Vitamin D Status as an Important Predictor of Preterm Birth in a Cohort of Black Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Variables and Instruments
2.3. Statistical Analysis
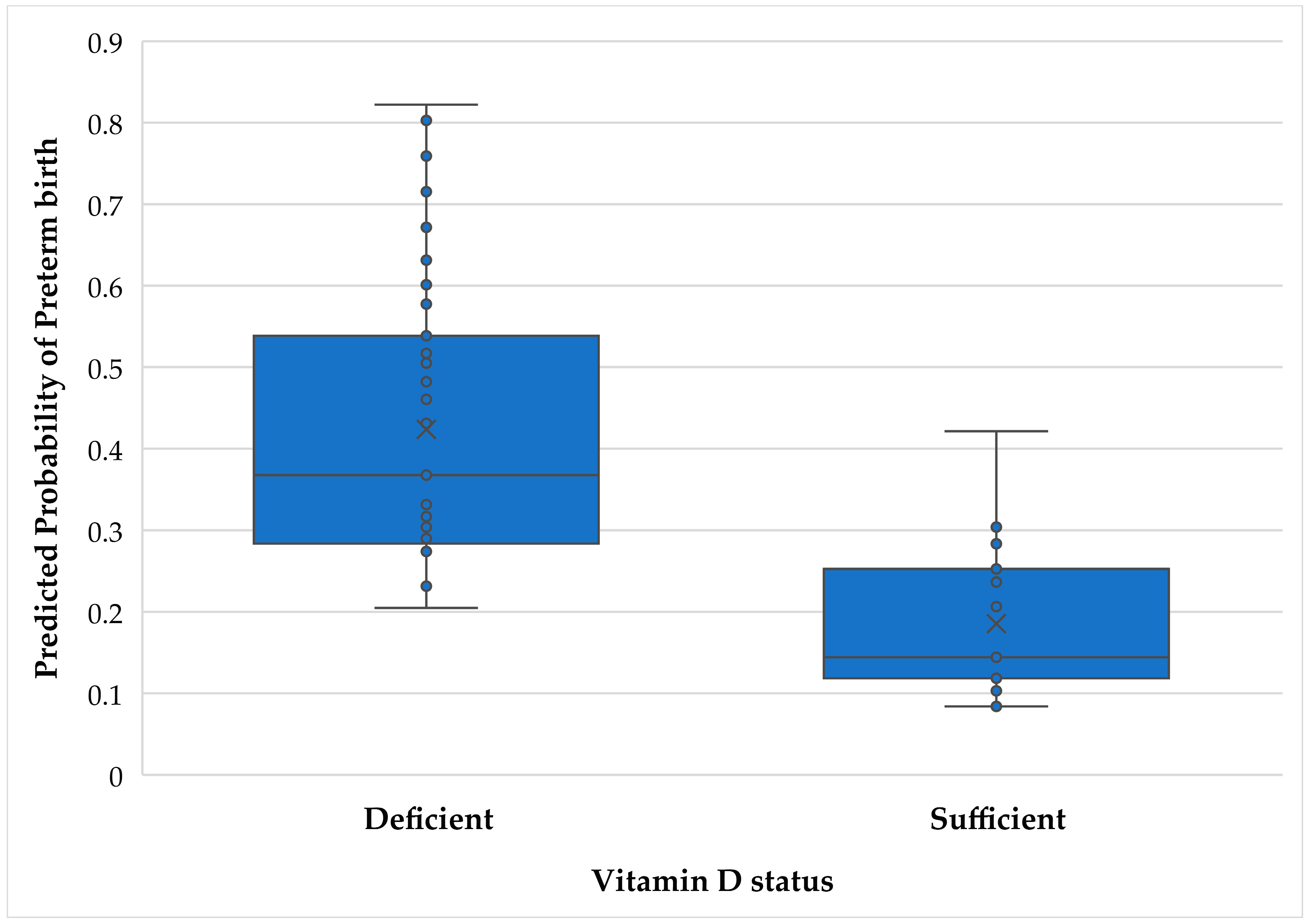
3. Results
3.1. Descriptive Characteristics of the Sample
3.2. Binary Logistic Regressions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamilton, B.E.; Martin, J.A.; Osterman, M.J.; Rossen, L.M. Births: Provisional Data for 2018. 2019. Available online: https://www.cdc.gov/nchs/data/vsrr/vsrr-007-508.pdf (accessed on 31 January 2023).
- Osterman, M.J.K.; Hamilton, B.E.; Martin, J.A.; Driscoll, A.K.; Valenzuela, C.P. Births: Final Data for 2021. Available online: https://www.cdc.gov/nchs/data/vsrr/vsrr020.pdf (accessed on 31 January 2023).
- Johnson, J.D.; Green, C.A.; Vladutiu, C.J.; Manuck, T.A. Racial Disparities in Prematurity Persist among Women of High Socioeconomic Status. Am. J. Obs. Gynecol. MFM 2020, 2, 100104. [Google Scholar] [CrossRef] [PubMed]
- McLemore, M.R.; Berkowitz, R.L.; Oltman, S.P.; Baer, R.J.; Franck, L.; Fuchs, J.; Karasek, D.A.; Kuppermann, M.; McKenzie-Sampson, S.; Melbourne, D.; et al. Risk and Protective Factors for Preterm Birth Among Black Women in Oakland, California. J. Racial Ethn. Heal. Disparities 2020, 8, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Amegah, A.K.; Klevor, M.K.; Wagner, C.L. Maternal vitamin D insufficiency and risk of adverse pregnancy and birth outcomes: A systematic review and meta-analysis of longitudinal studies. PLoS ONE 2017, 12, e0173605. [Google Scholar] [CrossRef]
- Wagner, C.L.; Baggerly, C.; McDonnell, S.; Baggerly, K.A.; French, C.B.; Baggerly, L.; Hamilton, S.A.; Hollis, B.W. Post-hoc analysis of vitamin D status and reduced risk of preterm birth in two vitamin D pregnancy cohorts compared with South Carolina March of Dimes 2009-2011 rates. J. Steroid Biochem. Mol. Biol. 2016, 155, 245–251. [Google Scholar] [CrossRef]
- Woo, J.; Giurgescu, C.; Wagner, C.L. Evidence of an Association Between Vitamin D Deficiency and Preterm Birth and Preeclampsia: A Critical Review. J. Midwifery Women’s Health 2019, 64, 613–629. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Baylin, A.; Levy, P.D. Vitamin D deficiency and insufficiency among US adults: Prevalence, predictors and clinical implications. Br. J. Nutr. 2018, 119, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2013; pp. 720–755. [Google Scholar]
- Hollis, B.W.; Wagner, C.L. New insights into the vitamin D requirements during pregnancy. Bone Res. 2017, 5, 17030. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Bouillon, R.; Carmeliet, G. Vitamin D insufficiency: Definition, diagnosis and management. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 669–684. [Google Scholar] [CrossRef]
- Cassidy-Bushrow, A.E.; Peters, R.M.; Johnson, D.A.; Li, J.; Rao, D.S. Vitamin D nutritional status and antenatal depressive symptoms in African American women. J. Women’s Health 2012, 21, 1189–1195. [Google Scholar] [CrossRef]
- Accortt, E.E.; Schetter, C.D.; Peters, R.M.; Cassidy-Bushrow, A.E. Lower prenatal vitamin D status and postpartum depressive symptomatology in African American women: Preliminary evidence for moderation by inflammatory cytokines. Arch. Women’s Ment. Health 2016, 19, 373–383. [Google Scholar] [CrossRef]
- Bobbitt, K.R.; Peters, R.M.; Li, J.; Rao, S.D.; Woodcroft, K.J.; Cassidy-Bushrow, A.E. Early pregnancy vitamin D and patterns of antenatal inflammation in African–American women. J. Reprod. Immunol. 2015, 107, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Fallah, M.; Askari, G.; Asemi, Z. Is vitamin D status associated with depression, anxiety and sleep quality in pregnancy: A systematic review. Adv. Biomed. Res. 2020, 9, 32. [Google Scholar]
- Accortt, E.E.; Lamb, A.; Mirocha, J.; Hobel, C.J. Vitamin D deficiency and depressive symptoms in pregnancy are associated with adverse perinatal outcomes. J. Behav. Med. 2018, 41, 680–689. [Google Scholar] [CrossRef]
- Lamb, A.R.; Lutenbacher, M.; Wallston, K.A.; Pepkowitz, S.H.; Holmquist, B.; Hobel, C.J. Vitamin D deficiency and depressive symptoms in the perinatal period. Arch. Women’s Ment. Health 2018, 21, 745–755. [Google Scholar] [CrossRef]
- Williams, J.A.; Romero, V.C.; Clinton, C.M.; Vazquez, D.M.; Marcus, S.M.; Chilimigras, J.L.; Hamilton, S.E.; Allbaugh, L.J.; Vahratian, A.M.; Schrader, R.M. Vitamin D levels and perinatal depressive symptoms in women at risk: A secondary analysis of the mothers, omega-3, and mental health study. BMC Pregnancy Childbirth 2016, 16, 203. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.K.; Mueller, M.; Hulsey, T.C.; Ebeling, M.D.; Wagner, C.L. An exploratory study of postpartum depression and vitamin D. J. Am. Psychiatr. Nurses Assoc. 2010, 16, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Giurgescu, C.; Misra, D.P. Psychosocial Factors and Preterm Birth Among Black Mothers and Fathers. MCN Am. J. Matern. Child Nurs. 2018, 43, 245–251. [Google Scholar] [CrossRef]
- Giurgescu, C.; Slaughter-Acey, J.C.; Templin, T.N.; Misra, D.P. The Impact of Symptoms of Depression and Walking on Gestational Age at Birth in African American Women. Womens Health Issues 2017, 27, 181–187. [Google Scholar] [CrossRef]
- Nutor, J.J.; Slaughter-Acey, J.C.; Giurgescu, C.; Misra, D. Symptoms of Depression and Preterm Birth Among Black Women. MCN. Am. J. Matern. Child Nurs. 2018, 43, 252. [Google Scholar] [CrossRef]
- Wagner, C.L.; Hollis, B.W. The implications of vitamin D status during pregnancy on mother and her developing child. Front. Endocrinol. 2018, 9, 500. [Google Scholar] [CrossRef] [PubMed]
- Tamblyn, J.; Hewison, M.; Wagner, C.; Bulmer, J.; Kilby, M. Immunological role of vitamin D at the maternal–fetal interface. J. Endocrinol. 2015, 224, R107–R121. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.M.; Kutmon, M.; Eijssen, L.; Hewison, M.; Evelo, C.T.; Coort, S.L. Pathway analysis of transcriptomic data shows immunometabolic effects of vitamin D. J. Mol. Endocrinol. 2018, 60, 95–108. [Google Scholar] [CrossRef]
- ACOG. Prediction and Prevention of Spontaneous Preterm Birth: ACOG Practice Bulletin Summary, Number 234. Obstet. Gynecol. 2021, 138, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.P.; Chawanpaiboon, S.; Moller, A.-B.; Watananirun, K.; Bonet, M.; Lumbiganon, P. The global epidemiology of preterm birth. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 52, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xu, G.; Sun, Y.; Du, Y.; Gao, R.; Snetselaar, L.G.; Santillan, M.K.; Bao, W. Association between maternal pre-pregnancy obesity and preterm birth according to maternal age and race or ethnicity: A population-based study. Lancet Diabetes Endocrinol. 2019, 7, 707–714. [Google Scholar] [CrossRef]
- Ju, A.C.; Heyman, M.B.; Garber, A.K.; Wojcicki, J.M. Maternal obesity and risk of preterm birth and low birthweight in Hawaii PRAMS, 2000–2011. Matern. Child Health J. 2018, 22, 893–902. [Google Scholar] [CrossRef]
- Moore, E.; Blatt, K.; Chen, A.; Van Hook, J.; DeFranco, E.A. Relationship of trimester-specific smoking patterns and risk of preterm birth. Am. J. Obstet. Gynecol. 2016, 215, 109.e101–109.e106. [Google Scholar] [CrossRef]
- Soneji, S.; Beltrán-Sánchez, H. Association of maternal cigarette smoking and smoking cessation with preterm birth. JAMA Netw. Open 2019, 2, e192514. [Google Scholar] [CrossRef]
- Berger, H.; Melamed, N.; Davis, B.M.; Hasan, H.; Mawjee, K.; Barrett, J.; McDonald, S.D.; Geary, M.; Ray, J.G. Impact of diabetes, obesity and hypertension on preterm birth: Population-based study. PLoS ONE 2020, 15, e0228743. [Google Scholar] [CrossRef]
- Premkumar, A.; Henry, D.E.; Moghadassi, M.; Nakagawa, S.; Norton, M.E. The interaction between maternal race/ethnicity and chronic hypertension on preterm birth. Am. J. Obstet. Gynecol. 2016, 215, 787.e781–787.e788. [Google Scholar] [CrossRef] [PubMed]
- Saadat, N.; Zhang, L.; Hyer, S.; Padmanabhan, V.; Woo, J.; Engeland, C.G.; Misra, D.P.; Giurgescu, C. Psychosocial and behavioral factors affecting inflammation among pregnant African American women. Brain Behav. Immun. Health 2022, 22, 100452. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.L.; Anderson, C.M.; Zhao, Y.; Ford, J.L.; Mackos, A.R.; Ohm, J.; Tan, A.; Saadat, N.; Misra, D.P.; Giurgescu, C. Epigenetic Implications of Neighborhood Disorder and Psychological Distress among Pregnant Black Women. West. J. Nurs. Res. 2023, 45, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine Committee on Nutritional Status During Pregnancy and Lactation. Nutrition During Pregnancy: Part I Weight Gain: Part II Nutrient Supplements; National Academies Press: Washington, DC, USA, 1990. [Google Scholar]
- Radloff, L.S. The CES-D scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Orr, S.T.; Blazer, D.G.; James, S.A.; Reiter, J.P. Depressive symptoms and indicators of maternal health status during pregnancy. J. Women’s Health 2007, 16, 535–542. [Google Scholar] [CrossRef]
- Hossein-nezhad, A.; Holick, M.F. Optimize dietary intake of vitamin D: An epigenetic perspective. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 567–579. [Google Scholar] [CrossRef]
- Hollis, B.W.; Wagner, C.L. Substantial Vitamin D Supplementation Is Required during the Prenatal Period to Improve Birth Outcomes. Nutrients 2022, 14, 899. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Simhan, H.N. Vitamin D may be a link to black-white disparities in adverse birth outcomes. Obstet. Gynecol. Surv. 2010, 65, 273. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Platt, R.W.; Simhan, H.N. Early-pregnancy vitamin D deficiency and risk of preterm birth subtypes. Obstet. Gynecol. 2015, 125, 439. [Google Scholar] [CrossRef]
- Lian, R.H.; Qi, P.A.; Yuan, T.; Yan, P.J.; Qiu, W.W.; Wei, Y.; Hu, Y.G.; Yang, K.H.; Yi, B. Systematic review and meta-analysis of vitamin D deficiency in different pregnancy on preterm birth: Deficiency in middle pregnancy might be at risk. Medicine 2021, 100, e26303. [Google Scholar] [CrossRef]
- Qin, L.L.; Lu, F.G.; Yang, S.H.; Xu, H.L.; Luo, B.A. Does Maternal Vitamin D Deficiency Increase the Risk of Preterm Birth: A Meta-Analysis of Observational Studies. Nutrients 2016, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, L.M.; Klebanoff, M.A.; Gernand, A.D.; Platt, R.W.; Parks, W.T.; Catov, J.M.; Simhan, H.N. Maternal vitamin D status and spontaneous preterm birth by placental histology in the US Collaborative Perinatal Project. Am. J. Epidemiol. 2014, 179, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Mirzakhani, H.; Litonjua, A.A.; McElrath, T.F.; O’Connor, G.; Lee-Parritz, A.; Iverson, R.; Macones, G.; Strunk, R.C.; Bacharier, L.B.; Zeiger, R.; et al. Early pregnancy vitamin D status and risk of preeclampsia. J. Clin. Investig. 2016, 126, 4702–4715. [Google Scholar] [CrossRef] [PubMed]
- Karras, S.N.; Wagner, C.L.; Castracane, V.D. Understanding vitamin D metabolism in pregnancy: From physiology to pathophysiology and clinical outcomes. Metabolism 2018, 86, 112–123. [Google Scholar] [CrossRef]
- Hollis, B.W.; Wagner, C.L. Vitamin D supplementation during pregnancy: Improvements in birth outcomes and complications through direct genomic alteration. Mol. Cell Endocrinol. 2017, 453, 113–130. [Google Scholar] [CrossRef]
- Giurgescu, C.; Engeland, C.G.; Templin, T.N. Symptoms of depression predict negative birth outcomes in African American women: A pilot study. J. Midwifery Women’s Health 2015, 60, 570–577. [Google Scholar] [CrossRef]
- Christian, L.M. At the forefront of psychoneuroimmunology in pregnancy: Implications for racial disparities in birth outcomes PART 1: Behavioral risks factors. Neurosci. Biobehav. Rev. 2020, 117, 319–326. [Google Scholar] [CrossRef]
- Roth, D.E.; Leung, M.; Mesfin, E.; Qamar, H.; Watterworth, J.; Papp, E. Vitamin D supplementation during pregnancy: State of the evidence from a systematic review of randomised trials. BMJ 2017, 359, j5237. [Google Scholar]
- Leow, S.M.; Di Quinzio, M.K.W.; Ng, Z.L.; Grant, C.; Amitay, T.; Wei, Y.; Hod, M.; Sheehan, P.M.; Brennecke, S.P.; Arbel, N.; et al. Preterm birth prediction in asymptomatic women at mid-gestation using a panel of novel protein biomarkers: The Prediction of PreTerm Labor (PPeTaL) study. Am. J. Obstet. Gynecol. MFM 2020, 2, 100084. [Google Scholar] [CrossRef]
- Heaney, R.P. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr. Rev. 2014, 72, 48–54. [Google Scholar] [CrossRef]
- McDonnell, S.L.; Baggerly, C.A.; Aliano, J.L.; French, C.B.; Wagner, C.L.; Sullivan, S.A.; Newman, R.B. 713: Prenatal correction of vitamin D deficiency is associated with substantial reduction in preterm birth. Am. J. Obstet. Gynecol. 2018, 218, S428–S429. [Google Scholar] [CrossRef]
- Sablok, A.; Batra, A.; Thariani, K.; Batra, A.; Bharti, R.; Aggarwal, A.R.; Kabi, B.; Chellani, H. Supplementation of vitamin D in pregnancy and its correlation with feto-maternal outcome. Clin. Endocrinol. 2015, 83, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Dawodu, A.; Saadi, H.F.; Bekdache, G.; Javed, Y.; Altaye, M.; Hollis, B.W. Randomized controlled trial (RCT) of vitamin D supplementation in pregnancy in a population with endemic vitamin D deficiency. J. Clin. Endocrinol. Metab. 2013, 98, 2337–2346. [Google Scholar] [CrossRef] [PubMed]
- Kiely, M.; Wagner, C.; Roth, D. Vitamin D in pregnancy: Where we are and where we should go. J. Steroid Biochem. Mol. Biol. 2020, 105669. [Google Scholar] [CrossRef] [PubMed]
- Newton, D.A.; Baatz, J.E.; Kindy, M.S.; Gattoni-Celli, S.; Shary, J.R.; Hollis, B.W.; Wagner, C.L. Vitamin D binding protein polymorphisms significantly impact vitamin D status in children. Pediatr. Res. 2019, 86, 662–669. [Google Scholar] [CrossRef]
- Karras, S.N.; Koufakis, T.; Fakhoury, H.; Kotsa, K. Deconvoluting the biological roles of Vitamin D-Binding protein during pregnancy: A both clinical and theoretical challenge. Front. Endocrinol. 2018, 9. [Google Scholar] [CrossRef]
- Wang, S.; Xin, X.; Luo, W.; Mo, M.; Si, S.; Shao, B.; Shen, Y.; Cheng, H.; Yu, Y. Association of vitamin D and gene variants in the vitamin D metabolic pathway with preterm birth. Nutrition 2021, 89. [Google Scholar] [CrossRef]
- Ganz, A.B.; Park, H.; Malysheva, O.V.; Caudill, M.A. Vitamin D binding protein rs7041 genotype alters vitamin D metabolism in pregnant women. FASEB J. 2018, 32, 2012. [Google Scholar] [CrossRef]
- Keith, J.N.; Nicholls, J.; Reed, A.; Kafer, K. The prevalence of self-reported lactose intolerance and the consumption of dairy foods among African American adults are less than expected. J. Natl. Med. Assoc. 2011, 103, 36–45. [Google Scholar] [CrossRef]
| Characteristics | Preterm Birth (n = 57) | Term Birth (n = 118) | p Value | Vitamin D Deficiency (n = 74) | Vitamin D Sufficiency (n = 101) | p Value |
|---|---|---|---|---|---|---|
| Mean ± SD | ||||||
| Maternal Age (years) | 27.77 ± 5.91 | 27.14 ± 5.83 | 0.501 | 27.66 ± 5.96 | 26.91 ± 5.70 | 0.398 |
| Gestational Age at Blood Draw (weeks) | 14.21 ± 4.88 | 13.93 ± 4.07 | 0.692 | 13.48 ± 4.07 | 14.77 ± 4.61 | 0.051 |
| Frequency (%) | ||||||
| Annual Household Income | 0.230 | 0.879 | ||||
| ≤US 10,000 | 27 (47.4%) | 49 (41.5%) | 46 (45.5%) | 30 (40.5%) | ||
| USD 10,000–19,999 | 10 (17.5%) | 17 (14.4%) | 14 (13.9%) | 13 (17.6%) | ||
| USD 20,000–29,999 | 14 (24.6%) | 24 (20.3%) | 22 (21.8%) | 16 (21.6%) | ||
| ≥USD 30,000 | 6 (10.5%) | 28(23.7%) | 19 (18.8%) | 15 (20.3%) | ||
| Level of Education | 0.294 | 0.685 | ||||
| Less than high school | 7 (12.3%) | 15 (12.7%) | 12 (11.9%) | 10 (13.5%) | ||
| High school or GED | 27 (47.4%) | 53 (44.9%) | 49 (48.5%) | 31 (41.9%) | ||
| >High school education | 23 (40.3%) | 50 (42.4%) | 40 (39.6%) | 33 (44.6%) | ||
| Marital status | 0.615 | 0.712 | ||||
| Married/live with partner | 24 (42.1%) | 45 (38.1%) | 41 (40.6%) | 28 (37.9%) | ||
| Work Status | 0.355 | 0.355 | ||||
| Currently working | 31 (54.4%) | 55 (46.6%) | 48 (47.5%) | 38 (51.4%) | ||
| Insurance | 0.676 | 0.474 | ||||
| Medicaid | 38 (66.7%) | 71 (60.2%) | 63 (62.4%) | 46 (62.2%) | ||
| Medicare | 6 (10.5%) | 11 (9.3%) | 12 (11.9%) | 5 (6.8%) | ||
| Medicaid + Medicare | 4 (7.0%) | 14 (11.9%) | 8 (7.9%) | 10 (13.5%) | ||
| Private or other | 9 (15.8%) | 22 (18.6%) | 18 (17.8%) | 13 (17.6%) | ||
| CES-D scores ≥ 23 | 13 (22.8%) | 31 (26.3%) | 0.621 | 28 (27.7%) | 16 (21.6%) | 0.358 |
| HDP diagnosis | 28 (49.1%) | 32 (27.1%) | 0.004 * | 35 (34.7%) | 25 (33.8%) | 0.905 |
| Obese (BMI ≥ 30 kg/m2) | 30 (52.6%) | 60 (50.8%) | 0.825 | 57 (56.4%) | 33 (44.6%) | 0.122 |
| Preterm Birth | ||||||
|---|---|---|---|---|---|---|
| Variables | Vitamin D Unadjusted Odds Ratio | Unweighted Adjusted Odds Ratio 1 | Weighted Adjusted Odds Ratio 1 | |||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Vitamin D deficiency (25(OH)D ≤ 20 ng/mL) | 2.80 ** | 1.40–5.59 | 3.00 ** | 1.47–6.15 | 2.74 ** | 1.35–5.54 |
| Model χ2 | 7.83 * | 9.09 * | 7.83 * | |||
| −2 log-likelihood | 211.75 | 202.66 | 237.00 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, J.; Guffey, T.; Dailey, R.; Misra, D.; Giurgescu, C. Vitamin D Status as an Important Predictor of Preterm Birth in a Cohort of Black Women. Nutrients 2023, 15, 4637. https://doi.org/10.3390/nu15214637
Woo J, Guffey T, Dailey R, Misra D, Giurgescu C. Vitamin D Status as an Important Predictor of Preterm Birth in a Cohort of Black Women. Nutrients. 2023; 15(21):4637. https://doi.org/10.3390/nu15214637
Chicago/Turabian StyleWoo, Jennifer, Thomas Guffey, Rhonda Dailey, Dawn Misra, and Carmen Giurgescu. 2023. "Vitamin D Status as an Important Predictor of Preterm Birth in a Cohort of Black Women" Nutrients 15, no. 21: 4637. https://doi.org/10.3390/nu15214637
APA StyleWoo, J., Guffey, T., Dailey, R., Misra, D., & Giurgescu, C. (2023). Vitamin D Status as an Important Predictor of Preterm Birth in a Cohort of Black Women. Nutrients, 15(21), 4637. https://doi.org/10.3390/nu15214637





