Absence of Effects of L-Arginine and L-Citrulline on Inflammatory Biomarkers and Oxidative Stress in Response to Physical Exercise: A Systematic Review with Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Registration
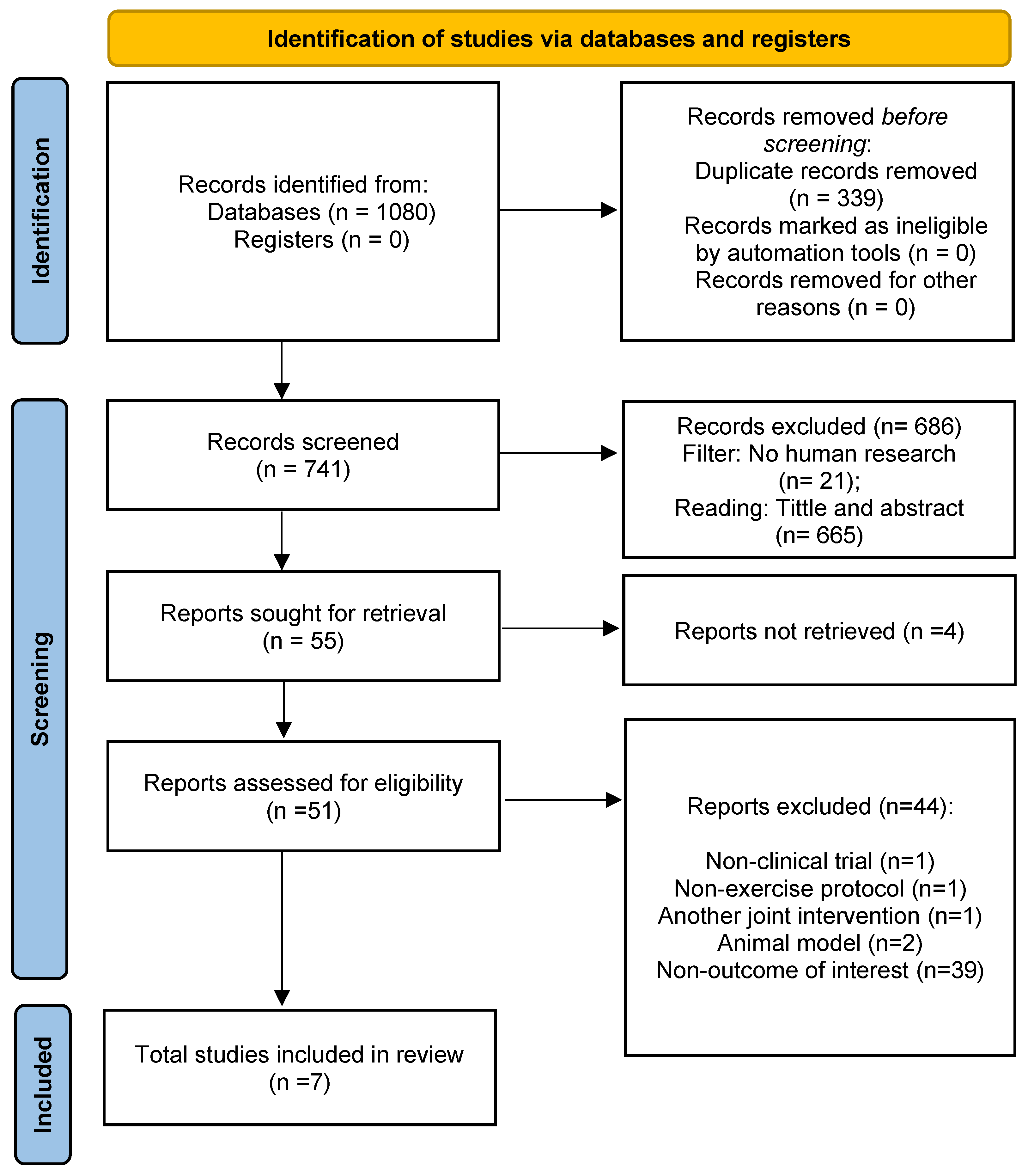
2.2. Search Strategy and Study Selection
2.3. Data Extraction
2.4. Assessment of the Risk of Bias
2.5. GRADE (Levels of Evidence)
2.6. Qualitative Analysis (Systematic Review)
2.7. Quantitative Analysis (Meta-Analysis)
3. Results
3.1. Description of Studies
3.2. Qualitative Analysis
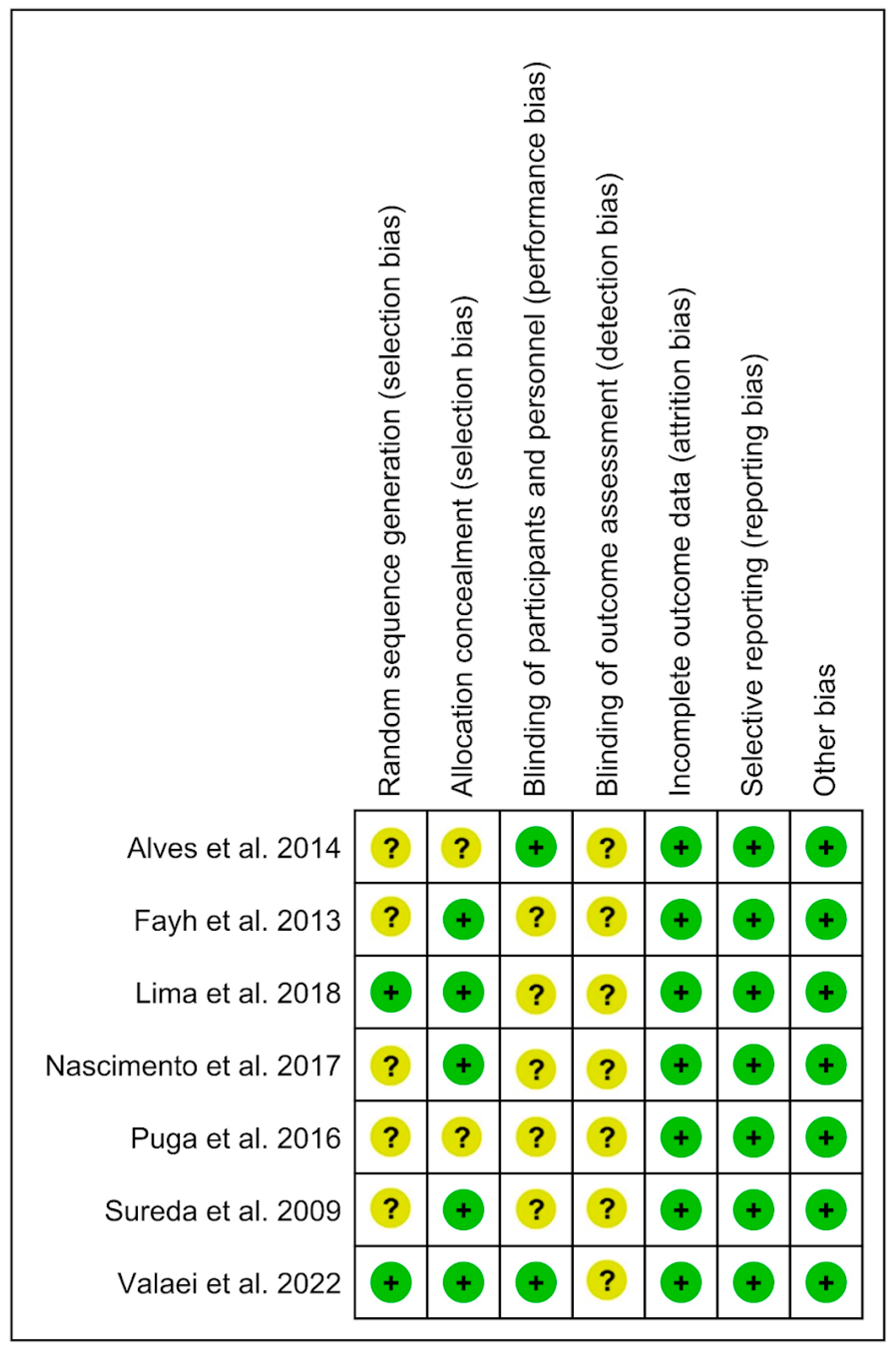
3.3. Analysis of the Risk of Bias
3.4. Quantitative Analysis
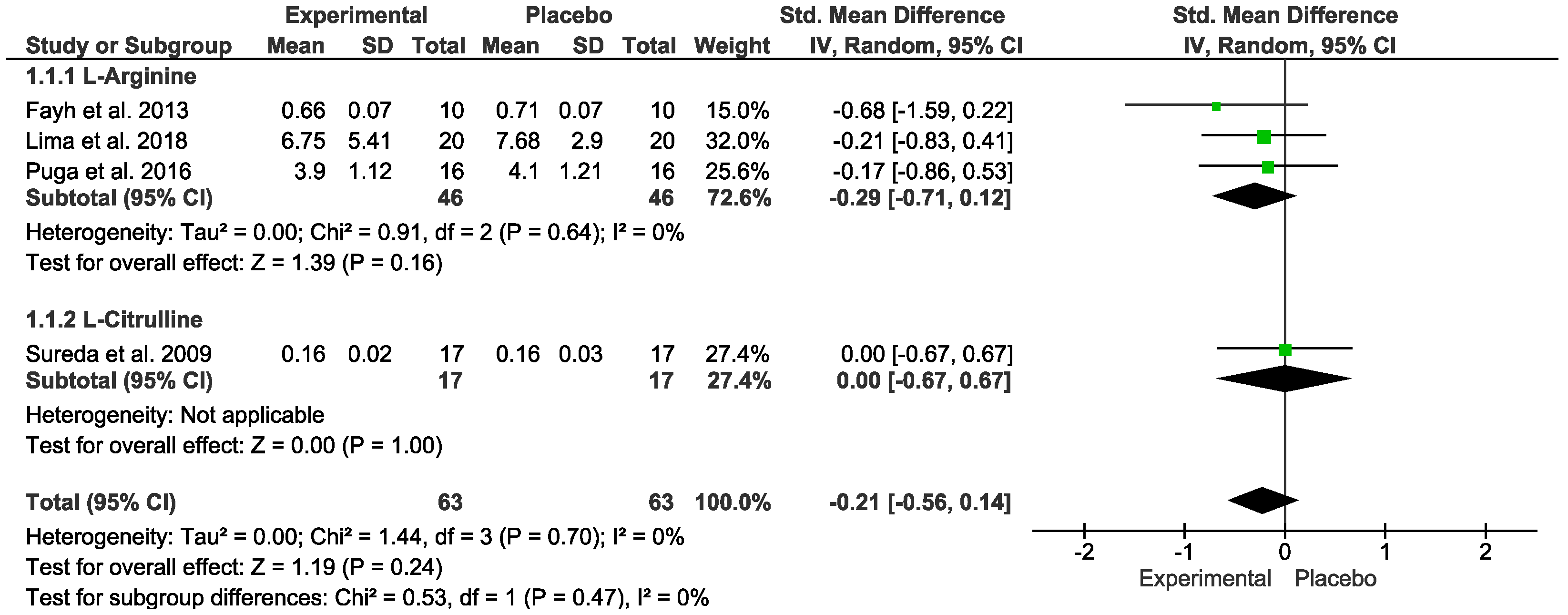
3.5. Oxidative Stress Markers
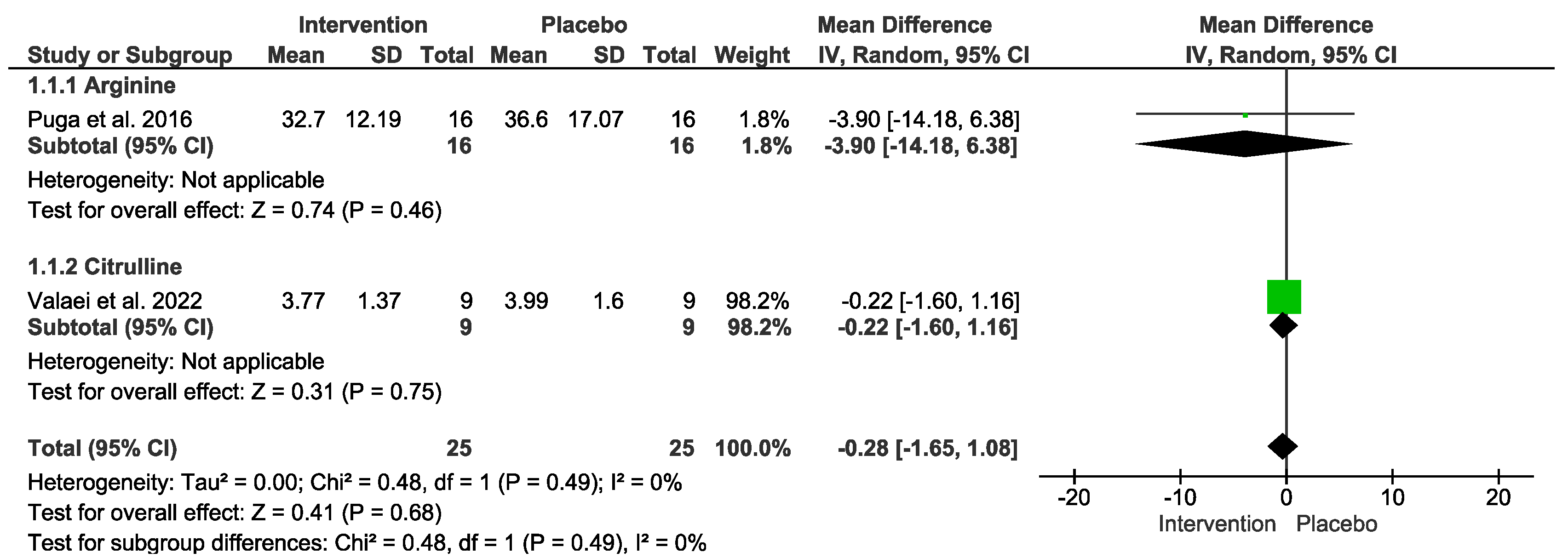
3.6. Antioxidants Markers
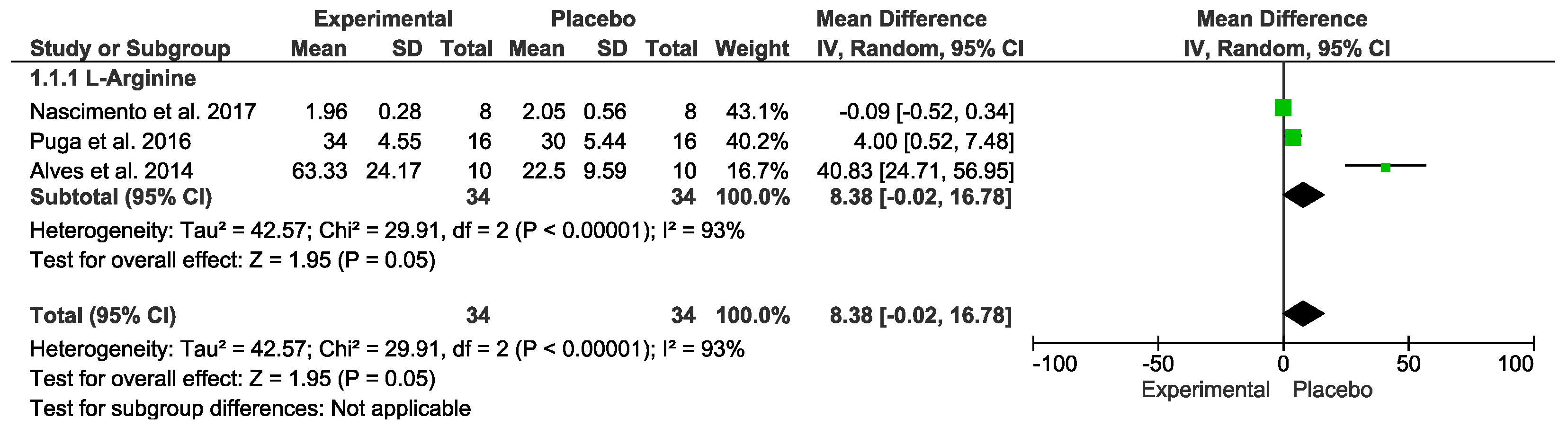
3.7. Inflammatory Markers
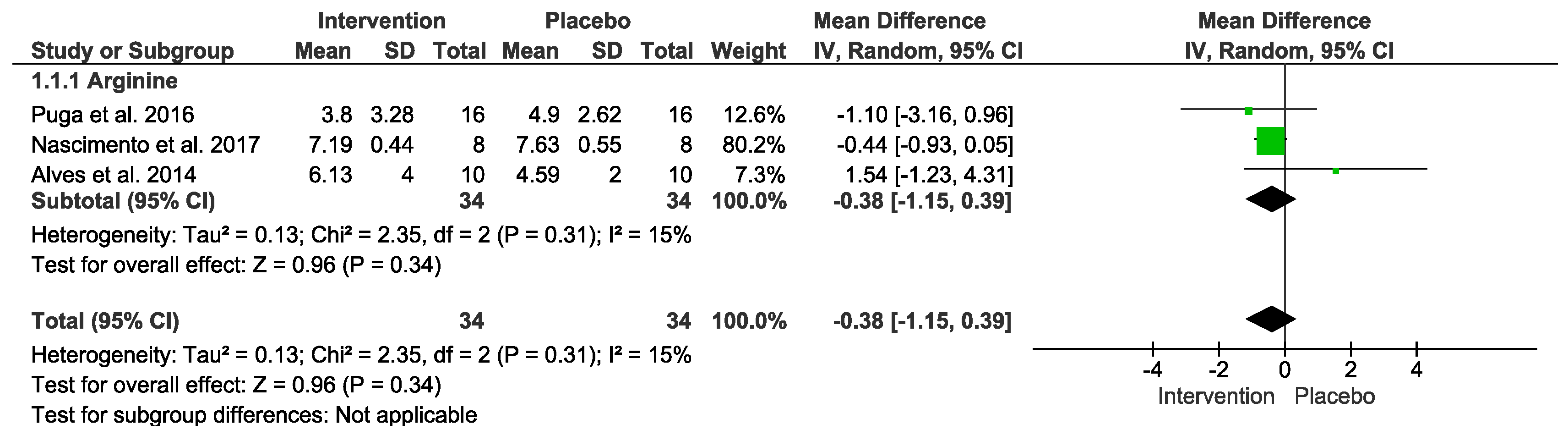
3.8. Anti-Inflammatory Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bloomer, R.J.; Goldfarb, A.H.; Wideman, L.; McKenzie, M.J.; Consitt, L.A. Effects of Acute Aerobic and Anaerobic Exercise on Blood Markers of Oxidative Stress. J. Strength Cond. Res. 2005, 19, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Exhaustive Exercise-Induced Neutrophil-Associated Tissue Damage and Possibility of its Prevention. J. Nanomed. Biother. Discov. 2017, 7, 156. [Google Scholar] [CrossRef]
- Pedersen, B.K. Muscles and Their Myokines. J. Exp. Biol. 2011, 214, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Stanelle, S.T.; McLaughlin, K.L.; Crouse, S.F. One Week of L-Citrulline Supplementation Improves Performance in Trained Cyclists. J. Strength Cond. Res. 2020, 34, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Bian, K.; Murad, F. Nitric Oxide Signaling in Vascular Biology. J. Am. Soc. Hypertens. 2007, 1, 17–29. [Google Scholar] [CrossRef]
- Daniela, M.; Catalina, L.; Ilie, O.; Paula, M.; Daniel-Andrei, I.; Ioana, B. Effects of Exercise Training on the Autonomic Nervous System with a Focus on Anti-Inflammatory and Antioxidants Effects. Antioxidants 2022, 11, 350. [Google Scholar] [CrossRef]
- Irrcher, I.; Ljubicic, V.; Hood, D.A. Interactions between ROS and AMP Kinase Activity in the Regulation of PGC-1 Transcription in Skeletal Muscle Cells. Am. J. Physiol. Cell Physiol. 2009, 296, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Ranchordas, M.K.; Rogerson, D.; Soltani, H.; Costello, J.T. Antioxidants for Preventing and Reducing Muscle Soreness after Exercise: A Cochrane Systematic Review. Br. J. Sports Med. 2020, 54, 74–78. [Google Scholar] [CrossRef]
- Academy of Nutrition and Dietetics (AND); Dietitians of Canada (DC); American College of Sports Medicine (ACSM). Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar] [CrossRef]
- Baar, K. Nutrition and the Adaptation to Endurance Training. Sports Med. 2014, 44, 5–12. [Google Scholar] [CrossRef]
- Draeger, C.L.; Naves, A.; Marques, N.; Baptistella, A.B.; Carnauba, R.A.; Paschoal, V.; Nicastro, H. Controversies of Antioxidant Vitamins Supplementation in Exercise: Ergogenic or Ergolytic Effects in Humans? J. Int. Soc. Sports Nutr. 2014, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Alves, G.N.; Tavares, A.M.V.; Vieira, P.J.C.; Sprinz, E.; Ribeiro, J.P. Oral L-Arginine Modulates Blood Lactate and Interleukin-6 after Exercise in HIV-Infected Men. Int. J. Sports Med. 2014, 35, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, M.A.; dos Santos Lira, F.; Punaro, G.R.; de Mello, M.T.; Tufik, S.; Higa, E.M.S. Short-Term l-Arginine Supplementation Attenuates Elevation of Interleukin 6 Level after Resistance Exercise in Overweight Men. Clin. Nutr. ESPEN 2017, 22, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Puga, G.M.; Novais, I.D.P.; Katsanos, C.S.; Zanesco, A. Combined Effects of Aerobic Exercise and L-Arginine Ingestion on Blood Pressure in Normotensive Postmenopausal Women: A Crossover Study. Life Sci. 2016, 151, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Fayh, A.P.T.; Krause, M.; Rodrigues-Krause, J.; Ribeiro, J.L.; Ribeiro, J.P.; Friedman, R.; Moreira, J.C.F.; Reischak-Oliveira, A. Effects of L-Arginine Supplementation on Blood Flow, Oxidative Stress Status and Exercise Responses in Young Adults with Uncomplicated Type I Diabetes. Eur. J. Nutr. 2013, 52, 975–983. [Google Scholar] [CrossRef]
- De Lima, F.F.; da Silva, T.F.; Neto, M.M.; Toscano, L.T.; da Silva, C.S.O.; Silva, A.S. Effect of L-Arginine Intake on Exercise-Induced Hypotension. Nutr. Hosp. 2018, 35, 1195–1200. [Google Scholar] [CrossRef]
- Sureda, A.; Córdova, A.; Ferrer, M.D.; Tauler, P.; Pérez, G.; Tur, J.A.; Pons, A. Effects of L-Citrulline Oral Supplementation on Polymorphonuclear Neutrophils Oxidative Burst and Nitric Oxide Production after Exercise. Free Radic. Res. 2009, 43, 828–835. [Google Scholar] [CrossRef]
- Valaei, K.; Mehrabani, J.; Wong, A. Effects of L-Citrulline Supplementation on Nitric Oxide and Antioxidant Markers after High-Intensity Interval Exercise in Young Men: A Randomized Controlled Trial. Br. J. Nutr. 2021, 127, 1303–1312. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated Guidance for Trusted Systematic Reviews: A New Edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef]
- De Carvalho, A.P.V.; Silva, V.; Grande, A.J. Avaliação Do Risco de Viés de Ensaios Clínicos Randomizados Pela Ferramenta Da Colaboração Cochrane. Diagn. Trat. 2013, 18, 38–44. [Google Scholar]
- Hultcrantz, M.; Rind, D.; Akl, E.A.; Treweek, S.; Mustafa, R.A.; Iorio, A.; Alper, B.S.; Meerpohl, J.J.; Murad, M.H.; Ansari, M.T.; et al. The GRADE Working Group clarifies the construct of certainty of evidence. J. Clin. Epidemiol. 2017, 87, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G. Quantifying Heterogeneity in a Meta-Analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; Cochrane: London, UK, 2019; pp. 1–694. [Google Scholar] [CrossRef]
- Radomski, M.W.; Palmer, R.M.J.; Moncada, S. Modulation of Platelet Aggregation by an L-Arginine-Nitric Oxide Pathway. Trends Pharmacol. Sci. 1991, 12, 87–88. [Google Scholar] [CrossRef]
- Banez, M.J.; Geluz, M.I.; Chandra, A.; Hamdan, T.; Biswas, O.S.; Bryan, N.S.; von Schwarz, E.R. A Systemic Review on the Antioxidant and Anti-Inflammatory Effects of Resveratrol, Curcumin, and Dietary Nitric Oxide Supplementation on Human Cardiovascular Health. Nutr. Res. 2020, 78, 11–26. [Google Scholar] [CrossRef]
- Cho, S.Y.; Chung, Y.S.; Yoon, H.K.; Roh, H.T. Impact of Exercise Intensity on Systemic Oxidative Stress, Inflammatory Responses, and Sirtuin Levels in Healthy Male Volunteers. Int. J. Environ. Res. Public Health 2022, 19, 11292. [Google Scholar] [CrossRef]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-Induced Oxidative Stress: Friend or Foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Bessa, A.L.; Oliveira, V.N.; Agostini, G.G.; Oliveira, R.J.S.; Oliveira, A.C.S.; White, G.E.; Wells, G.D.; Teixeira, D.N.S.; Espindola, F.S. Exercise Intensity and Recovery: Biomarkers of Injury, Inflammation, and Oxidative Stress. J. Strength Cond. Res. 2016, 30, 311–319. [Google Scholar] [CrossRef]
- Förstermann, U.; Li, H. Therapeutic Effect of Enhancing Endothelial Nitric Oxide Synthase (ENOS) Expression and Preventing ENOS Uncoupling. Br. J. Pharmacol. 2011, 164, 213–223. [Google Scholar] [CrossRef]
- Agarwal, U.; Didelija, I.C.; Yuan, Y.; Wang, X.; Marini, J.C. Supplemental Citrulline Is More Efficient Than Arginine in Increasing Systemic Arginine Availability in Mice. J. Nutr. 2017, 147, 596–602. [Google Scholar] [CrossRef]
- Windmueller, H.G.; Spaeth, A.E. Source and Fate of Circulating Citrulline. Am. J. Physiol. 1981, 241, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, K.A.; Mohammad, S.; Garneau, L.; McInnis, K.; Aguer, C.; Adamo, K.B. Examination of the Myokine Response in Pregnant and Non-Pregnant Women following an Acute Bout of Moderate-Intensity Walking. Front. Physiol. 2019, 10, 1188. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Song, X.; Wang, H.; Yan, Y.; Liu, B. The Role of Exercise-Induced Myokines in Promoting Angiogenesis. Front. Physiol. 2022, 13, 1787. [Google Scholar] [CrossRef] [PubMed]
| Author/ Years | Study Design | Sample | Age (Years) | Weight (kg) | Height(cm) | Body Fat (%) | Exercise Protocol | Average Peak Oxygen (mL/kg/min) | Intervention | Placebo |
|---|---|---|---|---|---|---|---|---|---|---|
| Alves et al., (2014) [12] | RCT (crossover) | 10 HIV-1-infected men on HAART | 47 ± 2 | 71 ± 4.7 | 1.68 ± 0.03 | Not reported | Maximal incremental cardiopulmonary exercise tests | 33 ± 8.8 (Arg) 33 ± 7.7 (Pla) | 20 g of L-arg diluted in 200 mL of water | Not reported |
| Fayh et al., 2013 [16] | RCT (parallel) | 10 young adult male subjects with uncomplicated type 1 diabetes and 20 matched control volunteers | Type I diabetics: 23.3 ± 1.73 Controls: 23.4 ± 0.59 | Type I diabetics: 72.3 ± 4.25 Controls: 75.2 ± 2.54 | Type I diabetics: 173.7 ± 2.18 Controls: 177.6 ± 1.81 | Type I diabetics: 19.06 ± 2.92 Controls: 17.8 ± 1.20 | Cycle ergometer test (during 45 min) | Type I diabetics: 37.1 ± 2.28 Controls: 45.4 ± 1.75 | L-Arginine supplementation consisted of oral ingestion of identical pills containing 7 g of L-Arginine-hydrochloride | Oral ingestion of identical pills containing either amide compound |
| Lima et al., 2018 [17] | RCT (crossover) | 20 diagnosed hypertensive patients | 51.47 ± 1.24 | Not reported | Not reported | Not reported | Treadmill exercise for 60 min (60–85% HRmax) | Not reported | Seven grams of lemon-flavor L-Arginine diluted in 100 mL of water | Seven grams of lemon-flavor placebo diluted in 100 mL of water |
| Puga et al., 2016 [14] | RCT (crossover) | 16 normotensive postmenopausal women | 57 ± 24 | Not reported | Not reported | Not reported | Treadmill for 30 min (maximal lactate steady state) | Not reported | 9 g of L-Arginine base (acid (2S)-2-amino-5-guanidopentanoic—Ajinomoto, Japan) | Placebo pill intake |
| Nascimento et al., 2017 [13] | RCT (crossover) | 8 obese hypertensive men | 46 ± 6 | 92.56 ± 9.9 | 171 ± 0.6 | 28.65 ± 8.58 | Resistance exercise session (intensity equal to 60% of 1 repetition maximum) | Not reported | Supplements of L-arg (Sigma® (Kanagawa, Japan)) gelatin capsules (6 g/day, 3 times per day and 2 g each time) were administered orally for one week | Placebo (starch) gelatin capsules (6 g/day, 3 times per day and 2 g each time) were administered orally for one week |
| Sureda et al., 2009 [18] | RCT (parallel) | 17 volunteer male pre-professional cyclists | 22.3 ± 3.71 | 70.6 ± 5.36 | Not reported | Not reported | The cycling stage was 137.1 km long (179 min) | 81.9 ± 10.72 | 6 g of citrulline–malate dissolved in lemon juice | The control group consumed the lemon juice vehicle alone |
| Valaei et al., 2022 [19] | RCT (crossover) | 9 trained young men | 21.41 ± 1.13 | 79.50 ± 9.35 | 183.44 ± 7.60 | 10.67 ± 1.52 | 10 min warm-up, which consisted of 5 min cycling (with the minimal workload, 50–60% of HRmax) and 5 min dynamic stretching (total body) exercise. After, participants performed twelve consecutive rounds of two-hand kettlebell swing exercise including (30 s of exercise and 30 s of rest) using a 16 kg kettlebell | Not reported | One hour before exercise, the participants consumed 12 g of L-Cit powder dissolved in 200 mL of water (L-Cit 1200 mg, NOW® Sports (Arden Hills, MN, USA)) | Placebo 12 g of maltodextrin with the same appearance, taste, smell and color |
| Outcome No. of Participants (Studies) | Anticipated Absolute Effects (95% CI) | Certainty | What Happens | |
|---|---|---|---|---|
| Comparison | Intervention (Difference) | |||
| Arginine or Citrulline vs. placebo after exercise (Oxidative Stress) № of participants: 63 (4 studies) | The Hedges’ g mean was 3.16 | SMD −0.21 [−0.56, 0.14] | ⊕⊕◯◯ LOW Due to serious risk of bias. Due to serious inconsistency. Due to strongly suspected publication bias. Upgraded because all plausible confounding would suggest spurious effect. | Intervention protocol before exercise presents no significant difference in oxidative stress index after exercise session. |
| Arginine or Citrulline vs. placebo after exercise (Antioxidants) № of participants: 25 (2 studies) | SOD mean was 20.29 | MD −0.28 [−1.65, 1.08] | ⊕◯◯◯ VERY LOW Due to serious risk of bias. Due to serious inconsistency. Due to serious imprecision. Due to strongly suspected publication bias. Upgraded because all plausible confounding would suggest spurious effect. | Intervention protocol before exercise presents no significant difference in antioxidants index after exercise session. |
| Arginine vs. placebo after exercise (Inflammatory) № of participants: 34 (3 studies) | IL−6 mean was 18.18 | MD 8.38 [−0.02, 16.78] | ⊕◯◯◯ VERY LOW Due to serious risk of bias. Due to serious inconsistency. Due to serious imprecision. Due to strongly suspected publication bias. Upgraded because all plausible confounding would suggest spurious effect. | Intervention protocol before exercise presents no significant difference in inflammatory index after exercise session. |
| Arginine vs. placebo after exercise (Anti-inflammatory) № of participants: 34 (3 studies) | IL−10 mean was 1.72 | MD −0.38 [−1.15, 0.39] | ⊕◯◯◯ VERY LOW Due to serious risk of bias. Due to serious inconsistency. Due to serious imprecision. Due to strongly suspected publication bias. Upgraded because all plausible confounding would suggest spurious effect. | Intervention protocol before exercise presents no significant difference in anti-inflammatory index after exercise session. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porto, A.A.; Gonzaga, L.A.; Benjamim, C.J.R.; Valenti, V.E. Absence of Effects of L-Arginine and L-Citrulline on Inflammatory Biomarkers and Oxidative Stress in Response to Physical Exercise: A Systematic Review with Meta-Analysis. Nutrients 2023, 15, 1995. https://doi.org/10.3390/nu15081995
Porto AA, Gonzaga LA, Benjamim CJR, Valenti VE. Absence of Effects of L-Arginine and L-Citrulline on Inflammatory Biomarkers and Oxidative Stress in Response to Physical Exercise: A Systematic Review with Meta-Analysis. Nutrients. 2023; 15(8):1995. https://doi.org/10.3390/nu15081995
Chicago/Turabian StylePorto, Andrey A., Luana A. Gonzaga, Cicero Jonas R. Benjamim, and Vitor E. Valenti. 2023. "Absence of Effects of L-Arginine and L-Citrulline on Inflammatory Biomarkers and Oxidative Stress in Response to Physical Exercise: A Systematic Review with Meta-Analysis" Nutrients 15, no. 8: 1995. https://doi.org/10.3390/nu15081995
APA StylePorto, A. A., Gonzaga, L. A., Benjamim, C. J. R., & Valenti, V. E. (2023). Absence of Effects of L-Arginine and L-Citrulline on Inflammatory Biomarkers and Oxidative Stress in Response to Physical Exercise: A Systematic Review with Meta-Analysis. Nutrients, 15(8), 1995. https://doi.org/10.3390/nu15081995