Influence of Strict Lockdown on Vitamin D Deficiency in Pregnant Women: A Word of Caution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.2.1. Evaluation of Vitamin D Levels
2.2.2. Covariate Assessment
2.2.3. Definition of Strict Lockdown (SL) Group
2.2.4. Assessment of Vitamin D-Specific UVB Dose in Study Geographical Area
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Study Participants
3.2. Distribution of Measured VD-UV Dose in Our Geographical Area
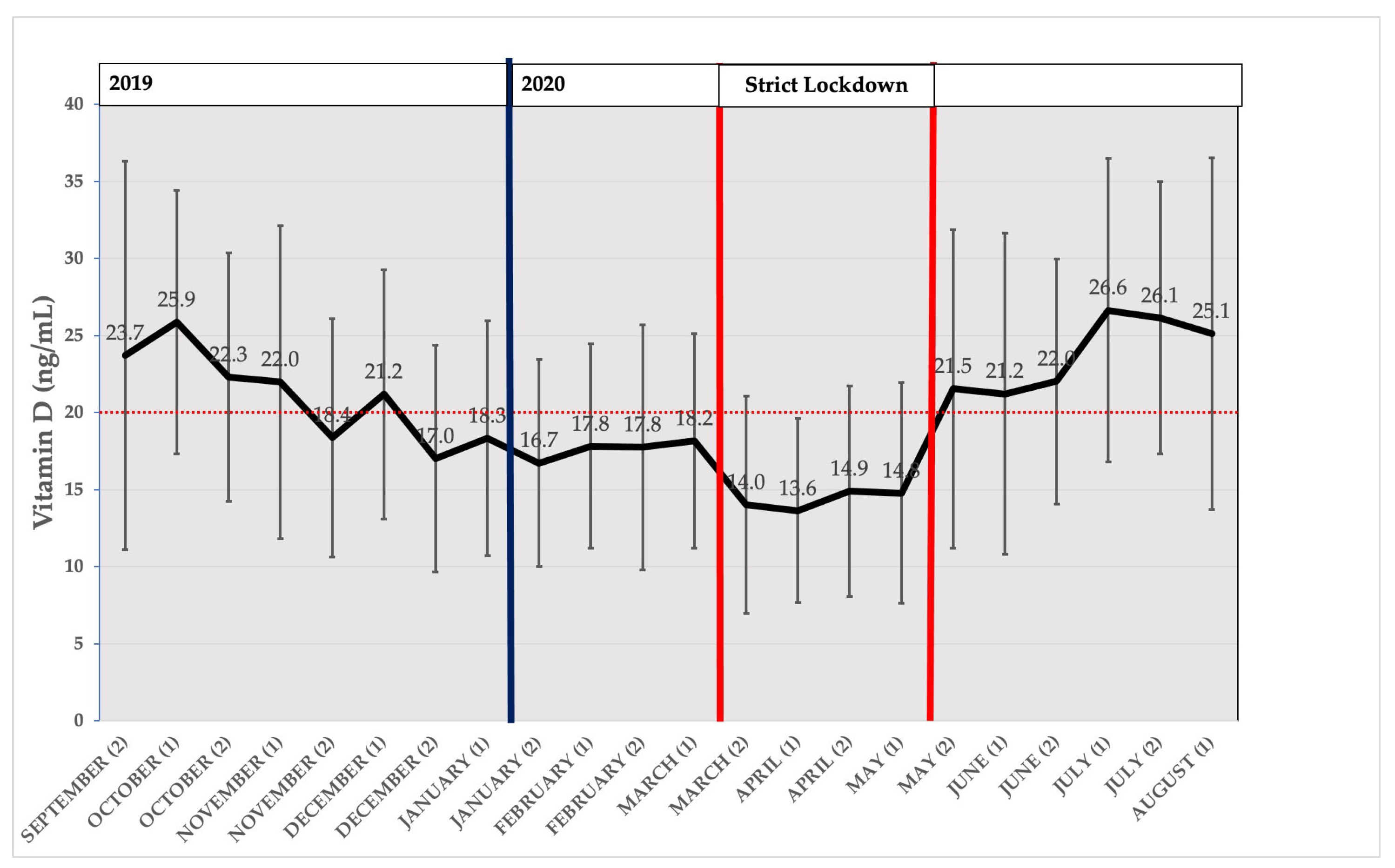
3.3. Seasonal Distribution of Average Measured Vitamin D
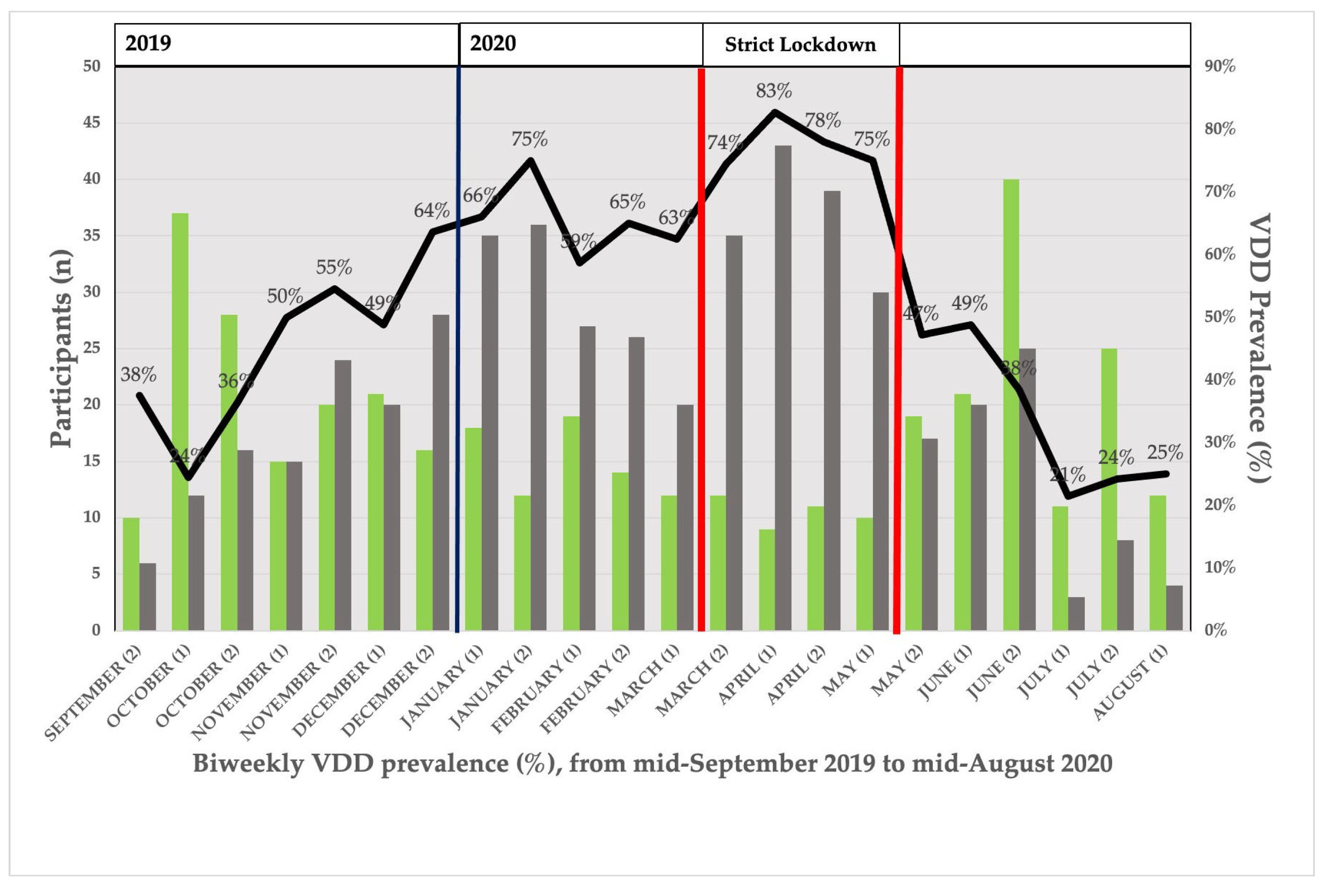
3.4. Seasonal Distribution of VDD Prevalence
3.5. Regression Analysis of 25(OH)D Levels and D-UVB Dose in Relation to SL Status
3.6. Cross-Sectional Study: Assessment of the Association of VDD and SL
3.7. Binary Logistic Model of VDD in Relation to SL Adjusted by Measured D-UVB Dose
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689S–1696S. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Hewison, M. Update in Vitamin D. J. Clin. Endocrinol. Metab. 2010, 95, 471–478. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, H.F. Vitamin D: Historical Overview. Vitam. Horm. 2016, 100, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.M.; Hart, K.H.; Williams, E.L.; Mendis, J.; Lanham-New, S.A.; Botelho, P.B. Vitamin D Supplementation and Sunlight Exposure on Serum Vitamin D Concentrations in 2 Parallel, Double-Blind, Randomized, Placebo-Controlled Trials. J. Nutr. 2021, 151, 3137–3150. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, M.; Kerr, P.E.; Slade, K.; Grant-Kels, J.E. Vitamin D and the skin. Clin. Dermatol. 2010, 28, 663–668. [Google Scholar] [CrossRef]
- Engelsen, O. The Relationship between Ultraviolet Radiation Exposure and Vitamin D Status. Nutrients 2010, 2, 482–495. [Google Scholar] [CrossRef]
- Khanna, T.; Shraim, R.; Zarkovic, M.; van Weele, M.; van Geffen, J.; Zgaga, L. Comprehensive Analysis of Seasonal and Geographical Variation in UVB Radiation Relevant for Vitamin D Production in Europe. Nutrients 2022, 14, 5189. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Nair, R.; Maseeh, A. Vitamin D: The “sunshine” vitamin. J. Pharmacol. Pharmacother. 2012, 3, 118–126. [Google Scholar] [CrossRef]
- Mata-Granados, J.; de Castro, M.D.L.; Gomez, J.Q. Inappropriate serum levels of retinol, α-tocopherol, 25 hydroxyvitamin D3 and 24,25 dihydroxyvitamin D3 levels in healthy Spanish adults: Simultaneous assessment by HPLC. Clin. Biochem. 2008, 41, 676–680. [Google Scholar] [CrossRef]
- Saraf, R.; Morton, S.M.B.; Camargo, C.A., Jr.; Grant, C.C. Global summary of maternal and newborn vitamin D status—A systematic review. Matern. Child Nutr. 2016, 12, 647–668. [Google Scholar] [CrossRef]
- Palacios, C.; Kostiuk, L.K.; Peña-Rosas, J.P. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019, 7, CD008873. [Google Scholar] [CrossRef]
- Zhang, Y.; Gong, Y.; Xue, H.; Xiong, J.; Cheng, G. Vitamin D and gestational diabetes mellitus: A systematic review based on data free of Hawthorne effect. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 784–793. [Google Scholar] [CrossRef]
- Urrutia, R.P.; Thorp, J.M. Vitamin D in pregnancy. Curr. Opin. Obstet. Gynecol. 2012, 24, 57–64. [Google Scholar] [CrossRef]
- Rostami, M.; Tehrani, F.R.; Simbar, M.; Bidhendi Yarandi, R.; Minooee, S.; Hollis, B.W.; Hosseinpanah, F. Effectiveness of Prenatal Vitamin D Deficiency Screening and Treatment Program: A Stratified Randomized Field Trial. J. Clin. Endocrinol. Metab. 2018, 103, 2936–2948. [Google Scholar] [CrossRef]
- Holick, M.F. A Call to Action: Pregnant Women In-Deed Require Vitamin D Supplementation for Better Health Outcomes. J. Clin. Endocrinol. Metab. 2019, 104, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.; Brach, M.; Trabelsi, K.; Chtourou, H.; Boukhris, O.; Masmoudi, L.; Bouaziz, B.; Bentlage, E.; How, D.; Ahmed, M.; et al. Effects of COVID-19 Home Confinement on Eating Behaviour and Physical Activity: Results of the ECLB-COVID19 International Online Survey. Nutrients 2020, 12, 1583. [Google Scholar] [CrossRef] [PubMed]
- Agüero-Domenech, N.; Jover, S.; Sarrión, A.; Baranda, J.; Quesada-Rico, J.A.; Pereira-Expósito, A.; Gil-Guillén, V.; Cortés-Castell, E.; García-Teruel, M.J. Vitamin D Deficiency and Gestational Diabetes Mellitus in Relation to Body Mass Index. Nutrients 2021, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Emmen, J.M.; Wielders, J.P.; Boer, A.-K.; Ouweland, J.M.V.D.; Vader, H.L. The new Roche Vitamin D Total assay: Fit for its purpose? Clin. Chem. Lab. Med. 2012, 50, 1969–1972. [Google Scholar] [CrossRef] [PubMed]
- Leino, A.; Turpeinen, U.; Koskinen, P. Automated Measurement of 25-OH Vitamin D3 on the Roche Modular E170 Analyzer. Clin. Chem. 2008, 54, 2059–2062. [Google Scholar] [CrossRef]
- Knudsen, C.S.; Nexo, E.; Højskov, C.S.; Heickendorff, L. Analytical validation of the Roche 25-OH Vitamin D Total assay. Clin. Chem. Lab. Med. 2012, 50, 1965–1968. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, E.M.I.; Ybern, M.L.G.; Botias, M.D.; De Osaba, J.B.M.; Sáez, I.H.; Ferrer, M.C.P. Comparación de las concentraciones de vitamina D por 3 métodos comerciales. Rev. Lab. Clín. 2013, 6, 2–9. [Google Scholar] [CrossRef]
- VDSCP List of Certified Participants|CDC. Available online: https://www.cdc.gov/labstandards/vdscp_participants.html (accessed on 22 November 2021).
- Gómez de Tejada Romero, M.; Sosa Henríquez, M.; del Pino Montes, J.; Jodar Gimeno, E.; Quesada Gómez, J.; Cancelo Hidalgo, M.; Díaz Curiel, M.; Mesa Ramos, M.; Muñoz Torres, M.; Carpintero Benítez, P.; et al. Documento de Posición Sobre Las Necesidades y Niveles Óptimos de Vitamina D Sociedad Española de Investigación Ósea y Del Metabolismo Mineral (SEIOMM) y Sociedades Afines. Rev. Osteoporos. Metab. Miner. 2011, 3, 53–64. [Google Scholar]
- Duran, A.; Martin, P.; Runkle, I.; Pérez, N.; Abad, R.; Fernández, M.; Del Valle, L.; Sanz, M.F.; Calle-Pascual, A.L. Benefits of self-monitoring blood glucose in the management of new-onset Type 2 diabetes mellitus: The St Carlos Study, a prospective randomized clinic-based interventional study with parallel groups. J. Diabetes 2010, 2, 203–211. [Google Scholar] [CrossRef] [PubMed]
- The Diabetes and Nutrition Study Group of the Spanish Diabetes Association Diabetes Nutrition and Complications Trial. Diabetes Care 2004, 27, 984–987. [CrossRef]
- Zempila, M.-M.; van Geffen, J.H.G.M.; Taylor, M.; Fountoulakis, I.; Koukouli, M.-E.; van Weele, M.; van der A, R.J.; Bais, A.; Meleti, C.; Balis, D. TEMIS UV product validation using NILU-UV ground-based measurements in Thessaloniki, Greece. Atmos. Meas. Tech. 2017, 17, 7157–7174. [Google Scholar] [CrossRef]
- Kelly, D.; Theodoratou, E.; Farrington, S.; Fraser, R.; Campbell, H.; Dunlop, M.; Zgaga, L. The contributions of adjusted ambient ultraviolet B radiation at place of residence and other determinants to serum 25-hydroxyvitamin D concentrations. Br. J. Dermatol. 2015, 174, 1068–1078. [Google Scholar] [CrossRef]
- O’sullivan, F.; Laird, E.; Kelly, D.; van Geffen, J.; van Weele, M.; McNulty, H.; Hoey, L.; Healy, M.; McCarroll, K.; Cunningham, C.; et al. Ambient UVB Dose and Sun Enjoyment Are Important Predictors of Vitamin D Status in an Older Population. J. Nutr. 2017, 147, 858–868. [Google Scholar] [CrossRef]
- O’neill, C.M.; Kazantzidis, A.; Ryan, M.J.; Barber, N.; Sempos, C.T.; Durazo-Arvizu, R.A.; Jorde, R.; Grimnes, G.; Eiriksdottir, G.; Gudnason, V.; et al. Seasonal Changes in Vitamin D-Effective UVB Availability in Europe and Associations with Population Serum 25-Hydroxyvitamin D. Nutrients 2016, 8, 533. [Google Scholar] [CrossRef]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef]
- Quesada-Gómez, J.M.; Diaz-Curiel, M.; Sosa-Henriquez, M.; Malouf-Sierra, J.; Nogues-Solan, X.; Gomez-Alonso, C.; Rodriguez-Mañas, L.; Neyro-Bilbao, J.L.; Cortes, X.; Delgadillo, J. Low calcium intake and inadequate vitamin D status in postmenopausal osteoporotic women. J. Steroid Biochem. Mol. Biol. 2013, 136, 175–177. [Google Scholar] [CrossRef]
- Werneke, U.; Gaughran, F.; Taylor, D.M. Vitamin D in the time of the coronavirus (COVID-19) pandemic—A clinical review from a public health and public mental health perspective. Ther. Adv. Psychopharmacol. 2021, 11, 1–27. [Google Scholar] [CrossRef]
- Webb, A.R.; Kline, L.; Holick, M.F. Influence of Season and Latitude on the Cutaneous Synthesis of Vitamin D3: Exposure to Winter Sunlight in Boston and Edmonton Will Not Promote Vitamin D3 Synthesis in Human Skin. J. Clin. Endocrinol. Metab. 1988, 67, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.S.; Rybchyn, M.S.; Abboud, M.; Brennan-Speranza, T.C.; Fraser, D.R. The Role of Skeletal Muscle in Maintaining Vitamin D Status in Winter. Curr. Dev. Nutr. 2019, 3, nzz087. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Smith, B.; Chung, E.A.; Watts, K.L.; Gonzalez, M.C.; Yang, S.; Heo, M.; Thomas, D.M.; Turner, D.; Bosy-Westphal, A.; et al. Phenotypic differences between people varying in muscularity. J. Cachexia Sarcopenia Muscle 2022, 13, 1100–1112. [Google Scholar] [CrossRef]
- Lippi, G.; Ferrari, A.; Targher, G. Is COVID-19 lockdown associated with vitamin D deficiency? Eur. J. Public Health 2021, 31, 278–279. [Google Scholar] [CrossRef]
- Yu, L.; Ke, H.-J.; Che, D.; Luo, S.-L.; Guo, Y.; Wu, J.-L. Effect of Pandemic-Related Confinement on Vitamin D Status Among Children Aged 0–6 Years in Guangzhou, China: A Cross-Sectional Study. Risk Manag. Healthc. Policy 2020, 13, 2669–2675. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.M.; Hart, K.H.; Lanham-New, S.A.; Botelho, P.B. Exploring the Impact of Individual UVB Radiation Levels on Serum 25-Hydroxyvitamin D in Women Living in High Versus Low Latitudes: A Cross-Sectional Analysis from the D-SOL Study. Nutrients 2020, 12, 3805. [Google Scholar] [CrossRef]
- Agudelo-Zapata, Y.; Maldonado-Acosta, L.M.; Sandoval-Alzate, H.F.; Poveda, N.E.; Garcés, M.F.; Cortés-Vásquez, J.A.; Linares-Vaca, A.F.; Mancera-Rodríguez, C.A.; Perea-Ariza, S.A.; Ramírez-Iriarte, K.Y.; et al. Serum 25-hydroxyvitamin D levels throughout pregnancy: A longitudinal study in healthy and preeclamptic pregnant women. Endocr. Connect. 2018, 7, 698–707. [Google Scholar] [CrossRef]

| Characteristics | Non-Strict Lockdown (n = 715, 80.7%) | Strict Lockdown (n = 171, 19.3%) | p-Value |
|---|---|---|---|
| Sociodemographic characteristics | |||
| Age (years), mean (SD) | 32.0 (5.8) | 31.7 (5.6) | 0.48 |
| Questionnaire (St. Carlos Study), mean (SD) | |||
| Physical activity | −0.7 (1.4) | −0.8 (1.4) | 0.26 |
| Nutritional status | 4.1 (3.6) | 3.2 (3.9) | 0.02 * |
| Lifestyle | 3.3 (4.1) | 2.3 (4.4) | <0.01 * |
| Maternal smoking habit, n (%) | 79 (11.0) | 12 (7.0) | 0.12 |
| Maternal hypothyroidism, n (%) | 158 (22.1) | 37 (21.6) | 0.90 |
| Ethnicity, n (%) | 0.12 | ||
| Caucasian | 626 (87.7) | 140 (82.8) | |
| South American | 44 (6.2) | 18 (10.7) | |
| Other | 44 (6.2) | 11 (6.5) | |
| Pregnancy-related characteristics | |||
| SAP, first trimester (mmHg), mean (SD) | 110.6 (11.7) | 111.7 (11.6) | 0.28 |
| DAP, first trimester (mmHg), mean (SD) | 68.8 (9.1) | 68.7 (7.8) | 0.78 |
| VDD, n (%) | 356 (50.1) | 133 (77.8) | <0.01 * |
| GDM, n (%) | 73 (10.2) | 20 (11.7) | 0.57 |
| BMI, mean (SD) | 24.7 (4.7) | 25.2 (4.8) | 0.25 |
| BMI group, n (%) | 0.12 | ||
| Normal (<25) | 441 (62.0) | 91 (53.5) | |
| Overweight (25–30) | 180 (25.3) | 54 (31.8) | |
| Obesity (>30) | 90 (12.7) | 25 (14.7) | |
| Parity, n (%) | 0.84 | ||
| Primigravida | 360 (50.3) | 86 (50.3) | |
| 2 pregnancies | 270 (37.8) | 62 (36.3) | |
| ≥3 pregnancies | 85 (11.9) | 23 (13.5) | |
| History of caesarean section, n (%) | 77 (10.8) | 21 (12.4) | 0.55 |
| Gestational hypothyroidism, n (%) | 158 (22.0) | 37 (21.6) | 0.90 |
| Blood test, mean (SD) | |||
| Vitamin D (ng/mL) | 20.5 (8.9) | 14.2 (6.7) | <0.01 * |
| C-reactive protein (mg/dL) | 5.8 (6.1) | 5.3 (4.9) | 0.22 |
| Ferritin (mg/dL) | 24.6 (25.3) | 24.5 (26.4) | 0.96 |
| Cholesterol (mg/dL) | 223.4 (37.4) | 228.4 (43.4) | 0.18 |
| HDL cholesterol (mg/dL) | 76.0 (15.1) | 75.0 (15.9) | 0.44 |
| LDL cholesterol (mg/dL) | 113.8 (32.0) | 115.5 (34.6) | 0.58 |
| Triglycerides (mg/dL) | 173.4 (63.7) | 193.5 (82.5) | <0.01 * |
| Fibrinogen (mg/dL) | 399.5 (56.2) | 391.1 (56.1) | 0.09 |
| Haemoglobin (g/dL) | 11.6 (0.9) | 11.6 (0.8) | 0.64 |
| Haematocrit (%) | 34.1 (2.5) | 34.4 (2.7) | 0.23 |
| Crude Model | Adjusted Model | ||||||
|---|---|---|---|---|---|---|---|
| POR | 95%CI | p-Value | POR | 95%CI | p-Value | ||
| Strict Lockdown: | No | 1 | 1 | ||||
| Yes | 3.5 | (2.4–5.1) | <0.01 * | 4.0 | (2.7–5.7) | <0.01 * | |
| D-UVB dose: | 0.9 | (0.9–0.9) | <0.01 * | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agüero-Domenech, N.; Bernabeu, E.; García-Valentín, A.; Sarrión, A.; Jover, S.; Baranda, J.; Cortés-Castell, E.; Gil-Guillén, V.; García-Teruel, M.J. Influence of Strict Lockdown on Vitamin D Deficiency in Pregnant Women: A Word of Caution. Nutrients 2023, 15, 1972. https://doi.org/10.3390/nu15081972
Agüero-Domenech N, Bernabeu E, García-Valentín A, Sarrión A, Jover S, Baranda J, Cortés-Castell E, Gil-Guillén V, García-Teruel MJ. Influence of Strict Lockdown on Vitamin D Deficiency in Pregnant Women: A Word of Caution. Nutrients. 2023; 15(8):1972. https://doi.org/10.3390/nu15081972
Chicago/Turabian StyleAgüero-Domenech, Nuria, Eduardo Bernabeu, Antonio García-Valentín, Ana Sarrión, Silvia Jover, Javier Baranda, Ernesto Cortés-Castell, Vicente Gil-Guillén, and María J. García-Teruel. 2023. "Influence of Strict Lockdown on Vitamin D Deficiency in Pregnant Women: A Word of Caution" Nutrients 15, no. 8: 1972. https://doi.org/10.3390/nu15081972





