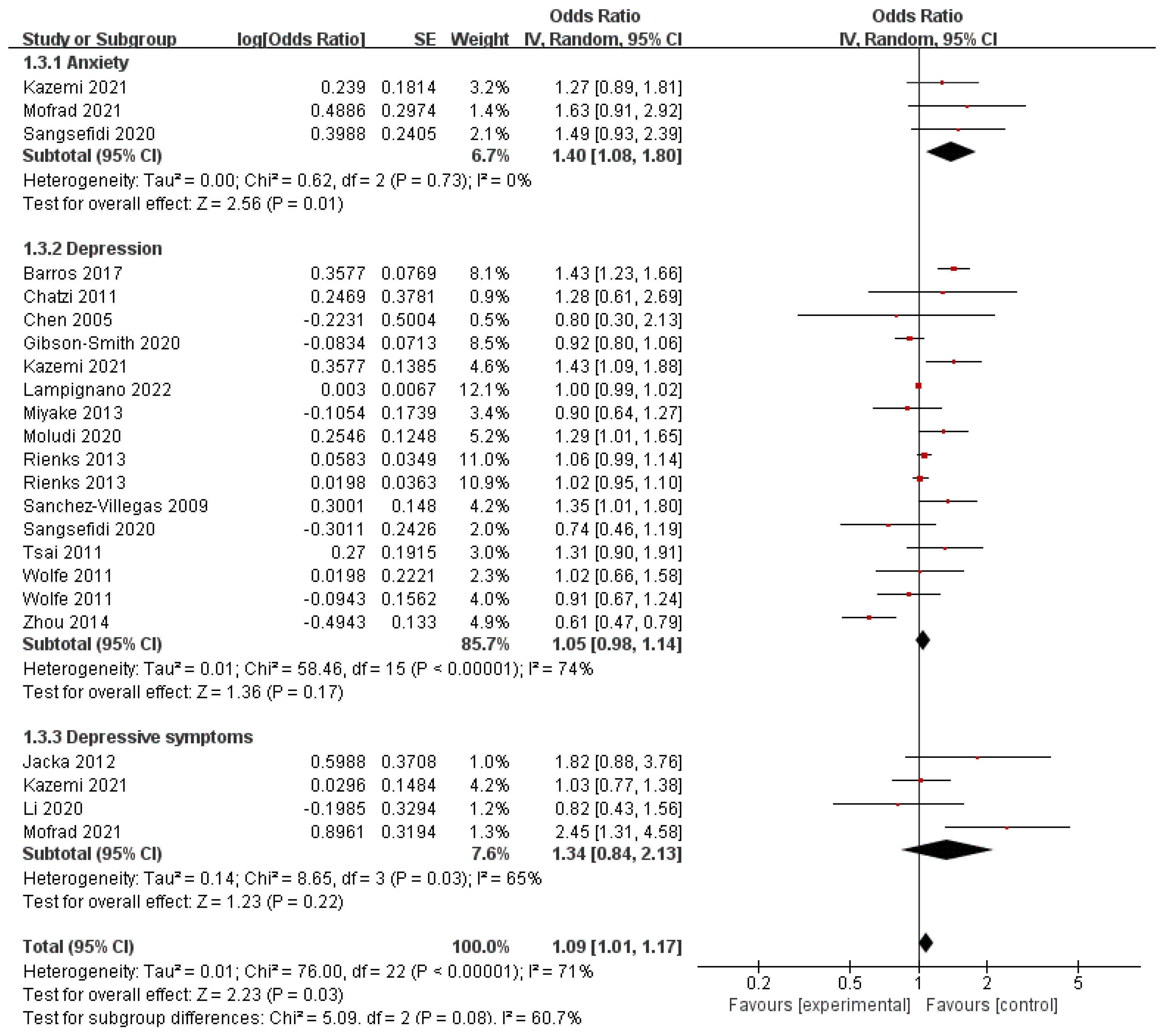
3.5. Red Meat and Behavioral Health Disorders
3.6. Refined Grain and Behavioral Health Disorders
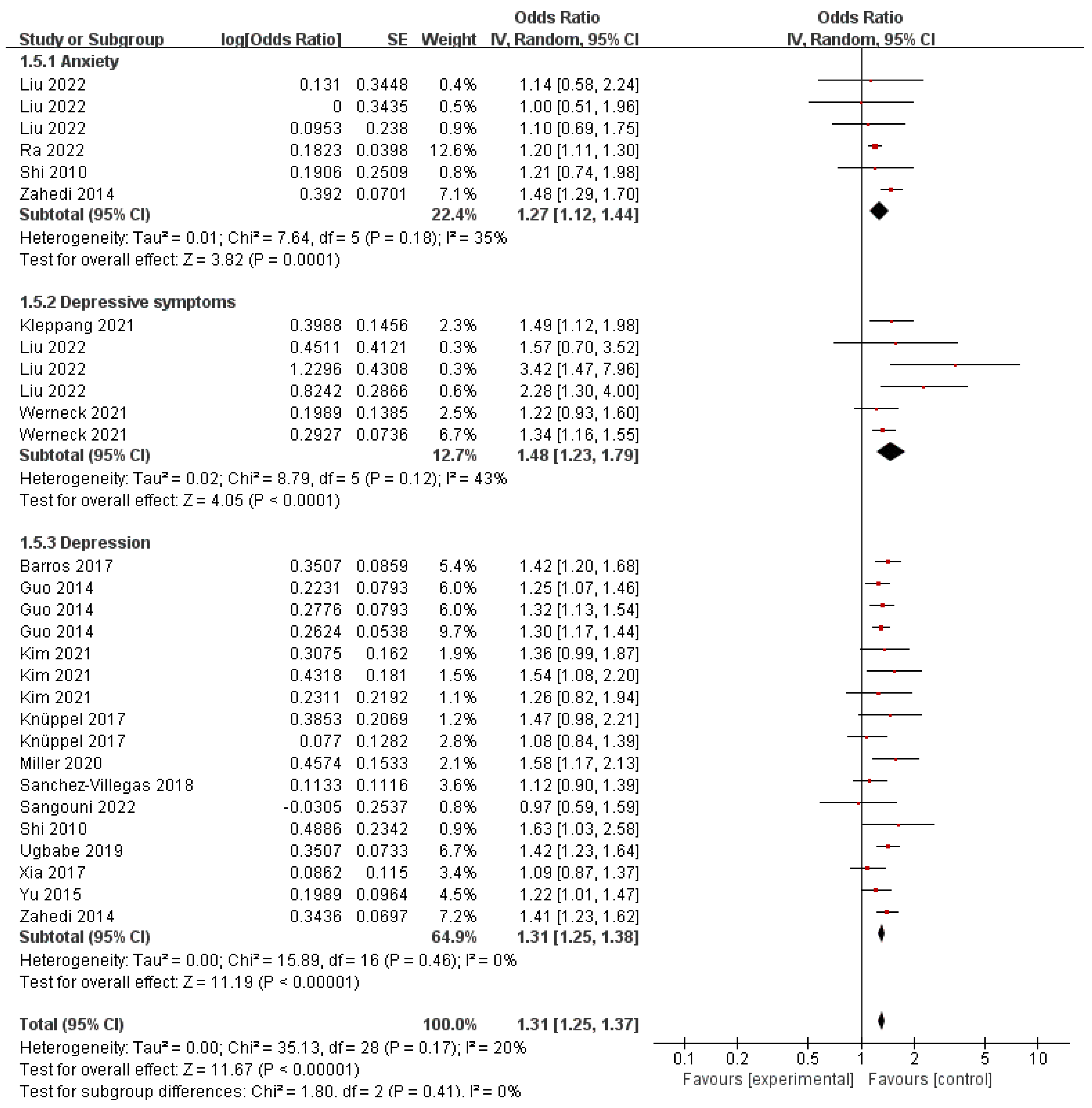
3.7. Sugar-Sweetened Beverage and Behavioral Health Disorders
3.8. High-Fat Dairy and Behavioral Health Disorders
3.9. Publication Bias and Sensitivity Analysis
3.10. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kris-Etherton, P.M.; Petersen, K.S.; Hibbeln, J.R.; Hurley, D.; Kolick, V.; Peoples, S.; Rodriguez, N.; Woodward-Lopez, G. Nutrition and behavioral health disorders: Depression and anxiety. Nutr. Rev. 2021, 79, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.B.d.A.; Lima, M.G.; Azevedo, R.C.S.d.; Medina, L.B.d.P.; Lopes, C.d.S.; Menezes, P.R.; Malta, D.C. Depression and health behaviors in Brazilian adults–PNS 2013. Rev. Saude Publica 2017, 51, 8s. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Fraile-Martínez, Ó.; García-Montero, C.; Alvarez-Mon, M.A.; Lahera, G.; Monserrat, J.; Llavero-Valero, M.; Gutiérrez-Rojas, L.; Molina, R.; Rodríguez-Jimenez, R. Biological role of nutrients, food and dietary patterns in the prevention and clinical management of major depressive disorder. Nutrients 2022, 14, 3099. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, G.S. Mechanisms and treatment of late-life depression. Transl. Psychiatry 2019, 9, 188. [Google Scholar] [CrossRef] [PubMed]
- Quirk, S.E.; Williams, L.J.; O’Neil, A.; Pasco, J.A.; Jacka, F.N.; Housden, S.; Berk, M.; Brennan, S.L. The association between diet quality, dietary patterns and depression in adults: A systematic review. BMC Psychiatry 2013, 13, 175. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hong, C.; Lee, G. Association between sugar-sweetened beverage consumption and depression and suicidal ideation among Korean adults: A cross-sectional study from the 2014 and 2016 Korean National Health and Nutrition Examination Survey (KNHANES). Nutr. Res. Pract. 2022, 16, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Gibson-Smith, D.; Bot, M.; Brouwer, I.A.; Visser, M.; Giltay, E.J.; Penninx, B.W. Association of food groups with depression and anxiety disorders. Eur. J. Nutr. 2020, 59, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Bi, B.; Zheng, L.; Li, Z.; Yang, H.; Song, H.; Sun, Y. The prevalence and risk factors for depression symptoms in a rural Chinese sample population. PLoS ONE 2014, 9, e99692. [Google Scholar] [CrossRef]
- Xu, Y.; Zeng, L.; Zou, K.; Shan, S.; Wang, X.; Xiong, J.; Zhao, L.; Zhang, L.; Cheng, G. Role of dietary factors in the prevention and treatment for depression: An umbrella review of meta-analyses of prospective studies. Transl. Psychiatry 2021, 11, 478. [Google Scholar] [CrossRef]
- Akbaraly, T.N.; Sabia, S.; Shipley, M.J.; Batty, G.D.; Kivimaki, M. Adherence to healthy dietary guidelines and future depressive symptoms: Evidence for sex differentials in the Whitehall II study. Am. J. Clin. Nutr. 2013, 97, 419–427. [Google Scholar] [CrossRef]
- Wu, P.-Y.; Lin, M.-Y.; Tsai, P.-S. Alternate healthy eating index and risk of depression: A meta-analysis and systemematic review. Nutr. Neurosci. 2020, 23, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, Y.; Chen, K.; Jing, Y.; He, J.; Sun, H.; Hu, X. Dietary inflammatory index and depression: A meta-analysis. Public Health Nutr. 2019, 22, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; Pasco, J.A.; Mykletun, A.; Williams, L.J.; Hodge, A.M.; O’Reilly, S.L.; Nicholson, G.C.; Kotowicz, M.A.; Berk, M. Association of Western and traditional diets with depression and anxiety in women. Am. J. Psychiatry 2010, 167, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Oddy, W.H.; Robinson, M.; Ambrosini, G.L.; Therese, A.; de Klerk, N.H.; Beilin, L.J.; Silburn, S.R.; Zubrick, S.R.; Stanley, F.J. The association between dietary patterns and mental health in early adolescence. Prev. Med. 2009, 49, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Park, Y.; Freedman, N.D.; Sinha, R.; Hollenbeck, A.R.; Blair, A.; Chen, H. Sweetened beverages, coffee, and tea and depression risk among older US adults. PLoS ONE 2014, 9, e94715. [Google Scholar] [CrossRef]
- Sangouni, A.A.; Ghayour-Mobarhan, M.; Mazidi, M.; Lane, K.E.; Khayyatzadeh, S.S. The association between intake of whole grain, refined grain, fast food and carbonated drinks with depression and quality of life in Iranian adolescent girls. Res. Sq. 2022. preprint. [Google Scholar]
- Wolfe, A.R.; Arroyo, C.; Tedders, S.H.; Li, Y.; Dai, Q.; Zhang, J. Dietary protein and protein-rich food in relation to severely depressed mood: A 10 year follow-up of a national cohort. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 232–238. [Google Scholar] [CrossRef]
- Hutton, B.; Catala-Lopez, F.; Moher, D. The PRISMA statement extension for systematic reviews incorporating network meta-analysis: PRISMA-NMA. Med. Clínica 2016, 147, 262–266. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Sugawara, N.; Yasui-Furukori, N.; Tsuchimine, S.; Kaneda, A.; Tsuruga, K.; Iwane, K.; Okubo, N.; Takahashi, I.; Kaneko, S. No association between dietary patterns and depressive symptoms among a community-dwelling population in Japan. Ann. Gen. Psychiatry 2012, 11, 24. [Google Scholar] [CrossRef]
- Park, S.-J.; Kim, M.-S.; Lee, H.-J. The association between dietary pattern and depression in middle-aged Korean adults. Nutr. Res. Pract. 2019, 13, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; Cherbuin, N.; Anstey, K.J.; Butterworth, P. Dietary patterns and depressive symptoms over time: Examining the relationships with socioeconomic position, health behaviours and cardiovascular risk. PLoS ONE 2014, 9, e87657. [Google Scholar] [CrossRef] [PubMed]
- Chocano-Bedoya, P.O.; O’Reilly, E.J.; Lucas, M.; Mirzaei, F.; Okereke, O.I.; Fung, T.T.; Hu, F.B.; Ascherio, A. Prospective study on long-term dietary patterns and incident depression in middle-aged and older women. Am. J. Clin. Nutr. 2013, 98, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Shakya, P.R.; Melaku, Y.A.; Page, A.; Gill, T.K. Association between dietary patterns and adult depression symptoms based on principal component analysis, reduced-rank regression and partial least-squares. Clin. Nutr. 2020, 39, 2811–2823. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Shin, D.; Song, W.O. Are dietary patterns associated with depression in US adults? J. Med. Food 2016, 19, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Moludi, J.; Moradinazar, M.; Hamzeh, B.; Najafi, F.; Soleimani, D.; Pasdar, Y. Depression relationship with dietary patterns and dietary inflammatory index in women: Result from ravansar cohort study. Neuropsychiatr. Dis. Treat. 2020, 16, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Nanri, A.; Kimura, Y.; Matsushita, Y.; Ohta, M.; Sato, M.; Mishima, N.; Sasaki, S.; Mizoue, T. Dietary patterns and depressive symptoms among Japanese men and women. Eur. J. Clin. Nutr. 2010, 64, 832–839. [Google Scholar] [CrossRef]
- Jacka, F.N.; Mykletun, A.; Berk, M.; Bjelland, I.; Tell, G.S. The association between habitual diet quality and the common mental disorders in community-dwelling adults: The Hordaland Health study. Psychosom. Med. 2011, 73, 483–490. [Google Scholar] [CrossRef]
- Le Port, A.; Gueguen, A.; Kesse-Guyot, E.; Melchior, M.; Lemogne, C.; Nabi, H.; Goldberg, M.; Zins, M.; Czernichow, S. Association between dietary patterns and depressive symptoms over time: A 10-year follow-up study of the GAZEL cohort. PLoS ONE 2012, 7, e51593. [Google Scholar] [CrossRef]
- Okubo, H.; Miyake, Y.; Sasaki, S.; Tanaka, K.; Murakami, K.; Hirota, Y. Dietary patterns during pregnancy and the risk of postpartum depression in Japan: The Osaka Maternal and Child Health Study. Br. J. Nutr. 2011, 105, 1251–1257. [Google Scholar] [CrossRef]
- Chatzi, L.; Melaki, V.; Sarri, K.; Apostolaki, I.; Roumeliotaki, T.; Georgiou, V.; Vassilaki, M.; Koutis, A.; Bitsios, P.; Kogevinas, M. Dietary patterns during pregnancy and the risk of postpartum depression: The mother–child ‘Rhea’ cohort in Crete, Greece. Public Health Nutr. 2011, 14, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, M.; Vafa, M.; Esmaillzadeh, A.; Feizi, A.; Majdzadeh, R.; Afshar, H.; Keshteli, A.H.; Adibi, P. Empirically derived dietary patterns in relation to psychological disorders. Public Health Nutr. 2016, 19, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Weng, T.-T.; Hao, J.-H.; Qian, Q.-W.; Cao, H.; Fu, J.-L.; Sun, Y.; Huang, L.; Tao, F.-B. Is there any relationship between dietary patterns and depression and anxiety in Chinese adolescents? Public Health Nutr. 2012, 15, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Miyaki, K.; Tsutsumi, A.; Hashimoto, H.; Kawakami, N.; Takahashi, M.; Shimazu, A.; Inoue, A.; Kurioka, S.; Kakehashi, M. Japanese dietary pattern consistently relates to low depressive symptoms and it is modified by job strain and worksite supports. J. Affect. Disord. 2013, 150, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Crawford, G.B.; Khedkar, A.; Flaws, J.A.; Sorkin, J.D.; Gallicchio, L. Depressive symptoms and self-reported fast-food intake in midlife women. Prev. Med. 2011, 52, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Villegas, A.; Toledo, E.; De Irala, J.; Ruiz-Canela, M.; Pla-Vidal, J.; Martínez-González, M.A. Fast-food and commercial baked goods consumption and the risk of depression. Public Health Nutr. 2012, 15, 424–432. [Google Scholar] [CrossRef]
- Liu, C.; Xie, B.; Chou, C.-P.; Koprowski, C.; Zhou, D.; Palmer, P.; Sun, P.; Guo, Q.; Duan, L.; Sun, X. Perceived stress, depression and food consumption frequency in the college students of China Seven Cities. Physiol. Behav. 2007, 92, 748–754. [Google Scholar] [CrossRef]
- Gougeon, L.; Payette, H.; Morais, J.; Gaudreau, P.; Shatenstein, B.; Gray-Donald, K. Dietary patterns and incidence of depression in a cohort of community-dwelling older Canadians. J. Nutr. Health Aging 2015, 19, 431–436. [Google Scholar] [CrossRef]
- Ra, J.S. Consumption of sugar-sweetened beverages and fast foods deteriorates adolescents’ mental health. Front. Nutr. 2022, 9, 1058190. [Google Scholar] [CrossRef]
- Xu, H.; Guo, J.; Wan, Y.; Zhang, S.; Yang, R.; Xu, H.; Ding, P.; Tao, F. Association between screen time, fast foods, sugar-sweetened beverages and depressive symptoms in Chinese adolescents. Front. Psychiatry 2020, 11, 458. [Google Scholar] [CrossRef]
- Liu, J.; Chen, T.; Chen, M.; Ma, Y.; Ma, T.; Gao, D.; Li, Y.; Ma, Q.; Chen, L.; Wang, X. Sugar-sweetened beverages and depressive and social anxiety symptoms among children and adolescents aged 7–17 years, stratified by body composition. Front. Nutr. 2022, 9, 888671. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.; Ettridge, K.; Wakefield, M.; Pettigrew, S.; Coveney, J.; Roder, D.; Durkin, S.; Wittert, G.; Martin, J.; Dono, J. Consumption of sugar-sweetened beverages, juice, artificially-sweetened soda and bottled water: An Australian population study. Nutrients 2020, 12, 817. [Google Scholar] [CrossRef] [PubMed]
- Ugbabe, O.A. Association between the Consumption of Sugar-Sweetened Beverages and Poor Mental Health among Adults in the United States. Prev. Chronic Dis. 2019, 18, 200574. [Google Scholar]
- Werneck, A.O.; Schuch, F.B.; Stubbs, B.; Oyeyemi, A.L.; Szwarcwald, C.L.; Vancampfort, D.; Silva, D.R. Independent and combined associations of sugar-sweetened beverage consumption, TV viewing, and physical activity with severe depressive symptoms among 59,402 adults. Braz. J. Psychiatry 2020, 43, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Villegas, A.; Zazpe, I.; Santiago, S.; Perez-Cornago, A.; Martinez-Gonzalez, M.A.; Lahortiga-Ramos, F. Added sugars and sugar-sweetened beverage consumption, dietary carbohydrate index and depression risk in the Seguimiento Universidad de Navarra (SUN) Project. Br. J. Nutr. 2018, 119, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Knüppel, A.; Shipley, M.J.; Llewellyn, C.H.; Brunner, E.J. Sugar intake from sweet food and beverages, common mental disorder and depression: Prospective findings from the Whitehall II study. Sci. Rep. 2017, 7, 6287. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; He, H.; Zhang, Q.; Wu, H.; Du, H.; Liu, L.; Wang, C.; Shi, H.; Xia, Y.; Guo, X. Soft drink consumption is associated with depressive symptoms among adults in China. J. Affect. Disord. 2015, 172, 422–427. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, N.; Yu, B.; Zhang, Q.; Liu, L.; Meng, G.; Wu, H.; Du, H.; Shi, H.; Guo, X. Dietary patterns are associated with depressive symptoms among Chinese adults: A case–control study with propensity score matching. Eur. J. Nutr. 2017, 56, 2577–2587. [Google Scholar] [CrossRef]
- Zahedi, H.; Kelishadi, R.; Heshmat, R.; Motlagh, M.E.; Ranjbar, S.H.; Ardalan, G.; Payab, M.; Chinian, M.; Asayesh, H.; Larijani, B. Association between junk food consumption and mental health in a national sample of Iranian children and adolescents: The CASPIAN-IV study. Nutrition 2014, 30, 1391–1397. [Google Scholar] [CrossRef]
- Shi, Z.; Taylor, A.W.; Wittert, G.; Goldney, R.; Gill, T.K. Soft drink consumption and mental health problems among adults in Australia. Public Health Nutr. 2010, 13, 1073–1079. [Google Scholar] [CrossRef]
- Sangsefidi, Z.S.; Mirzaei, M.; Hosseinzadeh, M. The relation between dietary intakes and psychological disorders in Iranian adults: A population-based study. BMC Psychiatry 2020, 20, 257. [Google Scholar] [CrossRef] [PubMed]
- Rienks, J.; Dobson, A.; Mishra, G. Mediterranean dietary pattern and prevalence and incidence of depressive symptoms in mid-aged women: Results from a large community-based prospective study. Eur. J. Clin. Nutr. 2013, 67, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.C.; Chang, T.-L.; Chi, S.-H. Frequent consumption of vegetables predicts lower risk of depression in older Taiwanese–results of a prospective population-based study. Public Health Nutr. 2012, 15, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, S.; Keshteli, A.H.; Saneei, P.; Afshar, H.; Esmaillzadeh, A.; Adibi, P. Red and white meat intake in relation to mental disorders in Iranian adults. Front. Nutr. 2021, 8, 710555. [Google Scholar] [CrossRef] [PubMed]
- Mofrad, M.D.; Mozaffari, H.; Sheikhi, A.; Zamani, B.; Azadbakht, L. The association of red meat consumption and mental health in women: A cross-sectional study. Complement. Ther. Med. 2021, 56, 102588. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wei, L.; Hu, Z.; Qin, X.; Copeland, J.R.; Hemingway, H. Depression in older people in rural China. Arch. Intern. Med. 2005, 165, 2019–2025. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Villegas, A.; Delgado-Rodríguez, M.; Alonso, A.; Schlatter, J.; Lahortiga, F.; Majem, L.S.; Martínez-González, M.A. Association of the Mediterranean dietary pattern with the incidence of depression: The Seguimiento Universidad de Navarra/University of Navarra follow-up (SUN) cohort. Arch. Gen. Psychiatry 2009, 66, 1090–1098. [Google Scholar] [CrossRef]
- Miyake, Y.; Tanaka, K.; Okubo, H.; Sasaki, S.; Arakawa, M. Fish and fat intake and prevalence of depressive symptoms during pregnancy in Japan: Baseline data from the Kyushu Okinawa Maternal and Child Health Study. J. Psychiatr. Res. 2013, 47, 572–578. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Li, S.; Zhang, D. Association between dietary protein intake and the risk of depressive symptoms in adults. Br. J. Nutr. 2020, 11, 1290–1301. [Google Scholar] [CrossRef]
- Jacka, F.N.; Pasco, J.A.; Williams, L.J.; Mann, N.; Hodge, A.; Brazionis, L.; Berk, M. Red meat consumption and mood and anxiety disorders. Psychother. Psychosom. 2012, 81, 196–198. [Google Scholar] [CrossRef]
- Lampignano, L.; Sardone, R.; D’Urso, F.; Altamura, M.; Piccininni, C.; Griseta, C.; Bortone, I.; Castellana, F.; Zupo, R.; Donghia, R. Processed meat consumption and the risk of incident late-onset depression: A 12-year follow-up of the Salus in Apulia Study. Age Ageing 2022, 51, afab257. [Google Scholar] [CrossRef] [PubMed]
- Kleppang, A.L.; de Ridder, K.; Haugland, S.H.; Stea, T.H. Physical activity, sugar-sweetened beverages, whole grain bread and insomnia among adolescents and psychological distress in adulthood: Prospective data from the population-based HUNT study. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, O.; Hassanzadeh-Keshteli, A.; Afshar, H.; Esmaillzadeh, A.; Adibi, P. The association of whole and refined grains consumption with psychological disorders among Iranian adults. Eur. J. Nutr. 2019, 58, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Sarsangi, P.; Sasanfar, B.; Dehghani, F.; Nadjarzadeh, A.; Esmaillzadeh, A.; Salehi-Abargouei, A.; Mirzaei, M. Substituting whole grains for refined grains and risk of developing psychological disorders in Iranian adults: YaHS and TAMYZ studies. Curr. Psychol. 2022, 42, 30250–30261. [Google Scholar] [CrossRef]
- Hockey, M.; Mohebbi, M.; Tolmunen, T.; Hantunen, S.; Tuomainen, T.-P.; Macpherson, H.; Jacka, F.N.; Virtanen, J.K.; Rocks, T.; Ruusunen, A. Associations between total dairy, high-fat dairy and low-fat dairy intake, and depressive symptoms: Findings from a population-based cross-sectional study. Eur. J. Nutr. 2023, 62, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhou, Y.; Yu, L.; Huang, X.; Huang, J.; Wang, K.; Liu, Z. Protective Effect of Eurotium cristatum Fermented Loose Dark Tea and Eurotium cristatum Particle on MAPK and PXR/AhR Signaling Pathways Induced by Electronic Cigarette Exposure in Mice. Nutrients 2022, 14, 2843. [Google Scholar] [CrossRef] [PubMed]
- Nucci, D.; Fatigoni, C.; Amerio, A.; Odone, A.; Gianfredi, V. Red and processed meat consumption and risk of depression: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2020, 17, 6686. [Google Scholar] [CrossRef]
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.C.; Byrne, M.L. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013, 11, 200. [Google Scholar] [CrossRef]
- Açik, M.; Çakiroğlu, F.P. Evaluating the relationship between inflammatory load of a diet and depression in young adults. Ecol. Food Nutr. 2019, 58, 366–378. [Google Scholar] [CrossRef]
- Lotrich, F.E. Inflammatory cytokine-associated depression. Brain Res. 2015, 1617, 113–125. [Google Scholar] [CrossRef]
- Gangwisch, J.E.; Hale, L.; St-Onge, M.-P.; Choi, L.; LeBlanc, E.S.; Malaspina, D.; Opler, M.G.; Shadyab, A.H.; Shikany, J.M.; Snetselaar, L. High glycemic index and glycemic load diets as risk factors for insomnia: Analyses from the Women’s Health Initiative. Am. J. Clin. Nutr. 2020, 111, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Currenti, W.; Angelino, D.; Mena, P.; Castellano, S.; Caraci, F.; Galvano, F.; Del Rio, D.; Ferri, R.; Grosso, G. Diet and mental health: Review of the recent updates on molecular mechanisms. Antioxidants 2020, 9, 346. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.; Barros, L.; Ferreira, I.C. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Martinowich, K.; Manji, H.; Lu, B. New insights into BDNF function in depression and anxiety. Nat. Neurosci. 2007, 10, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Quan, W.; Li, Y.; Jiao, Y.; Xue, C.; Liu, G.; Wang, Z.; He, Z.; Qin, F.; Zeng, M.; Chen, J. Simultaneous generation of acrylamide, β-carboline heterocyclic amines and advanced glycation ends products in an aqueous Maillard reaction model system. Food Chem. 2020, 332, 127387. [Google Scholar] [CrossRef]
- Wang, Y.; Kuang, Y.; Luo, J.; Zeng, M.; He, Z.; Shen, Q.; Chen, J.; Li, M.; Quan, W. Health effects of exposure to β-carboline heterocyclic amines: Insight into metabolic perturbations and biochemical analysis. Food Funct. 2023, 14, 4006–4016. [Google Scholar] [CrossRef]
- Zhang, H.; Mo, L.; Chen, X.; Li, M.; Li, M.; Xu, Y.; Zeng, M.; He, Z.; Shen, Q.; Chen, J. The effect of exogenous free Nε-(Carboxymethyl) Lysine on diabetes-associated cognitive dysfunction: Neuroinflammation, and metabolic disorders. Food Sci. Hum. Wellness 2023, 69, 783–793. [Google Scholar]
- Quan, W.; Li, M.; Jiao, Y.; Zeng, M.; He, Z.; Shen, Q.; Chen, J. Effect of dietary exposure to acrylamide on diabetes-associated cognitive dysfunction from the perspectives of oxidative damage, neuroinflammation, and metabolic disorders. J. Agric. Food Chem. 2022, 70, 4445–4456. [Google Scholar] [CrossRef]


| No. | Authors, Year, Country of Study | N = Subjects (Case) | Age; Year | Outcome Assessment (Diagnosis Criteria) | Quality Score | ||
|---|---|---|---|---|---|---|---|
| Type | Tool | Cut-Off | |||||
| 1 | Sugawara, 2012, [20] Japan | 791 (31) | 22–86 | Depression | CES-D | ≥16 | 5 |
| 2 | Park, 2019, [21] Korea | 338 (448) | 40–69 | Depression | BDI | ≥16 | 6 |
| 3 | Jacka, 2014, [22] Australia | 3663 (343) | 20–64 | Depressive symptoms | GDS | ≥6 | 6 |
| 4 | Chocano-Bedoya, 2013, [23] U.S | 50,605 (3002) | 50–77 | Depression | Clinical diagnosis | - | 8 |
| 5 | Shakya, 2020, [24] Australia | 1743 (86) | >24 | Depressive symptoms | CES-D | ≥16 | 6 |
| 6 | Kim, 2016, [25] U.S | 4180 (836) | 20–79 | Depression | PHQ-9 | ≥10 | 5 |
| 7 | Moludi, 2020, [26] Iran | 4630 (273) | 25–65 | Depression | Clinical diagnosis | - | 6 |
| 8 | Jacka, 2010, [13] Australia | 1046 (60) | 20–93 | Depressive symptoms/Anxiety | GHQ-12/SCID-I/NP | - | 5 |
| 9 | Nanri, 2010, [27] Japan | 521 (56) | 21–67 | Depression | CES-D | ≥16 | 5 |
| 10 | Jacka, 2011, [28] Norway | 3254 (281) | - | Depression/Anxiety | HADS | ≥8 | 6 |
| 11 | Le Port, 2012, [29] France | 9272 (630) | 35–50 | Depressive symptoms | CES-D | ≥17 (M) ≥23 (F) | 7 |
| 12 | Okubo, 2011, [30] Japan | 865 (121) | 29.9 | Depression | EPDS | ≥9 | 4 |
| 13 | Chatzi, 2011, [31] Greece | 529 (176) | - | Depression | EPDS | ≥13 | 4 |
| 14 | Hosseinzadeh, 2016, [32] Iran | 3846 (525) | 20–55 | Depression/Distress/Anxiety | HADS | ≥8 | 6 |
| 15 | Weng, 2012, [33] China | 5003 (560) | 11–16 | Depressive symptoms/Anxiety | DSRS/SCARED | ≥15 | 5 |
| 16 | Suzuki, 2013, [34] Japan | 2266 (167) | 21–65 | Depressive symptoms | K6 scale | ≥9 | 5 |
| No. | Authors, Year, Country of Study | N = Subjects (Case) | Age; Year | Outcome Assessment (Diagnosis Criteria) | Type | Quality Score | ||
|---|---|---|---|---|---|---|---|---|
| Type | Tool | Cut-off | ||||||
| 1 | Crawford, 2011, [35] U.S | 626 (155) | 45–54 | Depressive symptoms | CES-D | ≥16 | FFP | 4 |
| 2 | Villegas, 2012, [36] Spain | 10,374 (118) | - | Depression | SCID-I | - | FFP | 7 |
| 3 | Liu, 2007, [37] Norway | 2579 (368) | - | Depression | CES-D | ≥16 | FFP | 6 |
| 4 | Gougeon, 2015, [38] Canada | 1358 (170) | 67–84 | Depression | Geriatric Depression Scale | ≥11 | FFP | 5 |
| 5 | Ra, 2022, [39] Korea | 24,006 (19,806) | <18 | Depressive symptoms/Anxiety | Clinical diagnosis | - | SSB/FFP | 6 |
| 6 | Xu, 2020, [40] China | 14,500 (4217) | <20 | Depressive symptoms | CDI | ≥20 | SSB/FFP | 7 |
| 7 | Liu, 2022, [41] China | 1311 (183) | 7–17 | Depressive symptoms/Anxiety | CDI/SASC | ≥20 | SSB | 7 |
| 8 | Kim, 2021, [6] Korea | 5465 (739) | >20 | Depression | PHQ-9 | ≥5 | SSB | 5 |
| 9 | Miller, 2020, [42] Australia | 3430 (387) | - | Depression | Clinical diagnosis | - | SSB | 5 |
| 10 | Ugbabe, 2019, [43] U.S | 53,637 (10,597) | >18 | Depression | Clinical diagnosis | - | SSB | 7 |
| 11 | Werneck, 2021, [44] Spain | 25,920 (3715) | 42.9 | Depressive symptoms | PHQ-9 | ≥10 | SSB | 7 |
| 12 | Guo, 2014, [15] U.S | 10,524 (653) | 61.5 | Depression | Clinical diagnosis | - | SSB | 5 |
| 13 | Sanchez-Villegas, 2018, [45] Spain | 15,546 (769) | - | Depression | Clinical diagnosis | - | SSB | 5 |
| 14 | Knüppel, 2017, [46] UK | 9895 (1229) | 35–55 | Depression | CES-D | ≥16 | SSB | 6 |
| 15 | Yu, 2015, [47] China | 3667 (2565) | 42.5 | Depression | SDS | ≥40 | SSB | 5 |
| 16 | Barros, 2017, [2] Brazil | 49,025 (5144) | 37 | Depression | PHQ-9 | ≥20 | SSB/Red meat | 7 |
| 17 | Xia, 2017, [48] China | 2702 (1351) | 46.2 | Depression | SDS | ≥45 | SSB | 6 |
| 18 | Zahedi, 2014, [49] Iran | 13,486 (2794) | - | Depression/Anxiety | GSHS | - | SSB/FFP | 7 |
| 19 | Shi, 2010, [50] Australia | 4741 (326) | >16 | Depression/Anxiety | Clinical diagnosis/K10 | ≥22 | SSB | 6 |
| 20 | Sangsefidi, 2020, [51] Iran | 9965 (1651) | 20–69 | Depression/Anxiety | DASS 21 items | ≥10 | Red meat | 7 |
| 21 | Gibson-Smith, 2020, [7] Netherland | 1634 (414) | 18–65 | Depression | IDS/BAI/FEAR | - | Red meat/HFD | 5 |
| 22 | Rienks, 2013, [52] Australia | 8369 (1742) | 50–55 | Depression | CES-D | ≥10 | Red meat | 7 |
| 23 | Tsai, 2011, [53] Taiwan | 1609 (203) | >65 | Depression | CES-D | ≥10 | Red meat | 6 |
| 24 | Wolfe, 2011, [17] U.S | 1962 (223) | 25–74 | Depression | CES-D | ≥16 | Red meat | 6 |
| 25 | Kazemi, 2021, [54] Iran | 3362 (962) | 18–55 | Depression | HADS/GHQ | ≥4 | Red meat | 6 |
| 26 | Mofrad, 2021, [55] Iran | 482 (128) | 20–50 | Depressive symptoms | DASS 21 items | ≥10 | Red meat | 5 |
| 27 | Chen, 2005, [56] China | 1600 (142) | >60 | Depression | GMS | - | Red meat | 5 |
| 28 | Sanchez-Villegas, 2009, [57] Spain | 10,094 (480) | 37.2 | Depression | Clinical diagnosis | - | Red meat | 6 |
| 29 | Miyake, 2013, [58] Japan | 1745 | 31.2 | Depression | CES-D | ≥16 | Red meat | 5 |
| 30 | Zhou, 2014, [8] China | 11,473 | >65 | Depression | PHQ-9 | ≥10 | Red meat | 5 |
| 31 | Li, 2020, [59] U.S | 17,845 (1647) | 18–65 | Depressive symptoms | PHQ-9 | ≥10 | Red meat | 7 |
| 32 | Jacka, 2012, [60] Australia | 1046 (60) | 20–93 | Depressive symptoms | SCID-I/NP | - | Red meat | 5 |
| 33 | Lampignano, 2022, [61] Italy | 546 | - | Depression | DSM-IV-TR | - | Red meat | 5 |
| 34 | Kleppang, 2021, [62] Norway | 2230 | - | Depressive symptoms | CONOR-MHI | ≥2.15 | SSB | 5 |
| 35 | Sadeghi, 2017, [63] Iran | 1398 | 18–55 | Depression/Anxiety | HADS | - | Refined grain | 5 |
| 36 | Sarsangi, 2022, [64] Iran | 7574 (1333) | 20–70 | Depression/Anxiety | DASS 21 items | - | Refined grain | 7 |
| 37 | Sangouni, 2022, [16] Iran | 733 | 12–18 | Depression | BDI | ≥13 | Refined grain/SSB/FFP | 5 |
| 38 | Hockey, 2023, [65] Finland | 1600 (166) | 63 | Depression | DSM-III | ≥5 | HFD | 6 |
| 39 | Chatzi, 2011, [31] Greece | 529 (176) | - | Depression | EPDS | ≥13 | HFD/Red meat | 4 |
| Exposure and Outcomes | Factors | Variables | No. of Studies | RR (95% CI) | Test of Heterogeneity 1 | p 2 | |
|---|---|---|---|---|---|---|---|
| p | I2 (%) | ||||||
| WDP and depressive symptoms | Gender | Female | 4 | 1.06 (0.88–1.27) | 0.06 | 59 | 0.53 |
| Male | 3 | 1.36 (1.25–1.47) | 0.99 | 0 | <0.01 | ||
| WDP and depression | Gender | Female | 3 | 1.09 (0.95–1.27) | 0.42 | 0 | 0.23 |
| Male | 2 | 1.11 (0.98–1.24) | 0.68 | 0 | 0.29 | ||
| Location | Western countries | 3 | 1.08 (0.95–1.23) | 0.46 | 0 | 0.21 | |
| Eastern countries | 4 | 1.48 (1.12–1.96) | 0.15 | 43 | <0.01 | ||
| Red meat and depression | Number of participates | <2000 | 7 | 1.00 (0.99–1.02) | 0.62 | 0 | 0.71 |
| 2000–10,000 | 5 | 1.04 (0.97–1.12) | 0.18 | 36 | 0.28 | ||
| >10,000 | 2 | 1.41 (1.24–1.61) | 0.73 | 0 | <0.01 | ||
| Location | Western countries | 9 | 1.03 (0.98–1.07) | 0.73 | 0 | <0.01 | |
| Eastern countries | 6 | 1.41 (1.24–1.61) | 0.73 | 0 | <0.01 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Li, M.; Mo, L.; Luo, J.; Shen, Q.; Quan, W. Association between Western Dietary Patterns, Typical Food Groups, and Behavioral Health Disorders: An Updated Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2024, 16, 125. https://doi.org/10.3390/nu16010125
Zhang H, Li M, Mo L, Luo J, Shen Q, Quan W. Association between Western Dietary Patterns, Typical Food Groups, and Behavioral Health Disorders: An Updated Systematic Review and Meta-Analysis of Observational Studies. Nutrients. 2024; 16(1):125. https://doi.org/10.3390/nu16010125
Chicago/Turabian StyleZhang, Huang, Maiquan Li, Lan Mo, Jie Luo, Qingwu Shen, and Wei Quan. 2024. "Association between Western Dietary Patterns, Typical Food Groups, and Behavioral Health Disorders: An Updated Systematic Review and Meta-Analysis of Observational Studies" Nutrients 16, no. 1: 125. https://doi.org/10.3390/nu16010125
APA StyleZhang, H., Li, M., Mo, L., Luo, J., Shen, Q., & Quan, W. (2024). Association between Western Dietary Patterns, Typical Food Groups, and Behavioral Health Disorders: An Updated Systematic Review and Meta-Analysis of Observational Studies. Nutrients, 16(1), 125. https://doi.org/10.3390/nu16010125






