Effect of Rotavirus Infection and 2′-Fucosyllactose Administration on Rat Intestinal Gene Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design and Sample Collection
2.3. RNA Extraction and Microarray Procedure
2.4. Microarray Data Analysis
2.5. Validation of Gene Expression by Real-Time PCR
2.6. Statistical Analysis
3. Results
3.1. Clinical Results
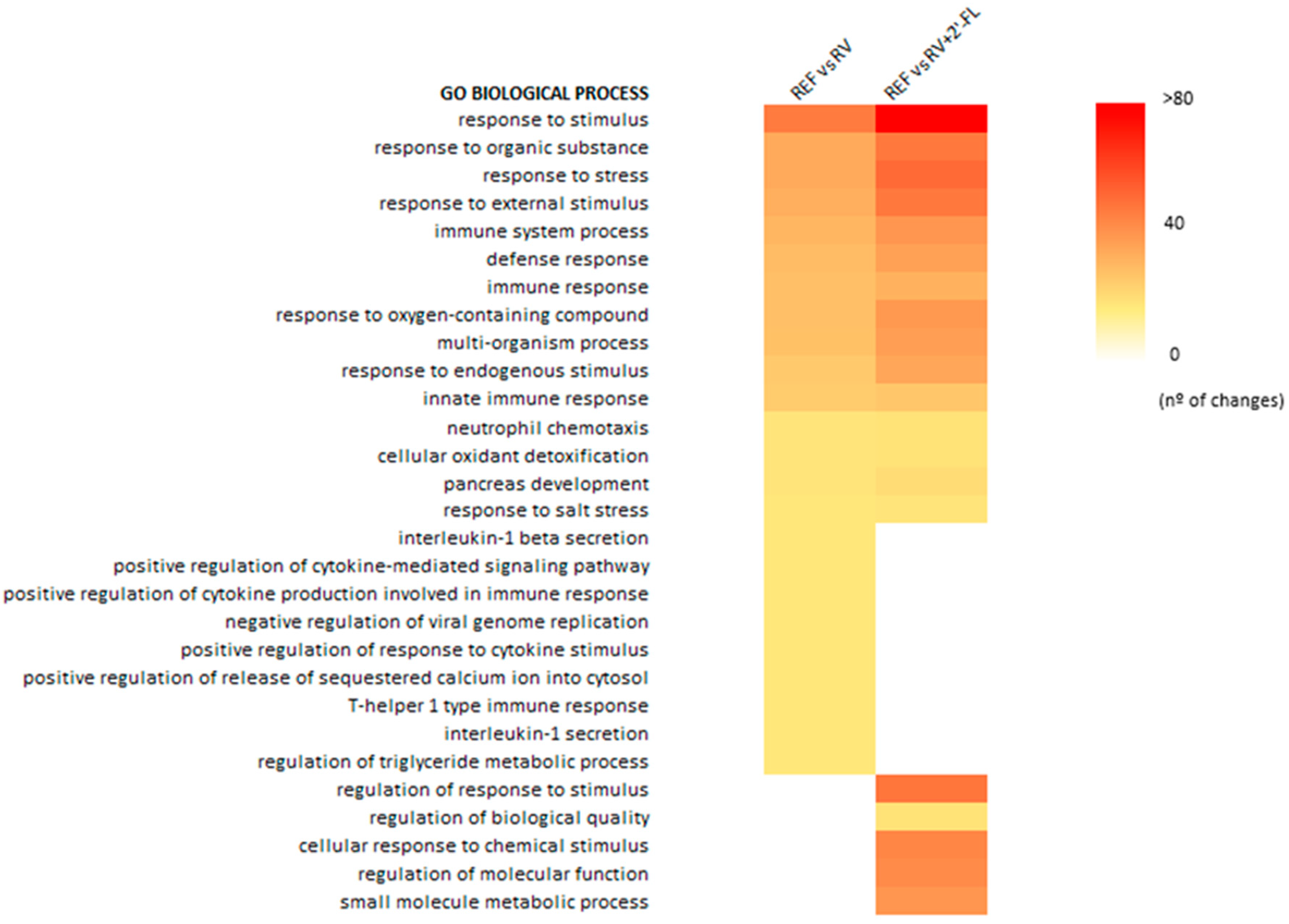
3.2. RV Effect on Overall Intestinal Rat Gene Expression
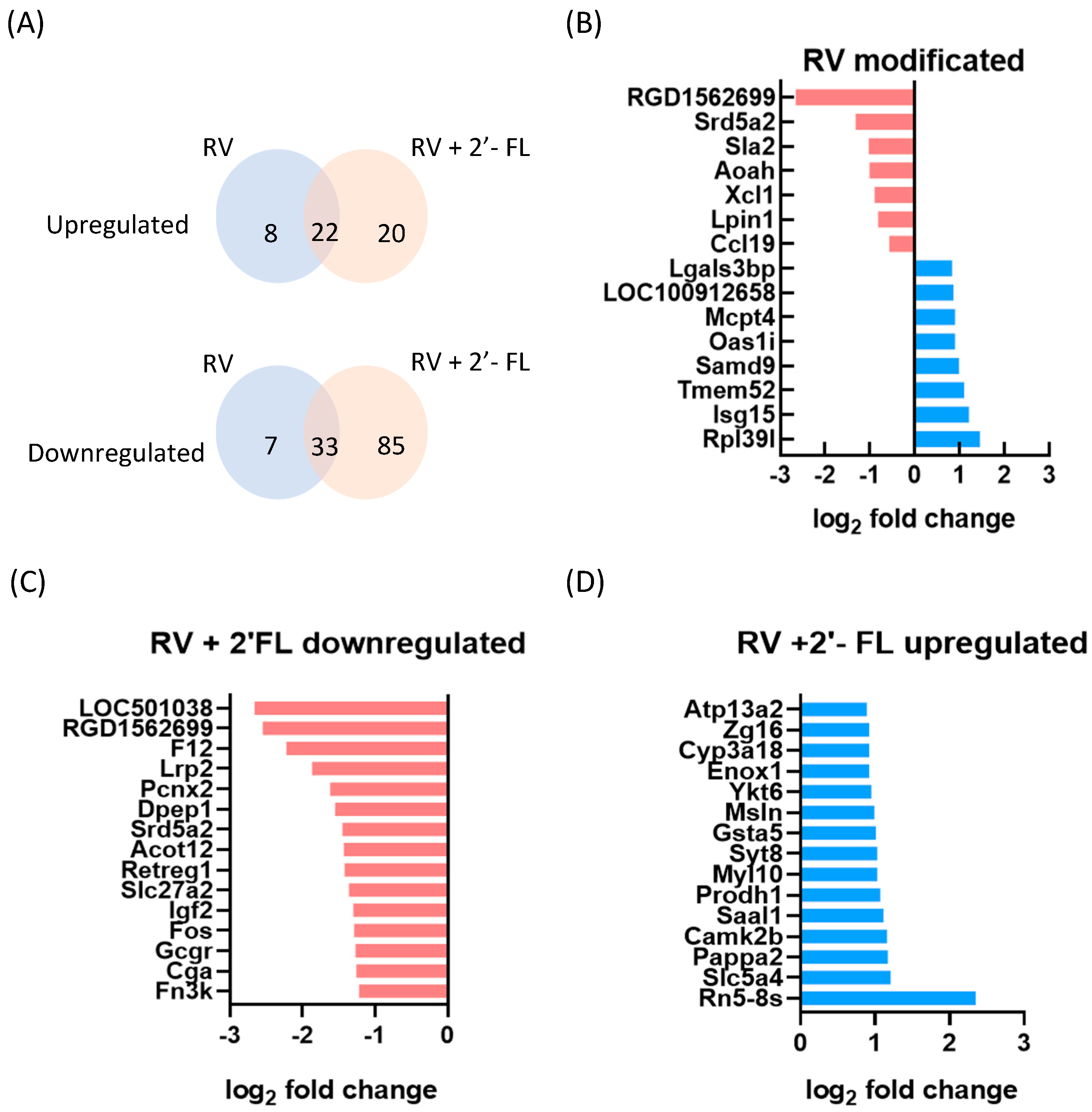
3.3. Gene Expression Changes Due to RV Infection and 2′-FL Supplementation
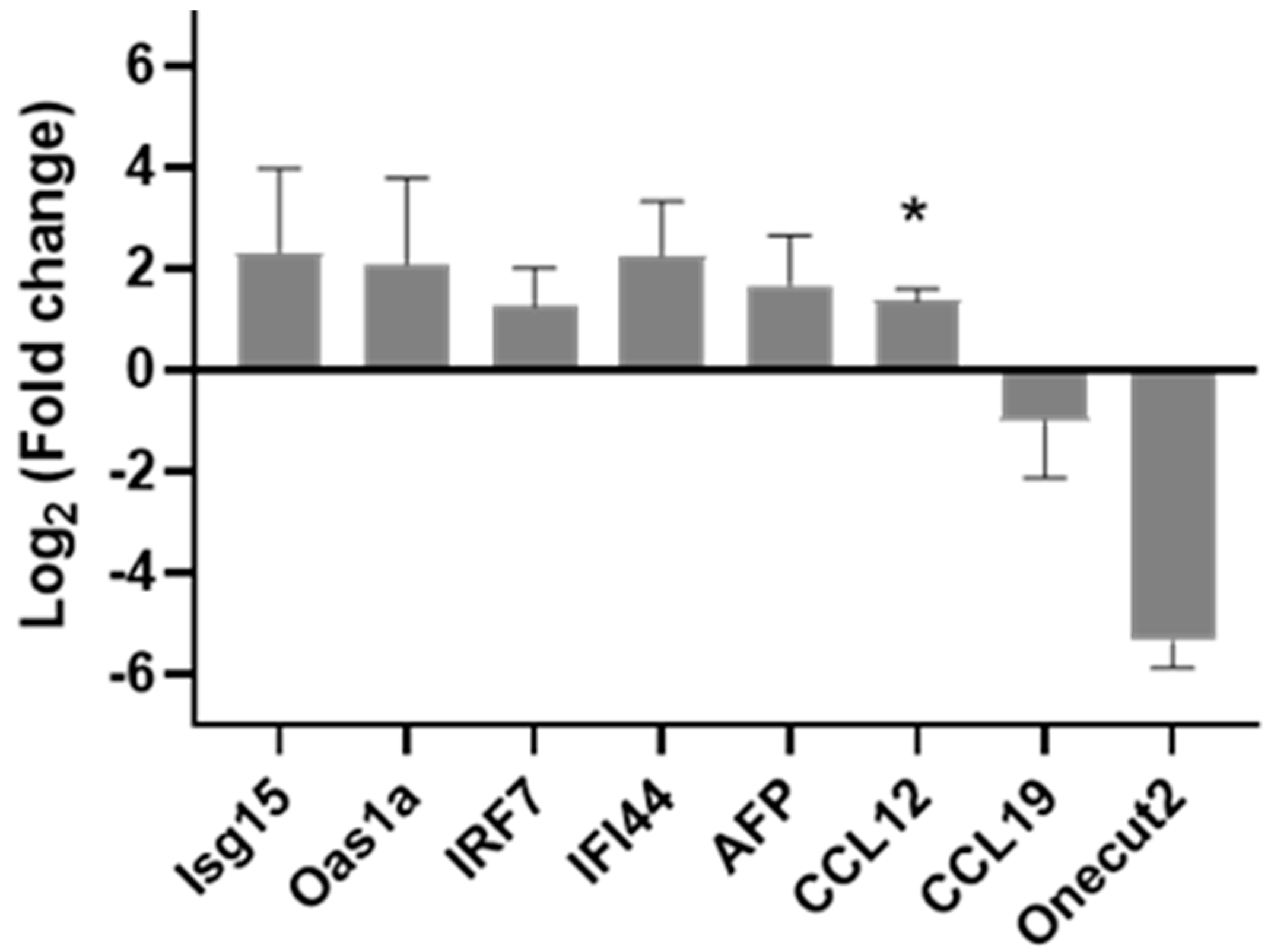
3.4. PCR Confirmation of Key Genes
3.5. Gene Expression Changes with 2′-FL Supplementation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Breastfeeding. Available online: https://www.who.int/health-topics/breastfeeding (accessed on 5 April 2022).
- Ballard, O.; Morrow, A.L. Human Milk Composition: Nutrients and Bioactive Factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Rio-Aige, K.; Azagra-Boronat, I.; Castell, M.; Selma-Royo, M.; Collado, M.C.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J. The Breast Milk Immunoglobulinome. Nutrients 2021, 13, 1810. [Google Scholar] [CrossRef] [PubMed]
- Picciano, M.F. Nutrient Composition of Human Milk. Pediatr. Clin. N. Am. 2001, 48, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Kirmiz, N.; Robinson, R.C.; Shah, I.M.; Barile, D.; Mills, D.A. Milk Glycans and Their Interaction with the Infant-Gut Microbiota. Annu. Rev. Food Sci. Technol. 2018, 9, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Castanys-Muñoz, E.; Martin, M.J.; Prieto, P.A. 2’-Fucosyllactose: An Abundant, Genetically Determined Soluble Glycan Present in Human Milk. Nutr. Rev. 2013, 71, 773–789. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; Fente, C.; Regal, P.; Lamas, A.; Lorenzo, M.P. Human Milk Oligosaccharides (HMOs) and Infant Microbiota: A Scoping Review. Foods 2021, 10, 1429. [Google Scholar] [CrossRef] [PubMed]
- Hegar, B.; Wibowo, Y.; Basrowi, R.W.; Ranuh, R.G.; Sudarmo, S.M.; Munasir, Z.; Atthiyah, A.F.; Widodo, A.D.; Supriatmo; Kadim, M.; et al. The Role of Two Human Milk Oligosaccharides, 2’-Fucosyllactose and Lacto-N-Neotetraose, in Infant Nutrition. Pediatr. Gastroenterol. Hepatol. Nutr. 2019, 22, 330–340. [Google Scholar] [CrossRef]
- Sodhi, C.P.; Wipf, P.; Yamaguchi, Y.; Fulton, W.B.; Kovler, M.; Niño, D.F.; Zhou, Q.; Banfield, E.; Werts, A.D.; Ladd, M.R.; et al. The Human Milk Oligosaccharides 2’-Fucosyllactose and 6’-Sialyllactose Protect against the Development of Necrotizing Enterocolitis by Inhibiting Toll-like Receptor 4 Signaling. Pediatr. Res. 2021, 89, 91–101. [Google Scholar] [CrossRef]
- Azagra-Boronat, I.; Massot-Cladera, M.; Knipping, K.; van’t Land, B.; Stahl, B.; Garssen, J.; Rodríguez-Lagunas, M.J.; Franch, À.; Castell, M.; Pérez-Cano, F.J. Supplementation with 2′-FL and ScGOS/LcFOS Ameliorates Rotavirus-Induced Diarrhea in Suckling Rats. Front. Cell. Infect. Microbiol. 2018, 8, 372. [Google Scholar] [CrossRef]
- Goehring, K.C.; Marriage, B.J.; Oliver, J.S.; Wilder, J.A.; Barrett, E.G.; Buck, R.H. Similar to Those Who Are Breastfed, Infants Fed a Formula Containing 2′-Fucosyllactose Have Lower Inflammatory Cytokines in a Randomized Controlled Trial. J. Nutr. 2016, 146, 2559–2566. [Google Scholar] [CrossRef]
- Berger, B.; Porta, N.; Foata, F.; Grathwohl, D.; Delley, M.; Moine, D.; Charpagne, A.; Siegwald, L.; Descombes, P.; Alliet, P.; et al. Linking Human Milk Oligosaccharides, Infant Fecal Community Types, and Later Risk To Require Antibiotics. mBio 2020, 11, e03196-19. [Google Scholar] [CrossRef] [PubMed]
- Esona, M.D.; Gautam, R. Rotavirus. Clin. Lab Med. 2015, 35, 363–391. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.E.; Burton, A.H.; Boschi-Pinto, C.; Steele, A.D.; Duque, J.; Parashar, U.D. 2008 Estimate of Worldwide Rotavirus-Associated Mortality in Children Younger than 5 Years before the Introduction of Universal Rotavirus Vaccination Programmes: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2012, 12, 136–141. [Google Scholar] [CrossRef]
- Frias, A.H.; Vijay-Kumar, M.; Gentsch, J.R.; Crawford, S.E.; Carvalho, F.A.; Estes, M.K.; Gewirtz, A.T. Intestinal Epithelia Activate Anti-Viral Signaling via Intracellular Sensing of Rotavirus Structural Components. Mucosal Immunol. 2010, 3, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Akira, S. Innate Immunity and Adjuvants. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2748–2755. [Google Scholar] [CrossRef]
- Morales-Ferré, C.; Azagra-Boronat, I.; Massot-Cladera, M.; Tims, S.; Knipping, K.; Garssen, J.; Knol, J.; Franch, À.; Castell, M.; Pérez-Cano, F.J.; et al. Preventive Effect of a Postbiotic and Prebiotic Mixture in a Rat Model of Early Life Rotavirus Induced-Diarrhea. Nutrients 2022, 14, 1163. [Google Scholar] [CrossRef]
- Hostetler, M.A.; Nakanishi, A.K.; Whiteman, P.J. Gastroenteritis: An Evidence Based Approach To Typical Vomiting, Diarrhea, and Dehydration. Pediatr. Emerg. Med. Pract. 2004, 1, 1–19. [Google Scholar]
- Pérez-Berezo, T.; Franch, A.; Ramos-Romero, S.; Castellote, C.; Pérez-Cano, F.J.; Castell, M. Cocoa-Enriched Diets Modulate Intestinal and Systemic Humoral Immune Response in Young Adult Rats. Mol. Nutr. Food Res. 2011, 55, S56–S66. [Google Scholar] [CrossRef]
- Massot-Cladera, M.; Franch, À.; Castell, M.; Pérez-Cano, F.J. Cocoa Polyphenols and Fiber Modify Colonic Gene Expression in Rats. Eur. J. Nutr. 2017, 56, 1871–1885. [Google Scholar] [CrossRef]
- Selga, E.; Pérez-Cano, F.J.; Franch, À.; Ramírez-Santana, C.; Rivero, M.; Ciudad, C.J.; Castellote, C.; Noé, V. Gene Expression Profiles in Rat Mesenteric Lymph Nodes upon Supplementation with Conjugated Linoleic Acid during Gestation and Suckling. BMC Genom. 2011, 12, 182. [Google Scholar] [CrossRef]
- Morales-Ferré, C.; Azagra-Boronat, I.; Massot-Cladera, M.; Franch, À.; Castell, M.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J. Sexual Dimorphism Has Low Impact on the Response against Rotavirus Infection in Suckling Rats. Vaccines 2020, 8, 345. [Google Scholar] [CrossRef] [PubMed]
- Rigo-Adrover, M.; Saldaña-Ruíz, S.; van Limpt, K.; Knipping, K.; Garssen, J.; Knol, J.; Franch, A.; Castell, M.; Pérez-Cano, F.J. A Combination of ScGOS/LcFOS with Bifidobacterium Breve M-16V Protects Suckling Rats from Rotavirus Gastroenteritis. Eur. J. Nutr. 2017, 56, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Azagra-Boronat, I.; Massot-Cladera, M.; Knipping, K.; Van‘T Land, B.; Tims, S.; Stahl, B.; Knol, J.; Garssen, J.; Franch, À.; Castell, M.; et al. Oligosaccharides Modulate Rotavirus-Associated Dysbiosis and TLR Gene Expression in Neonatal Rats. Cells 2019, 8, 876. [Google Scholar] [CrossRef] [PubMed]
- Amimo, J.O.; Raev, S.A.; Chepngeno, J.; Mainga, A.O.; Guo, Y.; Saif, L.; Vlasova, A.N. Rotavirus Interactions With Host Intestinal Epithelial Cells. Front. Immunol. 2021, 12, 793841. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Rajashekara, G.; Saif, L.J. Interactions between Human Microbiome, Diet, Enteric Viruses and Immune System: Novel Insights from Gnotobiotic Pig Research. Drug Discov. Today Dis. Models 2018, 28, 95–103. [Google Scholar] [CrossRef]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Polyphenols and Their Potential Role to Fight Viral Diseases: An Overview. Sci. Total Environ. 2021, 801, 149719. [Google Scholar] [CrossRef]
- Collinson, S.; Padua-Zamora, A.; Gv, G.; Li, C.; Lf, D.; Sj, A. Cochrane Library Cochrane Database of Systematic Reviews Probiotics for Treating Acute Infectious Diarrhoea (Review). Cochrane. Database Syst. Rev. 2020, 12, CD003048. [Google Scholar] [CrossRef]
- Nogacka, A.M.; Arboleya, S.; Nikpoor, N.; Auger, J.; Salazar, N.; Cuesta, I.; Mantecón, L.; Solís, G.; Gueimonde, M.; Tompkins, T.A.; et al. Influence of 2’-Fucosyllactose on the Microbiota Composition and Metabolic Activity of Fecal Cultures from Breastfed and Formula-Fed Infants at Two Months of Age. Microorganisms 2021, 9, 1478. [Google Scholar] [CrossRef]
- Li, M.; Monaco, M.H.; Wang, M.; Comstock, S.S.; Kuhlenschmidt, T.B.; Fahey, G.C.; Miller, M.J.; Kuhlenschmidt, M.S.; Donovan, S.M. Human Milk Oligosaccharides Shorten Rotavirus-Induced Diarrhea and Modulate Piglet Mucosal Immunity and Colonic Microbiota. ISME J. 2014, 8, 1609. [Google Scholar] [CrossRef]
- Laucirica, D.R.; Triantis, V.; Schoemaker, R.; Estes, M.K.; Ramani, S. Milk Oligosaccharides Inhibit Human Rotavirus Infectivity in MA104 Cells. J. Nutr. 2017, 147, 1709. [Google Scholar] [CrossRef]
- Colbère-Garapin, F.; Martin-Latil, S.; Blondel, B.; Mousson, L.; Pelletier, I.; Autret, A.; François, A.; Niborski, V.; Grompone, G.; Catonnet, G.; et al. Prevention and Treatment of Enteric Viral Infections: Possible Benefits of Probiotic Bacteria. Microbes Infect. 2007, 9, 1623–1631. [Google Scholar] [CrossRef] [PubMed]
- Sargeant, H.R.; Miller, H.M.; Shaw, M.A. Inflammatory Response of Porcine Epithelial IPEC J2 Cells to Enterotoxigenic E. Coli Infection Is Modulated by Zinc Supplementation. Mol. Immunol. 2011, 48, 2113–2121. [Google Scholar] [CrossRef] [PubMed]
- Ingle, H.; Peterson, S.T.; Baldridge, M.T. Distinct Effects of Type I and III Interferons on Enteric Viruses. Viruses 2018, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Villena, J.; Vizoso-Pinto, M.G.; Kitazawa, H. Intestinal Innate Antiviral Immunity and Immunobiotics: Beneficial Effects against Rotavirus Infection. Front. Immunol. 2016, 7, 563. [Google Scholar] [CrossRef]
- Elkhateeb, E.; Tag-El-Din-Hassan, H.T.; Sasaki, N.; Torigoe, D.; Morimatsu, M.; Agui, T. The Role of Mouse 2′,5′-Oligoadenylate Synthetase 1 Paralogs. Infect. Genet. Evol. 2016, 45, 393–401. [Google Scholar] [CrossRef]
- Dediego, M.L.; Nogales, A.; Martinez-Sobrido, L.; Topham, D.J. Interferon-Induced Protein 44 Interacts with Cellular FK506-Binding Protein 5, Negatively Regulates Host Antiviral Responses, and Supports Virus Replication. mBio 2019, 10, e01839-19. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, J.; Zhao, S.; Li, Y.; Zhang, Y.; Wang, Y.; Yan, Y.; Cheng, Y.; Sun, J. Goose IRF7 Is Involved in Antivirus Innate Immunity by Mediating IFN Activation. Dev. Comp. Immunol. 2022, 133, 104435. [Google Scholar] [CrossRef]
- Sherry, B. Rotavirus and Reovirus Modulation of the Interferon Response. J. Interferon Cytokine Res. 2009, 29, 559–567. [Google Scholar] [CrossRef]
- Santin, I.; Moore, F.; Grieco, F.A.; Marchetti, P.; Brancolini, C.; Eizirik, D.L. USP18 Is a Key Regulator of the Interferon-Driven Gene Network Modulating Pancreatic Beta Cell Inflammation and Apoptosis. Cell. Death Dis. 2012, 3, e419. [Google Scholar] [CrossRef]
- Malakhov, M.P.; Malakhova, O.A.; il Kim, K.; Ritchie, K.J.; Zhang, D.E. UBP43 (USP18) Specifically Removes ISG15 from Conjugated Proteins. J. Biol. Chem. 2002, 277, 9976–9981. [Google Scholar] [CrossRef]
- Ye, H.; Duan, X.; Yao, M.; Kang, L.; Li, Y.; Li, S.; Li, B.; Chen, L. USP18 Mediates Interferon Resistance of Dengue Virus Infection. Front. Microbiol. 2021, 12, 682380. [Google Scholar] [CrossRef] [PubMed]
- Malakhova, O.A.; Kim, K.I.; Luo, J.K.; Zou, W.; Kumar, K.G.S.; Fuchs, S.Y.; Shuai, K.; Zhang, D.E. UBP43 Is a Novel Regulator of Interferon Signaling Independent of Its ISG15 Isopeptidase Activity. EMBO J. 2006, 25, 2358–2367. [Google Scholar] [CrossRef] [PubMed]
- Carolina, D.; Palomino, T.; Marti, L.C. Chemokines and Immunity Quimiocinas e Imunidade. Einstein 2015, 13, 469–473. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, S.; Kou, L.; Tang, C.; Huang, R.; Pei, Z.; Li, Z. Ischemic Stroke Damages the Intestinal Mucosa and Induces Alteration of the Intestinal Lymphocytes and CCL19 MRNA in Rats. Neurosci. Lett. 2017, 658, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Kubo, Y.; Izumida, M.; Takahashi, E.; Kido, H.; Sato, K.; Yamaya, M.; Nishimura, H.; Nakayama, K.; Matsuyama, T. Enterokinase Enhances Influenza A Virus Infection by Activating Trypsinogen in Human Cell Lines. Front. Cell. Infect. Microbiol. 2018, 8, 91. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, D.; Fan, C.; Zhou, X.; Liu, Z.; Zheng, B.; Zhu, L.; Jin, Y. Novel Compound Heterozygous TMPRSS15 Gene Variants Cause Enterokinase Deficiency. Front. Genet. 2020, 11, 538778. [Google Scholar] [CrossRef]
- Shen, M.; Dong, C.; Ruan, X.; Yan, W.; Cao, M.; Pizzo, D.; Wu, X.; Yang, L.; Liu, L.; Ren, X.; et al. Chemotherapy-Induced Extracellular Vesicle MiRNAs Promote Breast Cancer Stemness by Targeting ONECUT2. Cancer Res. 2019, 79, 3608. [Google Scholar] [CrossRef] [PubMed]
- Kropp, P.A.; Gannon, M. Onecut Transcription Factors in Development and Disease. Trends. Dev. Biol. 2016, 9, 43–57. [Google Scholar]
- Christians, J.K.; Hoeflich, A.; Keightley, P.D. PAPPA2, an Enzyme That Cleaves an Insulin-Like Growth-Factor-Binding Protein, Is a Candidate Gene for a Quantitative Trait Locus Affecting Body Size in Mice. Genetics 2006, 173, 1547. [Google Scholar] [CrossRef]
- Andrew, M.; Liao, L.; Fujimoto, M.; Khoury, J.; Hwa, V.; Dauber, A. PAPPA2 as a Therapeutic Modulator of IGF-I Bioavailability: In Vivo and in Vitro Evidence. J. Endocr. Soc. 2018, 2, 646–656. [Google Scholar] [CrossRef]
- Azagra-Boronat, I.; Massot-Cladera, M.; Knipping, K.; Garssen, J.; ben Amor, K.; Knol, J.; Franch, À.; Castell, M.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J. Strain-Specific Probiotic Properties of Bifidobacteria and Lactobacilli for the Prevention of Diarrhea Caused by Rotavirus in a Preclinical Model. Nutrients 2020, 12, 498. [Google Scholar] [CrossRef] [PubMed]
- Wong, Q.W.L.; Li, J.; Ng, S.R.; Lim, S.G.; Yang, H.; Vardy, L.A. RPL39L Is an Example of a Recently Evolved Ribosomal Protein Paralog That Shows Highly Specific Tissue Expression Patterns and Is Upregulated in ESCs and HCC Tumors. RNA Biol. 2014, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Sun, P.; Liu, X.-Y.; Dong, D.; Du, J.; Gu, L.; Ge Min Chen, Y.-B.; Yun Gang Higher Vocational, L.; Ge, Y.-B. α-Fetoprotein Involvement during Glucocorticoid-Induced Precocious Maturation in Rat Colon. Available World J. Gastroenterol. 2011, 17, 2933–2940. [Google Scholar] [CrossRef] [PubMed]



| (A) Upregulated | (B) Downregulated | ||||
|---|---|---|---|---|---|
| Gene | RV | RV+2′-FL | Gene | RV | RV+2′-FL |
| Oas1a | 2.21 | 2.04 | Ccl19 | −0.57 * | - |
| Oas1k | 2.1 | 1.87 | Slpi | −0.77 | −1.06 |
| Usp18 | 2.06 | 1.75 | Lpin1 | −0.82 | - |
| Zbp1 | 1.71 | 1.79 | Alpk3 | −0.83 | −1.02 |
| Irf7 | 1.59 | 1.51 | LOC103691469 | −0.84 | −1.08 |
| Tmigd1 | 1.53 | 1.89 | Kng2l1 | −0.85 | −1.01 |
| Ifi44 | 1.52 | 1.38 | Gkap1 | −0.87 | −0.96 |
| Cfb | 1.51 | 1.58 | S100g | −0.88 | −0.97 |
| Rpl39l | 1.45 * | - | Xcl1 | −0.9 | - |
| Dhx58 | 1.4 | 1.2 | Abca8a | −0.94 | −1.14 |
| Ifi27 | 1.32 | 1.44 | Gpcpd1 | −0.95 | −0.93 |
| LOC679368 | 1.24 | 1.89 | Apoa4 | −0.95 | −0.95 |
| Isg15 | 1.21 | - | Ribc2 | −0.96 | −0.9 |
| LOC690082 | 1.19 | 1.03 | Selenop | −0.97 | −0.96 |
| Aqp3 | 1.17 | 1.36 | Aoah | −1.01 | - |
| Capn3 | 1.12 | 1.16 | Cyp3a62 | −1.03 | −1.2 |
| Rasa4 | 1.11 | 1.07 | Sla2 | −1.03 | - |
| Tmem52 | 1.1 | - | Fcgrt | −1.08 | −1.21 |
| Fyb2 | 1.07 | 1.05 | LOC102555026 | −1.09 | −1.67 |
| Chdh | 1.07 | 1.24 | Igfals | −1.11 | −1.35 |
| Samd9 | 1.06 | 1 | Gpx3 | −1.13 | −1.32 |
| Upk1b | 1.02 | 1.21 | Ephb6 | −1.13 | −1.2 |
| Samd9 | 0.99 | - | Cd36 | −1.17 | −1.1 |
| Ces1e | 0.93 | 0.92 | LOC691352 | −1.2 | −1.11 |
| MGC108823 | 0.91 | 0.92 | Ankrd29 | −1.23 | −1.44 |
| Oas1i | 0.9 | - | Ptprr | −1.23 | −1.28 |
| Mcpt4 | 0.9 | - | Hoxc11 | −1.26 | −1.41 |
| LOC100912658 | 0.86 | - | Tacr3 | −1.31 | −1.63 |
| Lgals3bp | 0.83 | - | Srd5a2 | −1.32 | - |
| Slc37a4 | 0.73 | 0.83 | C8g | −1.52 | −1.53 |
| Dapl1 | −1.54 | −1.82 | |||
| Ggh | −1.58 | −1.49 | |||
| Slc3a1 | −1.63 | −1.09 | |||
| Pdx1 | −1.66 | −1.8 | |||
| Kcne1 | −2.33 | −2.63 | |||
| Aqp8 | −2.35 | −2.85 | |||
| RGD1562699 | −2.66 | - | |||
| Afp | −3.29 * | −5.39 | |||
| Onecut2 | −3.75 | −3.71 | |||
| Tmprss15 | −5.13 | −5.86 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sáez-Fuertes, L.; Azagra-Boronat, I.; Massot-Cladera, M.; Knipping, K.; Garssen, J.; Franch, À.; Castell, M.; Pérez-Cano, F.J.; Rodríguez-Lagunas, M.J. Effect of Rotavirus Infection and 2′-Fucosyllactose Administration on Rat Intestinal Gene Expression. Nutrients 2023, 15, 1996. https://doi.org/10.3390/nu15081996
Sáez-Fuertes L, Azagra-Boronat I, Massot-Cladera M, Knipping K, Garssen J, Franch À, Castell M, Pérez-Cano FJ, Rodríguez-Lagunas MJ. Effect of Rotavirus Infection and 2′-Fucosyllactose Administration on Rat Intestinal Gene Expression. Nutrients. 2023; 15(8):1996. https://doi.org/10.3390/nu15081996
Chicago/Turabian StyleSáez-Fuertes, Laura, Ignasi Azagra-Boronat, Malén Massot-Cladera, Karen Knipping, Johan Garssen, Àngels Franch, Margarida Castell, Francisco J. Pérez-Cano, and María J. Rodríguez-Lagunas. 2023. "Effect of Rotavirus Infection and 2′-Fucosyllactose Administration on Rat Intestinal Gene Expression" Nutrients 15, no. 8: 1996. https://doi.org/10.3390/nu15081996
APA StyleSáez-Fuertes, L., Azagra-Boronat, I., Massot-Cladera, M., Knipping, K., Garssen, J., Franch, À., Castell, M., Pérez-Cano, F. J., & Rodríguez-Lagunas, M. J. (2023). Effect of Rotavirus Infection and 2′-Fucosyllactose Administration on Rat Intestinal Gene Expression. Nutrients, 15(8), 1996. https://doi.org/10.3390/nu15081996









