Quality of Life in Teduglutide-Treated Patients with Short Bowel Syndrome Intestinal Failure—A Nested Matched Pair Real-World Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Matching
2.4. Ethics and Informed Consent
2.5. Parenteral Support
2.6. Stool Characteristics
2.7. Sleep Disturbances
2.8. Bioelectrical Impedance Analysis
2.9. Blood Parameters
2.10. Quality of Life Assessment
2.11. Statistical Analysis
3. Results
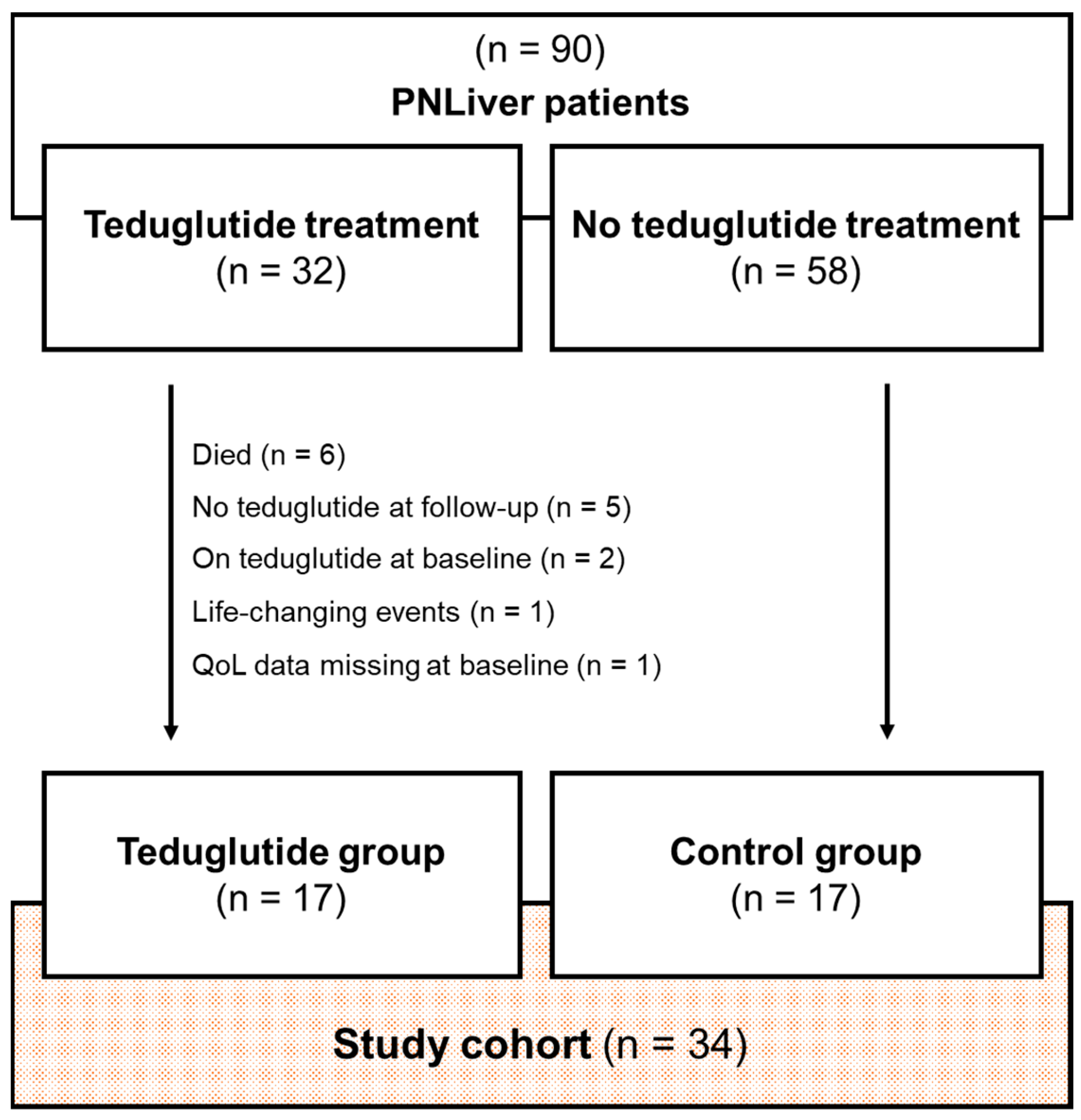
3.1. Study Cohort
3.2. Matching Characteristics
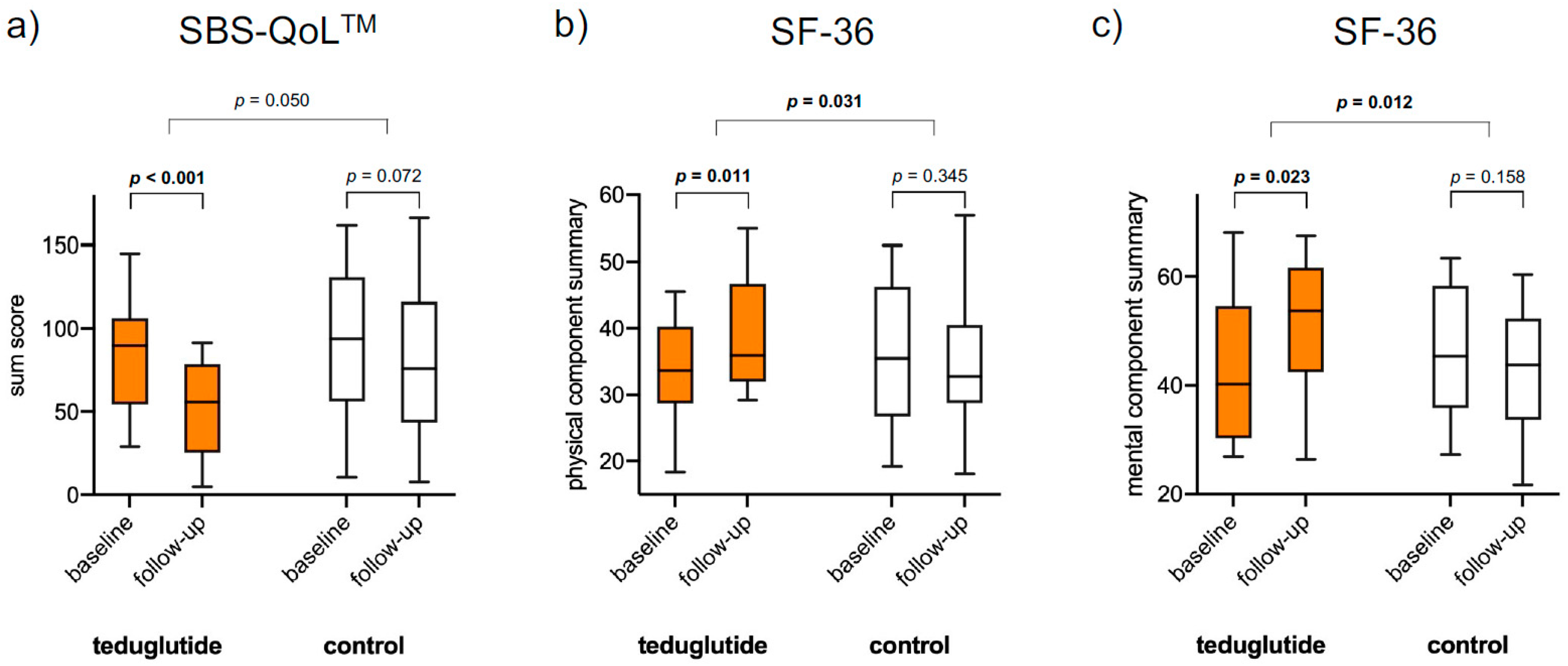
3.3. Quality of Life
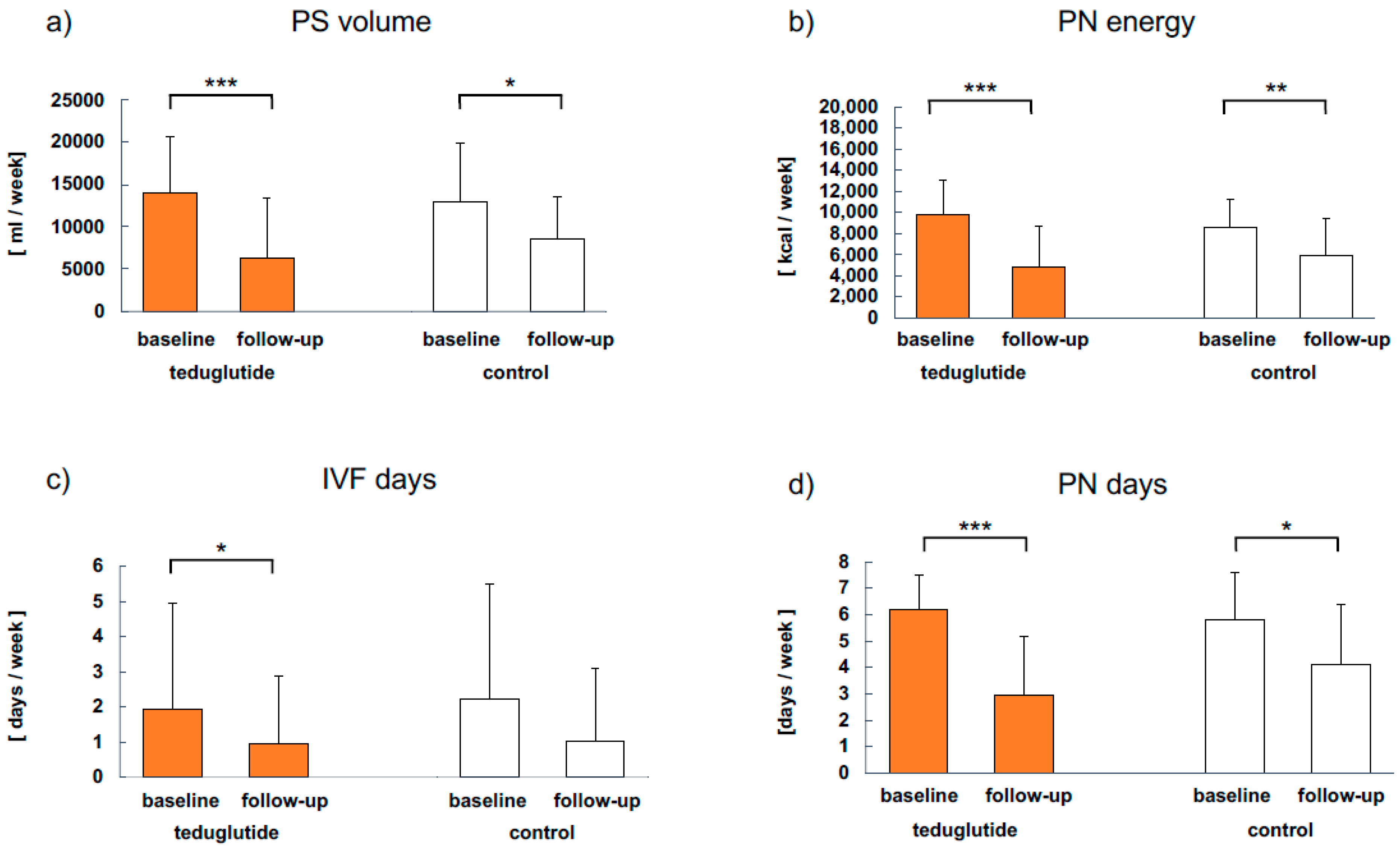
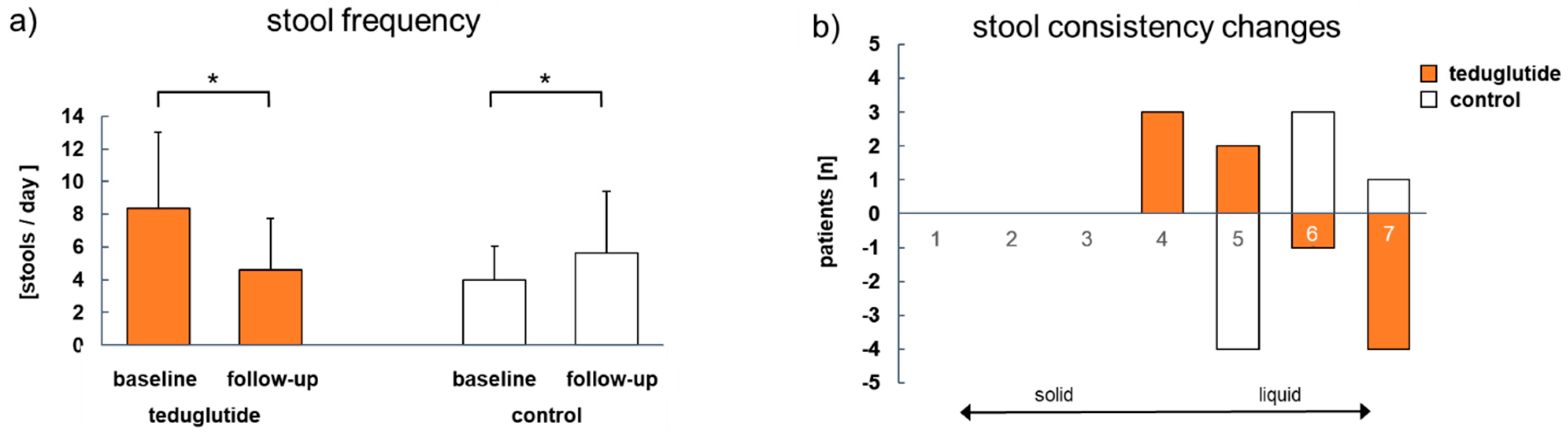
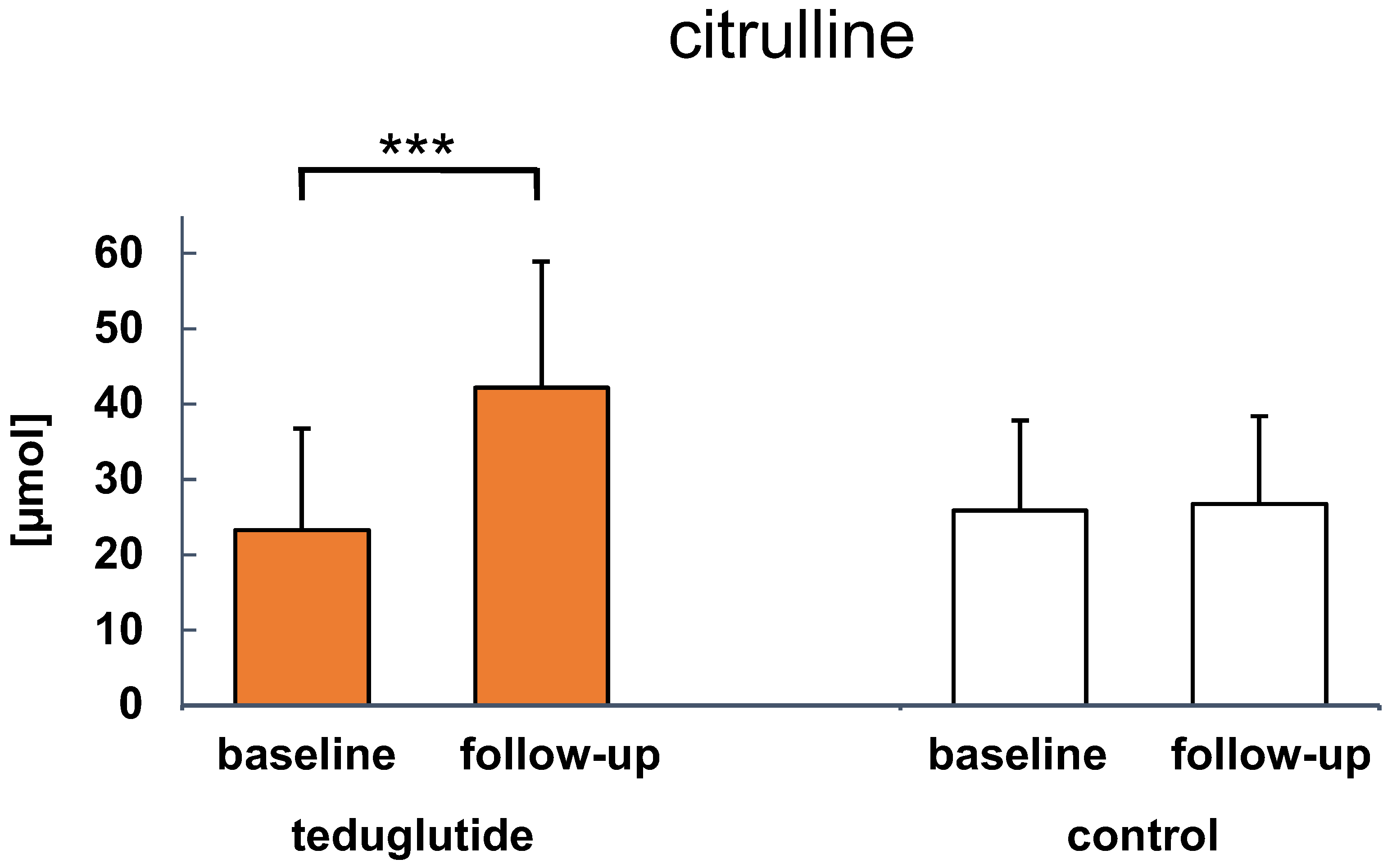
3.4. Clinical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Pironi, L. Translation of Evidence into Practice with Teduglutide in the Management of Adults with Intestinal Failure Due to Short-Bowel Syndrome: A Review of Recent Literature. J. Parenter. Enter. Nutr. 2019, 44, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Huisman-de Waal, G.; Schoonhoven, L.; Jansen, J.; Wanten, G.; van Achterberg, T. The impact of home parenteral nutrition on daily life-a review. Clin. Nutr. 2007, 26, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.F.; Smith, C.E. Clinical, social, and economic impacts of home parenteral nutrition dependence in short bowel syndrome. JPEN J. Parenter. Enteral. Nutr. 2014, 38 (Suppl. S1), 32s–37s. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, P.B.; Langholz, E.; Mortensen, P.B. Quality of life in patients receiving home parenteral nutrition. Gut 1999, 44, 844–852. [Google Scholar] [CrossRef]
- Blüthner, E.; Bednarsch, J.; Stockmann, M.; Karber, M.; Pevny, S.; Maasberg, S.; Gerlach, U.A.; Pascher, A.; Wiedenmann, B.; Pratschke, J.; et al. Determinants of Quality of Life in Patients with Intestinal Failure Receiving Long-Term Parenteral Nutrition Using the SF-36 Questionnaire: A German Single-Center Prospective Observational Study. J. Parenter. Enter. Nutr. 2019, 44, 291–300. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Pertkiewicz, M.; Forbes, A.; Pironi, L.; Gabe, S.M.; Joly, F.; Messing, B.; Loth, S.; Youssef, N.N.; Heinze, H.; et al. Quality of life in patients with short bowel syndrome treated with the new glucagon-like peptide-2 analogue teduglutide--analyses from a randomised, placebo-controlled study. Clin. Nutr. 2013, 32, 713–721. [Google Scholar] [CrossRef]
- Chen, K.; Mu, F.; Xie, J.; Kelkar, S.S.; Olivier, C.; Signorovitch, J.; Jeppesen, P.B. Impact of Teduglutide on Quality of Life among Patients with Short Bowel Syndrome and Intestinal Failure. J. Parenter. Enter. Nutr. 2020, 44, 119–128. [Google Scholar] [CrossRef]
- Blüthner, E.; Bednarsch, J.; Pape, U.-F.; Karber, M.; Maasberg, S.; Gerlach, U.A.; Pascher, A.; Wiedenmann, B.; Pratschke, J.; Stockmann, M. Advanced liver function assessment in patients with intestinal failure on long-term parenteral nutrition. Clin. Nutr. 2020, 39, 540–547. [Google Scholar] [CrossRef]
- Baxter, J.P.; Fayers, P.M.; Bozzetti, F.; Kelly, D.; Joly, F.; Wanten, G.; Jonkers, C.; Cuerda, C.; van Gossum, A.; Klek, S.; et al. An international study of the quality of life of adult patients treated with home parenteral nutrition. Clin. Nutr. 2019, 38, 1788–1796. [Google Scholar] [CrossRef]
- Nordsten, C.B.; Molsted, S.; Bangsgaard, L.; Fuglsang, K.A.; Brandt, C.F.; Niemann, M.J.; Jeppesen, P.B. High Parenteral Support Volume Is Associated with Reduced Quality of Life Determined by the Short-Bowel Syndrome Quality of Life Scale in Nonmalignant Intestinal Failure Patients. J. Parenter. Enter. Nutr. 2021, 45, 926–932. [Google Scholar] [CrossRef]
- Lewis, S.J.; Heaton, K.W. Stool Form Scale as a Useful Guide to Intestinal Transit Time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef]
- Maasberg, S.; Knappe-Drzikova, B.; Vonderbeck, D.; Jann, H.; Weylandt, K.H.; Grieser, C.; Pascher, A.; Schefold, J.C.; Pavel, M.; Wiedenmann, B.; et al. Malnutrition Predicts Clinical Outcome in Patients with Neuroendocrine Neoplasia. Neuroendocrinology 2017, 104, 11–25. [Google Scholar] [CrossRef]
- McHorney, C.A.; Ware, J.E., Jr.; Lu, J.F.; Sherbourne, C.D. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med. Care 1994, 32, 40–66. [Google Scholar] [CrossRef]
- Berghöfer, P.; Fragkos, K.C.; Baxter, J.P.; Forbes, A.; Joly, F.; Heinze, H.; Loth, S.; Pertkiewicz, M.; Messing, B.; Jeppesen, P.B. Development and validation of the disease-specific Short Bowel Syndrome-Quality of Life (SBS-QoL™) scale. Clin. Nutr. 2013, 32, 789–796. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Gilroy, R.; Pertkiewicz, M.; Allard, J.P.; Messing, B.; O’Keefe, S. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut 2011, 60, 902–914. [Google Scholar] [CrossRef]
- Pevny, S.; Maasberg, S.; Rieger, A.; Karber, M.; Blüthner, E.; Knappe-Drzikova, B.; Thurmann, D.; Büttner, J.; Weylandt, K.-H.; Wiedenmann, B.; et al. Experience with teduglutide treatment for short bowel syndrome in clinical practice. Clin. Nutr. 2019, 38, 1745–1755. [Google Scholar] [CrossRef]
- Greif, S.; Maasberg, S.; Wehkamp, J.; Fusco, S.; Zopf, Y.; Herrmann, H.J.; Lamprecht, G.; Jacob, T.; Schiefke, I.; von Websky, M.W.; et al. Long-term results of teduglutide treatment for chronic intestinal failure-Insights from a national, multi-centric patient home-care service program. Clin. Nutr. ESPEN 2022, 51, 222–230. [Google Scholar] [CrossRef]
- Schwartz, L.K.; O’Keefe, S.; Fujioka, K.; Gabe, S.M.; Lamprecht, G.; Pape, U.-F.; Li, B.; Youssef, N.N.; Jeppesen, P.B. Long-Term Teduglutide for the Treatment of Patients with Intestinal Failure Associated with Short Bowel Syndrome. Clin. Transl. Gastroenterol. 2016, 7, e142. [Google Scholar] [CrossRef]
- Lam, K.; Schwartz, L.; Batisti, J.; Iyer, K.R. Single-Center Experience with the Use of Teduglutide in Adult Patients with Short Bowel Syndrome. JPEN J. Parenter. Enteral. Nutr. 2018, 42, 225–230. [Google Scholar] [CrossRef]
- Burden, S.T.; Jones, D.J.; Gittins, M.; Ablett, J.; Taylor, M.; Mountford, C.; Tyrrell-Price, J.; Donnellan, C.; Leslie, F.; Bowling, T.; et al. Needs-based quality of life in adults dependent on home parenteral nutrition. Clin. Nutr. 2019, 38, 1433–1438. [Google Scholar] [CrossRef]
- Carlsson, E.; Berglund, B.; Nordgren, S. Living with an ostomy and short bowel syndrome: Practical aspects and impact on daily life. J. Wound Ostomy Cont. Nurs. 2001, 28, 96–105. [Google Scholar] [CrossRef]
- Amiot, A.; Messing, B.; Corcos, O.; Panis, Y.; Joly, F. Determinants of home parenteral nutrition dependence and survival of 268 patients with non-malignant short bowel syndrome. Clin. Nutr. 2013, 32, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Schoeler, M.; Klag, T.; Wendler, J.; Bernhard, S.; Adolph, M.; Kirschniak, A.; Goetz, M.; Malek, N.; Wehkamp, J. GLP-2 analog teduglutide significantly reduces need for parenteral nutrition and stool frequency in a real-life setting. Therap. Adv. Gastroenterol. 2018, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Harpain, F.; Schlager, L.; Hütterer, E.; Dawoud, C.; Kirchnawy, S.; Stift, J.; Krotka, P.; Stift, A. Teduglutide in short bowel syndrome patients: A way back to normal life? JPEN J. Parenter. Enteral. Nutr. 2022, 46, 300–309. [Google Scholar] [CrossRef]
- McLeod, L.D.; Coon, C.D.; Martin, S.A.; Fehnel, S.E.; Hays, R.D. Interpreting patient-reported outcome results: US FDA guidance and emerging methods. Expert Rev. Pharmacoecon. Outcomes Res. 2011, 11, 163–169. [Google Scholar] [CrossRef]
| Patient Characteristics | Teduglutide Group | Control Group |
|---|---|---|
| Number of patients | 17 | 17 |
| Female sex, n (%) | 9 (53%) | 15 (88%) |
| Age (years) | ||
| Mean ± SD | 48.2 ± 20.0 | 49.6 ± 17.2 |
| Median (IQR) | 53.3 (36) | 56.2 (28) |
| BMI (kg/m2) | ||
| Mean ± SD | 21.4 ± 3.4 | 20.4 ± 4.4 |
| Median (IQR) | 20.4 (5.1) | 20.2 (6.8) |
| Cause of major intestinal resection, n (%*) | ||
| Mesenteric ischemia | 5 (29%) | 7 (41%) |
| Inflammatory bowel disease (IBD) | 3 (18%) | 4 (24%) |
| Traumatic injury | 3 (18%) | 0 (0 %) |
| Adhesion ileus | 3 (18 %) | 3 (18%) |
| Surgical complications | 1 (6 %) | 2 (12%) |
| Other (motility disorder due to aganglionosis, benign tumor) | 2 (12%) | 1 (6%) |
| Length of remaining small bowel (cm) | ||
| Mean ± SD | 77 ± 37 | 111 ± 40 |
| Median (IQR) | 70 (60) | 104 (36) |
| Presence of ileocaecal valve, n (%) | 1 (6%) | 2 (12%) |
| Colon in continuity, n (%) | 15 (88%) | 11 (65%) |
| Duration of cIF (years) | ||
| Mean ± SD | 4.9 ± 5.1 | 3.7 ± 4.9 |
| Median (IQR) | 2.4 (7.0) | 1.5 (3.0) |
| Prescribed total parenteral volume (L/week) | ||
| Mean ± SD | 13.9 ± 6.6 | 12.9 ± 6.9 |
| Median (IQR) | 14.0 (12.7) | 14.0 (9.4) |
| Prescribed parenteral energy (kcal/kg/d) | ||
| Mean ± SD | 26.7 ± 6.6 | 28.2 ± 6.7 |
| Median (IQR) | 26.8 (9.6) | 27.3 (9.2) |
| Prescribed parenteral energy (kcal/week) | ||
| Mean ± SD | 9846 ± 3218 | 8615 ± 2693 |
| Median (IQR) | 10,514 (5645) | 9450 (5107) |
| PN-days (days/week) | ||
| Mean ± SD | 6 ± 1 | 6 ± 2 |
| Median (IQR) | 7 (2) | 7 (3) |
| IVF-infusions (number of fluid and electrolyte infusions/week) | ||
| Mean ± SD | 2 ± 3 | 2 ± 3 |
| Median (IQR) | 0 (5) | 0 (7) |
| Teduglutide Group n = 17 | Control Group n = 17 | ||||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| PN duration before baseline visit (years) | 1.3 | 4.3 | 1.0 | 1.6 | |
| SBS-Qol TM | Sum score | 89.9 | 51.9 | 93.9 | 74.8 |
| Subscale 1 | 60.1 | 34.0 | 59.7 | 57.4 | |
| Subscale 2 | 26.4 | 15.8 | 23.8 | 24.4 | |
| SF-36 | Physical component summary | 33.7 | 11.6 | 35.5 | 19.5 |
| Mental component summary | 40.2 | 22.6 | 45.3 | 22.5 | |
| Time to follow-up * (years) | 4.3 | 4.6 | 4.3 | 4.8 | |
| PS burden | PN days per week | 7 | 2 | 7 | 3 |
| PS volume per week (L) | 14.0 | 12.7 | 14.0 | 9.4 | |
| Bowel anatomy | Type 1, (n (%**)) | 2 (12%) | - | 6 (35%) | - |
| Type 2, (n (%**)) | 12 (71%) | - | 7 (41%) | - | |
| Type 3, (n (%**)) | 3 (18%) | - | 4 (24%) | - | |
| Teduglutide Group n = 17 | Control Group n = 17 | p-Value between Groups | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | Change | p-Value | Baseline | Follow-Up | Change | p-Value | |||
| SBS-QoLTM | Sum score | 84.1 | 51.0 | −33.1 | <0.001 | 89.7 | 79.2 | −10.5 | 0.072 | 0.050 |
| Subscale 1 | 58.8 | 34.6 | −24.2 | <0.001 | 63.8 | 54.2 | −9.6 | 0.109 | 0.063 | |
| Subscale 2 | 25.3 | 16.4 | −8.9 | 0.002 | 25.9 | 25.0 | −0.9 | 0.431 | 0.076 | |
| SF-36 | Physical component summary | 33.7 | 39.0 | 5.3 | 0.011 | 36.1 | 35.1 | −1.0 | 0.345 | 0.031 |
| Mental component summary | 43.5 | 51.2 | 7.7 | 0.023 | 45.6 | 42.7 | −2.9 | 0.158 | 0.012 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blüthner, E.; Pape, U.-F.; Tacke, F.; Greif, S. Quality of Life in Teduglutide-Treated Patients with Short Bowel Syndrome Intestinal Failure—A Nested Matched Pair Real-World Study. Nutrients 2023, 15, 1949. https://doi.org/10.3390/nu15081949
Blüthner E, Pape U-F, Tacke F, Greif S. Quality of Life in Teduglutide-Treated Patients with Short Bowel Syndrome Intestinal Failure—A Nested Matched Pair Real-World Study. Nutrients. 2023; 15(8):1949. https://doi.org/10.3390/nu15081949
Chicago/Turabian StyleBlüthner, Elisabeth, Ulrich-Frank Pape, Frank Tacke, and Sophie Greif. 2023. "Quality of Life in Teduglutide-Treated Patients with Short Bowel Syndrome Intestinal Failure—A Nested Matched Pair Real-World Study" Nutrients 15, no. 8: 1949. https://doi.org/10.3390/nu15081949
APA StyleBlüthner, E., Pape, U.-F., Tacke, F., & Greif, S. (2023). Quality of Life in Teduglutide-Treated Patients with Short Bowel Syndrome Intestinal Failure—A Nested Matched Pair Real-World Study. Nutrients, 15(8), 1949. https://doi.org/10.3390/nu15081949







