Relationship between Bone Mineral Density and Selected Parameters of Calcium-Phosphate Economy with Dietary Management and Metabolic Control in Polish Pediatric Patients with Classical Homocystinuria—A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Assessments
2.2.1. Dietary Assessment
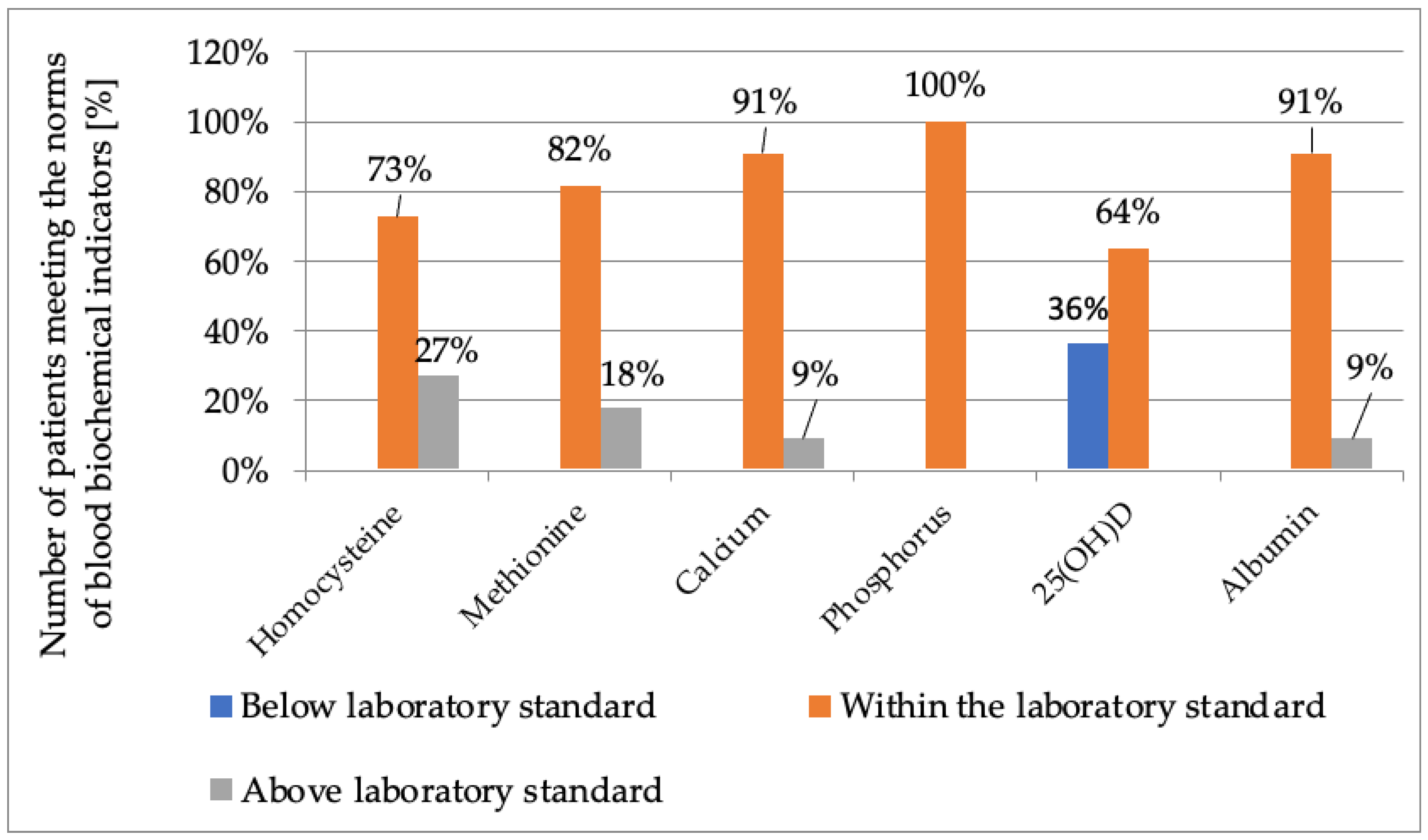
2.2.2. Biochemical Measures
2.2.3. Assessment of Bone Mineral Density
2.3. Ethical Permission
2.4. Statistical Analysis
3. Results
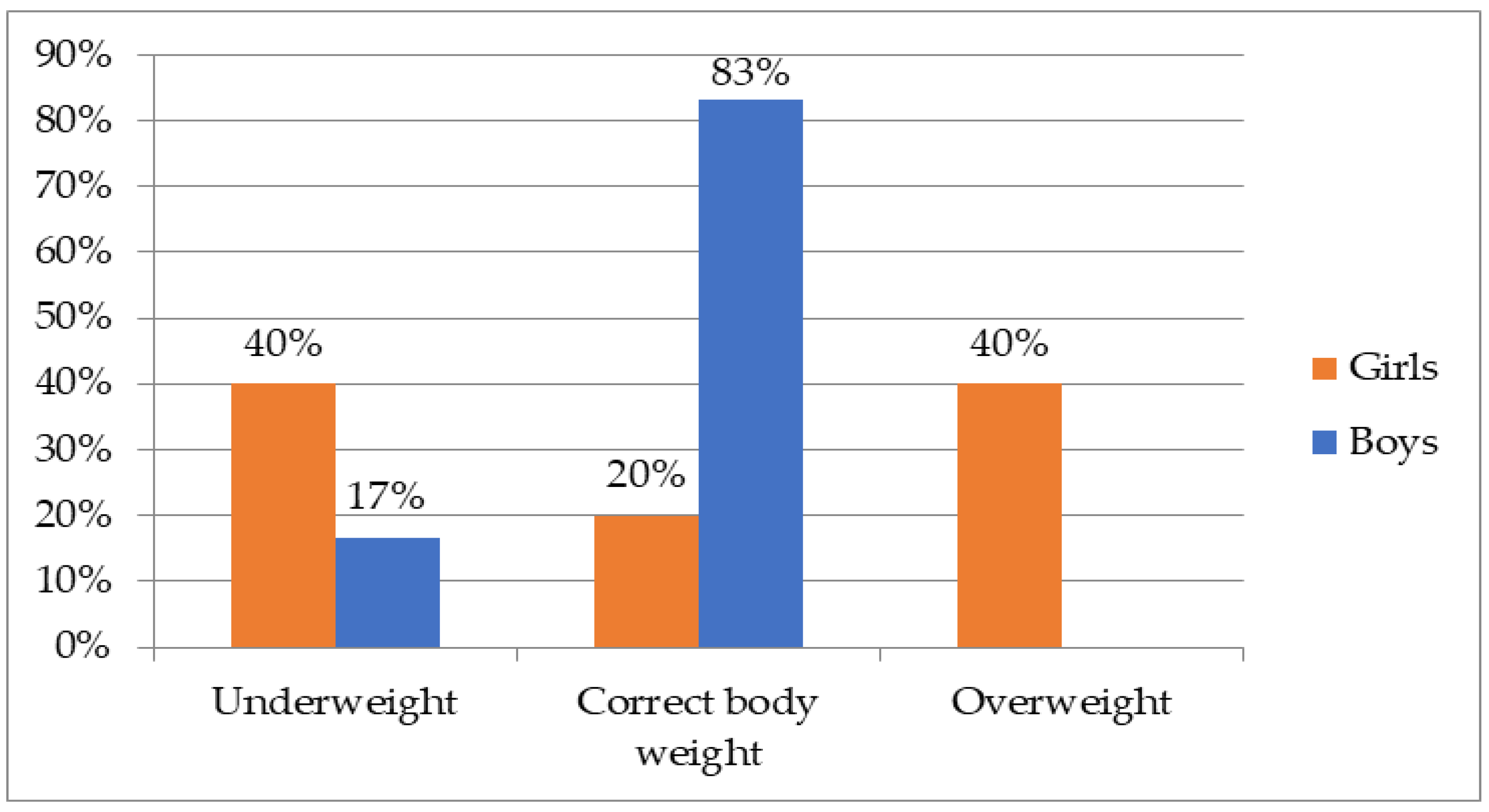
3.1. Characteristics of the Group
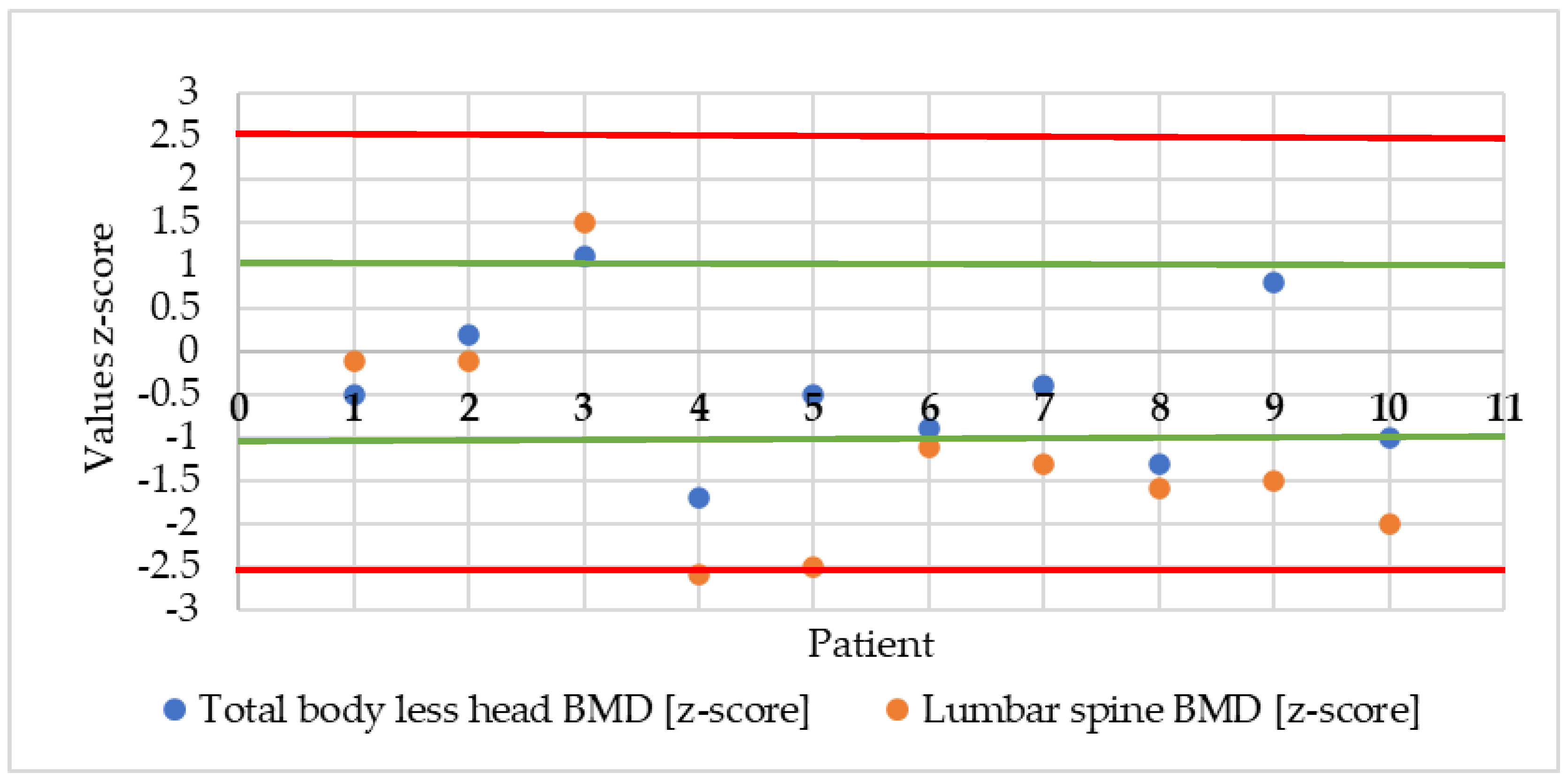
3.2. Selected Parameters of Bone Mineral Density
3.3. Relationships between Dietary Factors, Blood Concentrations of Minerals, Vitamins and Amino Acids, as Well as Age and BMI, and Mineral Density of the Whole Skeleton Excluding Head and Lumbar Region
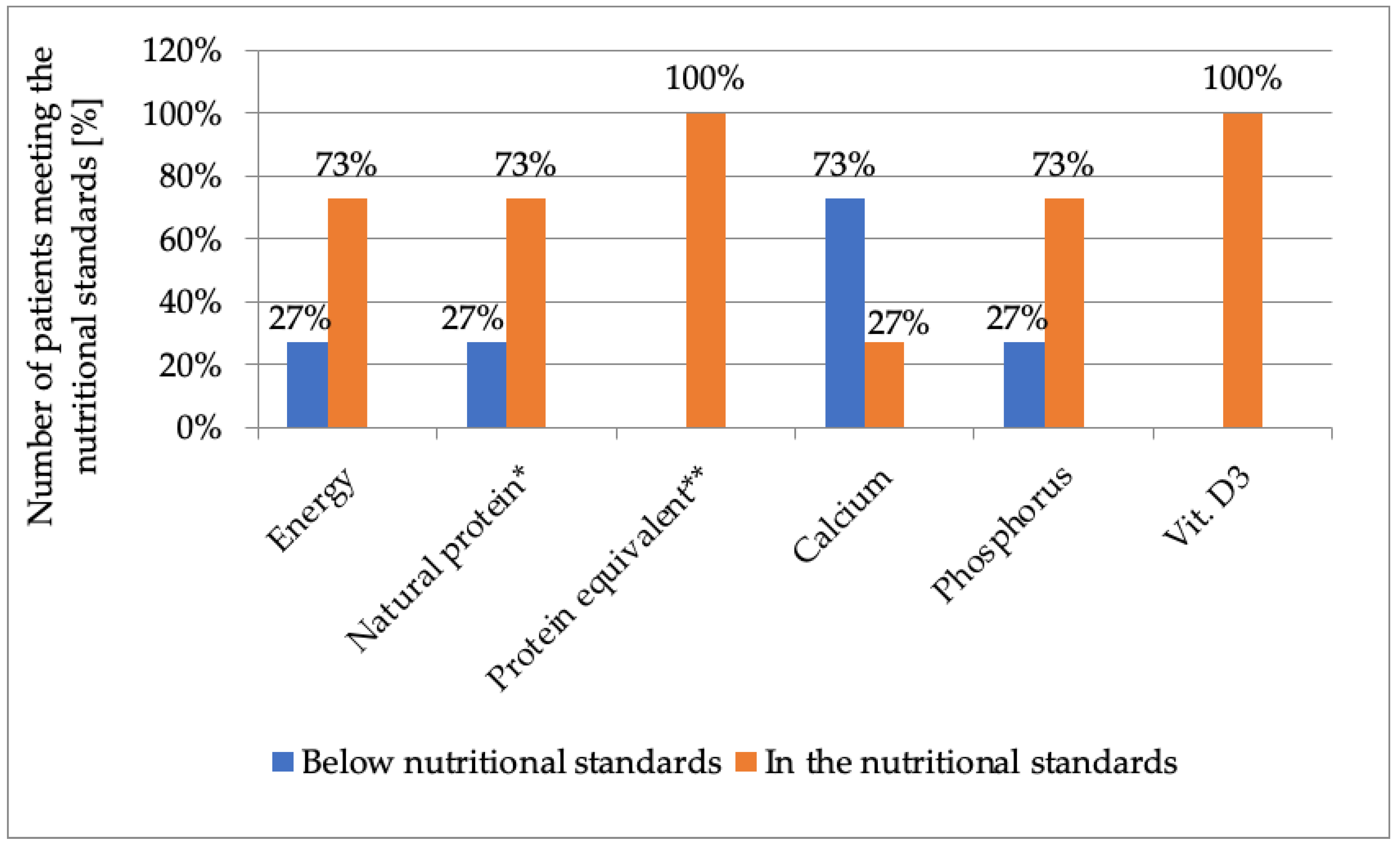
3.4. Energy Value and Intake of Selected Nutrients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zschocke, J.; Hoffmann, G.F. Vademecum Metabolicum; Milupa Metabolics GmbH & Co.: Friedrichsdorf, Germany, 2011. [Google Scholar]
- Valayannopoulos, V.; Schiff, M.; Guffon, N.; Nadjar, Y.; García-Cazorla, A.; Casanova, M.M.-P.; Cano, A.; Couce, M.L.; Dalmau, J.; Peña-Quintana, L.; et al. Betaine anhydrous in homocystinuria: Results from the RoCH registry. Orphanet J. Rare Dis. 2019, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadeq, D.W.; Nasrallah, G.K. The Spectrum of Mutations of Homocystinuria in the MENA Region. Genes 2020, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Ołtarzewski, M. Badania przesiewowe noworodków w Polsce. Postępy Neonatol. 2018, 24, 111–122. [Google Scholar] [CrossRef]
- Ehmke vel Emczyńska-Seliga, E. Leczenie dietetyczne w chorobach metabolicznych. Clin. Pediatr. 2015, 23, 6081–6085. [Google Scholar]
- Kraleva, D.; Evans, S.; Pinto, A.; Daly, A.; Ashmore, C.; Pointon-Bell, K.; Rocha, J.C.; MacDonald, A. Protein Labelling Accuracy for UK Patients with PKU Following a Low Protein Diet. Nutrients 2020, 12, 3440. [Google Scholar] [CrossRef] [PubMed]
- Kowalik, A. Leczenie dietetyczne we wrodzonych wadach metabolizmu. New Clin. 2012, 19, 22–25. [Google Scholar]
- Mönch, E.; Link, R. Diagnostik und Therapie bei angebornen Stoffwechselstrungen; SPS Publication: Heilbronn, Germany, 2002. [Google Scholar]
- Jarosz, M.; Rychlik, E.; Stoś, K.; Charzewska, J. Normy Żywienia dla Populacji Polski i ich Zastosowanie; Narodowy Instytut Zdrowia Publicznego—Państwowy Zakład Higieny: Warszawa, Poland, 2020.
- Handoom, B.; Megdad, E.; Al-Qasabi, D.; Al Mesned, M.; Hawary, R.; Al-Nufiee, S.; Al-Hassnan, Z.; Alsayed, M.D.; Eldali, A. The effects of low protein products availability on growth parameters and metabolic control in selected amino acid metabolism disorders patients. Int. J. Pediatr. Adolesc. Med. 2018, 5, 60–68. [Google Scholar] [CrossRef]
- Kowalik, A.; Narojek, L.; Sykut-Cegielska, J. Realizacja diety z ograniczeniem leucyny, izoleucyny i waliny u dzieci z chorobą syropu klonowego (MSUD). ROCZNIK PZH 2007, 58, 95–101. [Google Scholar]
- Weber, D.R.; Ii, C.C.; Brodsky, J.L.; Lindstrom, K.; Ficicioglu, C.; Kaplan, P.; Freehauf, C.L.; Levine, M. Low bone mineral density is a common finding in patients with homocystinuria. Mol. Genet. Metab. 2015, 117, 351–354. [Google Scholar] [CrossRef]
- Lim, J.S.; Lee, D.H. Changes in bone mineral density and body composition of children with well-controlled homocystinuria caused by CBS deficiency. Osteoporos. Int. 2013, 24, 2535–2538. [Google Scholar] [CrossRef]
- Pimentel, F.B.; Alves, R.C.; Costa, A.S.; Torres, D.; Almeida, M.F.; Oliveira, M.B.P. Phenylketonuria: Protein content and amino acids profile of dishes for phenylketonuric patients. The relevance of phenylalanine. Food Chem. 2014, 149, 144–150. [Google Scholar] [CrossRef] [PubMed]
- van Calcar, S.C.; Ney, D.M. Food Products Made with Glycomacropeptide, a Low-Phenylalanine Whey Protein, Provide a New Alternative to Amino Acid–Based Medical Foods for Nutrition Management of Phenylketonuria. J. Acad. Nutr. Diet. 2012, 112, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES): Anthropometry Procedures Manual; CDC: Atlanta, GA, USA, 2007; pp. 3–15. Available online: https://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_an.pdf (accessed on 22 April 2023).
- Kułaga, Z.; Różdżyńska-Świątkowska, A.; Grajda, A.; Gurzkowska, B.; Wojtyło, M.; Góźdź, M.; Świąder-Leśniak, A.; Litwin, M. Siatki centylowe dla oceny wzrastania i stanu odżywienia polskich dzieci i młodzieży od urodzenia do 18 roku życia. Standardy Medyczne/Pediatria 2015, 12, 119–135. [Google Scholar]
- Wajszczyk, B.; Chwojnowska, Z.; Nasiadko, D.; Rybaczuk, M.; Charzewska, J. Instrukcja korzystania z program Dieta 6.0. do Planowania i Bieżącej Oceny Żywienia Indywidualnego i Zbiorowego; Narodowy Instytut Zdrowia Publicznego—Państwowy Zakład Higieny: Warszawa, Poland, 2021.
- Marcdante, K.; Kliegman, R.; Jenson, H.; Behrman, R. Nelson Pediatria T.1 I T.2; Elsevier Urban & Partner: Wrocław, Poland, 2012. [Google Scholar]
- Shuhart, C.R.; Yeap, S.S.; Anderson, P.A.; Jankowski, L.G.; Lewiecki, E.M.; Morse, L.R.; Rosen, H.N.; Weber, D.R.; Zemel, B.S.; Shepherd, J.A. Executive Summary of the 2019 ISCD Position Development Conference on Monitoring Treatment, DXA Cross-Calibration and Least Significant Change, Spinal Cord Injury, Peri-Prosthetic and Orthopedic Bone Health, Transgender Medicine, and Pediatrics. J. Clin. Densitom. 2019, 22, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Purcell, O.; Coughlan, A.; Grant, T.; McNulty, J.; Clark, A.; Deverell, D.; Mayne, P.; Hughes, J.; Monavari, A.; Knerr, I.; et al. Growth Patterns in the Irish Pyridoxine Nonresponsive Homocystinuria Population and the Influence of Metabolic Control and Protein Intake. J. Nutr. Metab. 2017, 2017, 1–7. [Google Scholar] [CrossRef] [PubMed]
- De Biase, I.; Gherasim, C.; La’Ulu, S.L.; Asamoah, A.; Longo, N.; Yuzyuk, T. Laboratory evaluation of homocysteine remethylation disorders and classic homocystinuria: Long-term follow-up using a cohort of 123 patients. Clin. Chim. Acta 2020, 509, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Langeveld, M.; Hollak, C.E.M. Bone health in patients with inborn errors of metabolism. Rev. Endocr. Metab. Disord. 2018, 19, 81–92. [Google Scholar] [CrossRef]
- Van Meurs, J.B.; Dhonukshe-Rutten, R.A.; Pluijm, S.M.; Van Der Klift, M.; De Jonge, R.; Lindemans, J.; de Groot, L.C.; Hofman, A.; Witteman, J.C.; Van Leeuwen, J.P.; et al. Homocysteine Levels and the Risk of Osteoporotic Fracture. N. Engl. J. Med. 2004, 350, 2033–2041. [Google Scholar] [CrossRef]
- Sato, Y.; Honda, Y.; Iwamoto, J.; Kanoko, T.; Satoh, K. RETRACTED: Homocysteine as a predictive factor for hip fracture in stroke patients. Bone 2005, 36, 721–726. [Google Scholar] [CrossRef]
- Rusińska, A.; Płudowski, P.; Walczak, M.; Borszewska-Kornacka, M.K.; Bossowski, A.; Chlebna-Sokół, D.; Czech-Kowalska, J.; Dobrzańska, A.; Franek, E.; Helwich, E.; et al. Vitamin D Supplementation Guidelines for General Population and Groups at Risk of Vitamin D Deficiency in Poland—Recommendations of the Polish Society of Pediatric Endocrinology and Diabetes and the Expert Panel With Participation of National Specialist Consultants and Representatives of Scientific Societies—2018 Update. Front. Endocrinol. 2018, 9, 246. [Google Scholar] [CrossRef]
- Stroup, B.M.; Hansen, K.E.; Krueger, D.; Binkley, N.; Ney, D.M. Sex differences in body composition and bone mineral density in phenylketonuria: A cross-sectional study. Mol. Genet. Metab. Rep. 2018, 15, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Laine, C.M.; Laine, T. Diagnosis of Osteoporosis in Children and Adolescents. Eur. Endocrinol. 2010, 9, 141–144. [Google Scholar] [CrossRef]
- Geiger, K.E.; Koeller, D.M.; Harding, C.O.; Huntington, K.L.; Gillingham, M.B. Normal vitamin D levels and bone mineral density among children with inborn errors of metabolism consuming medical food–based diets. Nutr. Res. 2015, 36, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Poloni, S.; Leistner-Segal, S.; Bandeira, I.C.; D’Almeida, V.; de Souza, C.F.M.; Spritzer, P.M.; Castro, K.; Tonon, T.; Nalin, T.; Imbard, A.; et al. Body composition in patients with classical homocystinuria: Body mass relates to homocysteine and choline metabolism. Gene 2014, 546, 443–447. [Google Scholar] [CrossRef]
- Elshorbagy, A.K.; Nurk, E.; Gjesdal, C.G.; Tell, G.S.; Ueland, P.M.; Nygård, O.; Tverdal, A.; Vollset, S.E.; Refsum, H. Homocysteine, cysteine, and body composition in the Hordaland Homocysteine Study: Does cysteine link amino acid and lipid metabolism? Am. J. Clin. Nutr. 2008, 88, 738–746. [Google Scholar] [CrossRef]
- Modan-Moses, D.; Vered, I.; Schwartz, G.; Anikster, Y.; Abraham, S.; Segev, R.; Efrati, O. Peak bone mass in patients with phenylketonuria. J. Inherit. Metab. Dis. 2007, 30, 202–208. [Google Scholar] [CrossRef]
- Kose, E.; Arslan, N. Vitamin/mineral and micronutrient status in patients with classical phenylketonuria. Clin. Nutr. 2018, 38, 197–203. [Google Scholar] [CrossRef]
- Green, B.; Browne, R.; Firman, S.; Hill, M.; Rahman, Y.; Hansen, K.K.; Adam, S.; Skeath, R.; Hallam, P.; Herlihy, I.; et al. Nutritional and Metabolic Characteristics of UK Adult Phenylketonuria Patients with Varying Dietary Adherence. Nutrients 2019, 11, 2459. [Google Scholar] [CrossRef]
| Age (Years) | Polish Nutrition Standards (PZH 2020) 1 | Individuals with Classical Homocystinuria (HCU) 2 |
|---|---|---|
| Total Protein Intake | Total Protein Intake 2 | |
| 0–0.5 | 1.52 g/kg bw 3 | 2.1–2.3 g/kg bw |
| 0.5–1 | 1.60 g/kg bw | 2.0 g/kg bw |
| 1–3 | 1.17 g/kg bw | 22 g/d |
| 4 | 1.1 g/kg bw | |
| 5 | 32 g/d | |
| 6 | ||
| 7 | ||
| 8 | 40 g/d | |
| 9 | ||
| 10 | ||
| 11 | ||
| 15 | 55–60 g/d | |
| 16 | 0.95 g/kg bw | 50–60 g/d |
| 18 | ||
| 19 | 0.90 g/kg bw |
| n | Mean | SD | Median | Range | Confidence Intervals (CI: 5; 95) | ||
|---|---|---|---|---|---|---|---|
| Whole bone mineral density without head [z-score] | 10 | −0.42 | 0.9 | −0.5 | −1.7 | 1.1 | −1.02; 0.18 |
| Mineral density of the spine in the L2–L4 section [z-score] | 10 | −1.13 | −1.3 | −1.4 | −2.6 | 1.5 | −2.03; 0.23 |
| Mineral Density of Whole Bone without Head [z-Score] | Mineral Density of the L2–L4 Section [z-Score] | |||||||
|---|---|---|---|---|---|---|---|---|
| F-Ratio | p-Value | R2 | Standard Error of Estimation | F-Ratio | p-Value | R2 | Standard Error of Estimation | |
| Natural protein intake [g/d] | 5.37 1 | 0.0390 | 59.3% | 0.57 | ||||
| Magnesium intake [mg/d] | ||||||||
| Methionine intake [mg/d] | ||||||||
| Age [years] | 15.68 1 | 0.0049 | 86.7% | 0.325 | ||||
| BMI [kg/m2] | ||||||||
| 25-hydroxyvitamin D concentration in blood [ng/mL] | ||||||||
| 25-hydroxy vitamin D concentration in blood [ng/mL] | 101.37 1 | 0.0015 | 98.5% | 0.152 | ||||
| Calcium concentration in blood [mmol/L] | ||||||||
| Homocysteine concentration in blood [µmol/L] | ||||||||
| Methionine concentration in blood [µmol/L] | ||||||||
| Protein intake from amino acid preparation [g/d] | 7.44 2 | 0.0260 | 48.2% | 0.960 | ||||
| 25-Hydroxyvitamin D Concentration in Blood [ng/mL] | Energy Content of Diet [kcal/d] | BMI [kg/m2] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F-Ratio | p-Value | R2 | Standard Error of Estimation | F-Ratio | p-Value | R2 | Standard Error of Estimation | F-Ratio | p-Value | R2 | Standard Error of Estimation | |
| Vitamin D intake 2 [µg/d] | 5.90 | 0.038 | 39.6% | 6.54 | ||||||||
| Natural protein intake [g/d] | 14.8 | 0.004 | 72.2% | 322.89 | ||||||||
| Methionine concentration in blood [µmol/L] | 18.1 | 0.001 | 81.9% | 1.97 | ||||||||
| Parameter Examined | n | Mean | SD | Median | Range | Confidence Intervals (CI: 5; 95) | |
|---|---|---|---|---|---|---|---|
| Homocysteine (µmol/L) | 11 | 69.9 | 46.2 | 69.4 | 23.88 | 175.37 | 36.88; 102.92 |
| Methionine (µmol/L) | 11 | 83 | 79.5 | 62.4 | 33.5 | 316.5 | 26.15; 139.85 |
| Calcium (mmol/L) | 11 | 2.4 | 0.1 | 2.4 | 2.31 | 2.63 | 2.33; 2.47 |
| Phosphorus (mmol/L) | 11 | 1.5 | 0.1 | 1.5 | 1.31 | 1.67 | 1.41; 1.59 |
| 25-hydroxyvitamin D (ng/mL) | 11 | 34.0 | 8.0 | 32.3 | 23.9 | 48.1 | 28.29; 39.71 |
| Albumin (g/L) | 11 | 44.6 | 1.8 | 44.6 | 42 | 48 | 43.2; 46 |
| Dietary Intake | n | Mean | SD | Median | Range | Confidence Intervals (CI: 5; 95) | |
|---|---|---|---|---|---|---|---|
| Energy (kcal/d) | 11 | 1526.6 | 498.26 | 1448 | 875 | 2447 | 1191.9; 1861.3 |
| Protein from diet (g/d) | 11 | 12.68 | 9.41 | 8.3 | 6.2 | 35.09 | 6.38; 18.98 |
| Protein from amino acid preparations (g/d) | 11 | 24.34 | 9.08 | 20.0 | 10.0 | 45.0 | 18.24; 30.44 |
| Calcium intake from diet + supplementation (mg/d) | 11 | 759.1 | 333.2 | 631.0 | 473.0 | 1496.0 | 535.3; 928.9 |
| Phosphorus intake from diet (mg/d) | 11 | 708.5 | 186.1 | 701.0 | 397.7 | 1013.0 | 583.4; 833.6 |
| Vitamin D3 from diet + supplementation (µg/d) | 11 | 46.63 | 24.22 | 47.26 | 17.41 | 97.76 | 30.33; 62.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batycka, M.; Lange, E.; Ehmke vel Emczyńska-Seliga, E.; Jaworski, M.; Kobylińska, M.; Lech, N.; Samborowska, E.; Lipiński, P.; Perkowska, B.; Pokora, P.; et al. Relationship between Bone Mineral Density and Selected Parameters of Calcium-Phosphate Economy with Dietary Management and Metabolic Control in Polish Pediatric Patients with Classical Homocystinuria—A Preliminary Study. Nutrients 2023, 15, 2112. https://doi.org/10.3390/nu15092112
Batycka M, Lange E, Ehmke vel Emczyńska-Seliga E, Jaworski M, Kobylińska M, Lech N, Samborowska E, Lipiński P, Perkowska B, Pokora P, et al. Relationship between Bone Mineral Density and Selected Parameters of Calcium-Phosphate Economy with Dietary Management and Metabolic Control in Polish Pediatric Patients with Classical Homocystinuria—A Preliminary Study. Nutrients. 2023; 15(9):2112. https://doi.org/10.3390/nu15092112
Chicago/Turabian StyleBatycka, Małgorzata, Ewa Lange, Ewa Ehmke vel Emczyńska-Seliga, Maciej Jaworski, Maria Kobylińska, Natalia Lech, Emilia Samborowska, Patryk Lipiński, Barbara Perkowska, Paulina Pokora, and et al. 2023. "Relationship between Bone Mineral Density and Selected Parameters of Calcium-Phosphate Economy with Dietary Management and Metabolic Control in Polish Pediatric Patients with Classical Homocystinuria—A Preliminary Study" Nutrients 15, no. 9: 2112. https://doi.org/10.3390/nu15092112
APA StyleBatycka, M., Lange, E., Ehmke vel Emczyńska-Seliga, E., Jaworski, M., Kobylińska, M., Lech, N., Samborowska, E., Lipiński, P., Perkowska, B., Pokora, P., & Rokicki, D. (2023). Relationship between Bone Mineral Density and Selected Parameters of Calcium-Phosphate Economy with Dietary Management and Metabolic Control in Polish Pediatric Patients with Classical Homocystinuria—A Preliminary Study. Nutrients, 15(9), 2112. https://doi.org/10.3390/nu15092112









