Abstract
Phytochemicals are natural compounds found in food ingredients with a variety of health-promoting properties. Phytochemicals improve host health through their direct systematic absorption into the circulation and modulation of the gut microbiota. The gut microbiota increases the bioactivity of phytochemicals and is a symbiotic partner whose composition and/or diversity is altered by phytochemicals and affects host health. In this review, the interactions of phytochemicals with the gut microbiota and their impact on human diseases are reviewed. We describe the role of intestinal microbial metabolites, including short-chain fatty acids, amino acid derivatives, and vitamins, from a therapeutic perspective. Next, phytochemical metabolites produced by the gut microbiota and the therapeutic effect of some selected metabolites are reviewed. Many phytochemicals are degraded by enzymes unique to the gut microbiota and act as signaling molecules in antioxidant, anti-inflammatory, anticancer, and metabolic pathways. Phytochemicals can ameliorate diseases by altering the composition and/or diversity of the gut microbiota, and they increase the abundance of some gut microbiota that produce beneficial substances. We also discuss the importance of investigating the interactions between phytochemicals and gut microbiota in controlled human studies.
1. Introduction
The term holobiont describes the intimate relationship between the host and its microbiota. The gut microbiota has a marked influence on health and disease. Trillions of bacteria (and above 1000 species), archaea, fungi, and viruses reside in the human gastrointestinal tract [1]. Neonates inherit their microbiota from their mother during delivery and breastfeeding [2]. The composition and diversity of the gut microbiota change with age and are influenced by the host’s diet, antibiotics, xenobiotics, infections, genetics, epigenetics, immunity, lifestyle, and environment [3,4,5]. Among these factors, diet has the greatest impact on the gut microbiota [6,7,8,9,10]. The gut microbiota inhabiting the colon metabolizes undigested food material reaching the colon and produces a variety of metabolites which influence the composition of the gut microbiota [11,12]. Microbial metabolites are absorbed by the host and reach almost all organs, influencing the host metabolism and signaling. This dynamic relationship between diet, gut microbiota, and host has resulted in clinical trials of bacterial metabolites and gut bacterial species [13,14,15]. In the present review, we focus on the colonic microbiota since the interaction of the gut microbiota and phytochemicals mostly occurs in the colon.
Phytochemicals are chemical compounds found in plant-derived foods such as fruits, vegetables, cereals, teas, and mushrooms. Large number of phytochemicals are being researched with the purpose of developing drugs because many are generally safe, abundant in familiar food ingredients, and exert a health-promoting effect [16]. Many phytochemicals have poor bioavailability due to their molecular structure, solubility, stability, and other factors. Metabolism by the colonic microbiota transforms phytochemicals into compounds that are readily absorbable by colonic epithelial cells [17,18]. Such transformed phytochemicals can regulate the redox balance and metabolism and have antioxidant, antimicrobial, anti-inflammatory, and anticancer activities [16,19]. This association with the colonic microbiota causes inter-individual differences in the efficacy of phytochemicals (discussed later in this review). The composition and diversity of the colonic microbiota can be altered by phytochemicals. This alteration is considered to be associated with the amelioration of various diseases (Figure 1) [20,21,22].
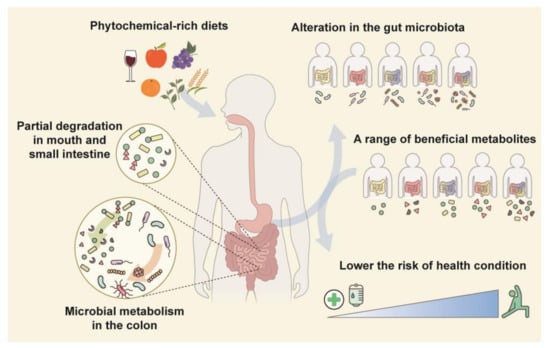
Figure 1.
Relationship between the colonic microbiota and phytochemicals. Most phytochemicals in fruits and vegetables are transformed by colonic-microbiota-derived enzymes. Phytochemical metabolites are absorbed into the host circulation and modulate colonic microbiota composition or diversity. An individual’s unique colonic microbiota produces a variety of metabolites which are linked to the risk of several diseases.
Phytochemicals and the colonic microbiota are potential therapeutic candidates for several diseases. Herein, we discuss the bioactivities of phytochemical metabolites produced by the colonic microbiota and highlight the role of the colonic microbiota as a mediator of the therapeutic effects of phytochemicals.
2. Role of the Colonic Microbiota in Human Health
2.1. Influence on Host Health
The colonic microbiota influences host health by continuously interacting with the host to promote digestion and the absorption of nutrients [23,24]; to produce energy sources [25], hormones [26], neurotransmitters [27], and vitamins [28]; to shape the immune system [29]; and to protect against pathogens and exogenous toxins [30,31]. These activities and their effects on human health are of such significance as to warrant the classification of the colonic microbiota as an organ.
The colonic microbiota influences metabolic homeostasis [32,33]. In C57BL/6J mice, Akkermansia muciniphila ameliorated the metabolic endotoxemia by secreting protein P9, which induced glucagon-like peptide-1 secretion by enteroendocrine cells [34]. In a recent a proof-of-concept exploratory study, the safety, tolerance, and metabolic effects of Akkermansia muciniphila were investigated. Daily oral supplementation of Akkermansia muciniphila for 3 months reduced the total cholesterol level and improved insulin sensitivity [35]. The gut–liver axis plays an important role in metabolic homeostasis. The gut microbiota helps to keep intestinal epithelial cells and tight junctions healthy and protects the liver from inflammatory substances and pathogens [36].
Furthermore, the colonic microbiota is a key component of the gut–brain axis and produces neurotransmitters and other metabolites that affect the brain [37,38]. The brain and the gut bidirectionally interact via central nervous system (CNS), hypothalamic–pituitary–adrenal (HPA) axis, endocrine system, and immune system [39]. Responding to factors such as stress and emotion, the HPA axis secretes several hormones which affect gut motility, digestion, and ultimately the ecosystem of the gut microbiota. Meanwhile, the colonic microbiota produces important neurotransmitters and metabolites such as serotonin and γ-aminobutyric acid (GABA), which modulate emotion and behavior and affect the neuronal signaling, digestive function, and immune system of the host [40]. A recent study showed that dysbiosis in pregnant mice seriously impaired fetal neurodevelopment. Thalamic explants from embryos of antibiotic-treated mothers at embryonic day 14.5 showed impaired axonogenesis; however, the number of axons was increased by treatment with maternal microbial metabolites [41].
The colonic microbiota is also involved in respiratory health [42]. The interaction between the gut and the lung is mostly mediated by the colonic microbiota’s ability to secure the immune system of the host [43]. Its involvement in COVID-19, which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been described [44]. SARS-CoV-2 invades upper respiratory cells and leads to asymptomatic, mild, or lethal respiratory infection in addition to gastrointestinal symptoms and gut dysbiosis [44,45,46,47]. Yeoh et al. showed that the abundance of Faecalibacterium prausnitzii and Bifidobacterium bifidum were inversely correlated with the severity of SARS-CoV-2 symptoms [46]. The reduction of Faecalibacterium prausnitzii was inversely correlated with the levels of inflammatory cytokines and post-acute-COVID-19 syndrome [46,48].
As we have seen above, many beneficial effects of the colonic microbiota are due to its immunomodulatory activity [49]. The gastrointestinal (GI) tract is a major site of immune surveillance. The colonic microbiota and its metabolites interact with immune cells in the GI tract; hence they are related to gut barrier function and immune-related GI diseases [50,51]. For example, Faecalibacterium prausnitzii is known to exert anti-inflammatory effects in Crohn’s disease and inflammatory bowel disease models by secreting proteins that inhibit the NF-κb pathway [52,53]. In a colon cancer animal model, Streptococcus thermophilus, Lactobacillus rhamnosus GG, and L. gallinarum exerted anticancer activities by inducing cell cycle arrest and apoptosis or by reducing the expression of inflammatory proteins [54,55]. The gut–heart axis also works through the gut microbiota’s association with the intestinal permeability and immune system [56]. The colonic microbiota reduces the risk of heart failure and coronary artery disease by suppressing inflammation and the total cholesterol level [57].
The term dysbiosis refers to an imbalance in the diversity and composition of the colonic microbiota. Dysbiosis is detrimental to the host’s growth and survival [58,59]. Germ-free mice have been shown to live with impaired cardiovascular [58], immunomodulatory [59,60], and neurological functions [61,62,63]. Young germ-free mice showed stunted growth and decreased somatotropic insulin-like growth factor 1 (IGF1) activity. These harmful effects were reversed through the colonization of the GI tract with lactobacilli that activated the somatotropic axis [64]. In a human study, dysbiosis caused by antibiotic treatment during the neonatal period was observed at 24 months old, suggesting a stunting of growth in the first 6 years of life [65]. Furthermore, the colonic bacterial composition was found to be related to the survival of patients with hemodialysis or cervical cancer and even to the survival of healthy seniors [66,67,68]. Notably, fecal microbiota transplantation from patients with obesity, irritable bowel syndrome, colitis, colorectal cancer, or schizophrenia to germ-free mice induced the same diseases or related physiological alterations in the mice [69,70,71,72,73]. Figure 2 illustrates the various roles of the colonic microbiota in the human body. To clarify causality and develop novel therapies, further mechanistic interventional studies controlling for diet, underlying diseases, and other factors affecting the gut microbiome are needed.
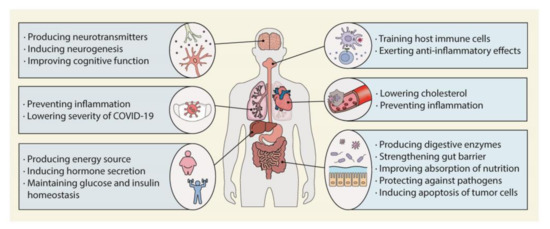
Figure 2.
Role of the colonic microbiota in human health. The colonic microbiota can promote human health by affecting almost every organ.
2.2. Production of Metabolites That Modulate Host Health
Diet is a major determinant in shaping the gut microbiota composition, diversity, and/or metabolic function [74,75,76,77]. The significance of diet is well-established in several studies which observed the change in the gut microbiota through a comparison of the Mediterranean diet (a healthy dietary pattern in the Mediterranean region which is composed of vegetables, fruits, fish, cereals, legumes, and olive oil) and the Western diet (which is rich in fat, proteins, and sugars) [78]. In a recent human study, 14 days of the Mediterranean diet did not significantly change the gut microbial composition but did change the microbial metabolic pathways when compared to the Western diet [79]. A long-term Mediterranean dietary lifestyle was highly associated with an increased level of SCFA-producing bacteria and a decreased level of L-Ruminococcus (Ruminococcus genus of Lachnospiraceae family), which is related to a high intake of fat and sugar [80].
The gut microbiota generates beneficial metabolites from the host diet. Using metagenomic shotgun sequencing, Visconti et al. analyzed blood samples from 859 individuals. Of 673 annotated metabolites in samples from more than 50 individuals, 309 (46%) were associated with the gut microbiota [81]. Several studies have evaluated the roles of gut microbial metabolites in human diseases [82,83,84,85]. Inosine, a colonic microbial metabolite of adenosine, enhanced the mucosal barrier in a colitis model and exhibited antitumor activity in several cancer models [86,87]. An impairment of cognitive function caused by sleep deprivation was recovered by resveratrol and grape seed polyphenol, but antibiotics disrupted this effect, indicating a role of the colonic microbiota [88]. The following section summarizes information on the production of short-chain fatty acids, amino acid metabolites, and vitamins by the colonic microbiota and their beneficial effects on human health.
2.2.1. Production of Short-Chain Fatty Acids
The colonic microbiota produces enzymes that degrade indigestible carbohydrates such as dietary fiber, inulin, fructo-oligosaccharides, and galacto-oligosaccharides, which are converted to short-chain fatty acids (SCFAs) including propionate, acetate, and butyrate [11]. SCFAs, especially butyrate, are the major energy sources for colonocytes and colonic bacteria. They also regulate host immunity, maintain gut homeostasis, accelerate mineral absorption, enhance gut barrier integrity, and protect the intestinal tract from antimicrobials [89,90,91,92,93,94]. Numerous studies have shown the potential of butyrate for cancer treatment [95,96,97]. For example, mice with intestinal carcinoma showed significantly decreased numbers and sizes of tumors after gavage administration of butyrate-producing bacteria [96]. It is noteworthy that there are contradicting studies [98]. Okumura et al. showed that the butyrate produced by Porphyromonas asaccharolytica and Porphyromonas gingivalis might induce colorectal tumorigenesis [99]. This discrepancy is associated with factors such as the tumor environment, mutations, and interaction with other metabolites, although the definite mechanisms remain unknown.
However, the health benefits of SCFAs have been described against many diseases [100]. The infection of mice with human sepsis pathogens significantly reduced their number of butyrate-producing bacteria and inhibited the NF-κB and interferon regulatory factor 3 (IRF3) signaling pathways. Levels of NF-κB and IRF3 were recovered by the introduction of butyrate through the transplantation of feces from healthy littermates [101]. Moreover, there is a relationship between SCFAs and neurological disorders such as Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, autism spectrum disorder (ASD), and depression [102,103,104,105]. In a study of children with ASD and transparent dysbiosis, the levels of SCFAs and dopamine metabolites were meaningfully lower compared to those of children without ASD. Intervention with probiotics and a fructo-oligosaccharide increased the levels of SCFAs and dopamine metabolites in children with ASD and markedly ameliorated symptoms of the disease [106]. Furthermore, colonic-microbiota-derived propionic acids are used in the endogenous synthesis of odd-chain fatty acids (OCFAs) in humans and rodents [107]. OCFAs, such as pentadecanoic acid (C15:0) and heptadecanoic acid (C17:0), are produced by rumen gut microbiota and consumed by humans in ruminant fat, such as beef and dairy products [108]. The circulating OCFA level is inversely related to the risk of metabolic and cardiovascular disease [109,110,111,112].
2.2.2. Production of Amino Acid Metabolites
The gut microbiota catabolizes dietary proteins, amino acids, and host-derived endogenous compounds, yielding polypeptides, derivatives of amino acids (i.e., amines, indoles, and phenolic compounds), ammonia, H2, CO2, and H2S [113,114,115]. The production of these metabolites has been reported to be influenced by the proteolytic environment and the composition of the gut microbiota [116,117]. Some of these metabolites exert harmful effects. For instance, in the gut of germ-free mice colonized with the colonic microbiota from patients with celiac disease, Pseudomonas aeruginosa catabolized gluten to peptides, which crossed the intestinal barrier and triggered immune responses, whereas Lactobacillus spp. from healthy controls degraded these peptides and showed reduced immune responses [118]. However, a range of colonic microbial metabolites derived from amino acids show beneficial effects on diseases. Tryptophan is converted into indole, tryptamine, and indole-3-propionic acid by the colonic microbiota [119,120]. Indole and indole-3-propionic acid stimulate glucagon-like peptide-1 secretion and improve glucose metabolism [121,122,123]. Furthermore, indole and indole-producing bacteria induce neurogenesis in the hippocampus and promote the apoptosis of colorectal cancer cells [55]. Tryptamine improves the gut transit time by increasing colon secretion and attenuates inflammatory bowel disease and autoimmune encephalomyelitis in animal models [124,125,126]. Some colonic microbiota, for example, Clostridium sporogenes, are involved in the biosynthesis of serotonin from tryptophan [127,128]. Serotonin is secreted into the GI tract and modulates peristalsis, inflammation, and gut epithelial development.
2.2.3. Production of Vitamins
Vitamins are precursors and/or coenzymes in diverse biochemical pathways, but some essential vitamins cannot be synthesized by humans. The colonic microbiota synthesizes vitamin K2 (menaquinone) from K1 (phylloquinone) and its derivative K3 (menadione), as well as B vitamins such as thiamine, riboflavin, niacin, pantothenic acid, pyridoxine, biotin, folates, and cobalamin [129,130]. The composition of the colonic microbiota can be affected by vitamins, subsequently affecting host immunity and gut homeostasis [131,132]. The relationship between the colonic microbial metabolism of vitamins and host disease is unclear, but a causal connection is proposed [28,133,134,135,136].
Next, we discuss the interactions of phytochemicals with the colonic microbiota and their effects on physiology and disease.
3. Therapeutic Effects of Phytochemical Metabolites Produced by the Colonic Microbiota
3.1. Phytochemical Metabolites Produced by the Colonic Microbiota
Phytochemicals are bioactive compounds abundant in plant-derived foods such as fruits, vegetables, grains, teas, and mushrooms. The most widely distributed phytochemicals are polyphenols, which have aromatic rings and hydroxyl groups [137]. Based on their structure, polyphenols are classified into flavonoids and non-flavonoids [138]. Flavonoids comprise flavones, flavonols, flavanols, flavanones, isoflavones, and anthocyanidin; non-flavonoids include phenolic acids, stilbenes, and lignans. Phenolic acids are subdivided into hydroxycinnamic acids and hydroxybenzoic acids [139]. Polyphenols have antioxidant, antimicrobial, anti-inflammatory, anticancer, cardioprotective, and neuroprotective activities, making them promising candidates for drug development [19,140,141,142,143,144].
The colonic microbiota degrades undigested polyphenols, enhancing their absorbability [139]. Colonic microbial enzymes mediate polyphenol deglycosylation, demethylation, dehydroxylation, ester cleavage, isomerization, ring fission, and decarboxylation [145,146]. Many phytochemicals are metabolized by different sets of colonic microbiota [147,148,149]. Ellagic acids are metabolized to urolithins by Clostridium spp., Ruminococcaceae, Eubacterium spp., Gordonibacter spp. and Ellagibacter isourolithinifaciens [12,150,151]. Daidzeins, isoflavones from soybeans, are converted into equols by Streptococcus intermedius, Bacteroides ovatus, and Ruminococcus productus [152]. Lignans are modified by various bacteria, e.g., Clostridium scindens, Eggerthella lenta, Clostridiales, and Lactonifactor longoviformis [153]. This indicates that phytochemical metabolites can be different among individuals eating the same diet because of individual colonic microbiota compositions. Various microbial metabolites derived from phenolic acids and interindividual differences have been reported [154,155]. Figure 3 illustrates several phytochemicals and their colonic microbial metabolites.
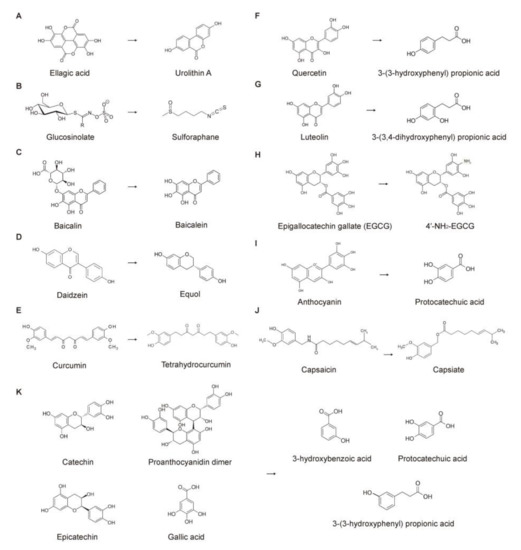
Figure 3.
Conversion of phytochemicals by the colonic microbiota. Biotransformation by (A) Clostridiales, Ruminococcaceae; (B) Enterococcus casseliflavus CP1, Bacteroides thetaiotaomicron; (C) Escherichia coli; (D) Streptococcus intermedius, Bacteroides ovatus, Ruminococcus productus; (E) Escherichia fergusonii, Escherichia coli; (F) Clostridium, Eubacterium; (G) Clostridium, Eubacterium; (H) not determined; (I) Bifidobacterium, Lactobacillus; (J) not determined; and (K) Bifidobacterium, Lactobacillus, Clostridium.
3.2. Therapeutic Effects of Phytochemical Metabolites
After fermentation by the colonic microbiota, polyphenol metabolites are absorbed by gut epithelial cells and exert beneficial effects on the host. In this section, based on reliable scientific evidence, we provide brief information on some selected phytochemical metabolites produced by the colonic microbiota and their beneficial effects on human diseases.
3.2.1. Urolithins (Metabolites of Ellagitannins and Ellagic Acids)
Ellagitannins—hexahydroxydiphenoic acid esters abundant in berries, walnuts, and pecans—are hydrolyzed to ellagic acids in the gut [156]. Ellagitannins and ellagic acids are metabolized through decarboxylation and dehydroxylation by the colonic microbiota, producing various intermediates (iso-urolithin A abd urolithin C·D·M5 and M6) and urolithin A and B, of which urolithin A is the most bioactive [157,158]. The bacterial taxa that produce urolithin A are unknown, but Clostridiales and Ruminococcaceae are candidates [157,159,160]. Urolithin A has health-promoting effects on inflammation, neurological disorders, cardiovascular diseases, and cancer [158,161,162,163]. Urolithin A inhibited CD4+ T cell proliferation by upregulating microRNA-10a-5p [164]. Urolithin A also suppressed the PI3-K/Akt/NF-κB pathway and proinflammatory factor production in vitro [165]. In Jurkat and K562 leukemia cells, urolithin A and B markedly increased the levels of metabolites that had been shown to promote cell death and induce metabolites associated with oxidative DNA damage [166]. Urolithin A increased senescence activity in a tumor-suppressor protein-p53-dependent manner in human colorectal cancer cells [167]. Furthermore, urolithin A exerted a cardioprotective effect by inhibiting myocardial fibrosis in vitro and in vivo in rats via the Nrf2 pathway [168]. The upregulation of Nrf2 by urolithin A exerted a protective effect in a rat colitis model by increasing tight junction proteins and enhancing gut barrier function [169]. Despite recent evidence showing beneficial effects of urolithin A, pharmacokinetics of urolithin A remains ambiguous. It is known that after absorption, urolithins undergo phase-II metabolism in epithelial cells [170,171]. As a result, conjugated urolithins (mainly glucuronides) are abundantly found in plasma, tissues, and urine. According to recent investigations, urolithin glucuronides had less or a lack of anti-inflammatory capacities compared to urolithins [172,173]. Further research work on the systemic action of urolithins and their conjugated metabolites after entering the circulation is required to clarify the logic of conflicting studies.
3.2.2. Sulforaphane (Metabolite of Glucosinolate)
Glucosinolates are abundant in cruciferous vegetables such as broccoli, cabbage, and kale. Sulforaphane (SFN) is converted from glucosinolate by myrosinase from plants or colonic microbiota such as Enterococcus casseliflavus CP1 and Bacteroides thetaiotaomicron [174,175]. After absorption, SFN is conjugated with glutathione to form dithiocarbamates through the mercapturic acid pathway [176]. Several studies have shown the presence of dithiocarbamates in plasma, tissues, and urine following SFN intake [177,178]. SFN has anticancer activity via an epigenetic mechanism [179,180]. Epigenetics refers to heritable alterations in gene expression without changes in DNA sequences. These alterations are reversible but can be preserved through mitotic or meiotic division and inherited [181]. Epigenetic modifications make changes in numerous molecular pathways linked to disease [182,183,184]. The major epigenetic mechanisms are DNA methylation, histone modification, and regulation by noncoding RNAs. In SFN-treated HepG2 cells, DNA hypermethylation was observed in transcription factors such as E2F3, THAP1, and ANKHD1, which are involved in cell proliferation, cell cycle progression, and apoptosis [185]. In contrast, the treatment of breast cancer cells with SFN resulted in global DNA hypomethylation, DNA methyltransferase 1 (DNMT1) and DNMT3B deactivation, cell cycle arrest, and senescence [186]. SFN also inhibits histone deacetylases (HDAC) [187,188,189]. SFN stimulated the tumor suppressors miR-9-3 in lung cancer cells by downregulating DNMT3a, HDAC1, HDAC3, HDAC6 [190], and p21 in breast cancer cells [191]. SFN reduced the expression and activity of HDACs and the viability of malignant melanoma cells [192]. The downregulation of HDAC5 by SFN blocked tumor growth in breast cancer cells [193]. In pancreatic cells, SFN decreased the expression of miR30a-3p, which restored connexin 43 expression, gap junction activity, and chemotherapy sensitivity [194]. SFN induced T-cell activation by affecting miR-155-5p and miR-194-5p signaling in the presence of pancreatic-cancer-derived antigens [195]. Furthermore, the tumor-suppressor gene RASAL2 was activated by the SFN-mediated upregulation of miR135b-5p in pancreatic ductal adenocarcinoma cells [196]. The effects of sulforaphane in various diseases are reviewed elsewhere [197,198,199].
3.2.3. Baicalein (Metabolite of Baicalin)
The flavone glycoside baicalin is found in herbs and teas and is hydrolyzed by the colonic microbiota to baicalein [200]. Evidence in the research is scarce, but E. coli converts baicalin to baicalein by hydrolysis [201]. The bioactivity and bioavailability of baicalein are greater than those of baicalin [202]. Baicalin and baicalein significantly inhibited the proliferation of HCT-116, HT-29, and SW-480 colon cancer cells, with baicalein having the greater effect; the parent form had a weaker antiproliferative effect on HCT-116 cells and negligible effects in the other two cell types [203]. In ApcMin/+ mice, the number of intestinal tumors and the levels of inflammatory cytokines were decreased by oral baicalein treatment [204]. Moreover, baicalein repressed tumor cell proliferation in a cervical cancer model and induced apoptosis in breast and thyroid cancer models by inhibiting AKT/mTOR signaling [205,206,207]. Baicalein inhibited the activities of HDAC-1 and HDAC-8 and induced proteasomal degradation of HDAC-1, thereby suppressing tumor cell growth and differentiation in in vitro and in vivo models of acute myeloid leukemia [208]. The epigenetic-modification-mediated anticancer activity of baicalein was also observed in a model of type-2-diabetes-induced liver cancer [209]. Baicalein also exerted therapeutic effects in models of Parkinson’s disease, Alzheimer’s disease [202,210,211,212], and metabolic diseases [213,214,215]. The inhibition of the NLRP3 inflammasome by baicalein attenuated osteoarthritis, Parkinson’s disease, acute liver injury, and hyperlipidemia [210,216,217,218,219]. A recent study showed that baicalein exerted substantial antiviral and anti-inflammatory effects in a COVID-19 model both in vitro and in vivo [220].
It is noteworthy that baicalin is poorly absorbed in the small and large intestines, while baicalein is absorbed well and further metabolized to oroxylin A or baicalin again [200,221]. This indicates the critical role of the colonic microbiota in the bioavailability of baicalin and the necessity of further investigations to elucidate the therapeutic effects of baicalein in relation to the interaction between baicalin, baicalein, and the colonic microbiota.
3.2.4. Equol (Metabolite of Daidzein)
Equols are generated from daidzeins, which are abundant in soybean, by the activities of colonic microbiota including Streptococcus intermedius, Bacteroides ovatus, and Ruminococcus productus [23]. After rapid absorption, equols are found mainly in their conjugated form (i.e., glucuronides) in circulation [222]. The pharmacokinetics of equols are still unclear. However, the bioavailability of equols changes in the enantiomeric forms of equols (i.e., R-equol, S-equol) [223,224]. The role of specific colonic microbiota in producing enantiomeric equols and the mode of action of each equol should be investigated further. As the most bioactive metabolite of daidzein, equol has antioxidant, antitumor, anti-inflammatory, and estrogen-like activities [225,226,227]. In some clinical trials, daidzein efficacy was affected by whether the participants produced equol, although the results were inconsistent [228,229]. The ability to produce equol is dependent on the composition of the colonic microbiota [230,231]. As a phytoestrogen, equol inhibited the tumor growth of B16 cells in a PAP-associated domain containing 5 (PAPD5)-dependent manner. Equol did not affect the tumor growth of PAPD5-ablated B16 cells. Interestingly, equol increased the growth of PAPD5-ablated breast cancer MCF-7 cells with high estrogen receptor expression [232]. Equol exerted a neuroprotective effect via an anti-inflammatory mechanism in vitro and in an animal model [233,234].
3.2.5. Tetrahydrocurcumin (Metabolite of Curcumin)
Tetrahydrocurcumin is a microbial metabolite of the turmeric polyphenol curcumin with equal or higher bioactivity compared to its parent compound. However, the mechanistic pathways underlying the therapeutic effects of tetrahydrocurcumin are unknown [235,236]. Escherichia fergusonii and E. coli produce tetrahydrocurcumin [237]. Tetrahydrocurcumin is known to exert therapeutic effects in cardiovascular diseases through its antioxidant property [238]. Tetrahydrocurcumin reportedly attenuates diabetes-induced cardiomyopathy by upregulating SIRT1, a histone deacetylase which increases the production of superoxide dismutase [239]. In cerebral ischemic/reperfusion (I/R), the DNA methylation level of CpG regions in the TIMP-2 promoter increases significantly. Tetrahydrocurcumin, a DNMT inhibitor suppressed I/R-mediated DNMT expression [240]. In a mouse model of myocardial I/R, tetrahydrocurcumin prevented heart failure and decreased autophagy and apoptosis by inducing the PI3K/AKT/mTOR pathway in H9c2 cells [241]. The anti-inflammatory activity of tetrahydrocurcumin protected brain microglial cells from lipopolysaccharide-induced injury [242]. Diverse aspects of tetrahydrocurcumin as a potential therapeutic agent are described well in the recent literature [243].
3.2.6. Propionic Acids (Metabolites of Quercetin, Catechin, and Luteolin)
Quercetin, a flavonoid with a bioavailability of <5%, is converted to 3-(3-hydroxyphenyl)propionic acid (3-HPPA) by colonic bacteria including Clostridium and Eubacterium [244]. It is thought that 3-HPPA is absorbed into the circulation by monocarboxylic acid transporters [245], and 3-HPPA exerts a greater vasodilatory effect than its parent compound [246,247]. Additionally, 3-HPPA reportedly improves bone health [248], and 3-HPPA regulated the cytoplasmic cAMP level, thus inhibiting osteoclastogenesis and osteoclastic bone resorption [249]. Furthermore, 3-HPPA and other propionic acids produced by the colonic microbiota demonstrate neuroprotective activity. α-synuclein is implicated in Parkinson’s disease and multiple system atrophy. It accumulates and spreads in the brain, leading to a worsening of disease symptoms; 3-HPPA prevented aggregation of α-synuclein and related symptoms in vitro [250]. In addition to quercetin, flavan-3-ols such as catechins in red wine are converted to 3-HPPA and other propionic acids by hydrolysis or oxidation by the colonic microbiota [251]. The metabolism of these derivatives in the liver yields diverse metabolites, which cross the blood–brain barrier and exert neuroprotective effects [252,253,254]. In a mouse model and human peripheral blood mononuclear cells, 3(3,4-dihydroxyphenyl) propionic acid from the mixture of Concord grape juice, grape seed extract and transresveratrol, reduced IL-6 expression by regulating DNA methylation in the IL-6 promoter region. IL-6 crosses the blood–brain barrier and influences synaptic plasticity, possibly leading to depressive disorder [255,256]. Among several phytochemicals, luteolin, a phytochemical abundant in citrus and lettuce, is known to be converted to 3(3,4-dihydroxyphenyl) propionic acid by colonic bacteria such as Clostridium and Eubacterium [145].
3.2.7. Other Phytochemical Metabolites Produced by the Colonic Microbiota
Zhang et al. reported that the colonic microbiota enhanced the metabolism of the green tea polyphenol epigallocatechin-3-gallate (EGCG) to 4′-NH2-EGCG, which scavenged the neurotoxic reactive carbonyl species methylglyoxal and inhibited the growth of HCT-116 and HT-29 human colon cancer cells [257]. Anthocyanins are a group of flavonoids abundant in blueberries and red wine with beneficial effects but low bioavailability [258]. Bifidobacterium and Lactobacillus degrade anthocyanins, yielding protocatechuic acid (PCA) and gallic acid [259], which have antioxidant, anticancer, and cardiovascular-protective effects [260,261]. The pharmacokinetics of PCA are still unknown. Nevertheless, Zheng et al. found that PCA is found in circulation in its free form [262]. The regulation of the PI3K/AKT signaling pathway by PCA attenuated type 2 diabetes in a rat model and prevented the apoptosis of human platelets in vitro [263,264]. Furthermore, PCA improved parameters related to the harmful state induced by chronic stress, including serum corticosterone, brain-derived neurotrophic factor, inflammatory cytokines, and TNF-α in a mouse model [265]. Capsiate, an analog of capsaicin from chili pepper, is produced by the colonic microbiota and ameliorates intestinal I/R injury by upregulating glutathione peroxidase 4 and inhibiting ferroptosis [266]. Ho et al. reported the neuroprotective activity of a polyphenol-rich cocoa preparation in gnotobiotic mice colonized by the colonic microbiota from a healthy human donor [267]. Polyphenols in the cocoa preparation included catechin, epicatechin, proanthocyanidin dimers, and gallic acid, which are fermented by Bifidobacterium, Lactobacillus, and Clostridium [268]. These polyphenols were converted into (+)-catechin, (−)-epicatechin, vanillic acid, 3-hydroxybenzoic acid (3-HBA), 3,4-dihydroxybenzoic acid (3,4-diHBA), and 3-(3-hydroxyphenyl)propionic acid (3-HPPA). Among them, 3-HBA, 3,4-diHBA, and 3-HPPA inhibited the assembly of α-synuclein in vitro and in a Drosophila model of Parkinson’s disease. As we have seen above, phytochemical metabolites can modulate various human diseases (Table 1), although the mechanisms need to be clarified further.

Table 1.
Phytochemical metabolites and their effects on human disease.
Although absorption is an important route by which phytochemicals enter the host, there are other ways. In the next section, we discuss phytochemicals’ modulation of the colonic microbiota and its impacts on various human diseases.
4. Modification of the Colonic Microbiota by Phytochemicals and Its Role in Diseases
The colonic microbiota is implicated in a variety of diseases [271,272,273,274,275]. Although little is known of the mechanisms underlying the relationships of polyphenols with the colonic microbiota, recent studies showed that the curative effects of phytochemicals on human diseases were mediated by an alteration in the composition of the colonic microbiota, altering the levels of microbial metabolites and/or the interaction between colonic microbiota and host epithelial cells, ultimately impacting host physiology and pathology. Figure 4 shows the therapeutic effects of phytochemicals in diseases linked to the modification of colonic microbiota.
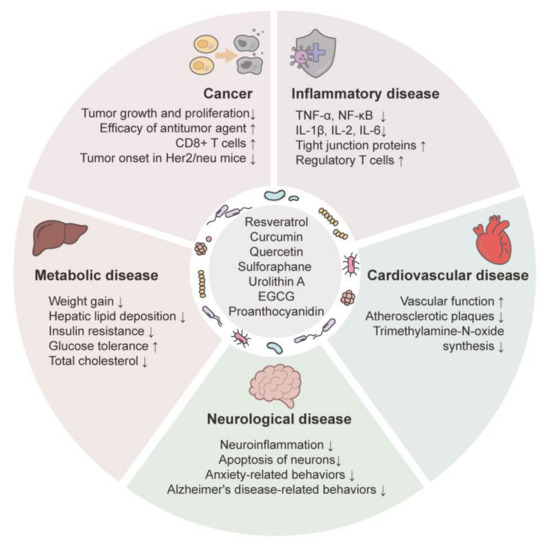
Figure 4.
Effects of phytochemicals on diseases via colonic microbiota modulation. Effects of phytochemicals on physiology and pathology indicate associations with the composition and diversity of colonic microbiota. Arrows indicate substantial increases (↑) and decreases (↓).
4.1. Modification of the Colonic Microbiota by Phytochemicals in Cancer
According to the WHO and NIH, cancer caused 10 million deaths worldwide in 2020 and imposed an economic burden of more than USD 21 billion on U.S. patients in 2019. Curcumin has antioxidant, anti-inflammatory, antibacterial, and antitumor effects, but its involvement in colonic-microbiota-mediated antitumor activity is unclear [276,277,278,279,280,281]. McFadden et al. suggested a causal link between normalization of the colonic microbiota composition, including an increased abundance of Lactobacillus, and the anticancer effects of curcumin in an IL10−/− mouse model of colorectal cancer [281]. In BALB/c mice with hepatocellular carcinoma, Wu et al. used a polyvinylpyrrolidone-based solid dispersion of Zn(II)–curcumin to overcome the low bioavailability of curcumin. The dispersion inhibited tumor growth and protected gut barrier function in a colonic-microbiota-dependent manner, decreasing the abundance of Firmicutes and increasing the abundance of Bacteroidetes [282]. The Firmicutes-to-Bacteroidetes ratio is a well-known marker for gut dysbiosis. Furthermore, curcumin enhanced the efficacy of 5-fluorouracil, but the effect was absent in antibiotic-treated mice, which also showed a significantly lower absorption of curcumin compared to untreated mice [283]. Green tea polyphenol significantly delayed the onset of estrogen-receptor-negative mammary tumors in Her2/neu transgenic mice, accompanied by a substantial increment in the level of SCFAs and the growth of Adlercreutzia and Lactobacillus [284]. Green tea catechins induce the proliferation of lactate-producing bacteria [285,286,287], which reportedly protect the gut mucosa [288,289]. Lactate produced by Bifidobacterium and Lactobacillus spp. Promoted the differentiation and proliferation of intestinal stem cells, thereby protecting the small intestine against radiation-induced injuries [290]. Ming et al. showed that EGCG increased Turicibacter and Lactobacillus and thereby protected the intestinal tract from radiation-induced injury in a mouse model [291]. Castalagin, a polyphenol from the camu camu berry, shifted the colonic microbial composition and promoted the growth of Ruminococcaceae, which was correlated with increased CD8+ T cells in the tumor microenvironment [292]. He et al. suggested that sulforaphane improved gut barrier function and anti-inflammatory activity by restoring cancer-related dysbiosis and thereby ameliorated bladder cancer in a mouse model, restoring Bacteroides fragilis and Clostridium cluster I [269]. Kaempferol is a flavonol found in vegetables and fruits. When fed to ApcMin/+ mice, kaempferol reduced intestinal polyps and proinflammatory cytokines and increased the levels of enzymes involved in bile acid synthesis (i.e., CYP8B1 and CYP27A1) [293]. Kaempferol reduced Anaerostipes, Desulfovibrio and Helicobacter, which are abundant in the colon of colorectal cancer patients, and downregulated the secondary bile acid synthesis pathways. The latter were correlated with decreased levels of bile-acid-producing bacteria such as Clostridium lavalense, Eubacterium desmolans, and Eubacterium rectale.
4.2. Modification of the Colonic Microbiota by Phytochemicals in Metabolic Diseases
Colonic-microbiota-mediated therapeutic effects of phytochemicals are especially noticeable in metabolic diseases. Rinott et al. compared the effect of diet on the colonic microbiota and the influence thereof on weight loss and glycemic control [294]. Autologous fecal microbiota transplantation showed that a polyphenol-rich Mediterranean diet induced the proliferation of beneficial bacteria such as Bacteroides massiliensis and Paraprevotella clara, attenuating weight gain and sustaining glycemic control. Oat is rich in β-glucans, which promote metabolic function, particularly cholesterol homeostasis and glucose control [295,296]. In a clinical trial involving hypercholesterolemic patients, oat consumption reduced the levels of total cholesterol and low-density lipoprotein cholesterol, which was correlated with the abundance of butyrate-producing Akkermansia muciniphila and Roseburia [297]. Resveratrol exerts beneficial effects on metabolic diseases [298,299,300,301,302]. When administered by gavage to high-fat diet (HFD)-fed mice, resveratrol decreased Desulfovibrio and Lachnospiraceae_NK4A136_group, which are related to obesity, and fecal transplantation from resveratrol-treated mice to HFD-fed mice increased the expression of tight junction proteins and the mRNA levels of fatty-acid oxidation and thermogenic genes [300]. Curcumin promoted the growth of SCFA-producing species such as Bacteroides, Akkermansia, Parabacteroides, Alistipes, and Alloprevotella and induced weight loss in mice with HFD-induced obesity and hepatic steatosis. PICRUSt analysis showed that altered bacterial gene expression was related to carbohydrate and lipid metabolism, glycolysis, gluconeogenesis, and bile secretion [303]. In a non-alcoholic fatty liver disease model, the proliferation of SCFA-producing bacteria such as Butyricicoccus and Lactobacillus was correlated with disease amelioration [304]. Curcumin ameliorated uric acid nephropathy in a rat model. Curcumin decreased Escherichia-Shigella and Bacteroides, which produce enzymes for the synthesis of indole, p-cresol, tryptophan, and tyrosine [305]. It should also be noted that p-cresol and some of indole derivatives, such as indole-3-acetic acid and indoxyl sulfate, are uremic toxins converted from tryptophan and tyrosine by the colonic microbiota [119,306]. The garlic phytochemical allicin altered the composition of the colonic microbiota by increasing the abundance of Bifidobacterium and Lactobacillus. The transplantation of an allicin-treated colonic microbiota to HFD-fed mice decreased adiposity, maintained glucose homeostasis, and ameliorated hepatic steatosis [307]. Similar anti-obesity effects and the modulation of the colonic microbiota were observed in glutamate-induced obese mice fed quercetin [308]. In combination with inulin (a non-digestible polysaccharide), isoquercetin, a glucoside of quercetin, maintained homeostasis of glucose metabolism and insulin resistance by normalizing the composition of the colonic microbiota, which is disrupted by a high-fat diet [309]. Proanthocyanidin from wild blueberry attenuated HFD-induced obesity by enriching Akkermansia muciniphila, leading to the proliferation of goblet cells [310]. Proanthocyanidin from grape seed extract increased the abundance of Prevotella, Clostridium XIVa, and Roseburia, which are butyrate-producing bacteria, leading to the amelioration of macrophage infiltration and decreased inflammatory cytokine levels [310,311].
4.3. Modification of the Colonic Microbiota by Phytochemicals in Inflammatory Diseases
In a mouse model of intestinal inflammation, EGCG modulated inflammation mediated by the colonic microbiota [312]. The oral administration of EGCG increased the abundance of the beneficial bacteria Akkermansia and was significantly correlated with SCFA production and IL-8 downregulation in colitis mice. This anti-inflammatory effect was supported by fecal microbiota transplantation from EGCG-treated mice to colitis mice [313]. In mice with HFD-induced obesity, EGCG not only increased the abundance of Akkermansia and other beneficial colonic bacteria, including Bacteroides and Parasutterella, but also regulated the levels of inflammatory factors such as IgA, major histocompatibility complex-II, and signaling molecules related to the NF-κB pathway, thus maintaining homeostasis [314]. In an animal model of lipopolysaccharide-induced systemic inflammation, EGCG suppressed the levels of inflammatory factors, including TNF-α and several interleukins, and reversed dysbiosis by decreasing the abundance of Enterobacteriales [315].
Resveratrol increased the abundance of Lactobacillus spp., especially Lactobacillus reuteri, and the survival rate by inhibiting proinflammatory cytokines and stimulating anti-inflammatory cytokines in mice with lethal acute respiratory distress syndrome caused by staphylococcal enterotoxin B [302]. Furthermore, resveratrol aids dysbiosis recovery by increasing Ruminococcus gnavus and Akkermansia muciniphila. The anti-inflammatory effect was supported by fecal transplantation from resveratrol-treated mice to colitis mice [316]. In colitis mice, Li et al. showed that the abundance of Lactobacillus and Bifidobacterium was recovered by resveratrol and was negatively correlated with the levels of IL-1β, IL-2, IL-6, TNF-α, and granulocyte-macrophage colony-stimulating factor [317]. In mice with allergic asthma, resveratrol increased the abundance of Bacteroides in the gut [318]. Butyrate produced by the colonic microbiota induced anti-inflammatory regulatory T cells, thus ameliorating asthma.
Espín et al. suggested that the anti-inflammatory activity of urolithin A was facilitated by Lactobacillus and Bifidobacterium in a rat model of colitis [319]. Hu et al. reported that PCA increased the abundance of Roseburia and Desulfovibrio and reduced the serum levels of IL-1β, IL-2, and IL-6 in lipopolysaccharide-treated piglets [320]. Vanillic acid, a metabolite of the anthocyanin cyanidin 3-glucoside, exerted an anti-inflammatory effect by reducing Prevotella 7, which showed a positive correlation with IL-1b, IL-2, and IL-6 [321].
Curcumin attenuated inflammation in BALB/c mice with colitis induced by dextran sulfate sodium [322]. In this study, a nano form of curcumin suppressed NF-κB activation, mRNA levels of inflammatory mediators, and the number of CD4+ Foxp3+ regulatory T-cells. These effects were accompanied by an increased abundance of Clostridium cluster IV and XIVa, which reportedly produce butyric acid and are implicated in the induction of mucosal regulatory T cells [323].
4.4. Modification of the Colonic Microbiota by Phytochemicals in Cardiovascular Diseases
The alteration of the colonic microbiota by phytochemicals is linked to cardiovascular diseases. The aronia berry is rich in polyphenols such as anthocyanins and exerts health-promoting effects [324]. In a clinical trial with healthy adults, aronia berry extract significantly improved endothelial function, which was correlated with an increased abundance of butyrate- and propionic-acid-producing colonic microbiota such as Dialister, Phascolarctobacterium, and Roseburia [325]. Propionic acid is known to exert a cardioprotective effect [326,327]. In a mouse model, resveratrol attenuated atherosclerosis by increasing Prevotella, Akkermansia, Lactobacillus, and Bifidobacterium, indicating a correlation with the inhibited microbial synthesis of trimethylamine-N-oxide (TMAO), a risk factor for atherosclerosis produced by the colonic microbiota from dietary choline [328]. Geraniin, an ellagitannin in Geranium thunbergia and other plants, enriched Bacteroides in C57BL/6J ApoE(−/−) mice and suppressed the plasma level of TMAO, resulting in decreased levels of proinflammatory cytokines and lipid uptake in macrophages [329]. In ApoE(−/−) mice, peanut skin extract, containing procyanidins, catechin, epicatechin and EGCG, reduced atherosclerotic plaques and the levels of TNF-α and IL-6, effects correlated with the abundance of beneficial bacteria such as Roseburia, Akkermansia, and Bifidobacterium [330].
4.5. Modification of the Colonic Microbiota by Phytochemicals in Neurological Diseases
There is a relationship between the colonic microbiota and neurological diseases such as Alzheimer’s disease, Parkinson’s disease, depression, anxiety, attention deficit hyperactivity disorder (ADHD), autism spectrum disorder, and schizophrenia [331,332,333,334,335,336,337]. Given the effects of phytochemicals on the colonic microbiota, they are likely to influence neurological disorders via the gut–brain axis. [38]. In a mouse model of dextran-sulfate-sodium-salt-induced anxiety, curcumin reversed anxiety-related harmful effects, such as anxiety-related behaviors, and the phosphatidylcholine level in the prefrontal cortex by upregulating Muribaculaceae [338]. In a mouse model of Alzheimer’s disease and SH-SY5Y cells, quercetin significantly attenuated AD-related parameters and symptoms. This attenuation was correlated with the abundance of Barnesiella, Lactobacillus, and Parasutterella [339]. Table 2 summarizes information on the modulation of colonic microbiota by phytochemicals and their therapeutic effects on human diseases.

Table 2.
Modulation of colonic microbiota by phytochemicals and their effects on diseases.
The influence of other factors (i.e., diet, antibiotics, acute stress, and exercise) must also be validated. Large clinical trials are necessitated by the marked inter-individual variability in colonic microbiota composition and activity in vitro and in vivo.
5. Conclusions
The gut microbiota constantly affects host health by digesting the host’s diet and producing various beneficial metabolites including SCFAs, indole, tryptamine, serotonin, and even essential vitamins such as Bs and K2. The gut microbiota also works as an essential degrader and enhances the bioactivity and bioavailability of many phytochemicals such as ellagitannin, curcumin, baicalin, and quercetin [158,202,247,342]. Following degradation by the gut microbiota, phytochemical metabolites have an impact on cellular signaling pathways (i.e., Nrf2 pathway, NF-κB pathway, PI3K/AKT/mTOR pathway, and the secondary bile acid synthesis pathway, etc.) [165,168,205,293]. These metabolites have shown therapeutic activities in wide range of diseases such as cancer, inflammatory diseases, cardiovascular diseases, Alzheimer’s disease, Parkinson’s disease, and depression.
Phytochemicals also affect the composition and diversity of the colon microbiota, thereby playing very important roles in the amelioration of various diseases. Phytochemicals themselves also affect the host by influencing the composition and diversity of the colon microbiota [343,344,345,346,347,348]. In other words, phytochemicals have been reported to enrich the beneficial microflora (i.e., butyrate-producing bacteria) that produce metabolites, playing a role as disease-ameliorating signaling molecules or enzymes [139]. However, the mechanism remains unknown in many cases; therefore, the profiling and functional analysis of the colonic microbiome and microbial metabolites to validate the underlying mechanism is a complex but high priority. It should not be overlooked that individual differences in the composition of the colonic microbiota affect the host’s phytopharmacology [20,349]. The influence of other factors (i.e., diet, antibiotics, acute stress, and exercise) must also be validated. Significant interindividual variability in the composition of the colonic microbiome and activity in vitro and in vivo necessitates large-scale clinical trials.
Collectively, this review presents up-to-date information on the role of the colon microbiome in human health which could be indispensable for future preclinical and clinical investigations.
Author Contributions
Conceptualization, C.K. and S.K.C.; writing—original draft, C.K. and S.K.C.; Writing—review and editing, M.K.E.; supervision, S.K.C.; All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2016R1A6A1A03012862).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Hullar, M.A.J.; Fu, B.C. Diet, the Gut Microbiome, and Epigenetics. Cancer J. 2014, 20, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Hagan, T.; Cortese, M.; Rouphael, N.; Boudreau, C.; Linde, C.; Maddur, M.S.; Das, J.; Wang, H.; Guthmiller, J.; Zheng, N.; et al. Antibiotics-Driven Gut Microbiome Perturbation Alters Immunity to Vaccines in Humans. Cell 2019, 178, 1313–1328.e13. [Google Scholar] [CrossRef]
- Von Schwartzenberg, R.J.; Bisanz, J.E.; Lyalina, S.; Spanogiannopoulos, P.; Ang, Q.Y.; Cai, J.; Dickmann, S.; Friedrich, M.; Liu, S.Y.; Collins, S.L.; et al. Caloric restriction disrupts the microbiota and colonization resistance. Nature 2021, 595, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Yoo, W.; Zieba, J.K.; Foegeding, N.J.; Torres, T.P.; Shelton, C.D.; Shealy, N.G.; Byndloss, A.J.; Cevallos, S.A.; Gertz, E.; Tiffany, C.R.; et al. High-fat diet-induced colonocyte dysfunction escalates microbiota-derived trimethylamine N-oxide. Science 2021, 373, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Sonnenburg, J.L.; Bäckhed, F. Diet–microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of Polyphenols on Gut Microbiota and Implications in Human Health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Peredo-Lovillo, A.; Romero-Luna, H.E.; Jiménez-Fernández, M. Health promoting microbial metabolites produced by gut microbiota after prebiotics metabolism. Food Res. Int. 2020, 136, 109473. [Google Scholar] [CrossRef] [PubMed]
- Feuerstadt, P.; Louie, T.J.; Lashner, B.; Wang, E.E.L.; Diao, L.; Bryant, J.A.; Sims, M.; Kraft, C.S.; Cohen, S.H.; Berenson, C.S.; et al. SER-109, an Oral Microbiome Therapy for Recurrent Clostridioides difficile Infection. N. Engl. J. Med. 2022, 386, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Valencia, P.M.; Richard, M.; Brock, J.; Boglioli, E. The human microbiome: Opportunity or hype? Nat. Rev. Drug Discov. 2017, 16, 823–824. [Google Scholar] [CrossRef]
- Upadhyay, S.; Dixit, M. Role of Polyphenols and Other Phytochemicals on Molecular Signaling. Oxidative Med. Cell. Longev. 2015, 2015, 504253. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M.V. Bioavailability of Phytochemicals and Its Enhancement by Drug Delivery Systems. Cancer Lett. 2013, 334, 133–141. [Google Scholar] [CrossRef]
- Williamson, G.; Clifford, M.N. Role of the small intestine, colon and microbiota in determining the metabolic fate of polyphenols. Biochem. Pharmacol. 2017, 139, 24–39. [Google Scholar] [CrossRef]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Das, S.; Patra, S.K.; Efferth, T.; Jena, M.; Bhutia, S.K. Dietary polyphenols in chemoprevention and synergistic effect in cancer: Clinical evidences and molecular mechanisms of action. Phytomedicine 2021, 90, 153554. [Google Scholar] [CrossRef]
- Iglesias-Aguirre, C.E.; Cortés-Martín, A.; Ávila-Gálvez, M.Á.; Giménez-Bastida, J.A.; Selma, M.V.; González-Sarrías, A.; Espín, J.C. Main drivers of (poly)phenol effects on human health: Metabolite production and/or gut microbiota-associated metabotypes? Food Funct. 2021, 12, 10324–10355. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, Q. Roles of the Polyphenol-Gut Microbiota Interaction in Alleviating Colitis and Preventing Colitis-Associated Colorectal Cancer. Adv. Nutr. 2021, 12, 546–565. [Google Scholar] [CrossRef] [PubMed]
- Nargeh, H.; Aliabadi, F.; Ajami, M.; Pazoki-Toroudi, H. Role of Polyphenols on Gut Microbiota and the Ubiquitin-Proteasome System in Neurodegenerative Diseases. J. Agric. Food Chem. 2021, 69, 6119–6144. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Skrypnik, K.; Suliburska, J. Association between the gut microbiota and mineral metabolism. J. Sci. Food Agric. 2018, 98, 2449–2460. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Jiang, Q.; Yin, Y. Butyrate in Energy Metabolism: There Is Still More to Learn. Trends Endocrinol. Metab. 2021, 32, 159–169. [Google Scholar] [CrossRef]
- Martin, A.M.; Sun, E.W.; Rogers, G.B.; Keating, D.J. The Influence of the Gut Microbiome on Host Metabolism Through the Regulation of Gut Hormone Release. Front. Physiol. 2019, 10, 428. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Das, P.; Babaei, P.; Nielsen, J. Metagenomic analysis of microbe-mediated vitamin metabolism in the human gut microbiome. BMC Genom. 2019, 20, 208. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341. [Google Scholar] [CrossRef]
- Rolhion, N.; Chassaing, B. When pathogenic bacteria meet the intestinal microbiota. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150504. [Google Scholar] [CrossRef]
- Koppel, N.; Maini Rekdal, V.; Balskus, E.P. Chemical transformation of xenobiotics by the human gut microbiota. Science 2017, 356, eaag2770. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Warmbrunn, M.V.; Nieuwdorp, M.; Clément, K. Metabolism and Metabolic Disorders and the Microbiome: The Intestinal Microbiota Associated With Obesity, Lipid Metabolism, and Metabolic Health-Pathophysiology and Therapeutic Strategies. Gastroenterology 2021, 160, 573–599. [Google Scholar] [CrossRef]
- Yang, G.; Wei, J.; Liu, P.; Zhang, Q.; Tian, Y.; Hou, G.; Meng, L.; Xin, Y.; Jiang, X. Role of the gut microbiota in type 2 diabetes and related diseases. Metab. Clin. Exp. 2021, 117, 154712. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Cho, C.H.; Yun, M.S.; Jang, S.J.; You, H.J.; Kim, J.H.; Han, D.; Cha, K.H.; Moon, S.H.; Lee, K.; et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 2021, 6, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Appleton, J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr. Med. 2018, 17, 28–32. [Google Scholar]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef] [PubMed]
- Vuong, H.E.; Pronovost, G.N.; Williams, D.W.; Coley, E.J.L.; Siegler, E.L.; Qiu, A.; Kazantsev, M.; Wilson, C.J.; Rendon, T.; Hsiao, E.Y. The maternal microbiome modulates fetal neurodevelopment in mice. Nature 2020, 586, 281–286. [Google Scholar] [CrossRef]
- Sencio, V.; Machado, M.G.; Trottein, F. The lung-gut axis during viral respiratory infections: The impact of gut dysbiosis on secondary disease outcomes. Mucosal Immunol. 2021, 14, 296–304. [Google Scholar] [CrossRef]
- Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019, 12, 843–850. [Google Scholar] [CrossRef]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Hayashi, Y.; Wagatsuma, K.; Nojima, M.; Yamakawa, T.; Ichimiya, T.; Yokoyama, Y.; Kazama, T.; Hirayama, D.; Nakase, H. The characteristics of gastrointestinal symptoms in patients with severe COVID-19: A systematic review and meta-analysis. J. Gastroenterol. 2021, 56, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Song, Z.G.; Liu, C.; Tan, S.; Lin, S.; Zhu, J.; Dai, F.H.; Gao, J.; She, J.L.; Mei, Z.; et al. Gut microbiome alterations and gut barrier dysfunction are associated with host immune homeostasis in COVID-19 patients. BMC Med. 2022, 20, 24. [Google Scholar] [CrossRef]
- Liu, Q.; Mak, J.W.Y.; Su, Q.; Yeoh, Y.K.; Lui, G.C.; Ng, S.S.S.; Zhang, F.; Li, A.Y.L.; Lu, W.; Hui, D.S.; et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut 2022, 71, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Chong, P.P.; Chin, V.K.; Looi, C.Y.; Wong, W.F.; Madhavan, P.; Yong, V.C. The Microbiome and Irritable Bowel Syndrome-A Review on the Pathophysiology, Current Research and Future Therapy. Front. Microbiol. 2019, 10, 1136. [Google Scholar] [CrossRef] [PubMed]
- Glassner, K.L.; Abraham, B.P.; Quigley, E.M.M. The microbiome and inflammatory bowel disease. J. Allergy Clin. Immunol. 2020, 145, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humarán, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef]
- Quévrain, E.; Maubert, M.A.; Michon, C.; Chain, F.; Marquant, R.; Tailhades, J.; Miquel, S.; Carlier, L.; Bermúdez-Humarán, L.G.; Pigneur, B.; et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut 2016, 65, 415–425. [Google Scholar] [CrossRef]
- Gamallat, Y.; Meyiah, A.; Kuugbee, E.D.; Hago, A.M.; Chiwala, G.; Awadasseid, A.; Bamba, D.; Zhang, X.; Shang, X.; Luo, F.; et al. Lactobacillus rhamnosus induced epithelial cell apoptosis, ameliorates inflammation and prevents colon cancer development in an animal model. Biomed. Pharm. 2016, 83, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, N.; Li, Q.; Chu, E.S.H.; Lau, H.C.H.; Fong, W.; Liu, W.; Liang, C.; Nakatsu, G.; Su, A.C.Y.; Coker, O.O.; et al. Lactobacillus gallinarum modulates the gut microbiota and produces anti-cancer metabolites to protect against colorectal tumourigenesis. Gut 2021. [Google Scholar] [CrossRef] [PubMed]
- Forkosh, E.; Ilan, Y. The heart-gut axis: New target for atherosclerosis and congestive heart failure therapy. Open Heart 2019, 6, e000993. [Google Scholar] [CrossRef]
- Troseid, M.; Andersen, G.O.; Broch, K.; Hov, J.R. The gut microbiome in coronary artery disease and heart failure: Current knowledge and future directions. EBioMedicine 2020, 52, 102649. [Google Scholar] [CrossRef]
- Edwards, J.M.; Roy, S.; Tomcho, J.C.; Schreckenberger, Z.J.; Chakraborty, S.; Bearss, N.R.; Saha, P.; McCarthy, C.G.; Vijay-Kumar, M.; Joe, B.; et al. Microbiota are critical for vascular physiology: Germ-free status weakens contractility and induces sex-specific vascular remodeling in mice. Vasc. Pharm. 2020, 125–126, 106633. [Google Scholar] [CrossRef]
- Winkler, E.S.; Shrihari, S.; Hykes, B.L., Jr.; Handley, S.A.; Andhey, P.S.; Huang, Y.S.; Swain, A.; Droit, L.; Chebrolu, K.K.; Mack, M.; et al. The Intestinal Microbiome Restricts Alphavirus Infection and Dissemination through a Bile Acid-Type I IFN Signaling Axis. Cell 2020, 182, 901–918.e18. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, W.H.; Li, S.X.; He, Z.M.; Zhu, W.L.; Ji, Y.B.; Wang, Z.; Zhu, X.M.; Yuan, K.; Bao, Y.P.; et al. Gut microbiota modulates the inflammatory response and cognitive impairment induced by sleep deprivation. Mol. Psychiatry 2021, 26, 6277–6292. [Google Scholar] [CrossRef]
- Wu, W.L.; Adame, M.D.; Liou, C.W.; Barlow, J.T.; Lai, T.T.; Sharon, G.; Schretter, C.E.; Needham, B.D.; Wang, M.I.; Tang, W.; et al. Microbiota regulate social behaviour via stress response neurons in the brain. Nature 2021, 595, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Morais, L.H.; Schreiber, H.L., 4th; Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Murciano-Brea, J.; Garcia-Montes, M.; Geuna, S.; Herrera-Rincon, C. Gut Microbiota and Neuroplasticity. Cells 2021, 10, 2084. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, M.; Makki, K.; Storelli, G.; Machuca-Gayet, I.; Srutkova, D.; Hermanova, P.; Martino, M.E.; Balmand, S.; Hudcovic, T.; Heddi, A.; et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science 2016, 351, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Uzan-Yulzari, A.; Turta, O.; Belogolovski, A.; Ziv, O.; Kunz, C.; Perschbacher, S.; Neuman, H.; Pasolli, E.; Oz, A.; Ben-Amram, H.; et al. Neonatal antibiotic exposure impairs child growth during the first six years of life by perturbing intestinal microbial colonization. Nat. Commun. 2021, 12, 443–444. [Google Scholar] [CrossRef]
- Lin, T.Y.; Wu, P.H.; Lin, Y.T.; Hung, S.C. Gut dysbiosis and mortality in hemodialysis patients. NPJ Biofilms Microbiomes 2021, 7, 20. [Google Scholar] [CrossRef]
- Sims, T.T.; El Alam, M.B.; Karpinets, T.V.; Dorta-Estremera, S.; Hegde, V.L.; Nookala, S.; Yoshida-Court, K.; Wu, X.; Biegert, G.W.G.; Delgado Medrano, A.Y.; et al. Gut Microbiome Diversity is an Independent Predictor of Survival in Cervical Cancer Patients Receiving Chemoradiation. Commun. Biol. 2021, 4, 237. [Google Scholar] [CrossRef]
- Wilmanski, T.; Diener, C.; Rappaport, N.; Patwardhan, S.; Wiedrick, J.; Lapidus, J.; Earls, J.C.; Zimmer, A.; Glusman, G.; Robinson, M.; et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat. Metab. 2021, 3, 274. [Google Scholar] [CrossRef]
- Trikha, S.R.J.; Lee, D.M.; Ecton, K.E.; Wrigley, S.D.; Vazquez, A.R.; Litwin, N.S.; Thomas, K.N.; Wei, Y.; Battson, M.L.; Johnson, S.A.; et al. Transplantation of an obesity-associated human gut microbiota to mice induces vascular dysfunction and glucose intolerance. Gut Microbes 2021, 13, 1940791. [Google Scholar] [CrossRef]
- De Palma, G.; Lynch, M.D.; Lu, J.; Dang, V.T.; Deng, Y.; Jury, J.; Umeh, G.; Miranda, P.M.; Pigrau Pastor, M.; Sidani, S.; et al. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci. Transl. Med. 2017, 9, eaaf6397. [Google Scholar] [CrossRef]
- Gao, T.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin Ameliorates Corticosterone-Mediated Oxidative Stress-Induced Colitis in Sleep-Deprived Mice Involving Gut Microbiota. Oxidative Med. Cell. Longev. 2021, 2021, 9981480. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.; Zhao, L.; Zhang, X.; Nakatsu, G.; Han, J.; Xu, W.; Xiao, X.; Kwong, T.N.Y.; Tsoi, H.; Wu, W.K.K.; et al. Gavage of Fecal Samples From Patients With Colorectal Cancer Promotes Intestinal Carcinogenesis in Germ-Free and Conventional Mice. Gastroenterology 2017, 153, 1621–1633.e6. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Guo, R.; Wang, W.; Ju, Y.; Wang, Q.; Ma, Q.; Sun, Q.; Fan, Y.; Xie, Y.; Yang, Z.; et al. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol. Psychiatry 2020, 25, 2905–2918. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e14. [Google Scholar] [CrossRef]
- Spencer, C.N.; McQuade, J.L.; Gopalakrishnan, V.; McCulloch, J.A.; Vetizou, M.; Cogdill, A.P.; Khan, M.A.W.; Zhang, X.; White, M.G.; Peterson, C.B.; et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science 2021, 374, 1632–1640. [Google Scholar] [CrossRef]
- Shi, H.; Ge, X.; Ma, X.; Zheng, M.; Cui, X.; Pan, W.; Zheng, P.; Yang, X.; Zhang, P.; Hu, M.; et al. A fiber-deprived diet causes cognitive impairment and hippocampal microglia-mediated synaptic loss through the gut microbiota and metabolites. Microbiome 2021, 9, 223. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, K.; Jia, Y.; Shi, J.; Tong, Z.; Fang, D.; Yang, B.; Su, C.; Li, R.; Xiao, X.; et al. Gut microbiome alterations in high-fat-diet-fed mice are associated with antibiotic tolerance. Nat. Microbiol. 2021, 6, 874–884. [Google Scholar] [CrossRef]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef]
- Barber, C.; Mego, M.; Sabater, C.; Vallejo, F.; Bendezu, R.A.; Masihy, M.; Guarner, F.; Espín, J.C.; Margolles, A.; Azpiroz, F. Differential Effects of Western and Mediterranean-Type Diets on Gut Microbiota: A Metagenomics and Metabolomics Approach. Nutrients 2021, 13, 2638. [Google Scholar] [CrossRef]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- Visconti, A.; Le Roy, C.I.; Rosa, F.; Rossi, N.; Martin, T.C.; Mohney, R.P.; Li, W.; de Rinaldis, E.; Bell, J.T.; Venter, J.C.; et al. Interplay between the human gut microbiome and host metabolism. Nat. Commun. 2019, 10, 4505. [Google Scholar] [CrossRef] [PubMed]
- Donia, M.S.; Fischbach, M.A. HUMAN MICROBIOTA. Small molecules from the human microbiota. Science 2015, 349, 1254766. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Li, Q.; Yi, H.; Kuang, T.; Tang, Y.; Fan, G. Gut microbiota-derived metabolites as key actors in type 2 diabetes mellitus. Biomed. Pharm. 2022, 149, 112839. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Nwe, P.K.; Yang, Y.; Rosen, C.E.; Bielecka, A.A.; Kuchroo, M.; Cline, G.W.; Kruse, A.C.; Ring, A.M.; Crawford, J.M.; et al. A Forward Chemical Genetic Screen Reveals Gut Microbiota Metabolites That Modulate Host Physiology. Cell 2019, 177, 1217–1231.e18. [Google Scholar] [CrossRef]
- Yang, W.; Cong, Y. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell Mol. Immunol. 2021, 18, 866–877. [Google Scholar] [CrossRef]
- Mager, L.F.; Burkhard, R.; Pett, N.; Cooke, N.C.A.; Brown, K.; Ramay, H.; Paik, S.; Stagg, J.; Groves, R.A.; Gallo, M.; et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 2020, 369, 1481–1489. [Google Scholar] [CrossRef]
- Li, D.; Feng, Y.; Tian, M.; Ji, J.; Hu, X.; Chen, F. Gut microbiota-derived inosine from dietary barley leaf supplementation attenuates colitis through PPARγ signaling activation. Microbiome 2021, 9, 83–87. [Google Scholar] [CrossRef]
- Frolinger, T.; Sims, S.; Smith, C.; Wang, J.; Cheng, H.; Faith, J.; Ho, L.; Hao, K.; Pasinetti, G.M. The gut microbiota composition affects dietary polyphenols-mediated cognitive resilience in mice by modulating the bioavailability of phenolic acids. Sci. Rep. 2019, 9, 3546-6. [Google Scholar] [CrossRef]
- Chen, J.; Vitetta, L. The Role of Butyrate in Attenuating Pathobiont-Induced Hyperinflammation. Immune Netw. 2020, 20, e15. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef]
- Van der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef]
- Kim, C.H. Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids. Cell Mol. Immunol. 2021, 18, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Beisner, J.; Filipe Rosa, L.; Kaden-Volynets, V.; Stolzer, I.; Gunther, C.; Bischoff, S.C. Prebiotic Inulin and Sodium Butyrate Attenuate Obesity-Induced Intestinal Barrier Dysfunction by Induction of Antimicrobial Peptides. Front. Immunol. 2021, 12, 678360. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Qu, F.; Chen, L.; Liu, C.; Zhang, M.; Ren, F.; Guo, H.; Zhang, H.; Ge, S.; Wu, C.; et al. SCFAs alleviated steatosis and inflammation in mice with NASH induced by MCD. J. Endocrinol. 2020, 245, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, C.; Villani, A.; Pisati, F.; Orsenigo, F.; Ulaszewska, M.; Latiano, T.P.; Potenza, A.; Andolfo, A.; Terracciano, F.; Tripodo, C.; et al. Butyrate, a postbiotic of intestinal bacteria, affects pancreatic cancer and gemcitabine response in in vitro and in vivo models. Biomed. Pharm. 2022, 151, 113163. [Google Scholar] [CrossRef]
- Chen, D.; Jin, D.; Huang, S.; Wu, J.; Xu, M.; Liu, T.; Dong, W.; Liu, X.; Wang, S.; Zhong, W.; et al. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota. Cancer Lett. 2020, 469, 456–467. [Google Scholar] [CrossRef]
- He, Y.; Fu, L.; Li, Y.; Wang, W.; Gong, M.; Zhang, J.; Dong, X.; Huang, J.; Wang, Q.; Mackay, C.R.; et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8(+) T cell immunity. Cell Metab. 2021, 33, 988–1000.e7. [Google Scholar] [CrossRef]
- Belcheva, A.; Irrazabal, T.; Robertson, S.J.; Streutker, C.; Maughan, H.; Rubino, S.; Moriyama, E.H.; Copeland, J.K.; Surendra, A.; Kumar, S.; et al. Gut microbial metabolism drives transformation of MSH2-deficient colon epithelial cells. Cell 2014, 158, 288–299. [Google Scholar] [CrossRef]
- Okumura, S.; Konishi, Y.; Narukawa, M.; Sugiura, Y.; Yoshimoto, S.; Arai, Y.; Sato, S.; Yoshida, Y.; Tsuji, S.; Uemura, K.; et al. Gut bacteria identified in colorectal cancer patients promote tumourigenesis via butyrate secretion. Nat. Commun. 2021, 12, 5674. [Google Scholar] [CrossRef]
- Gill, P.A.; van Zelm, M.C.; Muir, J.G.; Gibson, P.R. Review article: Short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment. Pharm. Ther. 2018, 48, 15–34. [Google Scholar] [CrossRef]
- Kim, S.M.; DeFazio, J.R.; Hyoju, S.K.; Sangani, K.; Keskey, R.; Krezalek, M.A.; Khodarev, N.N.; Sangwan, N.; Christley, S.; Harris, K.G.; et al. Fecal microbiota transplant rescues mice from human pathogen mediated sepsis by restoring systemic immunity. Nat. Commun. 2020, 11, 2354. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Bouzari, B.; Hosseini-Fard, S.R.; Mazaheri, M.; Ahmadyousefi, Y.; Abdi, M.; Jalalifar, S.; Karimitabar, Z.; Teimoori, A.; Keyvani, H.; et al. Role of microbiota-derived short-chain fatty acids in nervous system disorders. Biomed. Pharm. 2021, 139, 111661. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Wu, M.; Tian, T.; Mao, Q.; Zou, T.; Zhou, C.; Xie, J.; Chen, J. Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice. Transl. Psychiatry 2020, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Kidd, S.K.; Schneider, J.S. Protection of dopaminergic cells from MPP+-mediated toxicity by histone deacetylase inhibition. Brain Res. 2010, 1354, 172–178. [Google Scholar] [CrossRef]
- Wang, Y.; Li, N.; Yang, J.J.; Zhao, D.M.; Chen, B.; Zhang, G.Q.; Chen, S.; Cao, R.F.; Yu, H.; Zhao, C.Y.; et al. Probiotics and fructo-oligosaccharide intervention modulate the microbiota-gut brain axis to improve autism spectrum reducing also the hyper-serotonergic state and the dopamine metabolism disorder. Pharm. Res. 2020, 157, 104784. [Google Scholar] [CrossRef] [PubMed]
- Weitkunat, K.; Bishop, C.A.; Wittmüss, M.; Machate, T.; Schifelbein, T.; Schulze, M.B.; Klaus, S. Effect of Microbial Status on Hepatic Odd-Chain Fatty Acids Is Diet-Dependent. Nutrients 2021, 13, 1546. [Google Scholar] [CrossRef]
- Fievez, V.; Colman, E.; Castro-Montoya, J.M.; Stefanov, I.; Vlaeminck, B. Milk Odd- and Branched-Chain Fatty Acids as Biomarkers of Rumen Function—An Update. Anim. Feed. Sci. Technol. 2012, 172, 51–65. [Google Scholar] [CrossRef]
- White, M.J.; Armstrong, S.C.; Kay, M.C.; Perrin, E.M.; Skinner, A. Associations between milk fat content and obesity, 1999 to 2016. Pediatr. Obes. 2020, 15, e12612. [Google Scholar] [CrossRef]
- Imamura, F.; Fretts, A.; Marklund, M.; Ardisson Korat, A.V.; Yang, W.S.; Lankinen, M.; Qureshi, W.; Helmer, C.; Chen, T.A.; Wong, K.; et al. Fatty acid biomarkers of dairy fat consumption and incidence of type 2 diabetes: A pooled analysis of prospective cohort studies. PLoS Med. 2018, 15, e1002670. [Google Scholar] [CrossRef]
- Prada, M.; Wittenbecher, C.; Eichelmann, F.; Wernitz, A.; Drouin-Chartier, J.P.; Schulze, M.B. Association of the odd-chain fatty acid content in lipid groups with type 2 diabetes risk: A targeted analysis of lipidomics data in the EPIC-Potsdam cohort. Clin. Nutr. 2021, 40, 4988–4999. [Google Scholar] [CrossRef]
- Buziau, A.M.; Soedamah-Muthu, S.S.; Geleijnse, J.M.; Mishra, G.D. Total Fermented Dairy Food Intake Is Inversely Associated with Cardiovascular Disease Risk in Women. J. Nutr. 2019, 149, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Cummings, J.H.; Allison, C. Protein degradation by human intestinal bacteria. J. Gen. Microbiol. 1986, 132, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Davila, A.; Blachier, F.; Gotteland, M.; Andriamihaja, M.; Benetti, P.; Sanz, Y.; Tomé, D. Intestinal Luminal Nitrogen Metabolism: Role of the Gut Microbiota and Consequences for the Host. Pharmacol. Res. 2013, 68, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, A.; Bel Lassen, P.; Henricsson, M.; Wu, H.; Adriouch, S.; Belda, E.; Chakaroun, R.; Nielsen, T.; Bergh, P.O.; Rouault, C.; et al. Imidazole propionate is increased in diabetes and associated with dietary patterns and altered microbial ecology. Nat. Commun. 2020, 11, 5881. [Google Scholar] [CrossRef]
- Del Rio, B.; Redruello, B.; Fernandez, M.; Martin, M.C.; Ladero, V.; Alvarez, M.A. The biogenic amine tryptamine, unlike β-phenylethylamine, shows in vitro cytotoxicity at concentrations that have been found in foods. Food Chem. 2020, 331, 127303. [Google Scholar] [CrossRef]
- Caminero, A.; Galipeau, H.J.; McCarville, J.L.; Johnston, C.W.; Bernier, S.P.; Russell, A.K.; Jury, J.; Herran, A.R.; Casqueiro, J.; Tye-Din, J.A.; et al. Duodenal Bacteria From Patients With Celiac Disease and Healthy Subjects Distinctly Affect Gluten Breakdown and Immunogenicity. Gastroenterology 2016, 151, 670–683. [Google Scholar] [CrossRef]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef]
- Williams, B.B.; Van Benschoten, A.H.; Cimermancic, P.; Donia, M.S.; Zimmermann, M.; Taketani, M.; Ishihara, A.; Kashyap, P.C.; Fraser, J.S.; Fischbach, M.A. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe 2014, 16, 495–503. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, J.; Luo, L.; Gao, Y.; Bao, H.; Li, P.; Zhang, H. Research progress of indole compounds with potential antidiabetic activity. Eur. J. Med. Chem. 2021, 223, 113665. [Google Scholar] [CrossRef]
- Chimerel, C.; Emery, E.; Summers, D.K.; Keyser, U.; Gribble, F.M.; Reimann, F. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep. 2014, 9, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Abildgaard, A.; Elfving, B.; Hokland, M.; Wegener, G.; Lund, S. The microbial metabolite indole-3-propionic acid improves glucose metabolism in rats, but does not affect behaviour. Arch. Physiol. Biochem. 2018, 124, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, Y.; Williams, B.B.; Battaglioli, E.J.; Whitaker, W.R.; Till, L.; Grover, M.; Linden, D.R.; Akiba, Y.; Kandimalla, K.K.; Zachos, N.C.; et al. Gut Microbiota-Produced Tryptamine Activates an Epithelial G-Protein-Coupled Receptor to Increase Colonic Secretion. Cell Host Microbe 2018, 23, 775–785.e5. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, Y.; Jie, S.; Linden, D.R.; Ghatak, S.; Mars, R.A.T.; Williams, B.B.; Pu, M.; Sonnenburg, J.L.; Fischbach, M.A.; Farrugia, G.; et al. Bacterially Derived Tryptamine Increases Mucus Release by Activating a Host Receptor in a Mouse Model of Inflammatory Bowel Disease. iScience 2020, 23, 101798. [Google Scholar] [CrossRef] [PubMed]
- Dopkins, N.; Becker, W.; Miranda, K.; Walla, M.; Nagarkatti, P.; Nagarkatti, M. Tryptamine Attenuates Experimental Multiple Sclerosis Through Activation of Aryl Hydrocarbon Receptor. Front. Pharm. 2021, 11, 619265. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Milani, C.; de Giori, G.S.; Sesma, F.; van Sinderen, D.; Ventura, M. Bacteria as Vitamin Suppliers to Their Host: A Gut Microbiota Perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef]
- Lai, Y.; Masatoshi, H.; Ma, Y.; Guo, Y.; Zhang, B. Role of Vitamin K in Intestinal Health. Front. Immunol. 2022, 12, 791565. [Google Scholar] [CrossRef]
- Pham, V.T.; Fehlbaum, S.; Seifert, N.; Richard, N.; Bruins, M.J.; Sybesma, W.; Rehman, A.; Steinert, R.E. Effects of colon-targeted vitamins on the composition and metabolic activity of the human gut microbiome- a pilot study. Gut Microbes 2021, 13, 1875774. [Google Scholar] [CrossRef]
- Pham, V.T.; Dold, S.; Rehman, A.; Bird, J.K.; Steinert, R.E. Vitamins, the Gut Microbiome and Gastrointestinal Health in Humans. Nutr. Res. 2021, 95, 35–53. [Google Scholar] [CrossRef]
- Gustafsson, B.E.; Daft, F.S.; Mcdaniel, E.G.; Smith, J.C.; Fitzgerald, R.J. Effects of vitamin K-active compounds and intestinal microorganisms in vitamin K-deficient germfree rats. J. Nutr. 1962, 78, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Goto, R.; Fujimoto, M.; Okada, N.; Hardt, W.D. The Bactericidal Lectin RegIIIbeta Prolongs Gut Colonization and Enteropathy in the Streptomycin Mouse Model for Salmonella Diarrhea. Cell Host Microbe 2017, 21, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, M.A.Y.; Imhann, F.; Collij, V.; Fu, J.; Wijmenga, C.; Zhernakova, A.; Dijkstra, G.; Festen, E.A.M.; Gacesa, R.; Vich Vila, A.; et al. Anti-inflammatory Gut Microbial Pathways Are Decreased During Crohn’s Disease Exacerbations. J. Crohns Colitis 2019, 13, 1439–1449. [Google Scholar] [CrossRef]
- Belda, E.; Voland, L.; Tremaroli, V.; Falony, G.; Adriouch, S.; Assmann, K.E.; Prifiti, E.; Aron-Wisnewsky, J.; Debédat, J.; Le Roy, T.; et al. Impairment of Gut Microbial Biotin Metabolism and Host Biotin Status in Severe Obesity: Effect of Biotin and Prebiotic Supplementation on Improved Metabolism. Gut 2022, 71, 2463–2480. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Mithul Aravind, S.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef]
- Mishra, S.; Verma, S.S.; Rai, V.; Awasthee, N.; Chava, S.; Hui, K.M.; Kumar, A.P.; Challagundla, K.B.; Sethi, G.; Gupta, S.C. Long Non-Coding RNAs are Emerging Targets of Phytochemicals for Cancer and Other Chronic Diseases. Cell. Mol. Life Sci. 2019, 76, 1947–1966. [Google Scholar] [CrossRef]
- Griñán-Ferré, C.; Bellver-Sanchis, A.; Izquierdo, V.; Corpas, R.; Roig-Soriano, J.; Chillón, M.; Andres-Lacueva, C.; Somogyvári, M.; Sőti, C.; Sanfeliu, C.; et al. The pleiotropic neuroprotective effects of resveratrol in cognitive decline and Alzheimer’s disease pathology: From antioxidant to epigenetic therapy. Ageing Res. Rev. 2021, 67, 101271. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.; Ojcius, D.M.; Ko, Y.F.; Young, J.D. Phytochemicals as Prebiotics and Biological Stress Inducers. Trends Biochem. Sci. 2020, 45, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.S.; Qu, W. Dietary Apigenin promotes lipid catabolism, thermogenesis, and browning in adipose tissues of HFD-Fed mice. Food Chem. Toxicol. 2019, 133, 110780. [Google Scholar] [CrossRef]
- Xin, Y.; Bai, Y.; Jiang, X.; Zhou, S.; Wang, Y.; Wintergerst, K.A.; Cui, T.; Ji, H.; Tan, Y.; Cai, L. Sulforaphane prevents angiotensin II-induced cardiomyopathy by activation of Nrf2 via stimulating the Akt/GSK-3ß/Fyn pathway. Redox Biol. 2018, 15, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Braune, A.; Blaut, M. Bacterial species involved in the conversion of dietary flavonoids in the human gut. Gut Microbes 2016, 7, 216–234. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; García-Villalba, R.; González-Sarrías, A.; Selma, M.V.; Espín, J.C. Ellagic Acid Metabolism by Human Gut Microbiota: Consistent Observation of Three Urolithin Phenotypes in Intervention Trials, Independent of Food Source, Age, and Health Status. J. Agric. Food Chem. 2014, 62, 6535–6538. [Google Scholar] [CrossRef]
- Corona, G.; Kreimes, A.; Barone, M.; Turroni, S.; Brigidi, P.; Keleszade, E.; Costabile, A. Impact of lignans in oilseed mix on gut microbiome composition and enterolignan production in younger healthy and premenopausal women: An in vitro pilot study. Microb. Cell Fact. 2020, 19, 82. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.; García-Villalba, R.; Quartieri, A.; Raimondi, S.; Amaretti, A.; Leonardi, A.; Rossi, M. In vitro transformation of chlorogenic acid by human gut microbiota. Mol. Nutr. Food Res. 2014, 58, 1122–1131. [Google Scholar] [CrossRef]
- Selma, M.V.; Beltrán, D.; García-Villalba, R.; Espín, J.C.; Tomás-Barberán, F.A. Description of urolithin production capacity from ellagic acid of two human intestinal Gordonibacter species. Food Funct. 2014, 5, 1779–1784. [Google Scholar] [CrossRef]
- Beltrán, D.; Romo-Vaquero, M.; Espín, J.C.; Tomás-Barberán, F.A.; Selma, M.V. Ellagibacter isourolithinifaciens gen. nov., sp. nov., a New Member of the Family Eggerthellaceae, Isolated from Human Gut. Int. J. Syst. Evol. Microbiol. 2018, 68, 1707–1712. [Google Scholar] [CrossRef]
- Rowland, I.; Faughnan, M.; Hoey, L.; Wähälä, K.; Williamson, G.; Cassidy, A. Bioavailability of phyto-oestrogens. Br. J. Nutr. 2003, 89 (Suppl. 1), 45. [Google Scholar] [CrossRef]
- Quartieri, A.; García-Villalba, R.; Amaretti, A.; Raimondi, S.; Leonardi, A.; Rossi, M.; Tomàs-Barberàn, F. Detection of novel metabolites of flaxseed lignans in vitro and in vivo. Mol. Nutr. Food Res. 2016, 60, 1590–1601. [Google Scholar] [CrossRef] [PubMed]
- Bento-Silva, A.; Koistinen, V.M.; Mena, P.; Bronze, M.R.; Hanhineva, K.; Sahlstrøm, S.; Kitrytė, V.; Moco, S.; Aura, A.M. Factors affecting intake, metabolism and health benefits of phenolic acids: Do we understand individual variability? Eur. J. Nutr. 2020, 59, 1275–1293. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Fernández, M.; Young Tie Yang, P.; Ludwig, I.A.; Clifford, M.N.; Cid, C.; Rodriguez-Mateos, A. In vivo study of the bioavailability and metabolic profile of (poly)phenols after sous-vide artichoke consumption. Food Chem. 2022, 367, 130620. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M. Ellagitannins, Ellagic Acid and Their Derived Metabolites: A Review about Source, Metabolism, Functions and Health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- García-Villalba, R.; Beltrán, D.; Espín, J.C.; Selma, M.V.; Tomás-Barberán, F.A. Time Course Production of Urolithins from Ellagic Acid by Human Gut Microbiota. J. Agric. Food Chem. 2013, 61, 8797–8806. [Google Scholar] [CrossRef]
- Espín, J.C.; Larrosa, M.; García-Conesa, M.T.; Tomás-Barberán, F. Biological Significance of Urolithins, the Gut Microbial Ellagic Acid-Derived Metabolites: The Evidence So Far. Evid.-Based Complement. Altern. Med. 2013, 2013, 270418. [Google Scholar] [CrossRef]
- Singh, A.; D’Amico, D.; Andreux, P.A.; Dunngalvin, G.; Kern, T.; Blanco-Bose, W.; Auwerx, J.; Aebischer, P.; Rinsch, C. Direct supplementation with Urolithin A overcomes limitations of dietary exposure and gut microbiome variability in healthy adults to achieve consistent levels across the population. Eur. J. Clin. Nutr. 2022, 76, 297–308. [Google Scholar] [CrossRef]
- Selma, M.V.; Beltrán, D.; Luna, M.C.; Romo-Vaquero, M.; García-Villalba, R.; Mira, A.; Espín, J.C.; Tomás-Barberán, F.A. Isolation of Human Intestinal Bacteria Capable of Producing the Bioactive Metabolite Isourolithin A from Ellagic Acid. Front. Microbiol. 2017, 8, 1521. [Google Scholar] [CrossRef]
- D’Amico, D.; Andreux, P.A.; Valdés, P.; Singh, A.; Rinsch, C.; Auwerx, J. Impact of the Natural Compound Urolithin A on Health, Disease, and Aging. Trends Mol. Med. 2021, 27, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, S.A.; Abdulrahman, A.O.; Zamzami, M.A.; Khan, M.I. Urolithins: The Gut Based Polyphenol Metabolites of Ellagitannins in Cancer Prevention, a Review. Front. Nutr. 2021, 8, 647582. [Google Scholar] [CrossRef]
- Kujawska, M.; Jodynis-Liebert, J. Potential of the ellagic acid-derived gut microbiota metabolite-Urolithin A in gastrointestinal protection. World J. Gastroenterol. 2020, 26, 3170–3181. [Google Scholar] [CrossRef]
- Zhang, S.; Al-Maghout, T.; Cao, H.; Pelzl, L.; Salker, M.S.; Veldhoen, M.; Cheng, A.; Lang, F.; Singh, Y. Gut Bacterial Metabolite Urolithin A (UA) Mitigates Ca(2+) Entry in T Cells by Regulating miR-10a-5p. Front. Immunol. 2019, 10, 1737. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, W.; Kishi, H.; Yagasaki, K.; Ohhira, S. Urolithin A attenuates pro-inflammatory mediator production by suppressing PI3-K/Akt/NF-κB and JNK/AP-1 signaling pathways in lipopolysaccharide-stimulated RAW264 macrophages: Possible involvement of NADPH oxidase-derived reactive oxygen species. Eur. J. Pharm. 2018, 833, 411–424. [Google Scholar] [CrossRef]
- Alzahrani, A.M.; Shait Mohammed, M.R.; Alghamdi, R.A.; Ahmad, A.; Zamzami, M.A.; Choudhry, H.; Khan, M.I. Urolithin A and B Alter Cellular Metabolism and Induce Metabolites Associated with Apoptosis in Leukemic Cells. Int. J. Mol. Sci. 2021, 22, 5465. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Ávila-Gálvez, M.Á.; Espín, J.C.; González-Sarrías, A. The gut microbiota metabolite urolithin A, but not other relevant urolithins, induces p53-dependent cellular senescence in human colon cancer cells. Food Chem. Toxicol. 2020, 139, 111260. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Pei, J.; Wang, X.; Tai, S.; Tang, L.; Hu, X. Gut bacterial metabolite Urolithin A inhibits myocardial fibrosis through activation of Nrf2 pathway in vitro and in vivo. Mol. Med. 2022, 28, 19. [Google Scholar] [CrossRef]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef]
- Piwowarski, J.P.; Stanisławska, I.; Granica, S.; Stefańska, J.; Kiss, A.K. Phase II Conjugates of Urolithins Isolated from Human Urine and Potential Role of β-Glucuronidases in Their Disposition. Drug Metab. Dispos. 2017, 45, 657–665. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Giménez-Bastida, J.A.; García-Conesa, M.T.; Gómez-Sánchez, M.B.; García-Talavera, N.V.; Gil-Izquierdo, A.; Sánchez-Alvarez, C.; Fontana-Compiano, L.O.; Morga-Egea, J.P.; Pastor-Quirante, F. Aet al. Occurrence of urolithins, gut microbiota ellagic acid metabolites and proliferation markers expression response in the human prostate gland upon consumption of walnuts and pomegranate juice. Mol. Nutr. Food Res. 2010, 54, 311–322. [Google Scholar] [CrossRef] [PubMed]
- García-Villalba, R.; Giménez-Bastida, J.A.; Cortés-Martín, A.; Ávila-Gálvez, M.Á.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C.; González-Sarrías, A. Urolithins: A Comprehensive Update on their Metabolism, Bioactivity, and Associated Gut Microbiota. Mol. Nutr. Food Res. 2022, 66, e2101019. [Google Scholar] [CrossRef]
- Bobowska, A.; Granica, S.; Filipek, A.; Melzig, M.F.; Moeslinger, T.; Zentek, J.; Kruk, A.; Piwowarski, J.P. Comparative studies of urolithins and their phase II metabolites on macrophage and neutrophil functions. Eur. J. Nutr. 2021, 60, 1957–1972. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Spagnuolo, C.; Russo, G.L.; Skalicka-Woźniak, K.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Nrf2 targeting by sulforaphane: A potential therapy for cancer treatment. Crit. Rev. Food Sci. Nutr. 2018, 58, 1391–1405. [Google Scholar] [CrossRef]
- Wu, G.; Yan, Y.; Zhou, Y.; Duan, Y.; Zeng, S.; Wang, X.; Lin, W.; Ou, C.; Zhou, J.; Xu, Z. Sulforaphane: Expected to Become a Novel Antitumor Compound. Oncol. Res. 2020, 28, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or Sulforaphane: Is It the Source or Dose That Matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Dinkova-Kostova, A.T.; Wade, K.L.; Zhang, Y.; Shapiro, T.A.; Talalay, P. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: Pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin. Chim. Acta 2002, 316, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Cornblatt, B.S.; Ye, L.; Dinkova-Kostova, A.T.; Erb, M.; Fahey, J.W.; Singh, N.K.; Chen, M.S.; Stierer, T.; Garrett-Mayer, E.; Argani, P.; et al. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis 2007, 28, 1485–1490. [Google Scholar] [CrossRef]
- Traka, M.; Gasper, A.V.; Smith, J.A.; Hawkey, C.J.; Bao, Y.; Mithen, R.F. Transcriptome analysis of human colon Caco-2 cells exposed to sulforaphane. J. Nutr. 2005, 135, 1865–1872. [Google Scholar] [CrossRef]
- Myzak, M.C.; Karplus, P.A.; Chung, F.L.; Dashwood, R.H. A novel mechanism of chemoprotection by sulforaphane: Inhibition of histone deacetylase. Cancer Res. 2004, 64, 5767–5774. [Google Scholar] [CrossRef]
- Jablonka, E.; Raz, G. Transgenerational epigenetic inheritance: Prevalence, mechanisms, and implications for the study of heredity and evolution. Q. Rev. Biol. 2009, 84, 131–176. [Google Scholar] [CrossRef]
- Darwiche, N. Epigenetic mechanisms and the hallmarks of cancer: An intimate affair. Am. J. Cancer Res. 2020, 10, 1954–1978. [Google Scholar] [PubMed]
- Jiang, W.; Xia, T.; Liu, C.; Li, J.; Zhang, W.; Sun, C. Remodeling the Epigenetic Landscape of Cancer-Application Potential of Flavonoids in the Prevention and Treatment of Cancer. Front. Oncol. 2021, 11, 705903. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; He, C.; Wang, M.; Ma, X.; Mo, F.; Yang, S.; Han, J.; Wei, X. Targeting epigenetic regulators for cancer therapy: Mechanisms and advances in clinical trials. Signal Transduct. Target Ther. 2019, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, P.W.D.S.; Machado, A.R.T.; De Grandis, R.A.; Ribeiro, D.L.; Tuttis, K.; Morselli, M.; Aissa, A.F.; Pellegrini, M.; Antunes, L.M.G. Transcriptome and DNA methylation changes modulated by sulforaphane induce cell cycle arrest, apoptosis, DNA damage, and suppression of proliferation in human liver cancer cells. Food Chem. Toxicol. 2020, 136, 111047. [Google Scholar] [CrossRef]
- Lewinska, A.; Adamczyk-Grochala, J.; Deregowska, A.; Wnuk, M. Sulforaphane-Induced Cell Cycle Arrest and Senescence are accompanied by DNA Hypomethylation and Changes in microRNA Profile in Breast Cancer Cells. Theranostics 2017, 7, 3461–3477. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.; Clarke, J.D.; Dashwood, R.H. Dietary sulforaphane, a histone deacetylase inhibitor for cancer prevention. J. Nutr. 2009, 139, 2393–2396. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kee, H.J.; Jin, L.; Ryu, Y.; Sun, S.; Kim, G.R.; Jeong, M.H. Inhibition of class IIa histone deacetylase activity by gallic acid, sulforaphane, TMP269, and panobinostat. Biomed. Pharm. 2018, 101, 145–154. [Google Scholar] [CrossRef]
- Tortorella, S.M.; Royce, S.G.; Licciardi, P.V.; Karagiannis, T.C. Dietary Sulforaphane in Cancer Chemoprevention: The Role of Epigenetic Regulation and HDAC Inhibition. Antioxid. Redox Signal 2015, 22, 1382–1424. [Google Scholar] [CrossRef]
- Gao, L.; Cheng, D.; Yang, J.; Wu, R.; Li, W.; Kong, A.N. Sulforaphane epigenetically demethylates the CpG sites of the miR-9-3 promoter and reactivates miR-9-3 expression in human lung cancer A549 cells. J. Nutr. Biochem. 2018, 56, 109–115. [Google Scholar] [CrossRef]
- Royston, K.J.; Paul, B.; Nozell, S.; Rajbhandari, R.; Tollefsbol, T.O. Withaferin A and sulforaphane regulate breast cancer cell cycle progression through epigenetic mechanisms. Exp. Cell Res. 2018, 368, 67–74. [Google Scholar] [CrossRef]
- Mitsiogianni, M.; Trafalis, D.T.; Franco, R.; Zoumpourlis, V.; Pappa, A.; Panayiotidis, M.I. Sulforaphane and iberin are potent epigenetic modulators of histone acetylation and methylation in malignant melanoma. Eur. J. Nutr. 2021, 60, 147–158. [Google Scholar] [CrossRef]
- Cao, C.; Wu, H.; Vasilatos, S.N.; Chandran, U.; Qin, Y.; Wan, Y.; Oesterreich, S.; Davidson, N.E.; Huang, Y. HDAC5-LSD1 axis regulates antineoplastic effect of natural HDAC inhibitor sulforaphane in human breast cancer cells. Int. J. Cancer 2018, 143, 1388–1401. [Google Scholar] [CrossRef] [PubMed]
- Georgikou, C.; Yin, L.; Gladkich, J.; Xiao, X.; Sticht, C.; Torre, C.; Gretz, N.; Gross, W.; Schäfer, M.; Karakhanova, S.; et al. Inhibition of miR30a-3p by sulforaphane enhances gap junction intercellular communication in pancreatic cancer. Cancer Lett. 2020, 469, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Petrikova, E.; Gross, W.; Sticht, C.; Gretz, N.; Herr, I.; Karakhanova, S. Sulforaphane Promotes Dendritic Cell Stimulatory Capacity Through Modulation of Regulatory Molecules, JAK/STAT3- and MicroRNA-Signaling. Front. Immunol. 2020, 11, 589818. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Xiao, X.; Georgikou, C.; Luo, Y.; Liu, L.; Gladkich, J.; Gross, W.; Herr, I. Sulforaphane Induces miR135b-5p and Its Target Gene, RASAL2, thereby Inhibiting the Progression of Pancreatic Cancer. Mol. Ther. Oncolytics 2019, 14, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Mazarakis, N.; Snibson, K.; Licciardi, P.V.; Karagiannis, T.C. The potential use of l-sulforaphane for the treatment of chronic inflammatory diseases: A review of the clinical evidence. Clin. Nutr. 2020, 39, 664–675. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Kozumbo, W.J. The phytoprotective agent sulforaphane prevents inflammatory degenerative diseases and age-related pathologies via Nrf2-mediated hormesis. Pharm. Res. 2021, 163, 105283. [Google Scholar] [CrossRef]
- Cardozo, L.F.M.F.; Alvarenga, L.A.; Ribeiro, M.; Dai, L.; Shiels, P.G.; Stenvinkel, P.; Lindholm, B.; Mafra, D. Cruciferous vegetables: Rationale for exploring potential salutary effects of sulforaphane-rich foods in patients with chronic kidney disease. Nutr. Rev. 2021, 79, 1204–1224. [Google Scholar] [CrossRef]
- Akao, T.; Kawabata, K.; Yanagisawa, E.; Ishihara, K.; Mizuhara, Y.; Wakui, Y.; Sakashita, Y.; Kobashi, K. Baicalin, the predominant flavone glucuronide of scutellariae radix, is absorbed from the rat gastrointestinal tract as the aglycone and restored to its original form. J. Pharm. Pharm. 2000, 52, 1563–1568. [Google Scholar] [CrossRef]
- Kim, D.; Jang, I.; Lee, H.; Jung, E.; Lee, K. Metabolism of glycyrrhizin and baicalin by human intestinal bacteria. Arch. Pharm. Res. 1996, 19, 292–296. [Google Scholar] [CrossRef]
- Pan, L.; Cho, K.S.; Yi, I.; To, C.H.; Chen, D.F.; Do, C.W. Baicalein, Baicalin, and Wogonin: Protective Effects against Ischemia-Induced Neurodegeneration in the Brain and Retina. Oxidative Med. Cell. Longev. 2021, 2021, 8377362. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Zhang, C.F.; Chen, L.; Anderson, S.; Lu, F.; Yuan, C.S. Colon cancer chemopreventive effects of baicalein, an active enteric microbiome metabolite from baicalin. Int. J. Oncol. 2015, 47, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Zhang, C.F.; Luo, Y.; Yao, H.; Yu, C.; Chen, L.; Yuan, J.; Huang, W.H.; Wan, J.Y.; Zeng, J.; et al. Baicalein, an enteric microbial metabolite, suppresses gut inflammation and cancer progression in Apc(Min/+) mice. Clin. Transl. Oncol. 2020, 22, 1013–1022. [Google Scholar] [CrossRef]
- Hu, J.; Wang, R.; Liu, Y.; Zhou, J.; Shen, K.; Dai, Y. Baicalein Represses Cervical Cancer Cell Growth, Cell Cycle Progression and Promotes Apoptosis via Blocking AKT/mTOR Pathway by the Regulation of circHIAT1/miR-19a-3p Axis. Onco Targets Ther. 2021, 14, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Qiu, S.; Qin, J. Baicalein induced apoptosis and autophagy of undifferentiated thyroid cancer cells by the ERK/PI3K/Akt pathway. Am. J. Transl. Res. 2019, 11, 3341–3352. [Google Scholar] [PubMed]
- Yan, W.; Ma, X.; Zhao, X.; Zhang, S. Baicalein induces apoptosis and autophagy of breast cancer cells via inhibiting PI3K/AKT pathway in vivo and vitro. Drug. Des. Devel Ther. 2018, 12, 3961–3972. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, H.; Hu, P.; Qing, Y.; Wang, X.; Zhu, M.; Wang, H.; Wang, Z.; Xu, J.; Guo, Q.; et al. Natural HDAC-1/8 inhibitor baicalein exerts therapeutic effect in CBF-AML. Clin. Transl. Med. 2020, 10, e154. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yao, Q.; An, Y.; Fan, L.; Wang, J.; Li, H. Baicalin suppresses the progression of Type 2 diabetes-induced liver tumor through regulating METTL3/m(6)A/HKDC1 axis and downstream p-JAK2/STAT1/clevaged Capase3 pathway. Phytomedicine 2022, 94, 153823. [Google Scholar] [CrossRef]
- Hung, K.C.; Huang, H.J.; Wang, Y.T.; Lin, A.M. Baicalein attenuates α-synuclein aggregation, inflammasome activation and autophagy in the MPP(+)-treated nigrostriatal dopaminergic system in vivo. J. Ethnopharmacol. 2016, 194, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Z.; Wang, H.T.; Huang, H.J.; Lo, Y.L.; Lin, A.M. Neuroprotective Effects of Baicalein on Acrolein-induced Neurotoxicity in the Nigrostriatal Dopaminergic System of Rat Brain. Mol. Neurobiol. 2018, 55, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, S.K.; Uversky, V.N.; Chinnathambi, S. Baicalein inhibits heparin-induced Tau aggregation by initializing non-toxic Tau oligomer formation. Cell Commun. Signal 2021, 19, 16. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Lei, H.; Liu, Z.; Liu, Y.; Li, Y.; Qiu, Y.; Xu, L. Network pharmacology and molecular docking analysis on molecular targets: Mechanisms of baicalin and baicalein against hyperuricemic nephropathy. Toxicol. Appl. Pharm. 2021, 424, 115594. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Z.; Li, Y.; Yang, Y.; Li, L.; Jiang, Y.; Lin, C.; Cao, Y.; Zhou, P.; Tian, Y.; et al. Baicalein alleviates hyperuricemia by promoting uric acid excretion and inhibiting xanthine oxidase. Phytomedicine 2021, 80, 153374. [Google Scholar] [CrossRef]
- Baradaran Rahimi, V.; Askari, V.R.; Hosseinzadeh, H. Promising influences of Scutellaria baicalensis and its two active constituents, baicalin, and baicalein, against metabolic syndrome: A review. Phytother. Res. 2021, 35, 3558–3574. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Yuan, R.; Zhang, Z.; Liu, L.; Wang, X.; Song, X.; Ma, T.; Tang, J.; Liu, C.; Gao, L. Intra-articular Injection of Baicalein Inhibits Cartilage Catabolism and NLRP3 Inflammasome Signaling in a Posttraumatic OA Model. Oxidative Med. Cell. Longev. 2021, 2021, 6116890. [Google Scholar] [CrossRef] [PubMed]
- Rui, W.; Li, S.; Xiao, H.; Xiao, M.; Shi, J. Baicalein Attenuates Neuroinflammation by Inhibiting NLRP3/caspase-1/GSDMD Pathway in MPTP Induced Mice Model of Parkinson’s Disease. Int. J. Neuropsychopharmacol. 2020, 23, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Cui, Y.; Ji, H.; Yan, L.; Pei, D.; Qu, S. Baicalein attenuates acute liver injury by blocking NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 2021, 534, 212–218. [Google Scholar] [CrossRef]
- Wang, X.; Cai, H.; Chen, Z.; Zhang, Y.; Wu, M.; Xu, X.; Yang, L. Baicalein alleviates pyroptosis and inflammation in hyperlipidemic pancreatitis by inhibiting NLRP3/Caspase-1 pathway through the miR-192-5p/TXNIP axis. Int. Immunopharmacol. 2021, 101, 108315. [Google Scholar] [CrossRef]
- Song, J.; Zhang, L.; Xu, Y.; Yang, D.; Zhang, L.; Yang, S.; Zhang, W.; Wang, J.; Tian, S.; Yang, S.; et al. The comprehensive study on the therapeutic effects of baicalein for the treatment of COVID-19 in vivo and in vitro. Biochem. Pharm. 2021, 183, 114302. [Google Scholar] [CrossRef]
- Noh, K.; Kang, Y.; Nepal, M.R.; Jeong, K.S.; Oh, D.G.; Kang, M.J.; Lee, S.; Kang, W.; Jeong, H.G.; Jeong, T.C. Role of Intestinal Microbiota in Baicalin-Induced Drug Interaction and Its Pharmacokinetics. Molecules 2016, 21, 337. [Google Scholar] [CrossRef] [PubMed]
- Gardana, C.; Simonetti, P. Long-term kinetics of daidzein and its main metabolites in human equol-producers after soymilk intake: Identification of equol-conjugates by UPLC-orbitrap-MS and influence of the number of transforming bacteria on plasma kinetics. Int. J. Food Sci. Nutr. 2017, 68, 496–506. [Google Scholar] [CrossRef]
- Setchell, K.D.; Zhao, X.; Jha, P.; Heubi, J.E.; Brown, N.M. The pharmacokinetic behavior of the soy isoflavone metabolite S-(-)equol and its diastereoisomer R-(+)equol in healthy adults determined by using stable-isotope-labeled tracers. Am. J. Clin. Nutr. 2009, 90, 1029–1037. [Google Scholar] [CrossRef]
- Legette, L.L.; Prasain, J.; King, J.; Arabshahi, A.; Barnes, S.; Weaver, C.M. Pharmacokinetics of equol, a soy isoflavone metabolite, changes with the form of equol (dietary versus intestinal production) in ovariectomized rats. J. Agric. Food Chem. 2014, 62, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- Lin, I.C.; Yamashita, S.; Murata, M.; Kumazoe, M.; Tachibana, H. Equol suppresses inflammatory response and bone erosion due to rheumatoid arthritis in mice. J. Nutr. Biochem. 2016, 32, 101–106. [Google Scholar] [CrossRef]
- Danciu, C.; Avram, S.; Pavel, I.Z.; Ghiulai, R.; Dehelean, C.A.; Ersilia, A.; Minda, D.; Petrescu, C.; Moaca, E.A.; Soica, C. Main Isoflavones Found in Dietary Sources as Natural Anti-inflammatory Agents. Curr. Drug Targets 2018, 19, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Clerici, C.; Lephart, E.D.; Cole, S.J.; Heenan, C.; Castellani, D.; Wolfe, B.E.; Nechemias-Zimmer, L.; Brown, N.M.; Lund, T.D.; et al. S-equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am. J. Clin. Nutr. 2005, 81, 1072–1079. [Google Scholar] [CrossRef]
- Hazim, S.; Curtis, P.J.; Schär, M.Y.; Ostertag, L.M.; Kay, C.D.; Minihane, A.M.; Cassidy, A. Acute benefits of the microbial-derived isoflavone metabolite equol on arterial stiffness in men prospectively recruited according to equol producer phenotype: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 694–702. [Google Scholar] [CrossRef]
- Pawlowski, J.W.; Martin, B.R.; McCabe, G.P.; McCabe, L.; Jackson, G.S.; Peacock, M.; Barnes, S.; Weaver, C.M. Impact of equol-producing capacity and soy-isoflavone profiles of supplements on bone calcium retention in postmenopausal women: A randomized crossover trial. Am. J. Clin. Nutr. 2015, 102, 695–703. [Google Scholar] [CrossRef]
- Setchell, K.D.; Clerici, C. Equol: History, chemistry, and formation. J. Nutr. 2010, 140, 1355S–1362S. [Google Scholar] [CrossRef]
- Yuan, J.P.; Wang, J.H.; Liu, X. Metabolism of dietary soy isoflavones to equol by human intestinal microflora--implications for health. Mol. Nutr. Food Res. 2007, 51, 765–781. [Google Scholar] [CrossRef]
- Yamashita, S.; Lin, I.; Oka, C.; Kumazoe, M.; Komatsu, S.; Murata, M.; Kamachi, S.; Tachibana, H. Soy isoflavone metabolite equol inhibits cancer cell proliferation in a PAP associated domain containing 5-dependent and an estrogen receptor-independent manner. J. Nutr. Biochem. 2022, 100, 108910. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Gao, R.; Zhang, Y.; Jiang, N.; Chen, Y.; Sun, J.; Wang, Q.; Fan, B.; Liu, X.; Wang, F. S-equol, a metabolite of dietary soy isoflavones, alleviates lipopolysaccharide-induced depressive-like behavior in mice by inhibiting neuroinflammation and enhancing synaptic plasticity. Food Funct. 2021, 12, 5770–5778. [Google Scholar] [CrossRef]
- Johnson, S.L.; Park, H.Y.; Vattem, D.A.; Grammas, P.; Ma, H.; Seeram, N.P. Equol, a Blood-Brain Barrier Permeable Gut Microbial Metabolite of Dietary Isoflavone Daidzein, Exhibits Neuroprotective Effects against Neurotoxins Induced Toxicity in Human Neuroblastoma SH-SY5Y Cells and Caenorhabditis elegans. Plant Foods Hum. Nutr. 2020, 75, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Murugan, P.; Pari, L.; Rao, C.A. Effect of tetrahydrocurcumin on insulin receptor status in type 2 diabetic rats: Studies on insulin binding to erythrocytes. J. Biosci. 2008, 33, 63–72. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Deb, L.; Prasad, S. Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses. Molecules 2014, 20, 185–205. [Google Scholar] [CrossRef]
- Tan, S.; Rupasinghe, T.W.; Tull, D.L.; Boughton, B.; Oliver, C.; McSweeny, C.; Gras, S.L.; Augustin, M.A. Degradation of curcuminoids by in vitro pure culture fermentation. J. Agric. Food Chem. 2014, 62, 11005–11015. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ma, Y.; Zhang, C.; Chen, B.; Zhang, X.; Yu, X.; Shuai, H.; He, Q.; Ya, F. Tetrahydrocurcumin Downregulates MAPKs/cPLA2 Signaling and Attenuates Platelet Thromboxane A2 Generation, Granule Secretion, and Thrombus Growth. Thromb. Haemost. 2021, 122, 739–754. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhai, M.; Jiang, L.; Song, F.; Zhang, B.; Li, J.; Li, H.; Li, B.; Xia, L.; Xu, L.; et al. Tetrahydrocurcumin Ameliorates Diabetic Cardiomyopathy by Attenuating High Glucose-Induced Oxidative Stress and Fibrosis via Activating the SIRT1 Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 6746907. [Google Scholar] [CrossRef]
- Mondal, N.K.; Behera, J.; Kelly, K.E.; George, A.K.; Tyagi, P.K.; Tyagi, N. Tetrahydrocurcumin Epigenetically Mitigates Mitochondrial Dysfunction in Brain Vasculature during Ischemic Stroke. Neurochem. Int. 2019, 122, 120–138. [Google Scholar] [CrossRef]
- Chen, X.; Xie, Q.; Zhu, Y.; Xu, J.; Lin, G.; Liu, S.; Su, Z.; Lai, X.; Li, Q.; Xie, J.; et al. Cardio-protective effect of tetrahydrocurcumin, the primary hydrogenated metabolite of curcumin in vivo and in vitro: Induction of apoptosis and autophagy via PI3K/AKT/mTOR pathways. Eur. J. Pharm. 2021, 911, 174495. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.W.; Chen, T.C.; Yeh, J.H.; Tsou, S.C.; Wang, I.; Shen, T.J.; Chuang, C.J.; Chang, Y.Y. Suppressive Effect of Tetrahydrocurcumin on Pseudomonas aeruginosa Lipopolysaccharide-Induced Inflammation by Suppressing JAK/STAT and Nrf2/HO-1 Pathways in Microglial Cells. Oxidative Med. Cell. Longev. 2022, 2022, 4978556. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Chaturvedi, M.; Mishra, S.; Kumar, P.; Somvanshi, P.; Chaturvedi, R. Reductive metabolites of curcumin and their therapeutic effects. Heliyon 2020, 6, e05469. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Ge, C.; Li, W.; Li, R. 3-(3-Hydroxyphenyl)propionic acid, a microbial metabolite of quercetin, inhibits monocyte binding to endothelial cells via modulating E-selectin expression. Fitoterapia 2022, 156, 105071. [Google Scholar] [CrossRef]
- Konishi, Y.; Kobayashi, S. Microbial metabolites of ingested caffeic acid are absorbed by the monocarboxylic acid transporter (MCT) in intestinal Caco-2 cell monolayers. J. Agric. Food Chem. 2004, 52, 6418–6424. [Google Scholar] [CrossRef]
- Goldberg, D.M.; Yan, J.; Soleas, G.J. Absorption of Three Wine-Related Polyphenols in Three Different Matrices by Healthy Subjects. Clin. Biochem. 2003, 36, 79–87. [Google Scholar] [CrossRef]
- Najmanová, I.; Pourová, J.; Vopršalová, M.; Pilařová, V.; Semecký, V.; Nováková, L.; Mladěnka, P. Flavonoid metabolite 3-(3-hydroxyphenyl)propionic acid formed by human microflora decreases arterial blood pressure in rats. Mol. Nutr. Food Res. 2016, 60, 981–991. [Google Scholar] [CrossRef]
- Chen, J.R.; Wankhade, U.D.; Alund, A.W.; Blackburn, M.L.; Shankar, K.; Lazarenko, O.P. 3-(3-Hydroxyphenyl)-Propionic Acid (PPA) Suppresses Osteoblastic Cell Senescence to Promote Bone Accretion in Mice. JBMR Plus 2019, 3, e10201. [Google Scholar] [CrossRef]
- Zhao, H.; Lazarenko, O.P.; Chen, J.R. Hippuric acid and 3-(3-hydroxyphenyl) propionic acid inhibit murine osteoclastogenesis through RANKL-RANK independent pathway. J. Cell. Physiol. 2020, 235, 599–610. [Google Scholar] [CrossRef]
- Yamasaki, T.R.; Ono, K.; Ho, L.; Pasinetti, G.M. Gut Microbiome-Modified Polyphenolic Compounds Inhibit α-Synuclein Seeding and Spreading in α-Synucleinopathies. Front. Neurosci. 2020, 14, 398. [Google Scholar] [CrossRef]
- Esteban-Fernández, A.; Rendeiro, C.; Spencer, J.P.; Del Coso, D.G.; de Llano, M.D.; Bartolomé, B.; Moreno-Arribas, M.V. Neuroprotective Effects of Selected Microbial-Derived Phenolic Metabolites and Aroma Compounds from Wine in Human SH-SY5Y Neuroblastoma Cells and Their Putative Mechanisms of Action. Front. Nutr. 2017, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Boto-Ordóñez, M.; Rothwell, J.A.; Andres-Lacueva, C.; Manach, C.; Scalbert, A.; Urpi-Sarda, M. Prediction of the wine polyphenol metabolic space: An application of the Phenol-Explorer database. Mol. Nutr. Food Res. 2014, 58, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Qaiser, M.Z.; Begley, D.J.; Rice-Evans, C.A.; Abbott, N.J. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic. Biol. Med. 2004, 36, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ho, L.; Faith, J.; Ono, K.; Janle, E.M.; Lachcik, P.J.; Cooper, B.R.; Jannasch, A.H.; D’Arcy, B.R.; Williams, B.A.; et al. Role of intestinal microbiota in the generation of polyphenol-derived phenolic acid mediated attenuation of Alzheimer’s disease β-amyloid oligomerization. Mol. Nutr. Food Res. 2015, 59, 1025–1040. [Google Scholar] [CrossRef]
- Wang, J.; Hodes, G.E.; Zhang, H.; Zhang, S.; Zhao, W.; Golden, S.A.; Bi, W.; Menard, C.; Kana, V.; Leboeuf, M.; et al. Epigenetic modulation of inflammation and synaptic plasticity promotes resilience against stress in mice. Nat. Commun. 2018, 9, 477. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Blaze, J.; Haghighi, F.; Kim-Schulze, S.; Raval, U.; Trageser, K.J.; Pasinetti, G.M. Characterization of 3(3,4-dihydroxy-phenyl) propionic acid as a novel microbiome-derived epigenetic modifier in attenuation of immune inflammatory response in human monocytes. Mol. Immunol. 2020, 125, 172–177. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Ohland, C.; Jobin, C.; Sang, S. Microbiota facilitates the formation of the aminated metabolite of green tea polyphenol (-)-epigallocatechin-3-gallate which trap deleterious reactive endogenous metabolites. Free Radic. Biol. Med. 2019, 131, 332–344. [Google Scholar] [CrossRef]
- Fernandes, I.; Nave, F.; Gonçalves, R.; de Freitas, V.; Mateus, N. On the bioavailability of flavanols and anthocyanins: Flavanol-anthocyanin dimers. Food Chem. 2012, 135, 812–818. [Google Scholar] [CrossRef]
- Verediano, T.A.; Stampini Duarte Martino, H.; Dias Paes, M.C.; Tako, E. Effects of Anthocyanin on Intestinal Health: A Systematic Review. Nutrients 2021, 13, 1331. [Google Scholar] [CrossRef]
- Masella, R.; Santangelo, C.; D’Archivio, M.; Li Volti, G.; Giovannini, C.; Galvano, F. Protocatechuic acid and human disease prevention: Biological activities and molecular mechanisms. Curr. Med. Chem. 2012, 19, 2901–2917. [Google Scholar] [CrossRef]
- Forester, S.C.; Waterhouse, A.L. Gut metabolites of anthocyanins, gallic acid, 3-O-methylgallic acid, and 2,4,6-trihydroxybenzaldehyde, inhibit cell proliferation of Caco-2 cells. J. Agric. Food Chem. 2010, 58, 5320–5327. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Xiong, H.; Li, Q.; He, L.; Weng, H.; Ling, W.; Wang, D. Protocatechuic acid from chicory is bioavailable and undergoes partial glucuronidation and sulfation in healthy humans. Food Sci. Nutr. 2019, 7, 3071–3080. [Google Scholar] [CrossRef]
- Abdelmageed, M.E.; Shehatou, G.S.G.; Suddek, G.M.; Salem, H.A. Protocatechuic acid improves hepatic insulin resistance and restores vascular oxidative status in type-2 diabetic rats. Environ. Toxicol. Pharm. 2021, 83, 103577. [Google Scholar] [CrossRef] [PubMed]
- Ya, F.; Li, K.; Chen, H.; Tian, Z.; Fan, D.; Shi, Y.; Song, F.; Xu, X.; Ling, W.; Adili, R.; et al. Protocatechuic Acid Protects Platelets from Apoptosis via Inhibiting Oxidative Stress-Mediated PI3K/Akt/GSK3β Signaling. Thromb. Haemost. 2021, 121, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Thakare, V.N.; Lakade, S.H.; Mahajan, M.P.; Kulkarni, Y.P.; Dhakane, V.D.; Harde, M.T.; Patel, B.M. Protocatechuic acid attenuates chronic unpredictable mild stress induced-behavioral and biochemical alterations in mice. Eur. J. Pharm. 2021, 898, 173992. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Zhao, B.C.; Yang, X.; Lin, Z.B.; Sun, Q.S.; Wang, Y.F.; Yan, Z.Z.; Liu, W.F.; Li, C.; Hu, J.J.; et al. The gut microbiota metabolite capsiate promotes Gpx4 expression by activating TRPV1 to inhibit intestinal ischemia reperfusion-induced ferroptosis. Gut Microbes 2021, 13, 1902719. [Google Scholar] [CrossRef]
- Ho, L.; Zhao, D.; Ono, K.; Ruan, K.; Mogno, I.; Tsuji, M.; Carry, E.; Brathwaite, J.; Sims, S.; Frolinger, T.; et al. Heterogeneity in gut microbiota drive polyphenol metabolism that influences α-synuclein misfolding and toxicity. J. Nutr. Biochem. 2019, 64, 170–181. [Google Scholar] [CrossRef]
- Ansari, M.H.R.; Saher, S.; Parveen, R.; Khan, W.; Khan, I.A.; Ahmad, S. Role of Gut Microbiota Metabolism and Biotransformation on Dietary Natural Products to Human Health Implications with Special Reference to Biochemoinformatics Approach. J. Tradit. Complement. Med. 2022, 13, 150–160. [Google Scholar] [CrossRef]
- He, C.; Huang, L.; Lei, P.; Liu, X.; Li, B.; Shan, Y. Sulforaphane Normalizes Intestinal Flora and Enhances Gut Barrier in Mice with BBN-Induced Bladder Cancer. Mol. Nutr. Food Res. 2018, 62, e1800427. [Google Scholar] [CrossRef]
- Yu, X.; Li, H.; Zhu, M.; Hu, P.; Liu, X.; Qing, Y.; Wang, X.; Wang, H.; Wang, Z.; Xu, J.; et al. Involvement of p53 Acetylation in Growth Suppression of Cutaneous T-Cell Lymphomas Induced by HDAC Inhibition. J. Investig. Derm. 2020, 140, 2009–2022.e4. [Google Scholar] [CrossRef]
- Chi, M.; Ma, K.; Wang, J.; Ding, Z.; Li, Y.; Zhu, S.; Liang, X.; Zhang, Q.; Song, L.; Liu, C. The Immunomodulatory Effect of the Gut Microbiota in Kidney Disease. J. Immunol. Res. 2021, 2021, 5516035. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Battaglini, D.; Pimentel-Coelho, P.M.; Robba, C.; Dos Santos, C.C.; Cruz, F.F.; Pelosi, P.; Rocco, P.R.M. Gut Microbiota in Acute Ischemic Stroke: From Pathophysiology to Therapeutic Implications. Front. Neurol. 2020, 11, 598. [Google Scholar] [CrossRef] [PubMed]
- Long-Smith, C.; O’Riordan, K.J.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota-Gut-Brain Axis: New Therapeutic Opportunities. Annu. Rev. Pharm. Toxicol. 2020, 60, 477–502. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Gao, X.; Xia, G.; Chen, M.; Zeng, N.; Wang, S.; You, C.; Tian, X.; Di, H.; Tang, W.; et al. Rapid gut dysbiosis induced by stroke exacerbates brain infarction in turn. Gut 2021, 70, 1486–1494. [Google Scholar] [CrossRef]
- Feng, K.; Ge, Y.; Chen, Z.; Li, X.; Liu, Z.; Li, X.; Li, H.; Tang, T.; Yang, F.; Wang, X. Curcumin Inhibits the PERK-eIF2α-CHOP Pathway through Promoting SIRT1 Expression in Oxidative Stress-induced Rat Chondrocytes and Ameliorates Osteoarthritis Progression in a Rat Model. Oxidative Med. Cell. Longev. 2019, 2019, 8574386. [Google Scholar] [CrossRef]
- Cao, S.; Wang, C.; Yan, J.; Li, X.; Wen, J.; Hu, C. Curcumin ameliorates oxidative stress-induced intestinal barrier injury and mitochondrial damage by promoting Parkin dependent mitophagy through AMPK-TFEB signal pathway. Free Radic. Biol. Med. 2020, 147, 8–22. [Google Scholar] [CrossRef]
- Mukherjee, S.; Baidoo, J.N.E.; Fried, A.; Banerjee, P. Using curcumin to turn the innate immune system against cancer. Biochem. Pharm. 2020, 176, 113824. [Google Scholar] [CrossRef]
- Wu, R.; Wang, L.; Yin, R.; Hudlikar, R.; Li, S.; Kuo, H.D.; Peter, R.; Sargsyan, D.; Guo, Y.; Liu, X.; et al. Epigenetics/epigenomics and prevention by curcumin of early stages of inflammatory-driven colon cancer. Mol. Carcinog. 2020, 59, 227–236. [Google Scholar] [CrossRef]
- De, R.; Kundu, P.; Swarnakar, S.; Ramamurthy, T.; Chowdhury, A.; Nair, G.B.; Mukhopadhyay, A.K. Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob. Agents Chemother. 2009, 53, 1592–1597. [Google Scholar] [CrossRef]
- McFadden, R.M.; Larmonier, C.B.; Shehab, K.W.; Midura-Kiela, M.; Ramalingam, R.; Harrison, C.A.; Besselsen, D.G.; Chase, J.H.; Caporaso, J.G.; Jobin, C.; et al. The Role of Curcumin in Modulating Colonic Microbiota During Colitis and Colon Cancer Prevention. Inflamm. Bowel Dis. 2015, 21, 2483–2494. [Google Scholar] [CrossRef]
- Wu, R.; Mei, X.; Ye, Y.; Xue, T.; Wang, J.; Sun, W.; Lin, C.; Xue, R.; Zhang, J.; Xu, D. Zn(II)-curcumin solid dispersion impairs hepatocellular carcinoma growth and enhances chemotherapy by modulating gut microbiota-mediated zinc homeostasis. Pharm. Res. 2019, 150, 104454. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Kong, L.; Han, Y.; Zhang, S. Gut microbiota enhances the chemosensitivity of hepatocellular carcinoma to 5-fluorouracil in vivo by increasing curcumin bioavailability. Phytother. Res. 2021, 35, 5823–5837. [Google Scholar] [CrossRef]
- Sharma, M.; Arora, I.; Stoll, M.L.; Li, Y.; Morrow, C.D.; Barnes, S.; Berryhill, T.F.; Li, S.; Tollefsbol, T.O. Nutritional combinatorial impact on the gut microbiota and plasma short-chain fatty acids levels in the prevention of mammary cancer in Her2/neu estrogen receptor-negative transgenic mice. PLoS ONE 2020, 15, e0234893. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ye, T.; Chen, W.J.; Lv, Y.; Hao, Z.; Chen, J.; Zhao, J.Y.; Wang, H.P.; Cai, Y.K. Structural shift of gut microbiota during chemo-preventive effects of epigallocatechin gallate on colorectal carcinogenesis in mice. World J. Gastroenterol. 2017, 23, 8128–8139. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Kanaya, S.; Ishigami, T.; Hara, Y. The effects of tea catechins on fecal conditions of elderly residents in a long-term care facility. J. Nutr. Sci. Vitam. 1999, 45, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Zhang, X.; Miao, Y.; Cao, J.; Wu, Z.; Weng, P. The modulatory effect of (-)-epigallocatechin 3-O-(3-O-methyl) gallate (EGCG3″Me) on intestinal microbiota of high fat diet-induced obesity mice model. Food Res. Int. 2017, 92, 9–16. [Google Scholar] [CrossRef]
- Wang, Y.H.; Yao, N.; Wei, K.K.; Jiang, L.; Hanif, S.; Wang, Z.X.; Pei, C.X. The efficacy and safety of probiotics for prevention of chemoradiotherapy-induced diarrhea in people with abdominal and pelvic cancer: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2016, 70, 1246–1253. [Google Scholar] [CrossRef]
- Ciorba, M.A.; Riehl, T.E.; Rao, M.S.; Moon, C.; Ee, X.; Nava, G.M.; Walker, M.R.; Marinshaw, J.M.; Stappenbeck, T.S.; Stenson, W.F. Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut 2012, 61, 829–838. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, T.Y.; Kim, Y.; Lee, S.H.; Kim, S.; Kang, S.W.; Yang, J.Y.; Baek, I.J.; Sung, Y.H.; Park, Y.Y.; et al. Microbiota-Derived Lactate Accelerates Intestinal Stem-Cell-Mediated Epithelial Development. Cell Host Microbe 2018, 24, 833–846.e6. [Google Scholar] [CrossRef]
- Cai, S.; Xie, L.; Xu, J.; Zhou, H.; Yang, C.; Tang, L.; Tian, Y.; Li, M. (-)-Epigallocatechin-3-Gallate (EGCG) Modulates the Composition of the Gut Microbiota to Protect Against Radiation-Induced Intestinal Injury in Mice. Front. Oncol. 2022, 12, 848107. [Google Scholar] [CrossRef] [PubMed]
- Messaoudene, M.; Pidgeon, R.; Richard, C.; Ponce, M.; Diop, K.; Benlaifaoui, M.; Nolin-Lapalme, A.; Cauchois, F.; Malo, J.; Belkaid, W.; et al. A natural polyphenol exerts antitumor activity and circumvents anti-PD-1 resistance through effects on the gut microbiota. Cancer Discov. 2022, 12, 1070–1087. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Khan, I.; Huang, G.; Lu, Y.; Wang, L.; Liu, Y.; Lu, L.; Hsiao, W.L.W.; Liu, Z. Kaempferol acts on bile acid signaling and gut microbiota to attenuate the tumor burden in ApcMin/+ mice. Eur. J. Pharm. 2022, 918, 174773. [Google Scholar] [CrossRef] [PubMed]
- Rinott, E.; Youngster, I.; Yaskolka Meir, A.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Knights, D.; Tuohy, K.; Fava, F.; Scholz, M.U.; et al. Effects of Diet-Modulated Autologous Fecal Microbiota Transplantation on Weight Regain. Gastroenterology 2021, 160, 158–173.e10. [Google Scholar] [CrossRef] [PubMed]
- Joyce, S.A.; Kamil, A.; Fleige, L.; Gahan, C.G.M. The Cholesterol-Lowering Effect of Oats and Oat Beta Glucan: Modes of Action and Potential Role of Bile Acids and the Microbiome. Front. Nutr. 2019, 6, 171. [Google Scholar] [CrossRef]
- Zurbau, A.; Noronha, J.C.; Khan, T.A.; Sievenpiper, J.L.; Wolever, T.M.S. The effect of oat β-glucan on postprandial blood glucose and insulin responses: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2021, 75, 1540–1554. [Google Scholar] [CrossRef]
- Xu, D.; Feng, M.; Chu, Y.; Wang, S.; Shete, V.; Tuohy, K.M.; Liu, F.; Zhou, X.; Kamil, A.; Pan, D.; et al. The Prebiotic Effects of Oats on Blood Lipids, Gut Microbiota, and Short-Chain Fatty Acids in Mildly Hypercholesterolemic Subjects Compared With Rice: A Randomized, Controlled Trial. Front. Immunol. 2021, 12, 787797. [Google Scholar] [CrossRef]
- Hosseini, H.; Teimouri, M.; Shabani, M.; Koushki, M.; Babaei Khorzoughi, R.; Namvarjah, F.; Izadi, P.; Meshkani, R. Resveratrol alleviates non-alcoholic fatty liver disease through epigenetic modification of the Nrf2 signaling pathway. Int. J. Biochem. Cell Biol. 2020, 119, 105667. [Google Scholar] [CrossRef]
- Chung, J.Y.; Jeong, J.H.; Song, J. Resveratrol Modulates the Gut-Brain Axis: Focus on Glucagon-Like Peptide-1, 5-HT, and Gut Microbiota. Front. Aging Neurosci. 2020, 12, 588044. [Google Scholar] [CrossRef]
- Wang, P.; Gao, J.; Ke, W.; Wang, J.; Li, D.; Liu, R.; Jia, Y.; Wang, X.; Chen, X.; Chen, F.; et al. Resveratrol reduces obesity in high-fat diet-fed mice via modulating the composition and metabolic function of the gut microbiota. Free Radic. Biol. Med. 2020, 156, 83–98. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Alghetaa, H.; Mohammed, A.; Zhou, J.; Singh, N.; Nagarkatti, M.; Nagarkatti, P. Resveratrol-mediated attenuation of superantigen-driven acute respiratory distress syndrome is mediated by microbiota in the lungs and gut. Pharm. Res. 2021, 167, 105548. [Google Scholar] [CrossRef]
- Li, S.; You, J.; Wang, Z.; Liu, Y.; Wang, B.; Du, M.; Zou, T. Curcumin alleviates high-fat diet-induced hepatic steatosis and obesity in association with modulation of gut microbiota in mice. Food Res. Int. 2021, 143, 110270. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yao, Y.; Gao, P.; Bu, S. The Therapeutic Efficacy of Curcumin vs. Metformin in Modulating the Gut Microbiota in NAFLD Rats: A Comparative Study. Front. Microbiol. 2021, 11, 555293. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, H.; Guo, D.; Man, X.; Liu, J.; Li, J.; Luo, C.; Zhang, M.; Zhen, L.; Liu, X. Curcumin modulates gut microbiota and improves renal function in rats with uric acid nephropathy. Ren. Fail. 2021, 43, 1063–1075. [Google Scholar] [CrossRef]
- Mair, R.D.; Sirich, T.L.; Plummer, N.S.; Meyer, T.W. Characteristics of Colon-Derived Uremic Solutes. Clin. J. Am. Soc. Nephrol. 2018, 13, 1398–1404. [Google Scholar] [CrossRef]
- Zhang, C.; He, X.; Sheng, Y.; Yang, C.; Xu, J.; Zheng, S.; Liu, J.; Xu, W.; Luo, Y.; Huang, K. Allicin-induced host-gut microbe interactions improves energy homeostasis. FASEB J. 2020, 34, 10682–10698. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, X.; Xia, M.; Li, J.; Guo, A.Y.; Zhu, Y.; Yang, X. Quercetin Ameliorates Gut Microbiota Dysbiosis That Drives Hypothalamic Damage and Hepatic Lipogenesis in Monosodium Glutamate-Induced Abdominal Obesity. Front. Nutr. 2021, 8, 671353. [Google Scholar] [CrossRef]
- Tan, S.; Caparros-Martin, J.A.; Matthews, V.B.; Koch, H.; O’Gara, F.; Croft, K.D.; Ward, N.C. Isoquercetin and inulin synergistically modulate the gut microbiome to prevent development of the metabolic syndrome in mice fed a high fat diet. Sci. Rep. 2018, 8, 10100–10108. [Google Scholar] [CrossRef]
- Rodríguez-Daza, M.C.; Daoust, L.; Boutkrabt, L.; Pilon, G.; Varin, T.; Dudonné, S.; Levy, É.; Marette, A.; Roy, D.; Desjardins, Y. Wild blueberry proanthocyanidins shape distinct gut microbiota profile and influence glucose homeostasis and intestinal phenotypes in high-fat high-sucrose fed mice. Sci. Rep. 2020, 10, 2217. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, S.; Wang, J.; Shi, J.; Sun, Y.; Wang, W.; Ning, G.; Hong, J.; Liu, R. Grape seed proanthocyanidin extract ameliorates inflammation and adiposity by modulating gut microbiota in high-fat diet mice. Mol. Nutr. Food Res. 2017, 61, 1601082. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Liu, X.; Wang, Y.; Dong, J.; Wu, F.; Mackenzie, G.G.; Su, Z. (-)-Epigallocatechin-3-gallate mitigates cyclophosphamide-induced intestinal injury by modulating the tight junctions, inflammation and dysbiosis in mice. Food Funct. 2021, 12, 11671–11685. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Huang, S.; Li, T.; Li, N.; Han, D.; Zhang, B.; Xu, Z.Z.; Zhang, S.; Pang, J.; Wang, S.; et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome 2021, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, K.; Jing, N.; Kong, Q.; Yang, X. Epigallocatechin Gallate (EGCG) Promotes the Immune Function of Ileum in High Fat Diet Fed Mice by Regulating Gut Microbiome Profiling and Immunoglobulin Production. Front. Nutr. 2021, 8, 720439. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, G.; Tang, M.; Fang, J.; Jiang, H. Epigallocatechin Gallate Can Protect Mice From Acute Stress Induced by LPS While Stabilizing Gut Microbes and Serum Metabolites Levels. Front. Immunol. 2021, 12, 640305. [Google Scholar] [CrossRef]
- Alrafas, H.R.; Busbee, P.B.; Nagarkatti, M.; Nagarkatti, P.S. Resveratrol modulates the gut microbiota to prevent murine colitis development through induction of Tregs and suppression of Th17 cells. J. Leukoc. Biol. 2019, 106, 467–480. [Google Scholar] [CrossRef]
- Li, F.; Han, Y.; Cai, X.; Gu, M.; Sun, J.; Qi, C.; Goulette, T.; Song, M.; Li, Z.; Xiao, H. Dietary resveratrol attenuated colitis and modulated gut microbiota in dextran sulfate sodium-treated mice. Food Funct. 2020, 11, 1063–1073. [Google Scholar] [CrossRef]
- Alharris, E.; Mohammed, A.; Alghetaa, H.; Zhou, J.; Nagarkatti, M.; Nagarkatti, P. The Ability of Resveratrol to Attenuate Ovalbumin-Mediated Allergic Asthma Is Associated With Changes in Microbiota Involving the Gut-Lung Axis, Enhanced Barrier Function and Decreased Inflammation in the Lungs. Front. Immunol. 2022, 13, 805770. [Google Scholar] [CrossRef]
- Larrosa, M.; González-Sarrías, A.; Yáñez-Gascón, M.J.; Selma, M.V.; Azorín-Ortuño, M.; Toti, S.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J. Nutr. Biochem. 2010, 21, 717–725. [Google Scholar] [CrossRef]
- Hu, R.; He, Z.; Liu, M.; Tan, J.; Zhang, H.; Hou, D.X.; He, J.; Wu, S. Dietary protocatechuic acid ameliorates inflammation and up-regulates intestinal tight junction proteins by modulating gut microbiota in LPS-challenged piglets. J. Anim. Sci. Biotechnol. 2020, 11, 92–99. [Google Scholar] [CrossRef]
- Hu, R.; Wu, S.; Li, B.; Tan, J.; Yan, J.; Wang, Y.; Tang, Z.; Liu, M.; Fu, C.; Zhang, H.; et al. Dietary ferulic acid and vanillic acid on inflammation, gut barrier function and growth performance in lipopolysaccharide-challenged piglets. Anim. Nutr. 2022, 8, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Nishida, A.; Sugitani, Y.; Nishino, K.; Inatomi, O.; Sugimoto, M.; Kawahara, M.; Andoh, A. Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells. PLoS ONE 2017, 12, e0185999. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. Berry Phenolic Antioxidants-Implications for Human Health? Front. Pharm. 2018, 9, 78. [Google Scholar] [CrossRef]
- Istas, G.; Wood, E.; Le Sayec, M.; Rawlings, C.; Yoon, J.; Dandavate, V.; Cera, D.; Rampelli, S.; Costabile, A.; Fromentin, E.; et al. Effects of aronia berry (poly)phenols on vascular function and gut microbiota: A double-blind randomized controlled trial in adult men. Am. J. Clin. Nutr. 2019, 110, 316–329. [Google Scholar] [CrossRef]
- Bartolomaeus, H.; Balogh, A.; Yakoub, M.; Homann, S.; Markó, L.; Höges, S.; Tsvetkov, D.; Krannich, A.; Wundersitz, S.; Avery, E.G.; et al. Short-Chain Fatty Acid Propionate Protects From Hypertensive Cardiovascular Damage. Circulation 2019, 139, 1407–1421. [Google Scholar] [CrossRef]
- Haghikia, A.; Zimmermann, F.; Schumann, P.; Jasina, A.; Roessler, J.; Schmidt, D.; Heinze, P.; Kaisler, J.; Nageswaran, V.; Aigner, A.; et al. Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism. Eur. Heart J. 2022, 43, 518–533. [Google Scholar] [CrossRef]
- Chen, M.L.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.D.; Zhang, Q.Y.; Mi, M.T. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. mBio 2016, 7, 2210. [Google Scholar] [CrossRef]
- Lin, K.; Wang, X.; Li, J.; Zhao, P.; Xi, X.; Feng, Y.; Yin, L.; Tian, J.; Li, H.; Liu, X.; et al. Anti-atherosclerotic effects of geraniin through the gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway in mice. Phytomedicine 2022, 101, 154104. [Google Scholar] [CrossRef]
- Xu, M.; Lv, C.; Wang, H.; Lu, Q.; Ye, M.; Zhu, X.; Liu, R. Peanut skin extract ameliorates high-fat diet-induced atherosclerosis by regulating lipid metabolism, inflammation reaction and gut microbiota in ApoE(−/−) mice. Food Res. Int. 2022, 154, 111014. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef]
- Garcia-Gutierrez, E.; Narbad, A.; Rodríguez, J.M. Autism Spectrum Disorder Associated With Gut Microbiota at Immune, Metabolomic, and Neuroactive Level. Front. Neurosci. 2020, 14, 578666. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Cruz, N.J.; Kang, D.W.; Gandal, M.J.; Wang, B.; Kim, Y.M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J.; et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell 2019, 177, 1600–1618.e17. [Google Scholar] [CrossRef] [PubMed]
- Tengeler, A.C.; Dam, S.A.; Wiesmann, M.; Naaijen, J.; van Bodegom, M.; Belzer, C.; Dederen, P.J.; Verweij, V.; Franke, B.; Kozicz, T.; et al. Gut microbiota from persons with attention-deficit/hyperactivity disorder affects the brain in mice. Microbiome 2020, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Kalenik, A.; Kardaś, K.; Rahnama, A.; Sirojć, K.; Wolańczyk, T. Gut microbiota and probiotic therapy in ADHD: A review of current knowledge. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 110, 110277. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, G.; Feng, T.; Zhang, J.; Huang, X.; Wang, T.; Xie, Z.; Chu, X.; Yang, J.; Wang, H.; et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019, 29, 787–803. [Google Scholar] [CrossRef]
- Samochowiec, J.; Misiak, B. Gut microbiota and microbiome in schizophrenia. Curr. Opin. Psychiatry 2021, 34, 503–507. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, Y.; Chen, H.; Jiang, H.; Zhou, F.; Lv, B.; Xu, M. Curcumin Alleviates DSS-Induced Anxiety-Like Behaviors via the Microbial-Brain-Gut Axis. Oxidative Med. Cell. Longev. 2022, 2022, 6244757. [Google Scholar] [CrossRef]
- Xu, M.; Huang, H.; Mo, X.; Zhu, Y.; Chen, X.; Li, X.; Peng, X.; Xu, Z.; Chen, L.; Rong, S.; et al. Quercetin-3-O-Glucuronide Alleviates Cognitive Deficit and Toxicity in Aβ(1-42) -Induced AD-Like Mice and SH-SY5Y Cells. Mol. Nutr. Food Res. 2021, 65, e2000660. [Google Scholar] [CrossRef]
- Wang, Z.; Roberts, A.B.; Buffa, J.A.; Levison, B.S.; Zhu, W.; Org, E.; Gu, X.; Huang, Y.; Zamanian-Daryoush, M.; Culley, M.K.; et al. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell 2015, 163, 1585–1595. [Google Scholar] [CrossRef]
- Oliveira, T.G.; Chan, R.B.; Bravo, F.V.; Miranda, A.; Silva, R.R.; Zhou, B.; Marques, F.; Pinto, V.; Cerqueira, J.J.; Di Paolo, G.; et al. The impact of chronic stress on the rat brain lipidome. Mol. Psychiatry 2016, 21, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.L.; Chen, Y.Q.; Ma, B.H.; Yu, S.F.; Li, L.Y.; Zeng, Q.X.; Zhou, Y.T.; Wu, Y.F.; Liu, W.L.; Wan, J.B.; et al. Tetrahydrocurcumin, a major metabolite of curcumin, ameliorates allergic airway inflammation by attenuating Th2 response and suppressing the IL-4Rα-Jak1-STAT6 and Jagged1/Jagged2 -Notch1/Notch2 pathways in asthmatic mice. Clin. Exp. Allergy 2018, 48, 1494–1508. [Google Scholar] [CrossRef]
- Yin, R.; Kuo, H.; Hudlikar, R.; Sargsyan, D.; Li, S.; Wang, L.; Wu, R.; Kong, A. Gut microbiota, dietary phytochemicals and benefits to human health. Curr. Pharm. Rep. 2019, 5, 332–344. [Google Scholar] [CrossRef]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The Interactions between Polyphenols and Microorganisms, Especially Gut Microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Quintanar, L.; López Roa, R.I.; Quintero-Fabián, S.; Sánchez-Sánchez, M.A.; Vizmanos, B.; Ortuño-Sahagún, D. Phytochemicals That Influence Gut Microbiota as Prophylactics and for the Treatment of Obesity and Inflammatory Diseases. Mediat. Inflamm. 2018, 2018, 9734845. [Google Scholar] [CrossRef]
- Mukonowenzou, N.C.; Adeshina, K.A.; Donaldson, J.; Ibrahim, K.G.; Usman, D.; Erlwanger, K.H. Medicinal Plants, Phytochemicals, and Their Impacts on the Maturation of the Gastrointestinal Tract. Front. Physiol. 2021, 12, 684464. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Quiles, J.L.; Daglia, M.; Xiao, J.; Xu, B. Dietary phytochemicals modulate intestinal epithelial barrier dysfunction and autoimmune diseases. Food Front. 2021, 2, 357–382. [Google Scholar] [CrossRef]
- Dey, P. Gut microbiota in phytopharmacology: A comprehensive overview of concepts, reciprocal interactions, biotransformations and mode of actions. Pharm. Res. 2019, 147, 104367. [Google Scholar] [CrossRef]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).