Association between Polyphenol Intake and Lipid Profile of Adults and Elders in a Northeastern Brazilian Capital
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
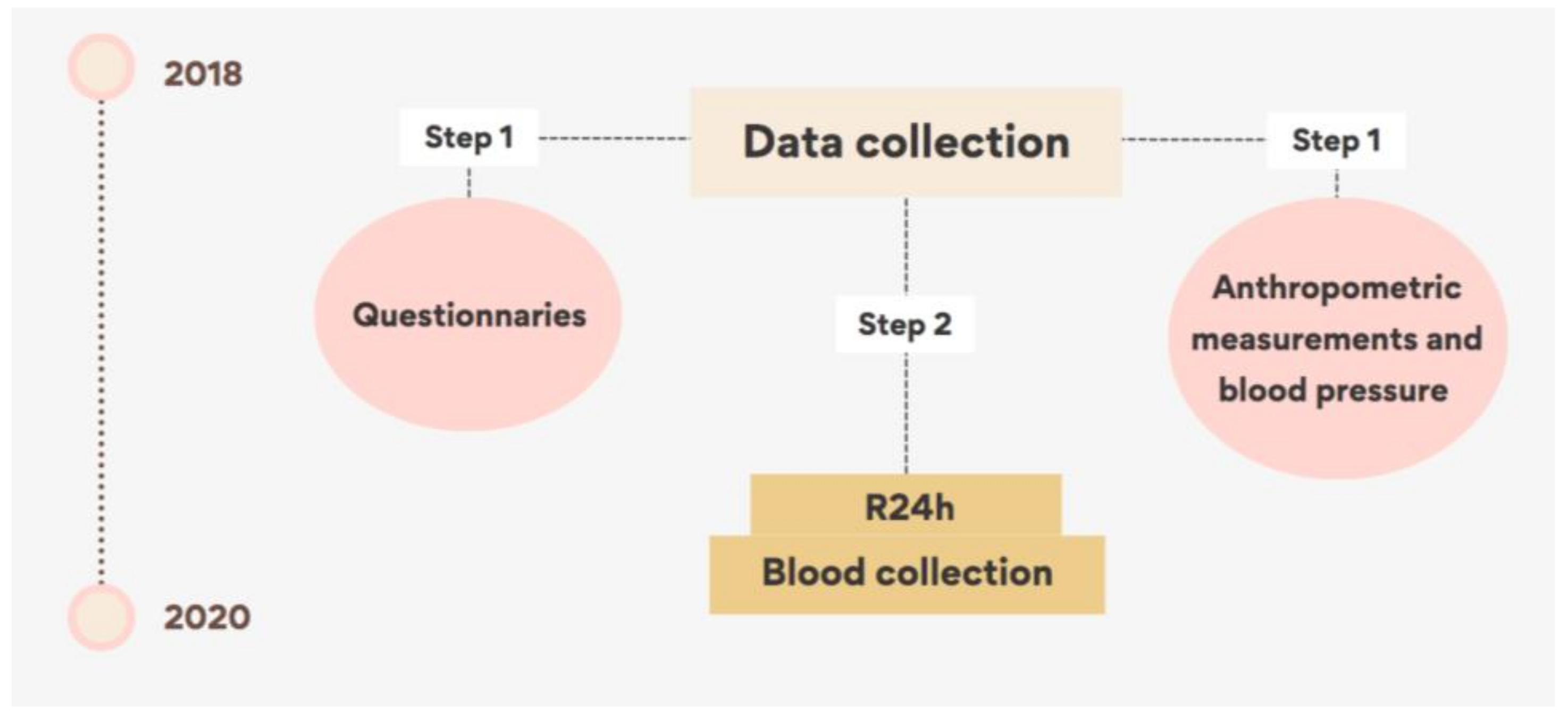
2.2. Data Collection
- Step 1—Quick list;
- Step 2—Forgotten list;
- Step 3—Time and occasion;
- Step 4—Detail and review;
- Step 5—Final review.
2.3. Estimated Intake of Polyphenols
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Noncommunicable Diseases N.D. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 14 September 2022).
- Bays, E.H.; Agarwala, A.; German, C.; Satish, P.; Iluyomade, A.; Dudum, R.; Thakkar, A.; Al Rifai, M.; Mehta, A.; Thobani, A.; et al. Ten things to know about ten cardiovascular disease risk factors—2022. Am. J. Prev. Cardiol. 2021, 10, 100342. [Google Scholar] [CrossRef]
- Rosa, C.D.O.B.; dos Santos, C.A.; Leite, J.I.A.; Caldas, A.P.S.; Bressan, J. Impact of Nutrients and Food Components on Dyslipidemias: What Is the Evidence? Adv. Nutr. Int. Rev. J. 2015, 6, 703–711. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Healthy Diet. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 29 October 2022).
- Becerra-Tomás, N.; Paz-Graniel, I.; Tresserra-Rimbau, A.; Martínez-González, M.; Barrubés, L.; Corella, D.; Muñoz-Martínez, J.; Romaguera, D.; Vioque, J.; Alonso-Gómez, M.; et al. Fruit consumption and cardiometabolic risk in the PREDIMED-plus study: A cross-sectional analysis. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1702–1713. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Liu, Y.-J.; Cai, L.-B.; Xu, F.-R.; Xie, T.; He, Q.-Q. Fruit and vegetable consumption and risk of cardiovascular disease: A meta-analysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2015, 57, 1650–1663. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remón, A.; Martínez-González, M.A.; de la Torre, R.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 639–647. [Google Scholar] [CrossRef]
- Medina-Remón, A.; Casas, R.; Tressserra-Rimbau, A.; Ros, E.; Martínez-González, M.A.; Fitó, M.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventos, R.M.; Estruch, R.; et al. Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: A substudy of the PREDIMED trial. Br. J. Clin. Pharmacol. 2016, 83, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Yuliwati, N.; Wiboworini, B.; Widyaningsih, V. The effect of strawberry, Rome beauty apple, and their combination on the level of low-density lipoprotein cholesterol of Type 2 diabetes mellitus patients. Natl. J. Physiol. Pharm. Pharmacol. 2020, 10, 1105. [Google Scholar] [CrossRef]
- Momose, Y.; Maeda-Yamamoto, M.; Nabetani, H. Systematic review of green tea epigallocatechin gallate in reducing low-density lipoprotein cholesterol levels of humans. Int. J. Food Sci. Nutr. 2016, 67, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Shrime, M.G.; Bauer, S.R.; McDonald, A.C.; Chowdhury, N.H.; Coltart, C.E.M.; Ding, E.L. Flavonoid-Rich Cocoa Consumption Affects Multiple Cardiovascular Risk Factors in a Meta-Analysis of Short-Term Studies. J. Nutr. 2011, 141, 1982–1988. [Google Scholar] [CrossRef] [PubMed]
- Castro-Barquero, S.; Tresserra-Rimbau, A.; Vitelli-Storelli, F.; Doménech, M.; Salas-Salvadó, J.; Martín-Sánchez, V.; Rubín-García, M.; Buil-Cosiales, P.; Corella, D.; Fitó, M.; et al. Dietary Polyphenol Intake is Associated with HDL-Cholesterol and A Better Profile of other Components of the Metabolic Syndrome: A PREDIMED-Plus Sub-Study. Nutrients 2020, 12, 689. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, M.; Miguel, M.; Aleixandre, A. Beneficial effects of polyphenols on cardiovascular disease. Pharmacol. Res. 2013, 68, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, R.D.; Carvalho, N.C.; Martin-Moreno, J.M.; Pimenta, A.M.; Lopes, A.C.S.; Gea, A.; Martinez-Gonzalez, M.A.; Bes-Rastrollo, M. Total polyphenol intake, polyphenol subtypes and incidence of cardiovascular disease: The SUN cohort study. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 69–78. [Google Scholar] [CrossRef]
- Miranda, A.M.; Steluti, J.; Fisberg, R.M.; Marchioni, D.M. Dietary intake and food contributors of polyphenols in adults and elderly adults of Sao Paulo: A population-based study. Br. J. Nutr. 2016, 115, 1061–1070. [Google Scholar] [CrossRef]
- Nascimento-Souza, M.A.; de Paiva, P.G.; Pérez-Jiménez, J.; Franceschini, S.D.C.C.; Ribeiro, A.Q. Estimated dietary intake and major food sources of polyphenols in elderly of Viçosa, Brazil: A population-based study. Eur. J. Nutr. 2016, 57, 617–627. [Google Scholar] [CrossRef]
- Carnauba, R.A.; Hassimotto, N.M.A.; Lajolo, F.M. Estimated dietary polyphenol intake and major food sources of the Brazilian population. Br. J. Nutr. 2020, 126, 441–448. [Google Scholar] [CrossRef]
- Correa, V.G.; Tureck, C.; Locateli, G.; Peralta, R.M.; Koehnlein, E.A. Estimate of consumption of phenolic compounds by Brazilian population. Rev. Nutr. 2015, 28, 185–196. [Google Scholar] [CrossRef]
- Knaze, V.; Rothwell, A.J.; Zamora-Ros, R.; Moskal, A.; Kyrø, C.; Jakszyn, P.; Skeie, G.; Weiderpass, E.; de Magistris, M.S.; Agnoli, C.; et al. A new food-composition database for 437 polyphenols in 19,899 raw and prepared foods used to estimate polyphenol intakes in adults from 10 European countries. Am. J. Clin. Nutr. 2018, 108, 517–524. [Google Scholar] [CrossRef]
- Rodrigues, L.A.R.L.; Silva, D.M.C.; Oliveira, E.A.R.; Lavôr, L.C.C.; Sousa, R.R.; Carvalho, R.B.N. Sampling plan and methodological aspects: Household health survey in Piauí. Rev. Saude Publica 2021, 55, 118. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Active Ageing: A Policy Framework. 2002. Available online: https://apps.who.int/iris/handle/10665/67215 (accessed on 11 February 2023).
- World Health Organization. Global Recommendations on Physical Activity for health. Geneva: WHO. 2010. Available online: http://whqlibdoc.who.int/publications/2010/9789241599979_eng.pdf (accessed on 10 June 2020).
- Quetelet, L.A.J. A treatise on man and the development of his faculties. Obes. Res. 1842, 2, 78–85. [Google Scholar]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Faludi, A.A.; Izar, M.; Saraiva, J.F.K.; Chacra, A.P.M.; Bianco, H.T.; Afiune Neto, A.; Bertolami, A.; Pereira, A.C.; Lottenberg, A.M.; Sposito, A.C.; et al. Update of the Brazilian Guideline on Dyslipidemias and Prevention of Atherosclerosis-2017. Arq. Bras. Cardiol. 2017, 109, 1–76. [Google Scholar] [PubMed]
- William, P.; Castelli, M.D.; Robert, D.; Abbott, P.H.D.; Patricia, M.; Mcnamara, A.B. Summary Estimates of Cholesterol Used to Predict Coronary Heart Disease. Circulation 1983, 67, 730–734. [Google Scholar]
- Gaziano, J.M.; Hennekens, C.H.; O’Donnell, C.J.; Breslow, J.L.; Buring, J.E. Fasting triglycerides, high-density lipoprotein, and risk of myocardial infarction. Circulation 1997, 96, 2520–2525. [Google Scholar] [CrossRef] [PubMed]
- Raper, N.; Perloff, B.; Ingwersen, L.; Steinfeldt, L.; Anand, J. An overview of USDA’s Dietary Intake Data System. J. Food Compos. Anal. 2004, 17, 545–555. [Google Scholar] [CrossRef]
- Moshfegh, A.J.; Rhodes, D.G.; Baer, D.J.; Murayi, T.; Clemens, J.C.; Rumpler, W.V.; Paul, D.R.; Sebastian, R.S.; Kuczynski, K.J.; Ingwersen, L.A.; et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am. J. Clin. Nutr. 2008, 88, 324–332. [Google Scholar] [CrossRef]
- Pinheiro, A.B.V.; Lacerda, E.M.D.A.; Benzecry, E.H.; Gomes, M.C.D.S.; Costa, V.M.D. Tabela para Avaliação de Consumo Alimentar em Medidas Caseiras, 5th ed.; Atheneu: São Paulo, Brazil, 2005. [Google Scholar]
- Fisberg, R.M.; Villar, B.S. Manual de Receitas e Medidas Caseiras para Cálculo de Inquéritos Alimentares: Manual Elaborado para Auxiliar o Processamento de Inquéritos); Signus: São Paulo, Brazil, 2002. [Google Scholar]
- Universidade Estadual de Campinas (UNICAMP). Tabela Brasileira de Composição de Alimentos;—TACO, 4th ed.; UNICAMP: Campinas, Brazil, 2011. [Google Scholar]
- Rothwell, J.; Urpi-Sarda, M.; Boto-Ordonez, M.; Knox, C.; Llorach, R.; Eisner, R.; Cruz, J.; Neveu, V.; Wishart, D.S.; Manach, C.; et al. Phenol-Explorer 2.0: A major update of the Phenol-Explorer database integrating data on polyphenol metabolism and pharmacokinetics in humans and experimental animals. Database 2012, 2012, bas031. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Pérez-Jiménez, J.; Neveu, V.; Medina-Remón, A.; M’Hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef]
- Del Bo’, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is there Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients 2019, 11, 1355. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Knaze, V.; Rothwell, J.A.; Hémon, B.; Moskal, A.; Overvad, K.; Tjønneland, A.; Kyrø, C.; Fagherazzi, G.; Boutron-Ruault, M.-C.; et al. Dietary polyphenol intake in Europe: The European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur. J. Nutr. 2015, 55, 1359–1375. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Biessy, C.; Rothwell, J.A.; Monge, A.; Lajous, M.; Scalbert, A.; López-Ridaura, R.; Romieu, I. Dietary polyphenol intake and their major food sources in the Mexican Teachers’ Cohort. Br. J. Nutr. 2018, 120, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, C.; Fukushima, Y.; Kishimoto, Y.; Suzuki-Sugihara, N.; Saita, E.; Takahashi, Y.; Kondo, K. Estimated Dietary Polyphenol Intake and Major Food and Beverage Sources among Elderly Japanese. Nutrients 2015, 7, 10269–10281. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Stepaniak, U.; Topor-Mądry, R.; Szafraniec, K.; Pająk, A. Estimated dietary intake and major food sources of polyphenols in the Polish arm of the HAPIEE study. Nutrition 2014, 30, 1398–1403. [Google Scholar] [CrossRef]
- Godos, J.; Marventano, S.; Mistretta, A.; Galvano, F.; Grosso, G. Dietary sources of polyphenols in the Mediterranean healthy Eating, Aging and Lifestyle (MEAL) study cohort. Int. J. Food Sci. Nutr. 2017, 68, 750–756. [Google Scholar] [CrossRef]
- Burkholder-Cooley, N.; Rajaram, S.; Haddad, E.; Fraser, G.E.; Jaceldo-Siegl, K. Comparison of polyphenol intakes according to distinct dietary patterns and food sources in the adventista health study-2 cohort. Br. J. Nutr. 2016, 115, 2162–2169. [Google Scholar] [CrossRef]
- Fukushima, Y.; Ohie, T.; Yonekawa, Y.; Yonemoto, K.; Aizawa, H.; Mori, Y.; Watanabe, M.; Takeuchi, M.; Hasegawa, M.; Taguchi, C.; et al. Coffee and Green Tea as a Large Source of Antioxidant Polyphenols in the Japanese Population. J. Agric. Food Chem. 2009, 57, 1253–1259. [Google Scholar] [CrossRef]
- Williamson, G.; Dionisi, F.; Renouf, M. Flavanols from green tea and phenolic acids from coffee: Critical quantitative evaluation of the pharmacokinetic data in humans after consumption of single doses of beverages. Mol. Nutr. Food Res. 2011, 55, 864–873. [Google Scholar] [CrossRef]
- Kolb, H.; Kempf, K.; Martin, S. Health Effects of Coffee: Mechanism Unraveled? Nutrients 2020, 12, 1842. [Google Scholar] [CrossRef]
- Lukitasari, M.; Rohman, M.S.; Nugroho, D.A.; Widodo, N.; Nugrahini, N.I.P. Cardiovascular protection effect of chlorogenic acid: Focus on the molecular mechanism. F1000Research 2020, 9, 1462. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.M.; Goulart, A.C.; Benseñor, I.M.; Lotufo, P.A.; Marchioni, D.M. Coffee consumption and risk of hypertension: A prospective analysis in the cohort study. Clin. Nutr. 2020, 40, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Frota, K.M.G.; Soares, R.A.M.; Arêas, J.A.G. Composição química do feijão caupi (Vigna unguiculata L. Walp), cultivar BRS-Milênio. Food Sci. Technol. 2008, 28, 470–476. [Google Scholar] [CrossRef]
- Cavalcanti, R.B.M.; Araújo, M.A.M.; Rocha, M.M.; Silva, K.J.D.; Moreira-Araújo, R.S.R. Effect of thermal processing on total polyphenol content in the grain of cowpea cultivars. Rev. Revista Ciência Agronômica 2017, 48, 806–810. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Fezeu, L.; Touvier, M.; Arnault, N.; Manach, C.; Hercberg, S.; Galan, P.; Scalbert, A. Dietary intake of 337 polyphenols in French adults. Am. J. Clin. Nutr. 2011, 93, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Karam, J.; Bibiloni, M.D.M.; Tur, J.A. Polyphenol estimated intake and dietary sources among older adults from Mallorca Island. PLoS ONE 2018, 13, e0191573. [Google Scholar] [CrossRef]
- Feng, S.; Yi, J.; Li, X.; Wu, X.; Zhao, Y.; Ma, Y.; Bi, J. Systematic Review of Phenolic Compounds in Apple Fruits: Compositions, Distribution, Absorption, Metabolism, and Processing Stability. J. Agric. Food Chem. 2021, 69, 7–27. [Google Scholar] [CrossRef]
- Vitale, M.; TOSCA.IT Study Group; Masulli, M.; Rivellese, A.A.; Bonora, E.; Cappellini, F.; Nicolucci, A.; Squatrito, S.; Antenucci, D.; Barrea, A.; et al. Dietary intake and major food sources of polyphenols in people with type 2 diabetes: The TOSCA.IT Study. Eur. J. Nutr. 2016, 57, 679–688. [Google Scholar] [CrossRef]
- Huang, Q.; Braffett, B.H.; Simmens, S.J.; Young, H.A.; Ogden, C.L. Dietary Polyphenol Intake in US Adults and 10-Year Trends: 2007–2016. J. Acad. Nutr. Diet. 2020, 120, 1821–1833. [Google Scholar] [CrossRef]
- Costa, J.C.; Canella, D.S.; Martins, A.P.B.; Levy, R.B.; Andrade, G.C.; Louzada, M.L.D.C. Consumo de frutas e associação com a ingestão de alimentos ultraprocessados no Brasil em 2008–2009. Ciência Saúde Coletiva 2021, 26, 1233–1244. [Google Scholar] [CrossRef]
- Assumpção, D.; Ruiz, A.M.P.; Borim, F.S.A.; Neri, A.L.; Malta, D.C.; Francisco, P.M.S.B. Eating Behavior of Older Adults with and without Diabetes: The Vigitel Survey, Brazil, 2016. Arq. Bras. Cardiol. 2016, 118, 388–397. [Google Scholar] [CrossRef]
- Sattar, N.; Preiss, D. Reverse Causality in Cardiovascular Epidemiological Research: More Common Than Imagined? Circulation 2017, 135, 2369–2372. [Google Scholar] [CrossRef] [PubMed]
- e Silva, D.M.C.; Santos, T.S.S.; Conde, W.L.; Slater, B. Estado nutricional e risco metabólico em adultos: Associação com a qualidade da dieta medida pela ESQUADA. Rev. Bras. Epidemiologia 2021, 24, e210019. [Google Scholar] [CrossRef] [PubMed]
- Aloo, S.-O.; Ofosu, F.K.; Kim, N.-H.; Kilonzi, S.M.; Oh, D.-H. Insights on Dietary Polyphenols as Agents against Metabolic Disorders: Obesity as a Target Disease. Antioxidants 2023, 12, 416. [Google Scholar] [CrossRef] [PubMed]
- Aali, Y.; Ebrahimi, S.; Shiraseb, F.; Mirzaei, K. The association between dietary polyphenol intake and cardiometabolic factors in overweight and obese women: A cross-sectional study. BMC Endocr. Disord. 2022, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Liao, W.; Xia, H.; Wang, S.; Sun, G. The Effect of Resveratrol on Blood Lipid Profile: A Dose-Response Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 3755. [Google Scholar] [CrossRef]
- Luna-Castillo, K.P.; Lin, S.; Muñoz-Valle, J.F.; Vizmanos, B.; López-Quintero, A.; Márquez-Sandoval, F. Functional Food and Bioactive Compounds on the Modulation of the Functionality of HDL-C: A Narrative Review. Nutrients 2021, 13, 1165. [Google Scholar] [CrossRef]
- Lorenzo, C.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
| Classes and Subclasses of Polyphenols | Food Groups | Contribution % of Main Foodstuffs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcoholic Beverages | Non-Alcoholic Beverages | Fruit | Vegetables | Cereals | Cocoa and Chocolate | Seeds | Oils | Seasonings | Total | ||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Total polyphenols | 25.27 (240.76) | 617.70 (603.40) | 41.58 (100.83) | 14.16 (33.99) | 10.48 (33.23) | 2.00 (17.01) | 364.70 (605.50) | 0.79 (8.90) | 1.56 (24.62) | 1006.53 (837.15) | Coffee (54.4.0%), Beans (35.1%), Apple (1.2%) |
| Flavonoids | 12.29 (116.70) | 8.21 (48.92) | 27.16 (71.66) | 2.81 (6.62) | 0.03 (0.27) | 1.99 (16.86) | 344.90 (589.50) | 0.00 (0.04) | 0.00 (0.08) | 397.90 (628.60) | Beans (86.6%), Beer (4.1%), Orange (2.0%) |
| Flavones | 1.37 (13.18) | 0.71 (5.37) | - | 0.51 (1.12) | 0.01 (0.15) | - | - | 0.00 (0.02) | - | 2.59 (14.29) | Potato (27.4%), Beer (21.6%), Orange Juice (6.6%) |
| Flavanols | 0.53 (5.06) | 0.96 (5.48) | 16.56 (48.92) | - | 0.00 (0.09) | 1.98 (16.81) | 324.80 (564.80) | - | 0.00 (0.01) | 349.30 (586.20) | Beans (93.0%), Banana (0.8%), Chocolate (0.4%) |
| Flavonols | 4.13 (39.79) | 0.57 (5.56) | 2.12 (21.37) | 1.79 (5.03) | - | 0.01 (0.08) | 18.60 (27.66) | - | 0.00 (0.07) | 27.21 (52.80) | Beans (67.7%), Beer (29.2%), Grapes (5.1%) |
| Flavanones | 5.66 (53.88) | 5.98 (39.03) | 8.48 (37.67) | 0.50 (1.10) | - | - | 0.00 (0.02) | 20.62 (75.67) | Orange (39.6%), Beer (22.2%), Orange Juice (14.6%) | ||
| Anthocyanins | - | - | - | 0.01 (0.19) | 0.02 (0.17) | - | 1.28 (7.47) | - | - | 1.31 (7.49) | Beans (97.7%), Corn (10.7%), Onion (1.1%) |
| Isoflavonoids | 0.61 (5.44) | - | - | - | - | - | 0.24 (0.51) | - | - | 0.85 (5.45) | Beer (71.8%), Beans (25.9%) |
| Dihydrochalcones | - | - | 0.37 (1.58) | - | - | - | - | - | - | 0.37 (1.58) | Apple (100.0%)) |
| Chalcones | 0.46 (4.46) | - | - | - | - | - | - | - | - | 0.46 (4.46) | Beer (100.0%) |
| Phenolics acids | 1.42 (13.58) | 450.40 (442.50) | 1.64 (25) | 5.05 (14.29) | 3.41 (30.05) | 0.01 (0.21) | 17.69 (32.07) | 0.65 (8.89) | 0.01 (0.08) | 619.63 (3057.40) | Coffee (95.0%), Grapes (0.2%), Beer (0.1%) |
| Hydroxybenzoic acids | 0.80 (7.71) | 1.25 (8.54) | - | 0.56 (2.11) | 0.44 (0.48) | 0.01 (0.12) | 0.07 (0.79) | 0.04 (0.25) | 0.003 (0.039) | 3.15 (11.59) | Beer (27.3%) Coffee (21.3%) Carrot (12.4%) |
| Hydroxycinnamic acids | 0.62 (5.88) | 449.20 (441.80) | 1.64 (6.25) | 4.49 (13.38) | 3.09 (30.63) | 0.01 (0.09) | 17.62 (31.99) | 0.60 (8.80) | 0.003 (0.038) | 615.90 (3057.30) | Coffee (95.4%), Beans (2.8%), Potatoes (0.4%) |
| Hydroxyphenylacetic acid | 0.05 (0.44) | - | 1.75 (14.01) | - | - | - | - | 0.00 (0.00) | - | 1.79 (14.01) | Grapes (62.2%), Beer (2.2%), Olive Oil (0.1%) |
| Stilbenes | - | - | 0.11 (1.055) | - | - | - | 0.00 (0.00) | - | 0.002 (0.033) | 0.11 (1.06) | Grape (95.3%), Vinegar (0.9%), Peanut (0.1%) |
| Lignans | 0.94 (9.34) | 42.81 (42.00) | 9.14 (19.61) | 5.98 (19.08) | 5.57 (6.64) | Traces | 6.83 (16.88) | 0.02 (0.21) | - | 71.23 (52.67) | Coffee (58.4%), Beans (6.8%), Banana (4.7%) |
| Other polyphenols | 7.57 (72.95) | 3.27 (3.65) | 0.04 (0.15) | 0.02 (0.14) | 1.65 (8.12) | 0.01 (0.14) | 0.00 (0.04) | 0.10 (0.86) | 0.00 (0.03) | 11.43 (67.53) | Beer (66.2%), Coffee (25.6%), Noodles (10.4%) |
| Classes and Subclasses of Polyphenols | Adults (n = 368) | Elderly (n = 133) | p-Value | ||
|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | ||
| Total polyphenols | 1120.01 (904.70) | 906.11 (494.11–1532.49) | 969.59 (712.02) | 870.71 (460.60–140.72) | 0.268 |
| Flavonoids | 415.99 (679.92) | 158.25 (11.83–541.29) | 348.95 (473.27) | 191.15 (13.71–447.15) | 0.860 |
| Flavones | 3.070 (16.51) | 0 (0–0.80) | 1.26 (3.66) | 0 (0–0.81) | 0.842 |
| Flavanols | 364.46 (628.43) | 93.69 (1.62–432.12) | 308.26 (452.82) | 137.69 (7.43–426.68) | 0.690 |
| Flavonols | 29.95 (59.66) | 13.40 (2.34–30.28) | 19.63 (24.20) | 13.79 (1.25–28.72) | 0.339 |
| Flavanones | 21.60 (81.52) | 0 (0–1.34) | 17.91 (56.60) | 0 (0–0.97) | 0.651 |
| Anthocyanins | 1.57 (8.52) | 0 (0–0) | 0.58 (3.15) | 0 (0–0) | 0.139 |
| Isoflavonoids | 1.073 (6.34) | 0.10 (0–0.28) | 0.25 (0.65) | 0.09 (0–0.21) | 0.634 |
| Dihydrochalcones | 0.46 (1.72) | 0 (0–0) | 0.14 (1.10) | 0 (0–0) | 0.015 |
| Chalcones | 0.61 (5.19) | 0 (0–0) | 0.04 (0.48) | 0 (0–0) | 0.230 |
| Phenolic acids | 489.50 (433.32) | 415.51 (185.08–692.44) | 454.83 (467.40) | 325.29 (128.27–629.29) | 0.159 |
| Hydroxybenzoic acids | 3.47 (12.53) | 0.85 (0.48–1.80) | 2.268 (8.46) | 0.61 (0.38–0.98) | 0.002 |
| Hidroxicinnamic acids | 486.03 (433.08) | 411.09 (171.00–688.13) | 452.56 (465.56) | 324.90 (127.77–661.02) | 0.177 |
| Hydroxyphenylacetic acids | 2.25 (16.09) | 0 (0–0) | 0.53 (4.62) | 0 (0–0) | 0.003 |
| Stilbenes | 0.14 (1.23) | 0 (0–0) | 0.003 (0.03) | 0 (0–0) | 0.408 |
| Lignans | 71.78 (52.78) | 58.94 (34.35–94.58) | 69.71 (52.52) | 55.28 (33.98–89.33) | 0.660 |
| Other polyphenols | 13.46 (78.72) | 3.34 (1.65–6.25) | 5.95 (10.97) | 3.30 (1.09–6.34) | 0.619 |
| Variables | n | Mean (SD) | Median | IQR | p-Value |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 167 | 1318.60 (1053.63) | 1078.60 | 628.63–1695.72 | <0.001 |
| Female | 334 | 960.34 (715.12) | 801.19 | 449.33–1322.33 | |
| Age group | |||||
| Adult | 368 | 1120.01 (904.70) | 906.11 | 494.11–1532.49 | 0.268 |
| Elder | 133 | 969.59 (712.02) | 870.71 | 460.60–1340.72 | |
| Income (minimum wages) | |||||
| <1 | 35 | 1231.74 (898.82) | 1075.23 | 616.50–1438.86 | |
| Between 1 and 2 | 294 | 1095.01 (943.68) | 845.75 | 460.60–1473.90 | |
| Between 3 and 4 | 98 | 1014.11 (710.98) | 915.96 | 494.11–1460.30 | 0.808 |
| Between 5 and 9 | 52 | 1042.71 (667.76) | 990.78 | 540.61–1383.14 | |
| Between 10 and 20 | 20 | 971.01 (625.41) | 801.01 | 455.37–1619.17 | |
| Smoke | |||||
| No | 387 | 1106.47 (898.79) | 912.68 | 493.99–1468.96 | 0.538 |
| Yes | 112 | 988.74 (710.72) | 863.08 | 453.68–1433.99 | |
| Alcoholic beverage | |||||
| Never had a drinking habit | 180 | 1052.26 (780.16) | 918.50 | 455.37–1465.21 | |
| Previously did drink, but does not drink currently | 118 | 1099.44 (871.18) | 835.82 | 501.82–1383.86 | 0.955 |
| Has a drinking habit | 201 | 1093.50 (871.18) | 950.30 | 498.02–1460.30 | |
| BMI | |||||
| <25 | 184 | 1105.82 (828.08) | 902.70 | 504.17–1498.04 | 0.442 |
| ≥25 | 317 | 1065.09 (878.18) | 892.29 | 445.55–1438.86 | |
| Physical activity | |||||
| Sedentary | 92 | 964.37 (767.40) | 841.18 | 378.04–1323.94 | 0.168 |
| Non-sedentary | 404 | 1106.86 (882.17) | 911.48 | 498.90–1492.77 | |
| Diastolic blood pressure (mmHg) | |||||
| <80 | 229 | 1066.31 (797.39) | 929.28 | 489.57–1431.67 | 0.853 |
| ≥80 | 267 | 1092.40 (916.72) | 870.71 | 461.41–1475.82 | |
| Systolic blood pressure (mmHg) | |||||
| <120 | 195 | 1079.88 (807.65) | 941.91 | 494.11–1461.46 | 0.710 |
| ≥120 | 301 | 1080.70 (898.26) | 865.60 | 475.79–1460.30 | |
| Total cholesterol | |||||
| <190 | 183 | 959.18 (851.01) | 705.06 | 374.51–1350.83 | 0.022 |
| ≥190 | 96 | 1080.99 (681.25) | 1066.01 | 522.84–1484.21 | |
| HDL-c | |||||
| >40 | 99 | 1,068.46 (813.61) | 972.68 | 463.24–1492.77 | 0.234 |
| ≤40 | 175 | 955.34 (70,907.39) | 788.85 | 426.16–1329.10 | |
| LDL-c | |||||
| <130 | 205 | 990.12 (851.77) | 720.05 | 394.16–1406.66 | 0.149 |
| ≥130 | 74 | 1031.50 (627.36) | 1016.37 | 499.99–1387.26 | |
| Triglycerides | |||||
| <150 | 142 | 941.94 (874.21) | 705.40 | 368.98–1240.64 | 0.020 |
| ≥150 | 137 | 1,062.41 (707.42) | 972.68 | 499.99–1485.42 | |
| Castelli Index 1 (TC/HDL) | |||||
| <3.5 | 70 | 999.53 (849.45) | 798.20 | 343.55–1492.77 | 0.747 |
| ≥3.5 | 204 | 995.07 (783.43) | 595.06 | 282.49–1022.68 | |
| Castelli Index 2 (LDL/HDL) | |||||
| ≤2.9 | 144 | 1031.47 (887.51) | 786.96 | 414.68–1450.48 | 0.982 |
| >2.9 | 227 | 957.16 (689.73) | 872.24 | 445.55–1329.10 | |
| Triglycerides/HDL | |||||
| ≤3.7 | 133 | 974.55 (862.52) | 820.05 | 379.92–1329.10 | 0.294 |
| >3.7 | 141 | 1016.64 (737.08) | 881.17 | 468.69–1438.86 |
| Variables (n = 274) | Total Cholesterol | HDL-c | Triglycerides | LDL-c |
|---|---|---|---|---|
| Rho (p-Value) | Rho (p-Value) | Rho (p-Value) | Rho (p-Value) | |
| Total polyphenols | 0.136 (0.023) | 0.044 (0.463) | 0.158 (0.008) | 0.105 (0.081) |
| Flavonoids | 0.066 (0.296) | 0.016 (0.795) | 0.009 (0.884) | 0.079 (0.217) |
| Flavones | 0.069 (0.251) | 0.171 (0.004) | −0.029 (0.623) | 0.045 (0.454) |
| Flavanols | 0.071 (0.266) | 0.026 (0.685) | −0.003 (0.960) | 0.085 (0.183) |
| Flavonols | 0.018 (0.763) | 0.035 (0.556) | 0.085 (0.160) | 0.002 (0.972) |
| Flavanones | 0.074 (0.220) | 0.139 (0.020) | −0.057 (0.342) | 0.070 (0.246) |
| Anthocyanins | −0.003 (0.951) | 0.021 (0.717) | 0.145 (0.015) | −0.080 (0.183) |
| Isoflavonoids | −0.007 (0.903) | −0.004 (0.945) | 0.140 (0.020) | −0.039 (0.517) |
| Dihydrochalcones | 0.040 (0.509) | 0.082 (0.175) | −0.054 (0.368) | 0.042 (0.489) |
| Chalcones | 0.029 (0.633) | 0.014 (0.808) | 0.032 (0.591) | 0.029 (0.628) |
| Phenolic acids | 0.127 (0.035) | 0.032 (0.590) | 0.165 (0.006) | 0.095 (0.115) |
| Hydroxybenzoic acids | 0.055 (0.359) | 0.095 (0.114) | 0.037 (0.535) | 0.024 (0.687) |
| Hydroxycinnamic acids | 0.126 (0.035) | 0.030 (0.619) | 0.167 (0.005) | 0.095 (0.115) |
| Hydroxyphenylacetic acids | 0.037 (0.535) | 0.125 (0.037) | −0.059 (0.322) | 0.037 (0.540) |
| Stilbenes | 0.090 (0.133) | 0.108 (0.072) | −0.074 (0.217) | 0.106 (0.078) |
| Lignans | 0.186 (0.001) | 0.104 (0.083) | 0.161 (0.007) | 0.149 (0.013) |
| Other polyphenols | 0.036 (0.572) | 0.024 (0.707) | 0.086 (0.176) | 0.042 (0.508) |
| Dyslipidemia (Hypercholesterolaemia, Isolated Hypertriglyceridaemia and Mixed Hyperlipidaemia) | |||||
|---|---|---|---|---|---|
| Classes and Subclasses of Polyphenols | No (n = 130) | Yes (n = 144) | p-Value | ||
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | ||
| Total polyphenols | 914.27 (871.53) | 700.28 (365.84–1199.75) | 1070.19 (722.94) | 987.34 (502.08–1501.42) | 0.007 |
| Flavonoids | 390.25 (711.20) | 176.20 (12.96–439.14) | 437.90 (489.49) | 101.23 (7.80–449.55) | 0.643 |
| Phenolic acids | 388.79 (386.73) | 327.06 (74.58–571.78) | 520.19 (437.34) | 452.64 (197.20–754.47) | 0.005 |
| Stilbenes | 0.04 (0.29) | 0 (0–0) | 0.001 (0.01) | 0 (0–0) | 0.233 |
| Lignans | 62.81 (51.54) | 53.17 (27.74–87.31) | 73.86 (47.15) | 63.91 (38.29–98.02) | 0.012 |
| Other polyphenols | 7.13 (18.83) | 3.34 (1.27–6.16) | 7.93 (29.61) | 3.34 (1.59–6.46) | 0.552 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Farias, L.M.; Lopes Rodrigues, L.A.R.; de Carvalho Lavôr, L.C.; de Lima, A.; Sampaio da Paz, S.M.R.; Pereira da Silva, J.D.; de Macêdo Gonçalves Frota, K.; Lucarini, M.; Durazzo, A.; Arcanjo, D.D.R.; et al. Association between Polyphenol Intake and Lipid Profile of Adults and Elders in a Northeastern Brazilian Capital. Nutrients 2023, 15, 2174. https://doi.org/10.3390/nu15092174
de Farias LM, Lopes Rodrigues LAR, de Carvalho Lavôr LC, de Lima A, Sampaio da Paz SMR, Pereira da Silva JD, de Macêdo Gonçalves Frota K, Lucarini M, Durazzo A, Arcanjo DDR, et al. Association between Polyphenol Intake and Lipid Profile of Adults and Elders in a Northeastern Brazilian Capital. Nutrients. 2023; 15(9):2174. https://doi.org/10.3390/nu15092174
Chicago/Turabian Stylede Farias, Luciana Melo, Lays Arnaud Rosal Lopes Rodrigues, Layanne Cristina de Carvalho Lavôr, Alessandro de Lima, Suzana Maria Rebêlo Sampaio da Paz, Jânyerson Dannys Pereira da Silva, Karoline de Macêdo Gonçalves Frota, Massimo Lucarini, Alessandra Durazzo, Daniel Dias Rufino Arcanjo, and et al. 2023. "Association between Polyphenol Intake and Lipid Profile of Adults and Elders in a Northeastern Brazilian Capital" Nutrients 15, no. 9: 2174. https://doi.org/10.3390/nu15092174
APA Stylede Farias, L. M., Lopes Rodrigues, L. A. R., de Carvalho Lavôr, L. C., de Lima, A., Sampaio da Paz, S. M. R., Pereira da Silva, J. D., de Macêdo Gonçalves Frota, K., Lucarini, M., Durazzo, A., Arcanjo, D. D. R., & de Carvalho e Martins, M. d. C. (2023). Association between Polyphenol Intake and Lipid Profile of Adults and Elders in a Northeastern Brazilian Capital. Nutrients, 15(9), 2174. https://doi.org/10.3390/nu15092174









