Vitamin K and the Visual System—A Narrative Review
Abstract
1. Introduction
2. Vitamin K Biology and the Visual System
2.1. Vitamin K Content and Delivery to Ocular and Related Tissue
2.2. Vitamin K-Dependent Proteins in the Ocular/Visual System
3. Vitamin K and Specific Ocular Conditions
3.1. Glaucoma
3.2. Cataracts
3.3. Schnyder Corneal Dystrophy
3.4. Retinal Disease
4. Non-Canonical Vitamin K Functions and Ferroptosis
5. Vitamin K, Higher Cortical Visual Processing Function, and Ocular Correlates
6. Discussion
7. Clinical Considerations
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scott, A.W.; Bressler, N.M.; Ffolkes, S.; Wittenborn, J.S.; Jorkasky, J. Public Attitudes About Eye and Vision Health. JAMA Ophthalmol. 2016, 134, 1111–1118. [Google Scholar] [CrossRef]
- Andersen, S.R. The Eye and Its Diseases in Ancient Egypt. Acta Ophthalmol. Scand. 1997, 75, 338–344. [Google Scholar] [CrossRef] [PubMed]
- The Writings of Hippocrates and Galen|Online Library of Liberty. Available online: https://oll.libertyfund.org/title/coxe-the-writings-of-hippocrates-and-galen (accessed on 3 October 2021).
- Papadopoulos, G.; Liarmakopoulou, A.; Zisoulis, E.; Tzimas, P.; Lena, A.; Kitsos, G. Treatment of Eye Diseases in the Hippocratic Era. Hell. J. Surg. 2018, 90, 143–145. [Google Scholar] [CrossRef]
- GBD 2019 Blindness and Vision Impairment Collaborators. Trends in Prevalence of Blindness and Distance and near Vision Impairment over 30 Years: An Analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e130. [Google Scholar] [CrossRef]
- Keel, S.; Cieza, A. Rising to the Challenge: Estimates of the Magnitude and Causes of Vision Impairment and Blindness. Lancet Glob. Health 2021, 9, e100. [Google Scholar] [CrossRef] [PubMed]
- Flaxman, A.D.; Wittenborn, J.S.; Robalik, T.; Gulia, R.; Gerzoff, R.B.; Lundeen, E.A.; Saaddine, J.; Rein, D.B.; Vision and Eye Health Surveillance System study Group. Prevalence of Visual Acuity Loss or Blindness in the US: A Bayesian Meta-Analysis. JAMA Ophthalmol. 2021, 139, 717–723. [Google Scholar] [CrossRef]
- Rein, D.B.; Wittenborn, J.S.; Zhang, P.; Sublett, F.; Lamuda, P.A.; Lundeen, E.A.; Saaddine, J. The Economic Burden of Vision Loss and Blindness in the United States. Ophthalmology 2021, 129, 369–378. [Google Scholar] [CrossRef]
- Assi, L.; Chamseddine, F.; Ibrahim, P.; Sabbagh, H.; Rosman, L.; Congdon, N.; Evans, J.; Ramke, J.; Kuper, H.; Burton, M.J.; et al. A Global Assessment of Eye Health and Quality of Life: A Systematic Review of Systematic Reviews. JAMA Ophthalmol. 2021, 139, 526–541. [Google Scholar] [CrossRef]
- Bailey, R.L.; West, K.P., Jr.; Black, R.E. The Epidemiology of Global Micronutrient Deficiencies. Ann. Nutr. Metab. 2015, 66, 22–33. [Google Scholar] [CrossRef]
- Harshman, S.G.; Shea, M.K. The Role of Vitamin K in Chronic Aging Diseases: Inflammation, Cardiovascular Disease, and Osteoarthritis. Curr. Nutr. Rep. 2016, 5, 90. [Google Scholar] [CrossRef]
- Roberts, S.B.; Silver, R.E.; Das, S.K.; Fielding, R.A.; Gilhooly, C.H.; Jacques, P.F.; Kelly, J.M.; Mason, J.B.; McKeown, N.M.; Reardon, M.A.; et al. Healthy Aging—Nutrition Matters: Start Early and Screen Often. Adv. Nutr. 2021, 12, 1438–1448. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.D.; Anderson, J.E.; Salem, M.N.; Bügel, S.G.; Fenech, M.; Mason, J.B.; Weber, P.; West, K.P.; Wilde, P.; Eggersdorfer, M.; et al. The Decline in Vitamin Research Funding: A Missed Opportunity? Curr. Dev. Nutr. 2017, 1, e000430. [Google Scholar] [CrossRef] [PubMed]
- Search of: VITAMIN|Eye Diseases—Search Details—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/results/details?cond=Eye+Diseases&term=VITAMIN&cntry=&state=&city=&dist=&Search=Search (accessed on 15 January 2022).
- Camacho-Barcia, M.L.; Bulló, M.; Garcia-Gavilán, J.F.; Ruiz-Canela, M.; Corella, D.; Estruch, R.; Fitó, M.; García-Layana, A.; Arós, F.; Fiol, M.; et al. Association of Dietary Vitamin K1 Intake With the Incidence of Cataract Surgery in an Adult Mediterranean Population: A Secondary Analysis of a Randomized Clinical Trial. JAMA Ophthalmol. 2017, 135, 657–661. [Google Scholar] [CrossRef]
- Taylor, H.R. LXIII Edward Jackson Memorial Lecture: Eye Care: Dollars and Sense. Am. J. Ophthalmol. 2007, 143, 1–8. [Google Scholar] [CrossRef]
- Andreatta, W.; Boukouvala, S.; Bansal, A. Combined Acute Haemolytic and Secondary Angle Closure Glaucoma Following Spontaneous Intraocular Haemorrhages in a Patient on Warfarin. Case Rep. Ophthalmol. 2016, 7, 233–238. [Google Scholar] [CrossRef] [PubMed]
- McCann, J.C.; Ames, B.N. Vitamin K, an Example of Triage Theory: Is Micronutrient Inadequacy Linked to Diseases of Aging? Am. J. Clin. Nutr. 2009, 90, 889–907. [Google Scholar] [CrossRef] [PubMed]
- Shearer, M.J.; Newman, P. Recent Trends in the Metabolism and Cell Biology of Vitamin K with Special Reference to Vitamin K Cycling and MK-4 Biosynthesis. J. Lipid Res. 2014, 55, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Olson, R.E.; Chao, J.; Graham, D.; Bates, M.W.; Lewis, J.H. Total Body Phylloquinone and Its Turnover in Human Subjects at Two Levels of Vitamin K Intake. Br. J. Nutr. 2002, 87, 543–553. [Google Scholar] [CrossRef]
- Suttie, J.W. VITAMIN K in Health and Disease, 1st ed.; CRC Press: Boca Raton, FL, USA, 2009; ISBN 978-0-429-11978-1. [Google Scholar]
- Ivanova, D.; Zhelev, Z.; Getsov, P.; Nikolova, B.; Aoki, I.; Higashi, T.; Bakalova, R. Vitamin K: Redox-Modulation, Prevention of Mitochondrial Dysfunction and Anticancer Effect. Redox Biol. 2018, 16, 352–358. [Google Scholar] [CrossRef]
- Nowicka, B.; Kruk, J. Occurrence, Biosynthesis and Function of Isoprenoid Quinones. Biochim. Biophys. Acta BBA-Bioenerg. 2010, 1797, 1587–1605. [Google Scholar] [CrossRef]
- Shearer, M.J.; Okano, T. Key Pathways and Regulators of Vitamin K Function and Intermediary Metabolism. Annu. Rev. Nutr. 2018, 38, 127–151. [Google Scholar] [CrossRef] [PubMed]
- Berkner, K.L.; Runge, K.W. Vitamin K-Dependent Protein Activation: Normal Gamma-Glutamyl Carboxylation and Disruption in Disease. Int. J. Mol. Sci. 2022, 23, 5759. [Google Scholar] [CrossRef]
- Mishima, E.; Ito, J.; Wu, Z.; Nakamura, T.; Wahida, A.; Doll, S.; Tonnus, W.; Nepachalovich, P.; Eggenhofer, E.; Aldrovandi, M.; et al. A Non-Canonical Vitamin K Cycle Is a Potent Ferroptosis Suppressor. Nature 2022, 608, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Tabb, M.M.; Sun, A.; Zhou, C.; Grün, F.; Errandi, J.; Romero, K.; Pham, H.; Inoue, S.; Mallick, S.; Lin, M.; et al. Vitamin K2 Regulation of Bone Homeostasis Is Mediated by the Steroid and Xenobiotic Receptor SXR. J. Biol. Chem. 2003, 278, 43919–43927. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, J.; Wang, Y.; Liu, Z.; Wu, Y. Ferroptosis Drives Photoreceptor Degeneration in Mice with Defects in All-Trans-Retinal Clearance. J. Biol. Chem. 2021, 296, 100187. [Google Scholar] [CrossRef]
- Stenflo, J.; Fernlund, P.; Egan, W.; Roepstorff, P. Vitamin K Dependent Modifications of Glutamic Acid Residues in Prothrombin. Proc. Natl. Acad. Sci. USA 1974, 71, 2730–2733. [Google Scholar] [CrossRef]
- Suttie, J.W. Synthesis of Vitamin K-Dependent Proteins. FASEB J. 1993, 7, 445–452. [Google Scholar] [CrossRef]
- Clapham, D.E. Calcium Signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef]
- Ferland, G. The Vitamin K-Dependent Proteins: An Update. Nutr. Rev. 1998, 56, 223–230. [Google Scholar] [CrossRef]
- Xiao, H.; Chen, J.; Duan, L.; Li, S. Role of Emerging Vitamin K-dependent Proteins: Growth Arrest-specific Protein 6, Gla-rich Protein and Periostin (Review). Int. J. Mol. Med. 2021, 47, 4835. [Google Scholar] [CrossRef]
- Carafoli, E.; Krebs, J. Why Calcium? How Calcium Became the Best Communicator. J. Biol. Chem. 2016, 291, 20849–20857. [Google Scholar] [CrossRef] [PubMed]
- Beato, S.; Toledo-Solís, F.J.; Fernández, I. Vitamin K in Vertebrates’ Reproduction: Further Puzzling Pieces of Evidence from Teleost Fish Species. Biomolecules 2020, 10, 1303. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.; Vermeer, C. Determination of Phylloquinone and Menaquinones in Food. Effect of Food Matrix on Circulating Vitamin K Concentrations. Haemostasis 2000, 30, 54147. [Google Scholar] [CrossRef]
- Elder, S.J.; Haytowitz, D.B.; Howe, J.; Peterson, J.W.; Booth, S.L. Vitamin K Contents of Meat, Dairy, and Fast Food in the U.S. Diet. J. Agric. Food Chem. 2006, 54, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, A.C.; Pavlic, A.; Petsophonsakul, P.; Halder, M.; Maresz, K.; Kramann, R.; Schurgers, L. Vitamin K2 Needs an RDI Separate from Vitamin K1. Nutrients 2020, 12, 1852. [Google Scholar] [CrossRef]
- Halder, M.; Petsophonsakul, P.; Akbulut, A.C.; Pavlic, A.; Bohan, F.; Anderson, E.; Maresz, K.; Kramann, R.; Schurgers, L. Vitamin K: Double Bonds beyond Coagulation Insights into Differences between Vitamin K1 and K2 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 896. [Google Scholar] [CrossRef]
- Shea, M.K.; Berkner, K.L.; Ferland, G.; Fu, X.; Holden, R.M.; Booth, S.L. Perspective: Evidence before Enthusiasm—A Critical Review of the Potential Cardiovascular Benefits of Vitamin K. Adv. Nutr. 2021, 12, 632–646. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on Micronutrient Deficiencies. Prevention of Micronutrient Deficiencies: Tools for Policymakers and Public Health Workers; Howson, C.P., Kennedy, E.T., Horwitz, A., Eds.; National Academies Press: Washington, DC, USA, 1998; ISBN 978-0-309-06029-5. [Google Scholar]
- Ioannidis, J. Implausible Results in Human Nutrition Research. BMJ 2013, 347, f6698. [Google Scholar] [CrossRef]
- Satija, A.; Yu, E.; Willett, W.; Hu, F.B. Understanding Nutritional Epidemiology and Its Role in Policy. Adv. Nutr. 2015, 6, 7492. [Google Scholar] [CrossRef]
- Brennan, L.; Hu, F.; Sun, Q. Metabolomics Meets Nutritional Epidemiology: Harnessing the Potential in Metabolomics Data. Metabolites 2021, 11, 709. [Google Scholar] [CrossRef]
- Booth, S.L.; Tucker, K.L.; McKeown, N.M.; Davidson, K.W.; Dallal, G.E.; Sadowski, J.A. Relationships between Dietary Intakes and Fasting Plasma Concentrations of Fat-Soluble Vitamins in Humans. J. Nutr. 1997, 127, 587–592. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dashti, H.S.; Shea, M.K.; Smith, C.E.; Tanaka, T.; Hruby, A.; Richardson, K.; Wang, T.J.; Nalls, M.A.; Guo, X.; Liu, Y.; et al. Meta-Analysis of Genome-Wide Association Studies for Circulating Phylloquinone Concentrations. Am. J. Clin. Nutr. 2014, 100, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Available online: https://pubmed.ncbi.nlm.nih.gov/25057538/ (accessed on 12 March 2023).
- Turck, D.; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Dietary Reference Values for Vitamin K. EFSA J. 2017, 15, e04780. [Google Scholar] [CrossRef] [PubMed]
- Kamao, M.; Suhara, Y.; Tsugawa, N.; Uwano, M.; Yamaguchi, N.; Uenishi, K.; Ishida, H.; Sasaki, S.; Okano, T. Vitamin K Content of Foods and Dietary Vitamin K Intake in Japanese Young Women. J. Nutr. Sci. Vitaminol. 2007, 53, 464–470. [Google Scholar] [CrossRef]
- USDA FoodData Central Component Search-Phylloquinone. Available online: https://fdc.nal.usda.gov/fdc-app.html#/?component=1185 (accessed on 18 March 2023).
- USDA FoodData Central Component Search-Menaquinone-4. Available online: https://fdc.nal.usda.gov/fdc-app.html#/?component=1183 (accessed on 18 March 2023).
- Regulska-Ilow, B.; Różańska, D.; Zatońska, K.; Szuba, A. Estimation of Vitamin K Content and Its Sources in the Diet of the Polish Participants of the PURE Study. Nutrients 2022, 14, 1917. [Google Scholar] [CrossRef] [PubMed]
- Felleman, D.J.; Van Essen, D.C. Distributed Hierarchical Processing in the Primate Cerebral Cortex. Cereb. Cortex 1991, 1, 1–47. [Google Scholar] [CrossRef]
- Moini, J.; Piran, P. Chapter 14—Visual System. In Functional and Clinical Neuroanatomy; Moini, J., Piran, P., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 417–466. ISBN 978-0-12-817424-1. [Google Scholar]
- Okano, T.; Shimomura, Y.; Yamane, M.; Suhara, Y.; Kamao, M.; Sugiura, M.; Nakagawa, K. Conversion of Phylloquinone (Vitamin K1) into Menaquinone-4 (Vitamin K2) in Mice: Two Possible Routes for Menaquinone-4 Accumulation in Cerebra of Mice. J. Biol. Chem. 2008, 283, 11270–11279. [Google Scholar] [CrossRef]
- Carrié, I.; Portoukalian, J.; Vicaretti, R.; Rochford, J.; Potvin, S.; Ferland, G. Menaquinone-4 Concentration Is Correlated with Sphingolipid Concentrations in Rat Brain. J. Nutr. 2004, 134, 167–172. [Google Scholar] [CrossRef]
- Hirota, Y.; Tsugawa, N.; Nakagawa, K.; Suhara, Y.; Tanaka, K.; Uchino, Y.; Takeuchi, A.; Sawada, N.; Kamao, M.; Wada, A.; et al. Menadione (Vitamin K3) Is a Catabolic Product of Oral Phylloquinone (Vitamin K1) in the Intestine and a Circulating Precursor of Tissue Menaquinone-4 (Vitamin K2) in Rats. J. Biol. Chem. 2013, 288, 33071. [Google Scholar] [CrossRef]
- Thijssen, H.H.W.; Vervoort, L.M.T.; Schurgers, L.J.; Shearer, M.J. Menadione Is a Metabolite of Oral Vitamin K. Br. J. Nutr. 2006, 95, 260–266. [Google Scholar] [CrossRef]
- Pardridge, W.M. CNS Drug Design Based on Principles of Blood-Brain Barrier Transport. J. Neurochem. 1998, 70, 1781–1792. [Google Scholar] [CrossRef] [PubMed]
- Vita, M.F.; Nagachar, N.; Avramidis, D.; Delwar, Z.M.; Cruz, M.H.; Siden, Å.; Paulsson, K.M.; Yakisich, J.S. Pankiller Effect of Prolonged Exposure to Menadione on Glioma Cells: Potentiation by Vitamin C. Investig. New Drugs 2011, 29, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.L.; Fu, X.; Karl, J.P.; Hernandez, C.J.; Mason, J.B.; DeBose-Boyd, R.A.; Booth, S.L. Multiple Dietary Vitamin K Forms Are Converted to Tissue Menaquinone-4 in Mice. J. Nutr. 2022, 152, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, H.H.; Drittij-Reijnders, M.J. Vitamin K Status in Human Tissues: Tissue-Specific Accumulation of Phylloquinone and Menaquinone-4. Br. J. Nutr. 1996, 75, 121–127. [Google Scholar] [CrossRef]
- Tanprasertsuk, J.; Ferland, G.; Johnson, M.A.; Poon, L.W.; Scott, T.M.; Barbey, A.K.; Barger, K.; Wang, X.-D.; Johnson, E.J. Concentrations of Circulating Phylloquinone, but Not Cerebral Menaquinone-4, Are Positively Correlated with a Wide Range of Cognitive Measures: Exploratory Findings in Centenarians. J. Nutr. 2020, 150, 82–90. [Google Scholar] [CrossRef]
- Tanprasertsuk, J.; Scott, T.M.; Barbey, A.K.; Barger, K.; Wang, X.-D.; Johnson, M.A.; Poon, L.W.; Vishwanathan, R.; Matthan, N.R.; Lichtenstein, A.H.; et al. Carotenoid-Rich Brain Nutrient Pattern Is Positively Correlated With Higher Cognition and Lower Depression in the Oldest Old With No Dementia. Front. Nutr. 2021, 8, 704691. [Google Scholar] [CrossRef]
- Fu, X.; Shea, M.K.; Dolnikowski, G.G.; Patterson, W.B.; Dawson-Hughes, B.; Holland, T.M.; Schneider, J.A.; Booth, S.L. Vitamin D and Vitamin K Concentrations in Human Brain Tissue Are Influenced by Freezer Storage Time: The Memory and Aging Project. J. Nutr. 2021, 151, 104–108. [Google Scholar] [CrossRef]
- Booth, S.L.; Shea, M.K.; Barger, K.; Leurgans, S.E.; James, B.D.; Holland, T.M.; Agarwal, P.; Fu, X.; Wang, J.; Matuszek, G.; et al. Association of Vitamin K with Cognitive Decline and Neuropathology in Community-dwelling Older Persons. Alzheimers Dement. Transl. Res. Clin. Interv. 2022, 8, 12255. [Google Scholar] [CrossRef]
- Arnott, J.A.; Planey, S.L. The Influence of Lipophilicity in Drug Discovery and Design. Expert Opin. Drug Discov. 2012, 7, 863–875. [Google Scholar] [CrossRef]
- Ji, Y.; Li, X.; Tso, P. Intestinal Fatty Acid Absorption. Immunol. Endocr. Metab. Agents Med. Chem. 2009, 9, 60–73. [Google Scholar] [CrossRef]
- Shearer, M.J.; Fu, X.; Booth, S.L. Vitamin K Nutrition, Metabolism, and Requirements: Current Concepts and Future Research. Adv. Nutr. 2012, 3, 182. [Google Scholar] [CrossRef]
- Lai, Y.; Masatoshi, H.; Ma, Y.; Guo, Y.; Zhang, B. Role of Vitamin K in Intestinal Health. Front. Immunol. 2022, 12, 791565. [Google Scholar] [CrossRef] [PubMed]
- Lamon-Fava, S.; Sadowski, J.A.; Davidson, K.W.; O’Brien, M.E.; McNamara, J.R.; Schaefer, E.J. Plasma Lipoproteins as Carriers of Phylloquinone (Vitamin K1) in Humans. Am. J. Clin. Nutr. 1998, 67, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Vermeer, C. Differential Lipoprotein Transport Pathways of K-Vitamins in Healthy Subjects. Biochim. Biophys. Acta 2002, 1570, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Jahn, C.E.; Leiss, O.; von Bergmann, K. Lipid Composition of Human Aqueous Humor. Ophthalmic Res. 1983, 15, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Cenedella, R.J. Lipoproteins and Lipids in Cow and Human Aqueous Humor. Biochim. Biophys. Acta 1984, 793, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.R.; Price, M.O.; Price, F.W.; Pardo, J.C.; Grandin, J.C.; You, J.; Wang, M.; Yoder, M.C. Proteomic Analysis of Human Aqueous Humor Using Multidimensional Protein Identification Technology. Mol. Vis. 2009, 15, 2740. [Google Scholar] [PubMed]
- Kodeboyina, S.K.; Lee, T.J.; Churchwell, L.; Ulrich, L.; Bollinger, K.; Bogorad, D.; Estes, A.; Zhi, W.; Sharma, S.; Sharma, A. The Constitutive Proteome of Human Aqueous Humor and Race Specific Alterations. Proteomes 2020, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Connor, W.E.; Duell, P.B.; Kean, R.; Wang, Y. The Prime Role of HDL to Transport Lutein into the Retina: Evidence from HDL-Deficient WHAM Chicks Having a Mutant ABCA1 Transporter. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4226–4231. [Google Scholar] [CrossRef]
- Li, B.; Vachali, P.; Chang, F.-Y.; Gorusupudi, A.; Arunkumar, R.; Shi, L.; Rognon, G.T.; Frederick, J.M.; Bernstein, P.S. HDL Is the Primary Transporter for Carotenoids from Liver to Retinal Pigment Epithelium in Transgenic ApoA-I-/-/Bco2-/- Mice. Arch. Biochem. Biophys. 2022, 716, 109111. [Google Scholar] [CrossRef]
- Yamanashi, Y.; Takada, T.; Kurauchi, R.; Tanaka, Y.; Komine, T.; Suzuki, H. Transporters for the Intestinal Absorption of Cholesterol, Vitamin E, and Vitamin K. J. Atheroscler. Thromb. 2017, 24, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Storti, F.; Raphael, G.; Griesser, V.; Klee, K.; Drawnel, F.; Willburger, C.; Scholz, R.; Langmann, T.; von Eckardstein, A.; Fingerle, J.; et al. Regulated Efflux of Photoreceptor Outer Segment-Derived Cholesterol by Human RPE Cells. Exp. Eye Res. 2017, 165, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Tserentsoodol, N.; Gordiyenko, N.V.; Pascual, I.; Lee, J.W.; Fliesler, S.J.; Rodriguez, I.R. Intraretinal Lipid Transport Is Dependent on High Density Lipoprotein-like Particles and Class B Scavenger Receptors. Mol. Vis. 2006, 12, 1319–1333. [Google Scholar] [PubMed]
- Gharahkhani, P.; Burdon, K.P.; Fogarty, R.; Sharma, S.; Hewitt, A.W.; Martin, S.; Law, M.H.; Cremin, K.; Bailey, J.N.C.; Loomis, S.J.; et al. Common Variants near ABCA1, AFAP1 and GMDS Confer Risk of Primary Open-Angle Glaucoma. Nat. Genet. 2014, 46, 1120–1125. [Google Scholar] [CrossRef]
- Nelsestuen, G.L.; Zytkovicz, T.H.; Howard, J.B. The Mode of Action of Vitamin K: Identification of γ-carboxyglutamic acid as a component of prothrombin. J. Biol. Chem. 1974, 249, 6347–6350. [Google Scholar] [CrossRef]
- Ferland, G. Vitamin K and the Nervous System: An Overview of Its Actions. Adv. Nutr. 2012, 3, 204–212. [Google Scholar] [CrossRef]
- Tissue Expression of GGCX—Summary—The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000115486-GGCX/tissue (accessed on 30 October 2022).
- Berkner, K.L.; Pudota, B.N. Vitamin K-Dependent Carboxylation of the Carboxylase. Proc. Natl. Acad. Sci. USA 1998, 95, 466. [Google Scholar] [CrossRef]
- Annis, D.S.; Ma, H.; Balas, D.M.; Kumfer, K.T.; Sandbo, N.; Potts, G.K.; Coon, J.J.; Mosher, D.F. Absence of Vitamin K-Dependent γ-Carboxylation in Human Periostin Extracted from Fibrotic Lung or Secreted from a Cell Line Engineered to Optimize γ-Carboxylation. PLoS ONE 2015, 10, e0135374. [Google Scholar] [CrossRef]
- Demirören, K.; Yavuz, H.; Cam, L. Intracranial Hemorrhage Due to Vitamin K Deficiency after the Newborn Period. Pediatr. Hematol. Oncol. 2004, 21, 585–592. [Google Scholar] [CrossRef]
- Bui Quoc, E.; Bonnet, D.; Bajolle, F. Vitamin K antagonist overdose induced blindness in an infant: An argument for a therapeutic educational program. Arch. Pediatr. Organe Off. Soc. Francaise Pediatr. 2012, 19, 22–26. [Google Scholar] [CrossRef]
- Holden, R. Spontaneous Hyphaema as a Result of Systemic Anticoagulation in Previously Abnormal Eyes. Postgrad. Med. J. 1991, 67, 1008. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Masri, I.; Smith, J.; Wride, N.; Ghosh, S. A Rare Case of Acute Angle Closure Due to Spontaneous Suprachoroidal Haemorrhage Secondary to Loss of Anti-Coagulation Control: A Case Report. BMC Ophthalmol. 2018, 18, 224. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, P.; Zigler, J.S.; Epstein, D.L.; Borrás, T. Identification and Isolation of Differentially Expressed Genes from Very Small Tissue Samples. BioTechniques 1999, 26, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, P.; Epstein, D.L.; Borrás, T. Characterization of Gene Expression in Human Trabecular Meshwork Using Single-Pass Sequencing of 1060 Clones. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3678–3693. [Google Scholar]
- Canfield, A.E.; Doherty, M.J.; Kelly, V.; Newman, B.; Farrington, C.; Grant, M.E.; Boot-Handford, R.P. Matrix Gla Protein Is Differentially Expressed During the Deposition of a Calcified Matrix by Vascular Pericytes. FEBS Lett. 2000, 487, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Borrás, T.; Cowley, D.O.; Asokan, P.; Pandya, K. Generation of a Matrix Gla (Mgp) Floxed Mouse, Followed by Conditional Knockout, Uncovers a New Mgp Function in the Eye. Sci. Rep. 2020, 10, 18583. [Google Scholar] [CrossRef] [PubMed]
- Borrás, T.; Smith, M.H.; Buie, L.K. A Novel Mgp-Cre Knock-in Mouse Reveals an Anticalcification/Antistiffness Candidate Gene in the Trabecular Meshwork and Peripapillary Scleral Region. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2203. [Google Scholar] [CrossRef]
- Asokan, P.; Mitra, R.N.; Periasamy, R.; Han, Z.; Borrás, T. A Naturally Fluorescent Mgp Transgenic Mouse for Angiogenesis and Glaucoma Longitudinal Studies. Investig. Ophthalmol. Vis. Sci. 2018, 59, 746. [Google Scholar] [CrossRef]
- Sarosiak, A.; Oziębło, D.; Udziela, M.; Vermeer, C.; Malejczyk, J.; Szaflik, J.P.; Ołdak, M. High Expression of Matrix Gla Protein in Schnyder Corneal Dystrophy Patients Points to an Active Role of Vitamin K in Corneal Health. Acta Ophthalmol. 2021, 99, e171–e177. [Google Scholar] [CrossRef]
- Collett, G.; Wood, A.; Alexander, M.Y.; Varnum, B.C.; Boot-Handford, R.P.; Ohanian, V.; Ohanian, J.; Fridell, Y.-W.; Canfield, A.E. Receptor Tyrosine Kinase Axl Modulates the Osteogenic Differentiation of Pericytes. Circ. Res. 2003, 92, 1123–1129. [Google Scholar] [CrossRef]
- Valverde, P.; Obin, M.S.; Taylor, A. Role of Gas6/Axl Signaling in Lens Epithelial Cell Proliferation and Survival. Exp. Eye Res. 2004, 78, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.O.; Prieto, A.L.; Obin, M.S.; Abrams, T.A.; Burgess, B.L.; Heeb, M.J.; Agnew, B.J. Outer Segment Phagocytosis by Cultured Retinal Pigment Epithelial Cells Requires Gas6. Exp. Eye Res. 2001, 73, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.O.; Obin, M.S.; Prieto, A.L.; Burgess, B.L.; Abrams, T.A. Gas6 Binding to Photoreceptor Outer Segments Requires Gamma-Carboxyglutamic Acid (Gla) and Ca2+ and Is Required for OS Phagocytosis by RPE Cells in Vitro. Exp. Eye Res. 2002, 75, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.O.; Obin, M.S.; Heeb, M.J.; Burgess, B.L.; Abrams, T.A. Both Protein S and Gas6 Stimulate Outer Segment Phagocytosis by Cultured Rat Retinal Pigment Epithelial Cells. Exp. Eye Res. 2005, 81, 581–591. [Google Scholar] [CrossRef]
- Ayala, A.; Warejcka, D.J.; Olague-Marchan, M.; Twining, S.S. Corneal Activation of Prothrombin to Form Thrombin, Independent of Vascular Injury. Investig. Ophthalmol. Vis. Sci. 2007, 48, 134–143. [Google Scholar] [CrossRef][Green Version]
- GBD 2019 Blindness and Vision Impairment Collaborators. Causes of Blindness and Vision Impairment in 2020 and Trends over 30 Years, and Prevalence of Avoidable Blindness in Relation to VISION 2020: The Right to Sight: An Analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144. [Google Scholar] [CrossRef]
- Casson, R.J.; Chidlow, G.; Wood, J.P.; Crowston, J.G.; Goldberg, I. Definition of Glaucoma: Clinical and Experimental Concepts. Clin. Experiment. Ophthalmol. 2012, 40, 341–349. [Google Scholar] [CrossRef]
- Kass, M.A.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.L.; Miller, J.P.; Parrish, R.K.; Wilson, M.R.; Gordon, M.O. The Ocular Hypertension Treatment Study: A Randomized Trial Determines That Topical Ocular Hypotensive Medication Delays or Prevents the Onset of Primary Open-Angle Glaucoma. Arch. Ophthalmol. Chic. 2002, 120, 701–713; discussion 829–830. [Google Scholar] [CrossRef]
- Heijl, A.; Leske, M.C.; Bengtsson, B.; Hyman, L.; Bengtsson, B.; Hussein, M. Early Manifest Glaucoma Trial Group Reduction of Intraocular Pressure and Glaucoma Progression: Results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. Chic. 2002, 120, 1268–1279. [Google Scholar] [CrossRef]
- Johnson, M. What Controls Aqueous Humour Outflow Resistance? Exp. Eye Res. 2006, 82, 545–557. [Google Scholar] [CrossRef]
- Acott, T.S.; Kelley, M.J. Extracellular Matrix in the Trabecular Meshwork. Exp. Eye Res. 2008, 86, 543. [Google Scholar] [CrossRef] [PubMed]
- Vranka, J.A.; Kelley, M.J.; Acott, T.S.; Keller, K.E. Extracellular Matrix in the Trabecular Meshwork: Intraocular Pressure Regulation and Dysregulation in Glaucoma. Exp. Eye Res. 2015, 133, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Wallin, R.; Olmsted-Davis, E.A.; Borrás, T. Matrix Gla Protein Function in Human Trabecular Meshwork Cells: Inhibition of Bmp2-Induced Calcification Process. Investig. Ophthalmol. Vis. Sci. 2006, 47, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.-F.; Huang, Q.-F.; Zhang, Z.-Y.; Van Keer, K.; Thijs, L.; Trenson, S.; Yang, W.-Y.; Cauwenberghs, N.; Mujaj, B.; Kuznetsova, T.; et al. Inactive Matrix Gla Protein Is a Novel Circulating Biomarker Predicting Retinal Arteriolar Narrowing in Humans. Sci. Rep. 2018, 8, 15088. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Yao, K.; Peng, F.; Zhao, B.; Chen, Z.; Chen, W.; Zhao, Y.; Zhang, H.; Wang, J. The Effect of Dietary Vitamin K1 Supplementation on Trabecular Meshwork and Retina in a Chronic Ocular Hypertensive Rat Model. Investig. Ophthalmol. Vis. Sci. 2020, 61, 40. [Google Scholar] [CrossRef]
- Ramdas, W.D.; Schouten, J.S.A.G.; Webers, C.A.B. The Effect of Vitamins on Glaucoma: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 359. [Google Scholar] [CrossRef]
- Coleman, A.L.; Stone, K.L.; Kodjebacheva, G.; Yu, F.; Pedula, K.L.; Ensrud, K.E.; Cauley, J.A.; Hochberg, M.C.; Topouzis, F.; Badala, F.; et al. Glaucoma Risk and the Consumption of Fruits and Vegetables among Older Women in the Study of Osteoporotic Fractures. Am. J. Ophthalmol. 2008, 145, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Moïse, M.M.; Benjamin, L.-M.; Doris, T.M.; Dalida, K.N.; Augustin, N.O. Role of Mediterranean Diet, Tropical Vegetables Rich in Antioxidants, and Sunlight Exposure in Blindness, Cataract and Glaucoma among African Type 2 Diabetics. Int. J. Ophthalmol. 2012, 5, 231–237. [Google Scholar] [CrossRef]
- Giaconi, J.A.; Yu, F.; Stone, K.L.; Pedula, K.L.; Ensrud, K.E.; Cauley, J.A.; Hochberg, M.C.; Coleman, A.L. The Association of Consumption of Fruits/Vegetables With Decreased Risk of Glaucoma Among Older African-American Women in the Study of Osteoporotic Fractures. Am. J. Ophthalmol. 2012, 154, 635–644. [Google Scholar] [CrossRef]
- Kang, J.H.; Willett, W.C.; Rosner, B.A.; Buys, E.; Wiggs, J.L.; Pasquale, L.R. Association of Dietary Nitrate Intake With Primary Open-Angle Glaucoma: A Prospective Analysis From the Nurses’ Health Study and Health Professionals Follow-up Study. JAMA Ophthalmol. 2016, 134, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.; Rao, S.K.; Ratra, V.; Liu, Y.; Mitchell, P.; King, J.; Tassignon, M.-J.; Jonas, J.; Pang, C.P.; Chang, D.F. Cataract. Nat. Rev. Dis. Primer 2015, 1, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Pakzad, R.; Yekta, A.; Aghamirsalim, M.; Pakbin, M.; Ramin, S.; Khabazkhoob, M. Global and Regional Prevalence of Age-Related Cataract: A Comprehensive Systematic Review and Meta-Analysis. Eye 2020, 34, 1357. [Google Scholar] [CrossRef]
- Hejtmancik, J.F.; Shiels, A. Overview of the Lens. Prog. Mol. Biol. Transl. Sci. 2015, 134, 119. [Google Scholar] [CrossRef] [PubMed]
- Gillies, M.; Brian, G.; La Nauze, J.; Le Mesurier, R.; Moran, D.; Taylor, H.; Ruit, S. Modern Surgery for Global Cataract Blindness: Preliminary Considerations. Arch. Ophthalmol. Chic. 1998, 116, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Heruye, S.H.; Maffofou Nkenyi, L.N.; Singh, N.U.; Yalzadeh, D.; Ngele, K.K.; Njie-Mbye, Y.-F.; Ohia, S.E.; Opere, C.A. Current Trends in the Pharmacotherapy of Cataracts. Pharmaceuticals 2020, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- López-Sobaler, A.M.; Aparicio, A.; Aranceta-Bartrina, J.; Gil, Á.; González-Gross, M.; Serra-Majem, L.; Varela-Moreiras, G.; Ortega, R.M. Overweight and General and Abdominal Obesity in a Representative Sample of Spanish Adults: Findings from the ANIBES Study. BioMed Res. Int. 2016, 2016, 8341487. [Google Scholar] [CrossRef]
- Online Food Calculator. Food Weight to Volume Conversions. Available online: https://www.aqua-calc.com/calculate/food-weight-to-volume (accessed on 12 January 2022).
- García-Layana, A.; Ciufo, G.; Toledo, E.; Martínez-González, M.; Corella, D.; Fitó, M.; Estruch, R.; Gómez-Gracia, E.; Fiol, M.; Lapetra, J.; et al. The Effect of a Mediterranean Diet on the Incidence of Cataract Surgery. Nutrients 2017, 9, 453. [Google Scholar] [CrossRef]
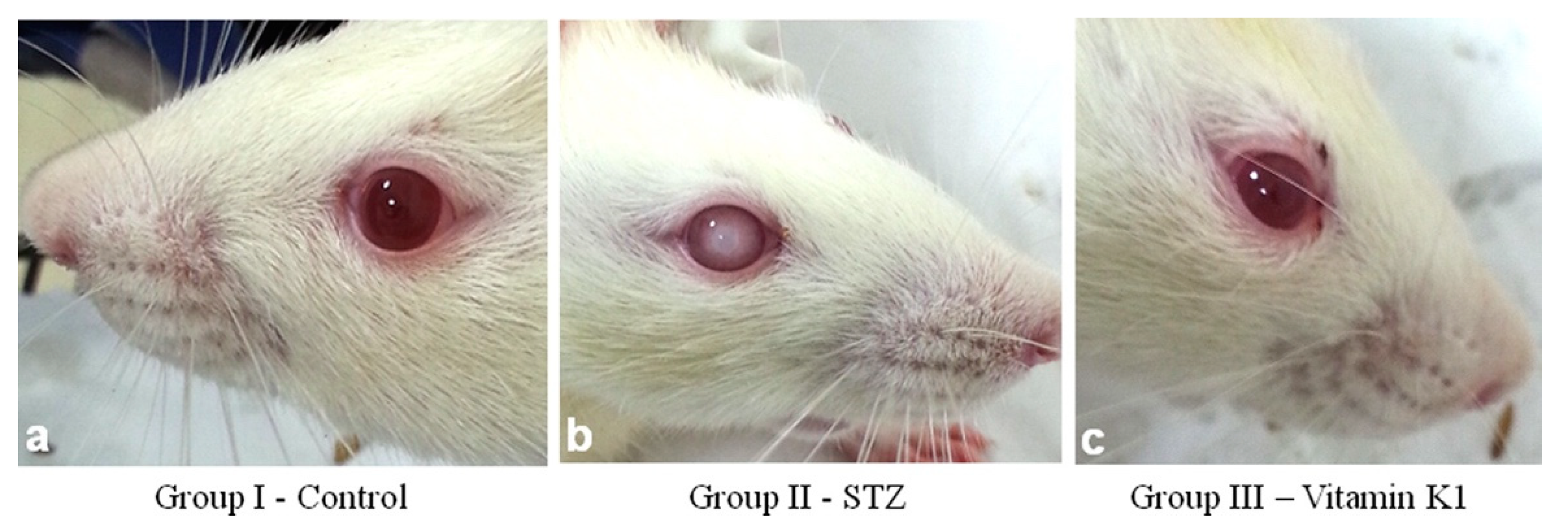
- Sai Varsha, M.K.N.; Raman, T.; Manikandan, R. Inhibition of Diabetic-Cataract by Vitamin K1 Involves Modulation of Hyperglycemia-Induced Alterations to Lens Calcium Homeostasis. Exp. Eye Res. 2014, 128, 73–82. [Google Scholar] [CrossRef]
- Varsha, M.K.N.S.; Thiagarajan, R.; Manikandan, R.; Dhanasekaran, G. Vitamin K1 Alleviates Streptozotocin-Induced Type 1 Diabetes by Mitigating Free Radical Stress, as Well as Inhibiting NF-ΚB Activation and INOS Expression in Rat Pancreas. Nutrition 2015, 31, 214–222. [Google Scholar] [CrossRef]
- Thiagarajan, R.; Varsha, M.K.N.S.; Srinivasan, V.; Ravichandran, R.; Saraboji, K. Vitamin K1 Prevents Diabetic Cataract by Inhibiting Lens Aldose Reductase 2 (ALR2) Activity. Sci. Rep. 2019, 9, 14684. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Su, D.; Liu, S.; Zheng, G.; Zhang, G.; Cui, T.; Ma, X.; Sun, Z.; Hu, S. RNA Sequencing and Bioinformatics Analysis of Human Lens Epithelial Cells in Age-Related Cataract. BMC Ophthalmol. 2021, 21, 152. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.D.; Sanderson, J. The Mechanisms of Calcium Homeostasis and Signalling in the Lens. Exp. Eye Res. 2009, 88, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, V.M.; Gao, J.; Minogue, P.J.; Jara, O.; Mathias, R.T.; Beyer, E.C. Connexin Mutants Compromise the Lens Circulation and Cause Cataracts through Biomineralization. Int. J. Mol. Sci. 2020, 21, 5822. [Google Scholar] [CrossRef]
- Burstyn-Cohen, T.; Hochberg, A. TAM Signaling in the Nervous System. Brain Plast. Amst. Neth. 2021, 7, 33–46. [Google Scholar] [CrossRef]
- Carnes, M.U.; Allingham, R.R.; Ashley-Koch, A.; Hauser, M.A. Transcriptome Analysis of Adult and Fetal Trabecular Meshwork, Cornea, and Ciliary Body Tissues by RNA Sequencing. Exp. Eye Res. 2018, 167, 91–99. [Google Scholar] [CrossRef]
- Sugimoto, M.; Kondo, M.; Yasuma, T.; D’Alessandro-Gabazza, C.N.; Toda, M.; Imai, H.; Nakamura, M.; Gabazza, E.C. Increased Expression of Protein S in Eyes with Diabetic Retinopathy and Diabetic Macular Edema. Sci. Rep. 2021, 11, 10449. [Google Scholar] [CrossRef]
- Sommer, A. Vitamin A Deficiency and Clinical Disease: An Historical Overview. J. Nutr. 2008, 138, 1835–1839. [Google Scholar] [CrossRef]
- Semba, R.D. On the ‘Discovery’ of Vitamin A. Ann. Nutr. Metab. 2012, 61, 192–198. [Google Scholar] [CrossRef]
- Wolf, G. The Discovery of the Visual Function of Vitamin A. J. Nutr. 2001, 131, 1647–1650. [Google Scholar] [CrossRef]
- Faulkner, R.; Jo, Y. Synthesis, Function, and Regulation of Sterol and Nonsterol Isoprenoids. Front. Mol. Biosci. 2022, 9, 1006822. [Google Scholar] [CrossRef] [PubMed]
- The Human Genome Project. Available online: https://www.genome.gov/human-genome-project (accessed on 12 November 2022).
- Stahl, A.; Smith, L.E.H. An Eye for Discovery. J. Clin. Investig. 2010, 120, 3008–3011. [Google Scholar] [CrossRef] [PubMed]
- Duggal, P.; Ibay, G.; Klein, A.P. Current Gene Discovery Strategies for Ocular Conditions. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7761–7770. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Porter, L.; Black, G. Personalized Ophthalmology. Clin. Genet. 2014, 86, 12389. [Google Scholar] [CrossRef]
- Garner, A.; Tripathi, R.C. Hereditary Crystalline Stromal Dystrophy of Schnyder. II. Histopathology and Ultrastructure. Br. J. Ophthalmol. 1972, 56, 400–408. [Google Scholar] [CrossRef][Green Version]
- Weiss, J.S.; Rodrigues, M.M.; Kruth, H.S.; Rajagopalan, S.; Rader, D.J.; Kachadoorian, H. Panstromal Schnyder’s Corneal Dystrophy: Ultrastructural and Histochemical Studies. Ophthalmology 1992, 99, 1072–1081. [Google Scholar] [CrossRef]
- Kruth, H.S. Accumulation of Unesterified Cholesterol in Limbal Cornea and Conjunctiva of Rabbits Fed a High-Cholesterol Diet. Detection with Filipin. Atherosclerosis 1987, 63, 1–6. [Google Scholar] [CrossRef]
- Weiss, J.S. Schnyder’s Dystrophy of the Cornea. A Swede-Finn Connection. Cornea 1992, 11, 93–101. [Google Scholar] [CrossRef]
- Weiss, J.S. Visual Morbidity in Thirty-four families with schnyder crystalline corneal dystrophy (an american ophthalmological society thesis). Trans. Am. Ophthalmol. Soc. 2007, 105, 616–648. [Google Scholar]
- Weiss, J.S.; Rodrigues, M.M.; Rajagopalan, S.; Kruth, H. Atypical Schnyder’s Crystalline Dystrophy of the Cornea: A Light and Electron Micoscopic Study [Abstract]. Proc. Int. Soc. Eye Res. 1990, 6, 198. [Google Scholar]
- Weiss, J.S.; Rodrigues, M.M.; Rajagopalan, S.; Kruth, H. Schnyder’s Corneal Dystrophy: Clinical, Ultrastructural, and Histo-Chemical Studies. Ophthalmology 1990, 97, 141. [Google Scholar]
- Orr, A.; Dubé, M.-P.; Marcadier, J.; Jiang, H.; Federico, A.; George, S.; Seamone, C.; Andrews, D.; Dubord, P.; Holland, S.; et al. Mutations in the UBIAD1 Gene, Encoding a Potential Prenyltransferase, Are Causal for Schnyder Crystalline Corneal Dystrophy. PLoS ONE 2007, 2, e685. [Google Scholar] [CrossRef] [PubMed]
- Yellore, V.S.; Khan, M.A.; Bourla, N.; Rayner, S.A.; Chen, M.C.; Sonmez, B.; Momi, R.S.; Sampat, K.M.; Gorin, M.B.; Aldave, A.J. Identification of Mutations in UBIAD1 Following Exclusion of Coding Mutations in the Chromosome 1p36 Locus for Schnyder Crystalline Corneal Dystrophy. Mol. Vis. 2007, 13, 1777–1782. [Google Scholar]
- Weiss, J.S.; Kruth, H.S.; Kuivaniemi, H.; Tromp, G.; White, P.S.; Winters, R.S.; Lisch, W.; Henn, W.; Denninger, E.; Krause, M.; et al. Mutations in the UBIAD1 Gene on Chromosome Short Arm 1, Region 36, Cause Schnyder Crystalline Corneal Dystrophy. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5007–5012. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Hirota, Y.; Sawada, N.; Yuge, N.; Watanabe, M.; Uchino, Y.; Okuda, N.; Shimomura, Y.; Suhara, Y.; Okano, T. Identification of UBIAD1 as a Novel Human Menaquinone-4 Biosynthetic Enzyme. Nature 2010, 468, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, M.L.; Bosley, A.D.; Weiss, J.S.; Kostiha, B.N.; Hirota, Y.; Brandt, W.; Esposito, D.; Kinoshita, S.; Wessjohann, L.; Morham, S.G.; et al. The UBIAD1 Prenyltransferase Links Menaquione-4 Synthesis to Cholesterol Metabolic Enzymes. Hum. Mutat. 2013, 34, 317–329, Corrected: Hum. Mutat. 2013, 34, 317–329. [Google Scholar] [CrossRef]
- Schumacher, M.M.; Elsabrouty, R.; Seemann, J.; Jo, Y.; DeBose-Boyd, R.A. The Prenyltransferase UBIAD1 Is the Target of Geranylgeraniol in Degradation of HMG CoA Reductase. eLife 2015, 4, 5560. [Google Scholar] [CrossRef]
- Schumacher, M.M.; Jun, D.-J.; Jo, Y.; Seemann, J.; DeBose-Boyd, R.A. Geranylgeranyl-Regulated Transport of the Prenyltransferase UBIAD1 between Membranes of the ER and Golgi. J. Lipid Res. 2016, 57, 1286–1299. [Google Scholar] [CrossRef]
- Schumacher, M.M.; Jun, D.-J.; Johnson, B.M.; DeBose-Boyd, R.A. UbiA Prenyltransferase Domain-Containing Protein-1 Modulates HMG-CoA Reductase Degradation to Coordinate Synthesis of Sterol and Nonsterol Isoprenoids. J. Biol. Chem. 2018, 293, 312–323. [Google Scholar] [CrossRef]
- Jo, Y.; Hamilton, J.S.; Hwang, S.; Garland, K.; Smith, G.A.; Su, S.; Fuentes, I.; Neelam, S.; Thompson, B.M.; McDonald, J.G.; et al. Schnyder Corneal Dystrophy-Associated UBIAD1 Inhibits ER-Associated Degradation of HMG CoA Reductase in Mice. eLife 2019, 8, e44396. [Google Scholar] [CrossRef]
- Jiang, S.-Y.; Tang, J.-J.; Xiao, X.; Qi, W.; Wu, S.; Jiang, C.; Hong, J.; Xu, J.; Song, B.-L.; Luo, J. Schnyder Corneal Dystrophy-Associated UBIAD1 Mutations Cause Corneal Cholesterol Accumulation by Stabilizing HMG-CoA Reductase. PLoS Genet. 2019, 15, e1008289. [Google Scholar] [CrossRef]
- Jun, D.-J.; Schumacher, M.M.; Hwang, S.; Kinch, L.N.; Grishin, N.V.; DeBose-Boyd, R.A. Schnyder Corneal Dystrophy-Associated UBIAD1 Is Defective in MK-4 Synthesis and Resists Autophagy-Mediated Degradation. J. Lipid Res. 2020, 61, 746–757. [Google Scholar] [CrossRef]
- Dong, F.; Jin, X.; Boettler, M.; Sciulli, H.; Abu-Asab, M.; Greco, C.; Wang, S.; Hu, Y.-C.; Campos, M.; Jackson, S.; et al. A Mouse Model of Schnyder Corneal Dystrophy with the N100S Point Mutation. Sci. Rep. 2018, 8, 10219. [Google Scholar] [CrossRef] [PubMed]
- Sarosiak, A.; Ołdak, M. Functional Characterization of the UBIAD1 Protein: The Nodal Point for Vitamin K and Cholesterol Synthesis. From Corneal Dystrophies to Lifestyle Diseases. Postepy Hig. Med. Dosw. 2018, 72, 116–130. [Google Scholar] [CrossRef]
- Sarosiak, A.; Udziela, M.; Ścieżyńska, A.; Oziębło, D.; Wawrzynowska, A.; Szaflik, J.P.; Ołdak, M. Clinical Diversity in Patients with Schnyder Corneal Dystrophy-a Novel and Known UBIAD1 Pathogenic Variants. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 2018, 256, 2127–2134. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, L.; Négrier, C.; Boukerche, H. Protein S: A Multifunctional Anticoagulant Vitamin K-Dependent Protein at the Crossroads of Coagulation, Inflammation, Angiogenesis, and Cancer. Crit. Rev. Oncol. Hematol. 2013, 88, 637–654. [Google Scholar] [CrossRef]
- Ireland, L.; Luckett, T.; Schmid, M.C.; Mielgo, A. Blockade of Stromal Gas6 Alters Cancer Cell Plasticity, Activates NK Cells, and Inhibits Pancreatic Cancer Metastasis. Front. Immunol. 2020, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, D.; Shanahan, C.M. Molecular Mechanisms Mediating Vascular Calcification: Role of Matrix Gla Protein (Review Article). Nephrology 2006, 11, 455–461. [Google Scholar] [CrossRef]
- Chen, X.; Furukawa, N.; Jin, D.-Y.; Liu, Y.; Stafford, D.W.; Williams, C.M.; Suhara, Y.; Tie, J.-K. Naturally Occurring UBIAD1 Mutations Differentially Affect Menaquinone Biosynthesis and Vitamin K-Dependent Carboxylation. FEBS J. 2021, 289, 2613–2627. [Google Scholar] [CrossRef]
- Jin, D.-Y.; Chen, X.; Liu, Y.; Williams, C.; Pedersen, L.; Stafford, D.; Tie, J.-K. A Genome-Wide CRISPR-Cas9 Knockout Screen Reveals FSP1 as Warfarin-Resistant Vitamin K Reductase 2022. Available online: https://assets.researchsquare.com/files/rs-2039668/v1_covered.pdf?c=1663010298 (accessed on 31 January 2023).
- Jonas, J.B.; Cheung, C.M.G.; Panda-Jonas, S. Updates on the Epidemiology of Age-Related Macular Degeneration. Asia-Pac. J. Ophthalmol. 2017, 6, 493–497. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global Prevalence of Age-Related Macular Degeneration and Disease Burden Projection for 2020 and 2040: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group. The Relationship of Dietary Carotenoid and Vitamin A, E, and C Intake With Age-Related Macular Degeneration in a Case-Control Study: AREDS Report No. 22. Arch. Ophthalmol. 2007, 125, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Agrón, E.; Mares, J.; Clemons, T.E.; Swaroop, A.; Chew, E.Y.; Keenan, T.D.L. Dietary Nutrient Intake and Progression to Late Age-Related Macular Degeneration in the Age-Related Eye Disease Studies 1 and 2. Ophthalmology 2021, 128, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Search of: Vitamin|Retina—List Results—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/results?cond=retina&term=vitamin&cntry=&state=&city=&dist=&Search=Search (accessed on 14 November 2022).
- Search of: Vitamin k|Retina-List Results—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/results?cond=retina&term=vitamin+k&cntry=&state=&city=&dist= (accessed on 14 November 2022).
- Wei, F.-F.; Trenson, S.; Verhamme, P.; Vermeer, C.; Staessen, J.A. Vitamin K–Dependent Matrix Gla Protein as Multifaceted Protector of Vascular and Tissue Integrity. Hypertension 2019, 73, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Dalmeijer, G.W.; van der Schouw, Y.T.; Magdeleyns, E.; Ahmed, N.; Vermeer, C.; Beulens, J.W.J. The Effect of Menaquinone-7 Supplementation on Circulating Species of Matrix Gla Protein. Atherosclerosis 2012, 225, 397–402. [Google Scholar] [CrossRef]
- Wong, T.Y.; Klein, R.; Nieto, F.J.; Klein, B.E.K.; Sharrett, A.R.; Meuer, S.M.; Hubbard, L.D.; Tielsch, J.M. Retinal Microvascular Abnormalities and 10-Year Cardiovascular Mortality: A Population-Based Case-Control Study. Ophthalmology 2003, 110, 933–940. [Google Scholar] [CrossRef]
- Wong, T.Y.; Klein, R.; Sharrett, A.R.; Duncan, B.B.; Couper, D.J.; Tielsch, J.M.; Klein, B.E.K.; Hubbard, L.D. Retinal Arteriolar Narrowing and Risk of Coronary Heart Disease in Men and Women. The Atherosclerosis Risk in Communities Study. JAMA 2002, 287, 1153–1159. [Google Scholar] [CrossRef]
- Cheung, C.Y.; Ikram, M.K.; Chen, C.; Wong, T.Y. Imaging Retina to Study Dementia and Stroke. Prog. Retin. Eye Res. 2017, 57, 89–107. [Google Scholar] [CrossRef]
- Simes, D.C.; Viegas, C.S.B.; Araújo, N.; Marreiros, C. Vitamin K as a Diet Supplement with Impact in Human Health: Current Evidence in Age-Related Diseases. Nutrients 2020, 12, 138. [Google Scholar] [CrossRef]
- Popa, D.-S.; Bigman, G.; Rusu, M.E. The Role of Vitamin K in Humans: Implication in Aging and Age-Associated Diseases. Antioxidants 2021, 10, 566. [Google Scholar] [CrossRef]
- Khalil, Z.; Alam, B.; Akbari, A.R.; Sharma, H. The Medical Benefits of Vitamin K2 on Calcium-Related Disorders. Nutrients 2021, 13, 691. [Google Scholar] [CrossRef] [PubMed]
- Schor, A.M.; Allen, T.D.; Canfield, A.E.; Sloan, P.; Schor, S.L. Pericytes Derived from the Retinal Microvasculature Undergo Calcification in Vitro. J. Cell Sci. 1990, 97, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Villa-Bellosta, R. New Insights into Endogenous Mechanisms of Protection against Arterial Calcification. Atherosclerosis 2020, 306, 68–74. [Google Scholar] [CrossRef]
- Doherty, M.J.; Ashton, B.A.; Walsh, S.; Beresford, J.N.; Grant, M.E.; Canfield, A.E. Vascular Pericytes Express Osteogenic Potential In Vitro and In Vivo. J. Bone Miner. Res. 1998, 13, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Collett, G.D.M.; Sage, A.P.; Kirton, J.P.; Alexander, M.Y.; Gilmore, A.P.; Canfield, A.E. Axl/Phosphatidylinositol 3-Kinase Signaling Inhibits Mineral Deposition by Vascular Smooth Muscle Cells. Circ. Res. 2007, 100, 502–509. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Ayyadurai, S.; Zlokovic, B.V. Pericytes of the Neurovascular Unit: Key Functions and Signaling Pathways. Nat. Neurosci. 2016, 19, 771–783. [Google Scholar] [CrossRef]
- Simó, R.; Stitt, A.W.; Gardner, T.W. Neurodegeneration in Diabetic Retinopathy: Does It Really Matter? Diabetologia 2018, 61, 1902–1912. [Google Scholar] [CrossRef]
- Nandrot, E.F.; Dufour, E.M. Mertk in Daily Retinal Phagocytosis: A History in the Making. In Retinal Degenerative Diseases: Laboratory and Therapeutic Investigations; Anderson, R.E., Hollyfield, J.G., LaVail, M.M., Eds.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2010; pp. 133–140. ISBN 978-1-4419-1399-9. [Google Scholar]
- Penberthy, K.K.; Lysiak, J.J.; Ravichandran, K.S. Re-Thinking Phagocytes: Clues from the Retina and Testes. Trends Cell Biol. 2018, 28, 317. [Google Scholar] [CrossRef]
- Kolbrink, B.; von Samson-Himmelstjerna, F.; Messtorff, M.; Riebeling, T.; Nische, R.; Schmitz, J.; Bräsen, J.; Kunzendorf, U.; Krautwald, S. Vitamin K1 Inhibits Ferroptosis and Counteracts a Detrimental Effect of Phenprocoumon in Experimental Acute Kidney Injury. Cell. Mol. Life Sci. CMLS 2022, 79, 387. [Google Scholar] [CrossRef]
- Hirschhorn, T.; Stockwell, B.R. Vitamin K: A New Guardian against Ferroptosis. Mol. Cell 2022, 82, 3760–3762. [Google Scholar] [CrossRef]
- Mukai, K.; Itoh, S.; Morimoto, H. Stopped-Flow Kinetic Study of Vitamin E Regeneration Reaction with Biological Hydroquinones (Reduced Forms of Ubiquinone, Vitamin K, and Tocopherolquinone) in Solution. J. Biol. Chem. 1992, 267, 22277–22281. [Google Scholar] [CrossRef] [PubMed]
- Vervoort, L.; Ronden, J.; Thijssen, H. The Potent Antioxidant Activity of the Vitamin K Cycle in Microsomal Lipid Peroxidation. Biochem. Pharmacol. 1997, 54, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Milo, R. The Distribution of Cellular Turnover in the Human Body. Nat. Med. 2021, 27, 45–48. [Google Scholar] [CrossRef]
- Aktipis, C.A.; Boddy, A.M.; Jansen, G.; Hibner, U.; Hochberg, M.E.; Maley, C.C.; Wilkinson, G.S. Cancer across the Tree of Life: Cooperation and Cheating in Multicellularity. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140219. [Google Scholar] [CrossRef]
- West, S.A.; Griffin, A.S.; Gardner, A. Evolutionary Explanations for Cooperation. Curr. Biol. 2007, 17, R661–R672. [Google Scholar] [CrossRef] [PubMed]
- Hanna, C.; Bicknell, D.S.; O’brien, J.E. Cell Turnover in the Adult Human Eye. Arch. Ophthalmol. 1961, 65, 695–698. [Google Scholar] [CrossRef]
- Barishak, Y.R. Embryology of the Eye and Its Adnexae. Dev. Ophthalmol. 1992, 24, 1–142. [Google Scholar]
- Lynnerup, N.; Kjeldsen, H.; Heegaard, S.; Jacobsen, C.; Heinemeier, J. Radiocarbon Dating of the Human Eye Lens Crystallines Reveal Proteins without Carbon Turnover throughout Life. PLoS ONE 2008, 3, e1529. [Google Scholar] [CrossRef]
- Hughes, J.R.; Levchenko, V.A.; Blanksby, S.J.; Mitchell, T.W.; Williams, A.; Truscott, R.J. No Turnover in Lens Lipids for the Entire Human Lifespan. eLife 2015, 4, e06003. [Google Scholar] [CrossRef]
- Sundaram, K.S.; Fan, J.H.; Engelke, J.A.; Foley, A.L.; Suttie, J.W.; Lev, M. Vitamin K Status Influences Brain Sulfatide Metabolism in Young Mice and Rats. J. Nutr. 1996, 126, 2746–2751. [Google Scholar] [CrossRef]
- Borchman, D.; Yappert, M.C.; Afzal, M. Lens Lipids and Maximum Lifespan. Exp. Eye Res. 2004, 79, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, Y.; Steller, H. Live to Die Another Way: Modes of Programmed Cell Death and the Signals Emanating from Dying Cells. Nat. Rev. Mol. Cell Biol. 2015, 16, 3999. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular Mechanisms of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Mishima, E.; Conrad, M. Nutritional and Metabolic Control of Ferroptosis. Available online: https://www.annualreviews.org/doi/abs/10.1146/annurev-nutr-062320-114541 (accessed on 7 August 2022).
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Non-Apoptotic Cell Death. Cell 2012, 149, 1060. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R. Ferroptosis Turns 10: Emerging Mechanisms, Physiological Functions, and Therapeutic Applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Goya Grocin, A.; Xavier da Silva, T.N.; Panzilius, E.; Scheel, C.H.; et al. FSP1 Is a Glutathione-Independent Ferroptosis Suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ Oxidoreductase FSP1 Acts Parallel to GPX4 to Inhibit Ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef]
- Braasch-Turi, M.M.; Koehn, J.T.; Crans, D.C. Chemistry of Lipoquinones: Properties, Synthesis, and Membrane Location of Ubiquinones, Plastoquinones, and Menaquinones. Int. J. Mol. Sci. 2022, 23, 12856. [Google Scholar] [CrossRef]
- Lowenthal, J.; MacFarlane, J.A. The nature of the antagonism between vitamin K and indirect anticoagulants. J. Pharmacol. Exp. Ther. 1964, 143, 273–277. [Google Scholar]
- Wallin, R.; Hutson, S. Vitamin K-Dependent Carboxylation. Evidence That at Least Two Microsomal Dehydrogenases Reduce Vitamin K1 to Support Carboxylation. J. Biol. Chem. 1982, 257, 1583–1586. [Google Scholar] [CrossRef]
- Tie, J.-K.; Stafford, D.W. Structural and Functional Insights into Enzymes of the Vitamin K Cycle. J. Thromb. Haemost. JTH 2016, 14, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sheng, S.; Wang, W.; Dai, J.; Zhong, Y.; Ren, J.; Jiang, K.; Li, S.; Bian, X.; Liu, L. Molecular Mechanisms of Iron Mediated Programmed Cell Death and Its Roles in Eye Diseases. Front. Nutr. 2022, 9, 844757. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Tsui, M.G.; Tsang, J.K.W.; Goit, R.K.; Yao, K.-M.; So, K.-F.; Lam, W.-C.; Lo, A.C.Y. Involvement of FSP1-CoQ10-NADH and GSH-GPx-4 Pathways in Retinal Pigment Epithelium Ferroptosis. Cell Death Dis. 2022, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Hao, C.; Huangfu, J.; Srinivasagan, R.; Zhang, X.; Fan, X. Aging Lens Epithelium Is Susceptible to Ferroptosis. Free Radic. Biol. Med. 2021, 167, 94–108. [Google Scholar] [CrossRef]
- Morris, M.C.; Wang, Y.; Barnes, L.L.; Bennett, D.A.; Dawson-Hughes, B.; Booth, S.L. Nutrients and Bioactives in Green Leafy Vegetables and Cognitive Decline: Prospective Study. Neurology 2018, 90, e214. [Google Scholar] [CrossRef]
- Kiely, A.; Ferland, G.; Ouliass, B.; O’Toole, P.W.; Purtill, H.; O’Connor, E.M. Vitamin K Status and Inflammation Are Associated with Cognition in Older Irish Adults. Nutr. Neurosci. 2020, 23, 591–599. [Google Scholar] [CrossRef]
- Bigman, G.; Rusu, M.E. Low Dietary Intakes of Vitamin K and Leafy Green Vegetables Are Individually Associated With Low Cognitive Functioning in A National Sample of U.S. Older Adults. Curr. Dev. Nutr. 2021, 5, 1306. [Google Scholar] [CrossRef]
- McCann, A.; Jeffery, I.B.; Ouliass, B.; Ferland, G.; Fu, X.; Booth, S.L.; Tran, T.T.T.; O’Toole, P.W.; O’Connor, E.M. Exploratory Analysis of Covariation of Microbiota-Derived Vitamin K and Cognition in Older Adults. Am. J. Clin. Nutr. 2019, 110, 1404–1415. [Google Scholar] [CrossRef]
- Alisi, L.; Cao, R.; De Angelis, C.; Cafolla, A.; Caramia, F.; Cartocci, G.; Librando, A.; Fiorelli, M. The Relationships Between Vitamin K and Cognition: A Review of Current Evidence. Front. Neurol. 2019, 10, 239. [Google Scholar] [CrossRef]
- Wilson, R.; Boyle, P.; Yu, L.; Barnes, L.; Sytsma, J.; As, B.; Bennett, D.; Schneider, J. Temporal Course and Pathologic Basis of Unawareness of Memory Loss in Dementia. Neurology 2015, 85, 984–991. [Google Scholar] [CrossRef]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E. Clinical Diagnosis of Alzheimer’s Disease: Report of the NINCDS-ADRDA Work Group under the Auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 1. [Google Scholar] [CrossRef] [PubMed]
- Greene, J.D.W. Apraxia, Agnosias, and Higher Visual Function Abnormalities. J. Neurol. Neurosurg. Psychiatry 2005, 76, v25–v34. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pelak, V.; van Stavern, G.; Moss, H. Higher Cortical Dysfunction Presenting as Visual Symptoms in Neurodegenerative Diseases. Front. Neurol. 2020, 11, 679. [Google Scholar] [CrossRef]
- Garobbio, S.; Pilz, K.S.; Kunchulia, M.; Herzog, M.H. No Common Factor Underlying Decline of Visual Abilities in Mild Cognitive Impairment. Exp. Aging Res. 2022, 49, 183–200. [Google Scholar] [CrossRef]
- Pratt, J.; Radulescu, P.; Guo, R.; Al-Aidroos, N.; Abrams, R. Biological Motion Captures Attention. J. Vis. 2010, 10, 120. [Google Scholar] [CrossRef]
- Harding, A.J.; Broe, G.A.; Halliday, G.M. Visual Hallucinations in Lewy Body Disease Relate to Lewy Bodies in the Temporal Lobe. Brain J. Neurol. 2002, 125, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Matar, E.; Phillips, J.R.; Martens, K.A.E.; Halliday, G.M.; Lewis, S.J.G. Impaired Color Discrimination-A Specific Marker of Hallucinations in Lewy Body Disorders. J. Geriatr. Psychiatry Neurol. 2019, 32, 257–264. [Google Scholar] [CrossRef]
- Fuller-Thomson, E.; Nowaczynski, A.; MacNeil, A. The Association Between Hearing Impairment, Vision Impairment, Dual Sensory Impairment, and Serious Cognitive Impairment: Findings from a Population-Based Study of 5.4 Million Older Adults. J. Alzheimers Dis. Rep. 2022, 6, 211–222. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Shi, C.; Shen, M.; Lu, F. Advances in Retina Imaging as Potential Biomarkers for Early Diagnosis of Alzheimer’s Disease. Transl. Neurodegener. 2021, 10, 6. [Google Scholar] [CrossRef]
- Jeevakumar, V.; Sefton, R.; Chan, J.; Gopinath, B.; Liew, G.; Shah, T.M.; Siette, J. Association between Retinal Markers and Cognition in Older Adults: A Systematic Review. BMJ Open 2022, 12, e054657. [Google Scholar] [CrossRef]
- Hofmeijer, J.; van Putten, M.J.A.M. Ischemic Cerebral Damage: An Appraisal of Synaptic Failure. Stroke 2012, 43, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, S.B.; de Ruyter van Steveninck, R.R.; Anderson, J.C. The Metabolic Cost of Neural Information. Nat. Neurosci. 1998, 1, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Niven, J.E.; Laughlin, S.B. Energy Limitation as a Selective Pressure on the Evolution of Sensory Systems. J. Exp. Biol. 2008, 211, 1792–1804. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.; Zhang, X.; Ling, X.; Bui, C.; Wang, Y.; Ip, P.; Chu, W.; Chen, L.; Tham, C.; Yam, J.; et al. Vitamin D and Ocular Diseases: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 4226. [Google Scholar] [CrossRef] [PubMed]
- Sajovic, J.; Meglič, A.; Glavač, D.; Markelj, Š.; Hawlina, M.; Fakin, A. The Role of Vitamin A in Retinal Diseases. Int. J. Mol. Sci. 2022, 23, 1014. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G.; Olson, C.; Euritt, C.; Koulen, P. Molecular Mechanisms Underlying the Therapeutic Role of Vitamin E in Age-Related Macular Degeneration. Front. Neurosci. 2022, 16, 890021. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Jialal, I. Biochemistry, Fat Soluble Vitamins; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Clarke, P.; Shearer, M.; Card, D.; Nichols, A.; Ponnusamy, V.; Mahaveer, A.; Voong, K.; Dockery, K.; Holland, N.; Mulla, S.; et al. Exclusively Breastmilk-Fed Preterm Infants Are at High Risk of Developing Subclinical Vitamin K Deficiency despite Intramuscular Prophylaxis at Birth. J. Thromb. Haemost. JTH 2022, 20, 15874. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Vitamin Tolerance of Animals; The National Academies Press: Washington, DC, USA, 1987; ISBN 978-0-309-03728-0. [Google Scholar]
- Suttie, J.W.; Zempleni, J.; Gregory, J.F.G., III; Stover, P.J. Handbook of Vitamins, 5th ed.; CRC Press: Boca Raton, FL, USA, 2013; ISBN 978-0-429-18955-5. [Google Scholar]
- Ames, B.N. Prolonging Healthy Aging: Longevity Vitamins and Proteins. Proc. Natl. Acad. Sci. USA 2018, 115, 10836. [Google Scholar] [CrossRef]
- Shea, M.K.; Booth, S.L. Concepts and Controversies in Evaluating Vitamin K Status in Population-Based Studies. Nutrients 2016, 8, 8. [Google Scholar] [CrossRef]
- Westerman, K.; Kelly, J.M.; Ordovás, J.M.; Booth, S.L.; DeMeo, D.L. Epigenome-Wide Association Study Reveals a Molecular Signature of Response to Phylloquinone (Vitamin K1) Supplementation. Epigenetics 2020, 15, 859–870. [Google Scholar] [CrossRef]
- Shea, M.; Benjamin, E.; Dupuis, J.; Massaro, J.; Jacques, P.; D’Agostino, R.; Ordovas, J.; O’Donnell, C.; Dawson-Hughes, B.; Vasan, R.; et al. Genetic and Non-Genetic Correlates of Vitamins K and D. Eur. J. Clin. Nutr. 2009, 63, 1602959. [Google Scholar] [CrossRef] [PubMed]
- Research Portfolio Online Reporting Tools (RePORT) Estimates of NIH Funding for Various Research, Condition, and Disease Categories (RCDC). Available online: https://report.nih.gov/funding/categorical-spending#/ (accessed on 22 January 2023).
- NIH Office of Budget Supplemental Tables NIH—Office of Budget–Budget Request FY. 2023. Available online: https://officeofbudget.od.nih.gov/pdfs/FY23/br/Overview%20of%20FY%202023%20Supplementary%20Tables.pdf (accessed on 27 January 2023).
- Booth, S.L. RePORT-Vitamins D and K and Neuropathologically-Defined Alzheimer and Other Dementias in Older Persons. Available online: https://reporter.nih.gov/search/kehAwJ-zEE24u-RBTtLAcw/project-details/9193206 (accessed on 27 January 2023).
- Shea, M.K.; Booth, S.L. Vitamin K. Adv. Nutr. 2022, 13, 350. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, E.; Lengyel, I.; Parravano, M.; Biagini, I.; Veldsman, M.; Badhwar, A.; Betts, M.; Cherubini, A.; Llewellyn, D.J.; Lourida, I.; et al. Ocular Biomarkers for Alzheimer Disease Dementia: An Umbrella Review of Systematic Reviews and Meta-Analyses. JAMA Ophthalmol. 2023, 141, 84. [Google Scholar] [CrossRef] [PubMed]
- Barrett-Young, A. An Eye on Clinical Translation-Considerations for Research Into Ocular Biomarkers of Alzheimer Disease and Related Dementias. JAMA Ophthalmol. 2023, 141, 4959. [Google Scholar] [CrossRef]
- Hoeft, B.; Weber, P.; Eggersdorfer, M. Micronutrients—A Global Perspective on Intake, Health Benefits and Economics. Int. J. Vitam. Nutr. Res. 2012, 82, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Victor, L.; Fulgoni, I.I.I. Foods, Fortificants, and Supplements: Where Do Americans Get Their Nutrients? J. Nutr. 2011, 141, 1847. [Google Scholar] [CrossRef]
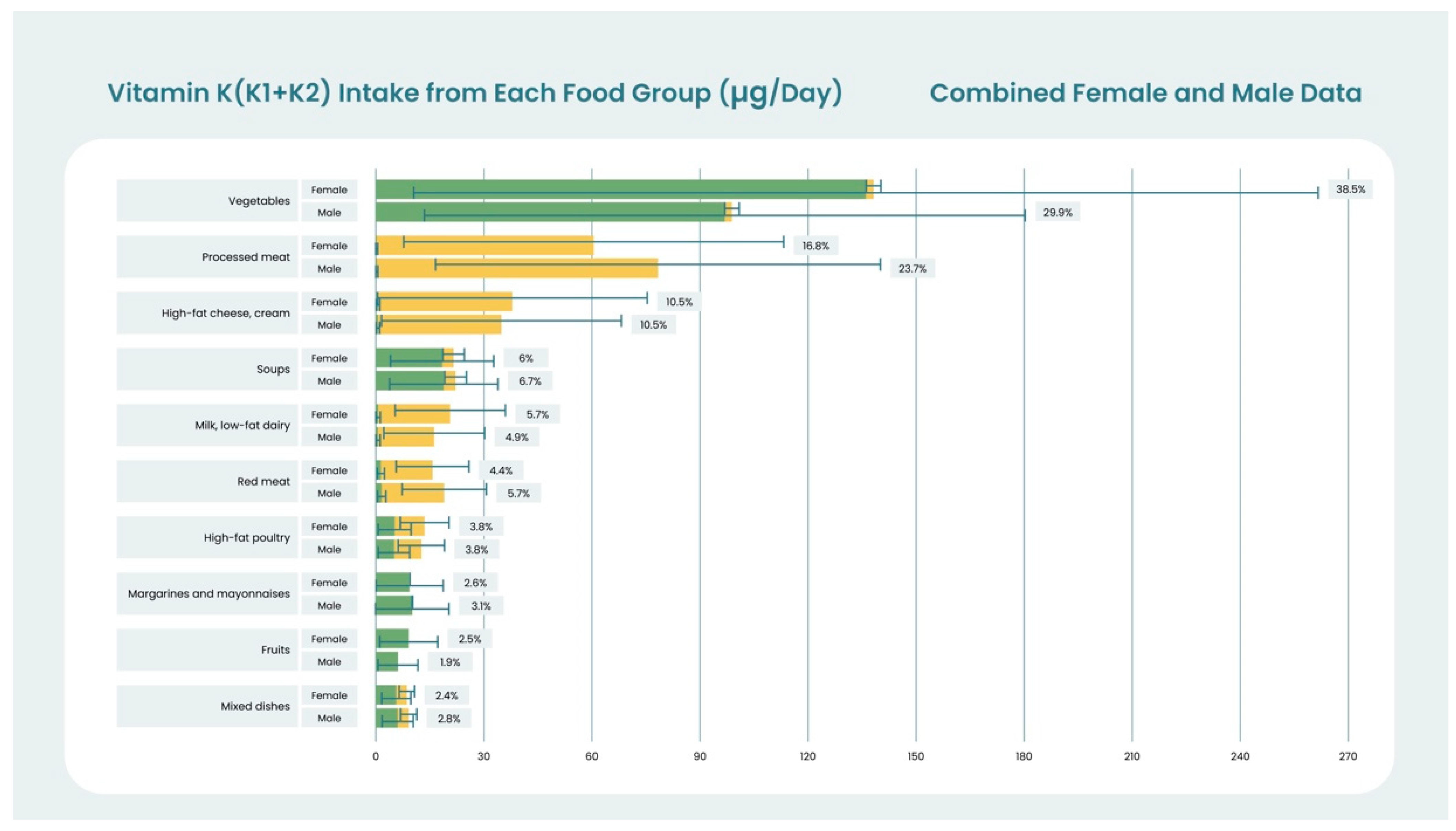
| Phylloquinone-Rich Food Description | Serving Weight (g) | PK (μg/100g) | Serving Measure | Serving PK (μg) |
|---|---|---|---|---|
| Spinach, raw | 340 | 483 | 1 bunch | 1640 |
| Cabbage, cooked, boiled, without salt | 1262 | 109 | 1 head | 1370 |
| Endive, raw | 513 | 231 | 1 head | 1180 |
| Turnip greens, frozen, cooked, drained | 220 | 519 | 10 oz package | 1140 |
| Broccoli, cooked | 437 | 256 | 1 bunch cooked | 1120 |
| Collards, frozen, chopped, cooked | 170 | 623 | 1 cup, chopped | 1060 |
| Parsley, fresh | 60 | 1640 | 1 cup chopped | 984 |
| Kale, frozen, unprepared | 284 | 334 | 1 package (10 oz) | 947 |
| Menaquinone-4-Rich Food Description | Serving Weight (g) | MK (μg/100g) | Serving Measure | Serving MK4 (μg) |
| Kielbasa, fully cooked, grilled | 20.1 | 367 | 1 link | 73.8 |
| Pepperoni, beef and pork, sliced | 41.7 | 85 | 1 serving (3 oz) | 35.4 |
| Butter, whipped, with salt | 20.9 | 151 | 1 cup | 31.6 |
| Chicken, broilers or fryers, drumstick | 35.7 | 85 | 1 serving (3oz) | 30.3 |
| Meatballs, frozen, Italian style | 28.1 | 85 | 1 serving (3 oz) | 23.9 |
| Frankfurter, meat and poultry, cooked | 35.6 | 48 | 1 frankfurter | 17.1 |
| Baby food, meat, turkey, junior | 18.7 | 68 | 1 container | 12.7 |
| Baby food, meat, turkey, junior | 18.7 | 68 | 1 container | 12.7 |
| Sausage, turkey, breakfast links, mild | 36.6 | 27.9 | 1 link | 10.2 |
| Salami, cooked, beef and pork | 28 | 12.3 | 1 slice round | 3.4 |
| Cheese, cheddar | 9.3 | 105 | 1 cup | 9.8 |
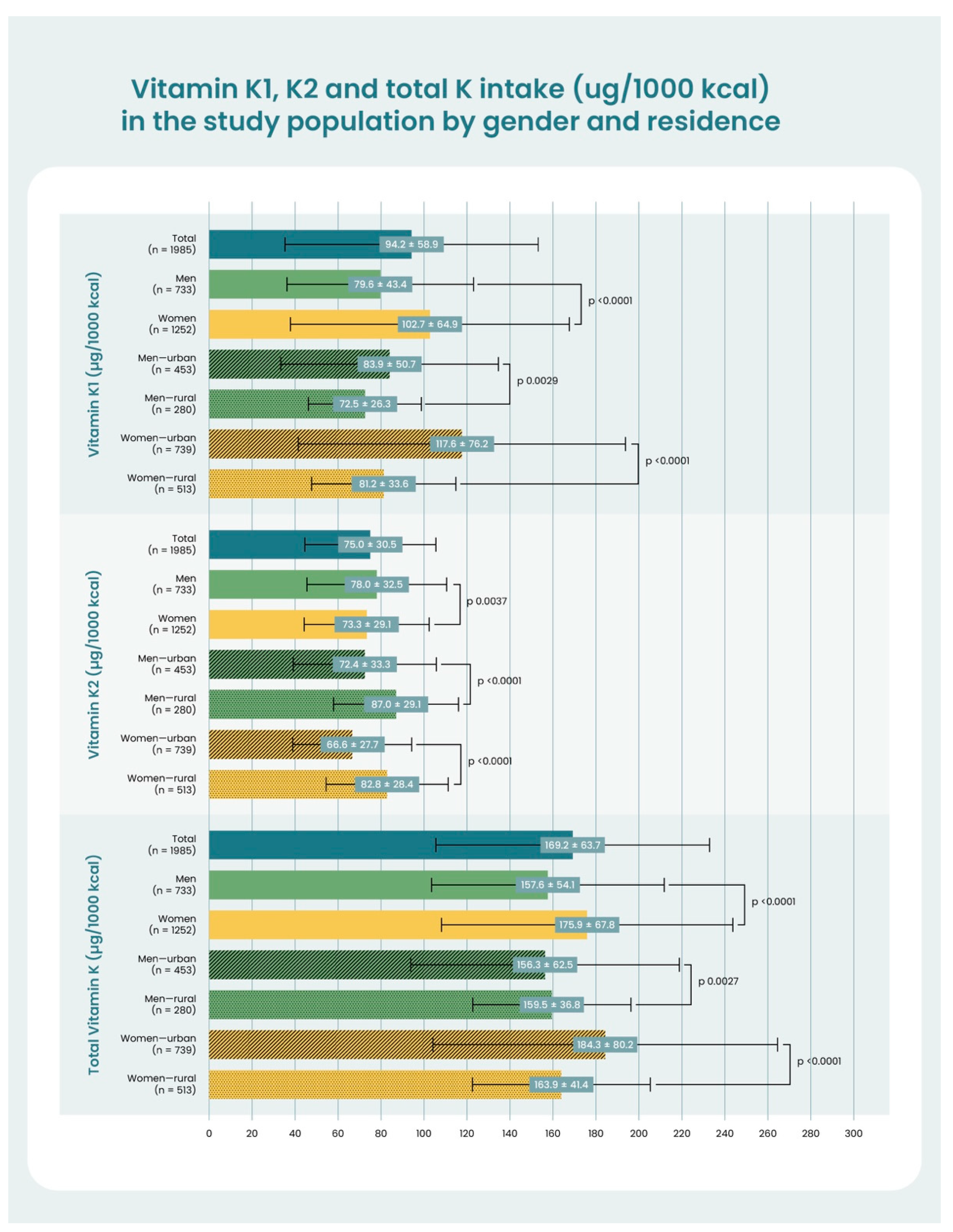
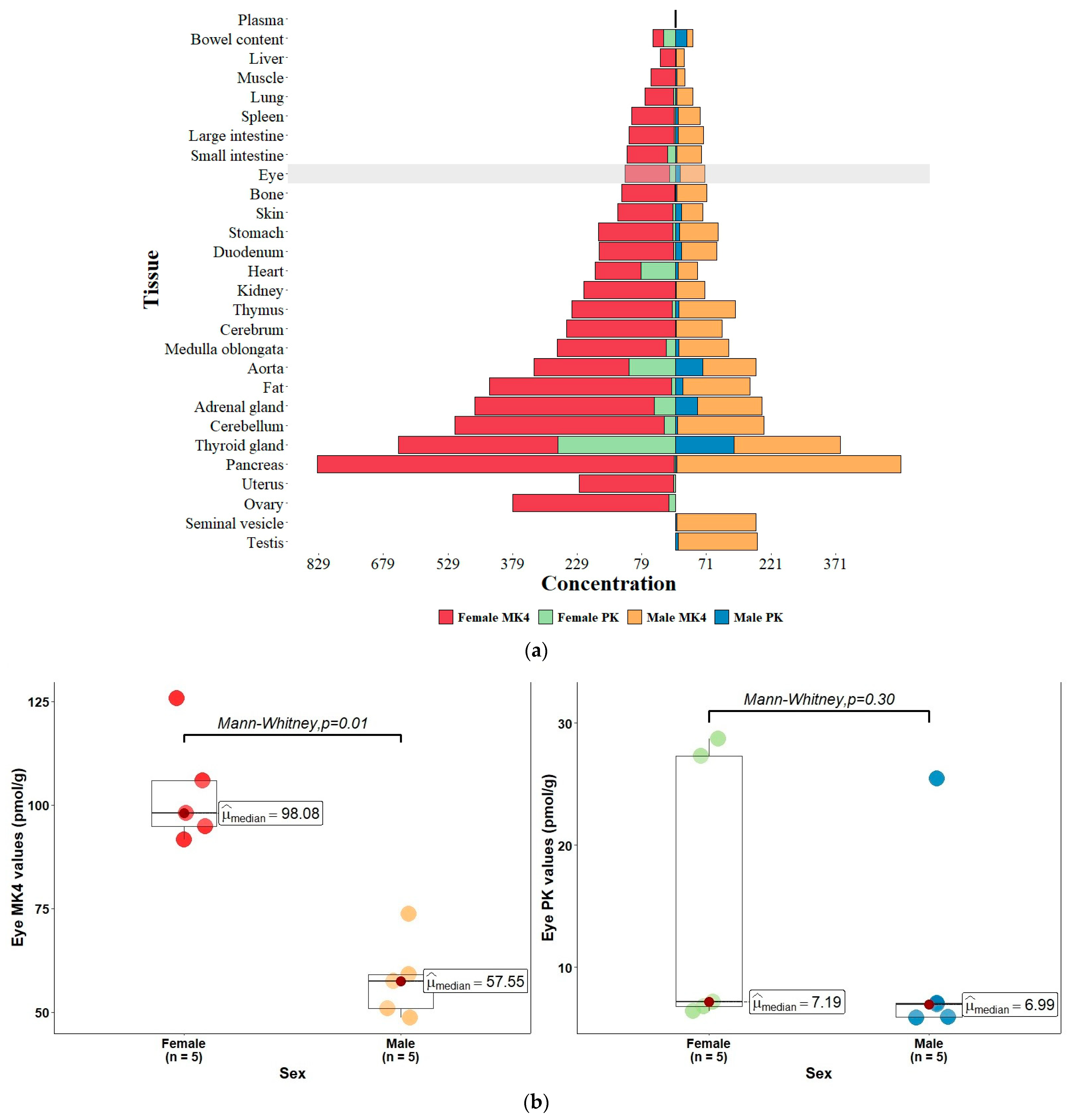
| Menaquinone-4 (MK-4) | Phylloquinone (PK) | |||||
|---|---|---|---|---|---|---|
| Tissue | Female (n = 5) | Male (n = 5) | Female:Male Ratio | Female (n = 5) | Male (n = 5) | Female:Male Ratio |
| Eye tissue | ||||||
| Eye | 103.3 ± 6.1 | 58.0 ± 4.4 | 1.8 (p = 0.01) | 15.3 ± 5.2 | 10.3 ± 3.8 | 1.5 (p = 0.3) |
| Brain tissue | ||||||
| Cerebellum | 487.7 ± 28.2 | 200.5 ± 17.5 | 2.4 | 26.2 ± 21.5 | 4.8 ± 1.8 | 5.4 |
| Medulla oblongata | 253.3 ± 5.5 | 116.2 ± 8.3 | 2.1 | 22.4 ± 15.5 | 7.6 ± 2.8 | 2.9 |
| Cerebrum | 252.5 ± 10.3 | 106.0 ± 8.2 | 2.3 | 1.1 ± 0.4 | 1.4 ± 0.7 | 0.8 |
| Other tissues in descending female MK-4 concentration | ||||||
| Pancreas | 829.4 ± 56.7 | 520.1 ± 47.4 | 1.6 | 3.2 ± 0.4 | 3.0 ± 0.6 | 1.0 |
| Fat | 423.0 ± 60.1 | 155.2 ± 37.2 | 2.7 | 10.3 ± 2.1 | 17.4 ± 7.5 | 0.6 |
| Adrenal gland | 417.5 ± 139.6 | 148.6 ± 14.7 | 2.8 | 50.7 ± 10.6 | 50.7 ± 10.9 | 1 |
| Thyroid gland | 370.3 ± 64.0 | 247.3 ± 30.4 | 1.4 | 274.8 ± 140.3 | 134.9 ± 45.5 | 2.0 |
| Ovary | 363.4 ± 35.6 | NA | 16.2 ± 3.4 | NA | ||
| Thymus | 232.5 ± 12.0 | 131.2 ± 5.5 | 1.8 | 9.0 ± 3.9 | 7.5 ± 1.2 | 1.2 |
| Aorta | 220.0 ± 8.8 | 124.0 ± 13.7 | 1.7 | 109.2 ± 45.2 | 62.2 ± 32.6 | 1.7 |
| Uterus | 219.6 ± 31.8 | NA | 4.9 ± 1.2 | NA | ||
| Kidney | 212.7 ± 20.8 | 66.1 ± 4.9 | 3.2 | 1.7 ± 0.2 | 1.1 ± 0.1 | 1.5 |
| Duodenum | 172.8 ± 11.5 | 82.7 ± 6.0 | 2.1 | 5.0 ± 0.5 | 13.2 ± 8.8 | 0.4 |
| Stomach | 171.7 ± 14.4 | 90.4 ± 6.1 | 1.9 | 7.7 ± 1.1 | 7.8 ± 1.2 | 0.8 |
| Skin | 128.2 ± 26.2 | 50.2 ± 7.5 | 2.5 | 6.1 ± 1.3 | 12.7 ± 6.2 | 0.5 |
| Bone | 122.7 ± 10.2 | 69.8 ± 9.2 | 1.7 | 2.2 ± 0.5 | 3.2 ± 1.4 | 0.7 |
| Heart | 107.7 ± 10.0 | 46.4 ± 4.2 | 2.3 | 80.4 ± 76.3 | 4.9 ± 2.3 | 16 |
| Large intestine | 106.1 ± 13.1 | 60.1 ± 5.4 | 1.7 | 3.1 ± 0.7 | 5.5 ± 2.1 | 0.6 |
| Spleen | 100.6 ± 9.0 | 50.9 ± 5.0 | 2 | 3.9 ± 0.3 | 4.6 ± 0.9 | 0.8 |
| Small intestine | 93.6 ± 6.8 | 57.8 ± 7.8 | 1.6 | 19.7 ± 16.7 | 3.1 ± 0.6 | 6.3 |
| Lung | 67.1 ± 5.4 | 38.1 ± 4.2 | 1.8 | 5.5 ± 2.9 | 2.1 ± 0.4 | 2.5 |
| Muscle | 56.3 ± 7.5 | 19.1 ± 1.5 | 2.9 | 1.4 ± 0.2 | 2.2 ± 0.8 | 0.4 |
| Liver | 35.0 ± 2.2 | 18.2 ± 2.7 | 1.9 | 1.5 ± 0.2 | 1.2 ± 0.1 | 1.1 |
| Bowel content | 24.6 ± 4.7 | 13.6 ± 3.7 | 1.8 | 27.8 ± 2.1 | 24.9 ± 2.9 | 1.1 |
| Plasma | 1.2 ± 0.1 | 0.7 ± 0.1 | 1.7 | 0.6 ± 0.0 | 0.6 ± 0.0 | 1 |
| Testis | 185.3 ± 3.3 | NA | 5.5 ± 2.5 | NA | ||
| Seminal vesicle | 184.2 ± 9.1 | NA | 2.2 ± 0.6 | NA | ||
| Author, Year Country [Ref] | Location | Protein [Number of Gla Residues] | Expression | Function |
|---|---|---|---|---|
| Sarosiak et al., 2021 Poland [98] | Human cornea | Matrix Gla Protein (MGP) [5 Gla] | Epithelium, stromal fibroblasts, and keratinocytes | Essentially unknown, possible maintenance of calcium homeostasis and anti-calcification role. Possible role in calcium signaling of epithelial proliferation and differentiation [52] |
| Borrás et al., 2000–2021 USA [93,95,96,97] | Human and mouse trabecular meshwork (TM), peripapillary sclera (SC), retinal vascular smooth muscle cells (VSMC) | MGP [5 Gla] | TM, SC, capillaries, and pericytes | Essential to maintain physiological IOP |
| Ayala et al., 2007 USA [104] | Human cornea | Prothrombin [10 Gla] | All layers of the cornea, intracellularly and in extracellular matrix | Production of thrombin, corneal wound healing, regulation of growth factors, and other signaling molecules via protease-activated receptors. |
| Hall et al., 2001–2005 USA [101,102,103] | Rat photoreceptor outer segments (POS), retinal pigment epithelium (RPE) | Growth Arrest Specific 6 (Gas6) [11 Gla], Protein S (PROS1) [11 Gla] | RPE, POS | Phagocytosis of POS by the overlying RPE via vitamin K-dependent gamma-carboxylation of Gas6 and PS in a Ca++-mediated linking of POS to RPE Mer receptors for phagocytosis |
| Valverde et al., 2004 USA [100] | Vitreous humor | Gas6 [11 Gla] | Bovine vitreous humor | Gas6/Axl signaling in lens epithelial cells support cell growth and survival. |
| Collett et al., 2003 UK [99] | Bovine retinal capillary pericytes | Gas6 [11 Gla] | Bovine retinal capillary pericytes | Interactions in regulating osteogenesis and diseases involving ectopic calcification. |
| Canfield et al., 2000 UK [94] | Bovine retinal capillary pericytes | MGP [5 Gla] | Bovine retinal capillary pericytes | Regulation of cell differentiation and calcification |
| Author, Year, Country [Ref] | Subjects | Design | Intervention | Findings |
|---|---|---|---|---|
| Coleman et al., 2008 USA [116] | 1155 women with glaucoma in at least one eye | Observational Cross-sectional cohort | Block Food Frequency Questionnaire | Decreased glaucoma risk by 69% (odds ratio [OR], 0.31; 95% confidence interval [CI], 0.11 to 0.91) in women who consumed ≥one serving per month of collard greens and kale compared with those who consumed <than one serving per month. |
| Moïse et al., 2012 Africa [117] | 500 African type II diabetics | Cross-sectional design | Mediterranean-style dietary score (MSDPS) using the Harvard semi quantitative FFQ adapted for Africa. | Regular intake of the Mediterranean Diet and the consumption of locally grown vegetables including Brassica Rapa, dry beans, Abelmoschus esculentus, and Musa acuminata significantly reduced the absolute risk of glaucoma. |
| Giaconi et al., 2012 USA [118] | 584 African-American women participants in the Study of Osteoporotic Fractures | Observational Cross-sectional cohort | The Block Food Frequency Questionnaire | >1 serving/week compared to ≤1 serving/month of collard greens/kale decreased the odds of glaucoma by 57% (OR = 0.43; 95% CI: 0.21–0.85) |
| Kang et al., 2016 USA [119] | Prospective cohorts of the Nurses’ Health Study (63,893 women; 1984–2012) and the Health Professionals Follow-up Study (41,094 men; 1986–2012) | Prospective cross-sectional observational study | Primary exposure was dietary nitrate intake. Information on diet and potential confounders was updated with validated questionnaires | Very robust study demonstrating greater intake of dietary nitrate and green leafy vegetables was associated with a 20% to 30% lower POAG risk; and a 40–50% lower risk for POAG with early paracentral VF loss at diagnosis, in 1483 incident cases identified in 63,893 women and 41,094 men followed for more than 25 years. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mong, M.A. Vitamin K and the Visual System—A Narrative Review. Nutrients 2023, 15, 1948. https://doi.org/10.3390/nu15081948
Mong MA. Vitamin K and the Visual System—A Narrative Review. Nutrients. 2023; 15(8):1948. https://doi.org/10.3390/nu15081948
Chicago/Turabian StyleMong, Michael A. 2023. "Vitamin K and the Visual System—A Narrative Review" Nutrients 15, no. 8: 1948. https://doi.org/10.3390/nu15081948
APA StyleMong, M. A. (2023). Vitamin K and the Visual System—A Narrative Review. Nutrients, 15(8), 1948. https://doi.org/10.3390/nu15081948





