Vitamin D Status Determines Cardiometabolic Effects of Cabergoline in Women with Elevated Prolactin Levels: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
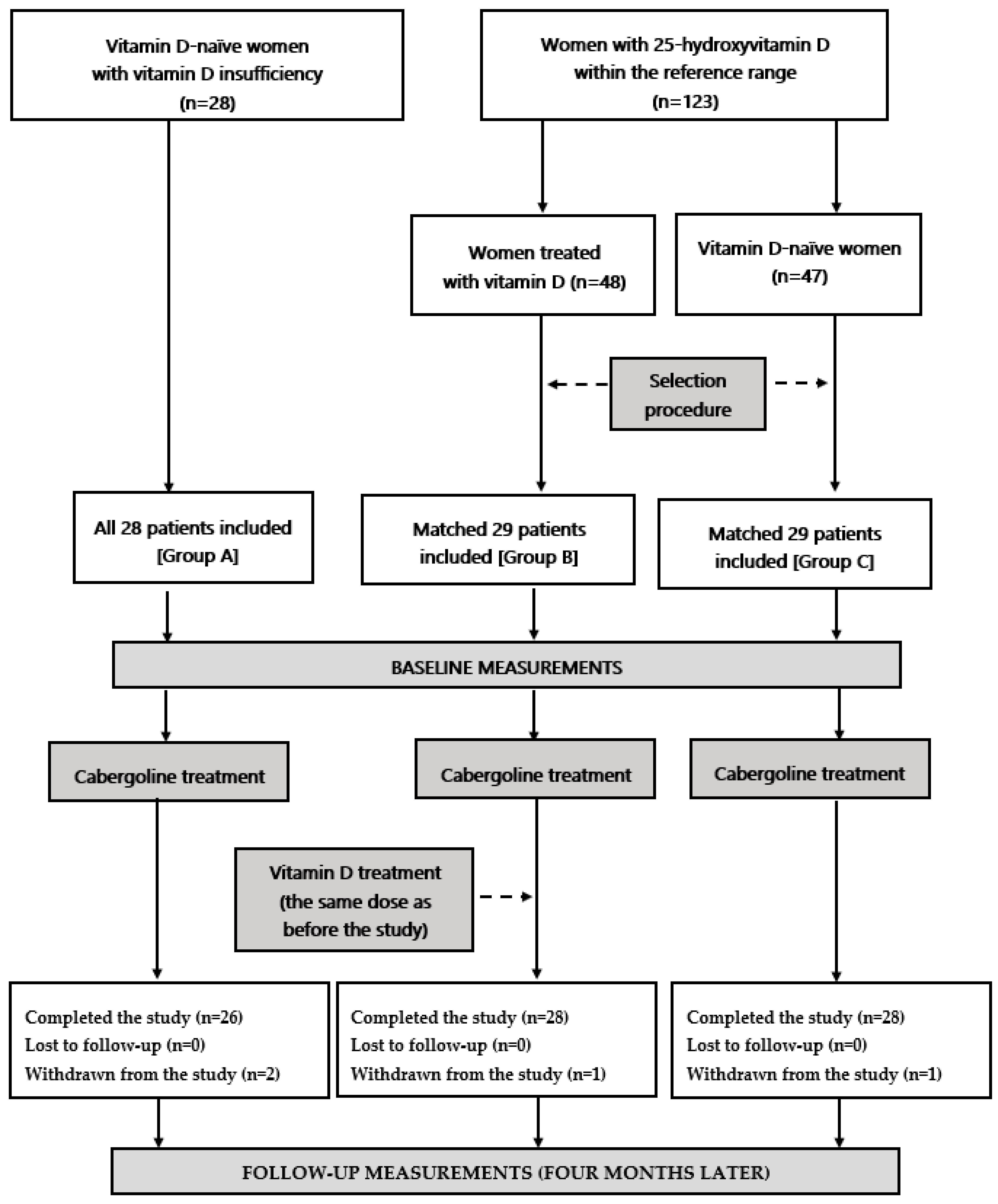
2.1. Patients
2.2. Study Design
2.3. Laboratory Assays
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Auriemma, R.S.; De Alcubierre, D.; Pirchio, R.; Pivonello, R.; Colao, A. The effects of hyperprolactinemia and its control on metabolic diseases. Expert Rev. Endocrinol. Metab. 2018, 13, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Pirchio, R.; Graziadio, C.; Colao, A.; Pivonello, R.; Auriemma, R.S. Metabolic effects of prolactin. Front. Endocrinol. 2022, 13, 1015520. [Google Scholar] [CrossRef] [PubMed]
- Gierach, M.; Bruska-Sikorska, M.; Rojek, M.; Junik, R. Hyperprolactinemia and insulin resistance. Endokrynol. Pol. 2022, 73, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Auriemma, R.S.; De Alcubierre, D.; Pirchio, R.; Pivonello, R.; Colao, A. Glucose abnormalities associated to prolactin secreting pituitary adenomas. Front. Endocrinol. 2019, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.B.; Li, C.L.; He, D.S.; Mao, Z.G.; Liu, D.H.; Fan, X.; Hu, B.; Zhu, Y.H.; Wang, H.J. Increased carotid intima media thickness is associated with prolactin levels in subjects with untreated prolactinoma: A pilot study. Pituitary 2014, 17, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Reuwer, A.Q.; van Eijk, M.; Houttuijn-Bloemendaal, F.M.; van der Loos, C.M.; Claessen, N.; Teeling, P.; Kastelein, J.J.; Hamann, J.; Goffin, V.; von der Thüsen, J.H.; et al. The prolactin receptor is expressed in macrophages within human carotid atherosclerotic plaques: A role for prolactin in atherogenesis? J. Endocrinol. 2011, 208, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, D.; Deyneli, O.; Akpinar, I.; Yildiz, E.; Gözü, H.; Sezgin, O.; Haklar, G.; Akalin, S. Endothelial function, insulin sensitivity and inflammatory markers in hyperprolactinemic premenopausal women. Eur. J. Endocrinol. 2003, 149, 187–193. [Google Scholar] [CrossRef]
- Krysiak, R.; Szkróbka, W.; Okopień, B. Different effects of atorvastatin on cardiometabolic risk factors in young women with and without hyperprolactinemia. J. Clin. Pharmacol. 2019, 59, 83–89. [Google Scholar] [CrossRef]
- Erem, C.; Kocak, M.; Nuhoglu, I.; Yılmaz, M.; Ucuncu, O. Blood coagulation, fibrinolysis and lipid profile in patients with prolactinoma. Clin. Endocrinol. 2010, 73, 502–507. [Google Scholar] [CrossRef]
- Haring, R.; Friedrich, N.; Völzke, H.; Vasan, R.S.; Felix, S.B.; Dörr, M.; Meyer zu Schwabedissen, H.E.; Nauck, M.; Wallaschofski, H. Positive association of serum prolactin concentrations with all-cause and cardiovascular mortality. Eur. Heart J. 2014, 35, 1215–1221. [Google Scholar] [CrossRef]
- Raaz, D.; Wallaschofski, H.; Stumpf, C.; Yilmaz, A.; Cicha, I.; Klinghammer, L. Increased prolactin in acute coronary syndromes as putative co-activator of ADP-stimulated P-selectin expression. Horm. Metab. Res. 2006, 38, 767–772. [Google Scholar] [CrossRef]
- Wallaschofski, H.; Lohmann, T.; Hild, E.; Kobsar, A.; Siegemund, A.; Spilcke-Liss, E.; Hentschel, B.; Stumpf, C.; Daniel, W.G.; Garlichs, C.D.; et al. Enhanced platelet activation by prolactin in patients with ischemic stroke. Thromb. Haemost. 2006, 96, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Szkróbka, W.; Okopień, B. Different effects of fenofibrate on cardiometabolic risk factors in young women with and without hyperprolactinemia. Pharmacol. Rep. 2019, 71, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Auriemma, R.S.; Pirchio, R.; De Alcubierre, D.; Pivonello, R.; Colao, A. Dopamine agonists: From the 1970s to today. Neuroendocrinology 2019, 109, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Greenman, Y.; Tordjman, K.; Stern, N. Increased body weight associated with prolactin secreting pituitary adenomas: Weight loss with normalization of prolactin levels. Clin. Endocrinol. 1998, 48, 547–553. [Google Scholar] [CrossRef]
- Berinder, K.; Nyström, T.; Höybye, C.; Hall, K.; Hulting, A.L. Insulin sensitivity and lipid profile in prolactinoma patients before and after normalization of prolactin by dopamine agonist therapy. Pituitary 2011, 14, 199–207. [Google Scholar] [CrossRef]
- dos Santos Silva, C.M.; Barbosa, F.R.; Lima, G.A.; Warszawski, L.; Fontes, R.; Domingues, R.C.; Gadelha, M.R. BMI and metabolic profile in patients with prolactinoma before and after treatment with dopamine agonists. Obesity 2011, 19, 800–805. [Google Scholar] [CrossRef]
- Ciresi, A.; Amato, M.C.; Guarnotta, V.; Lo Castro, F.; Giordano, C. Higher doses of cabergoline further improve metabolic parameters in patients with prolactinoma regardless of the degree of reduction in prolactin levels. Clin. Endocrinol. 2013, 79, 845–852. [Google Scholar] [CrossRef]
- Auriemma, R.S.; Galdiero, M.; Vitale, P.; Granieri, L.; Lo Calzo, F.; Salzano, C.; Ferreri, L.; Pivonello, C.; CariatI, F.; Coppola, G.; et al. Effect of cabergoline on metabolism in prolactinomas. Neuroendocrinology 2013, 98, 299–310. [Google Scholar] [CrossRef]
- Krysiak, R.; Okopień, B. Different effects of cabergoline and bromocriptine on metabolic and cardiovascular risk factors in patients with elevated prolactin levels. Basic Clin. Pharmacol. Toxicol. 2015, 116, 251–256. [Google Scholar] [CrossRef]
- Inancli, S.S.; Usluogullari, A.; Ustu, Y.; Caner, S.; Tam, A.A.; Ersoy, R.; Cakir, B. Effect of cabergoline on insulin sensitivity, inflammation, and carotid intima media thickness in patients with prolactinoma. Endocrine 2013, 44, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Brophy, J.M.; Suissa, S.; Renoux, C. Risks of cardiac valve regurgitation and heart failure associated with ergot- and non-ergot-derived dopamine agonist use in patients with Parkinson’s disease: A systematic review of observational studies. CNS Drugs 2015, 29, 985–998. [Google Scholar] [CrossRef] [PubMed]
- Daniel, J.B.; de Farias Costa, P.R.; Pereira, M.; Oliveira, A.M. Vitamin D deficiency and cardiometabolic risk factors in adolescents: Systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2022, 23, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Podzolkov, V.I.; Pokrovskaya, A.E.; Panasenko, O.I. Vitamin D deficiency and cardiovascular pathology. Ter. Arkh. 2018, 90, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Bolívar, V.; García-Fontana, B.; García-Fontana, C.; Muñoz-Torres, M. Mechanisms involved in the relationship between vitamin D and insulin resistance: Impact on clinical practice. Nutrients 2021, 13, 3491. [Google Scholar] [CrossRef]
- Daraghmeh, A.H.; Bertoia, M.L.; Al-Qadi, M.O.; Abdulbaki, A.M.; Roberts, M.B.; Eaton, C.B. Evidence for the vitamin D hypothesis: The NHANES III extended mortality follow-up. Atherosclerosis 2016, 25, 96–101. [Google Scholar] [CrossRef]
- Gouni-Berthold, I.; Berthold, H.K. Vitamin D and vascular disease. Curr. Vasc. Pharmacol. 2021, 19, 250–268. [Google Scholar] [CrossRef]
- Brewer, L.C.; Michos, E.D.; Reis, J.P. Vitamin D in atherosclerosis, vascular disease, and endothelial function. Curr. Drug Targets 2011, 12, 54–60. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; Costela-Ruiz, V.J.; García-Recio, E.; De Luna-Bertos, E.; Ruiz, C.; Illescas-Montes, R. Role of vitamin D in the metabolic syndrome. Nutrients 2021, 13, 830. [Google Scholar] [CrossRef]
- Krysiak, R.; Kowalcze, K.; Okopień, B. Vitamin D status determines the impact of metformin on circulating prolactin levels in premenopausal women. J. Clin. Pharm. Ther. 2021, 46, 1349–1356. [Google Scholar] [CrossRef]
- Krysiak, R.; Szkróbka, W.; Okopień, B. Alternative treatment strategies in women poorly tolerating moderate doses of bromocriptine. Exp. Clin. Endocrinol. Diabetes 2017, 125, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Gilowska, M.; Okopień, B. Different cardiometabolic effects of atorvastatin in men with normal vitamin D status and vitamin D insufficiency. Clin. Cardiol. 2016, 39, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Naci, H.; Brugts, J.J.; Fleurence, R.; Tsoi, B.; Toor, H.; Ades, A.E. Comparative benefits of statins in the primary and secondary prevention of major coronary events and all-cause mortality: A network meta-analysis of placebo-controlled and active-comparator trials. Eur. J. Prev. Cardiol. 2013, 20, 641–657. [Google Scholar] [CrossRef]
- Krysiak, R.; Kowalcze, K.; Okopień, B. The impact of vitamin D status on cardiometabolic effects of fenofibrate in women with atherogenic dyslipidemia. Clin. Exp. Pharmacol. Physiol. 2021, 48, 186–194. [Google Scholar] [CrossRef]
- Krysiak, R.; Kowalska, B.; Szkróbka, W.; Okopień, B. The association between macroprolactin levels and vitamin D status in premenopausal women with macroprolactinemia: A pilot study. Exp. Clin. Endocrinol. Diabetes 2015, 123, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Marek, B.; Okopień, B. Cardiometabolic risk factors in young women with macroprolactinaemia. Endokrynol. Pol. 2019, 70, 336–341. [Google Scholar] [CrossRef]
- Gozdzik, A.; Barta, J.L.; Weir, A.; Cole, D.E.; Vieth, R.; Whiting, S.J.; Parra, E.J. Serum 25-hydroxyvitamin D concentrations fluctuate seasonally in young adults of diverse ancestry living in Toronto. J. Nutr. 2010, 140, 2213–2220. [Google Scholar] [CrossRef]
- Fahie-Wilson, M.; Smith, T.P. Determination of prolactin: The macroprolactin problem. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 725–742. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Arslan, M.S.; Sahin, M.; Karakose, M.; Tutal, E.; Topaloglu, O.; Ucan, B.; Demirci, T.; Caliskan, M.; Ozdemir, S.; Ozbek, M.; et al. Serum levels of fibroblast growth factor-23, osteoprotegerin, and receptor activator of nuclear factor kappa B ligand in patients with prolactinoma. Endocr. Pract. 2017, 23, 266–270. [Google Scholar] [CrossRef]
- Aboelnaga, M.M.; Abdullah, N.; El Shaer, M. 5-hydroxyvitamin D correlation with prolactin levels and adenoma size in female patients with newly diagnosed prolactin secreting adenoma. Endocr. Metab. Immune Disord. Drug Targets 2017, 17, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Czech, M.P. The GLUT4 glucose transporter. Cell Metab. 2007, 5, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Tavares, G.; Marques, D.; Barra, C.; Rosendo-Silva, D.; Costa, A.; Rodrigues, T.; Gasparini, P.; Melo, B.F.; Sacramento, J.F.; Seiça, R.; et al. Dopamine D2 receptor agonist, bromocriptine, remodels adipose tissue dopaminergic signalling and upregulates catabolic pathways, improving metabolic profile in type 2 diabetes. Mol. Metab. 2021, 51, 101241. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Vitamin D up-regulates glucose transporter 4 (GLUT4) translocation and glucose utilization mediated by cystathionine-γ-lyase (CSE) activation and H2S formation in 3T3L1 adipocytes. J. Biol. Chem. 2012, 287, 42324–42332. [Google Scholar] [CrossRef] [PubMed]
- Garbossa, S.G.; Folli, F. Vitamin D, sub-inflammation and insulin resistance. A window on a potential role for the interaction between bone and glucose metabolism. Rev. Endocr. Metab. Disord. 2017, 18, 243–258. [Google Scholar] [CrossRef]
- Scott, D.L.; Wolfe, F.; Huizinga, T.W. Rheumatoid arthritis. Lancet 2010, 376, 1094–1108. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Zhang, C.; Chen, H.Y.; Zhang, Z.J.; Ji, Z.F.; Yue, T.; Dai, X.M.; Zhu, Q.; Ma, L.L.; He, D.Y.; et al. Dopamine receptor DR2 expression in B cells is negatively correlated with disease activity in rheumatoid arthritis patients. Immunobiology 2015, 220, 323–330. [Google Scholar] [CrossRef]
- Haeckel, R.; Wosniok, W. The phenomenon of regression-toward-the-mean. Clin. Chem. Lab. Med. 2004, 42, 241–242. [Google Scholar] [CrossRef]
| Variable | Group A | Group B | Group C | p-Value | ||
|---|---|---|---|---|---|---|
| A vs. B | A vs. C | B vs. C | ||||
| Number (n) | 26 | 28 | 28 | - | - | - |
| Age (years) | 32 ± 7 | 31 ± 7 | 32 ± 6 | 0.6021 | 1.0000 | 0.5628 |
| Smokers (%)/Number of cigarettes a day (n)/Duration of smoking (months) | 35/8 ± 5/125 ± 48 | 32/9 ± 5/131 ± 46 | 36/8 ± 4/120 ± 42 | 0.8081 | 0.7656 | 0.4046 |
| Reasons for prolactin excess (%): microprolactinoma/drug-induced hyperprolactinemia/traumatic brain injury/empty sella syndrome/idiopathic | 31/38/15/8/8 | 29/39/14/11/7 | 25/36/18/11/11 | 0.7875 | 0.8015 | 0.8268 |
| Oligomenorrhea (%) a,b | 77 | 71 | 75 | 0.3245 | 0.7405 | 0.5240 |
| BMI (kg/m2) | 24.0 ± 5.2 | 23.8 ± 5.0 | 23.6 ± 4.8 | 0.8860 | 0.7698 | 0.8792 |
| Waist circumference (cm) | 84 ± 9 | 83 ± 8 | 83 ± 7 | 0.6674 | 0.6492 | 1.0000 |
| Systolic blood pressure (mmHg) | 132 ± 14 | 130 ± 15 | 129 ± 16 | 0.6124 | 0.4641 | 0.8103 |
| Diastolic blood pressure (mmHg) | 86 ± 7 | 85 ± 7 | 84 ± 8 | 0.6021 | 0.3296 | 0.6207 |
| Variable | Group A | Group B | Group C |
|---|---|---|---|
| Total prolactin (ng/mL) | |||
| Baseline | 55.1 ± 12.1 | 56.7 ± 12.5 | 57.3 ± 11.8 |
| Follow-up | 19.1 ± 6.7 *#$ | 11.6 ± 5.0 $ | 12.0 ± 5.6 $ |
| Monomeric prolactin (ng/mL) | |||
| Baseline | 52.0 ± 11.7 | 53.2 ± 12.1 | 53.9 ± 11.4 |
| Follow-up | 16.3 ± 5.6 *#$ | 8.5 ± 4.0 $ | 8.8 ± 4.2 $ |
| Macroprolactin (ng/mL) | |||
| Baseline | 3.1 ± 1.2 | 3.5 ± 1.7 | 3.4 ± 1.4 |
| Follow-up | 2.8 ± 0.9 | 3.1 ± 1.5 | 3.2 ± 1.2 |
| 25-hydroxyvitamin D (nmol/L) | |||
| Baseline | 61.8 ± 5.8 *# | 107.2 ± 18.5 | 112.4 ± 17.2 |
| Follow-up | 62.4 ± 6.0 *# | 111.2 ± 17.5 | 114.0 ± 16.5 |
| Estradiol (pmol/L) | |||
| Baseline | 125 ± 40 | 134 ± 42 | 129 ± 37 |
| Follow-up | 203 ± 62 $ | 229 ± 70 $ | 220 ± 68 $ |
| Glucose (mg/dL) | |||
| Baseline | 93 ± 14 | 91 ± 11 | 92 ± 12 |
| Follow-up | 90 ± 10 | 86 ± 11 | 86 ± 12 |
| HOMA1-IR | |||
| Baseline | 3.5 ± 0.9 | 3.4 ± 1.0 | 3.5 ± 0.8 |
| Follow-up | 3.0 ± 0.8 *#$ | 2.2 ± 0.7 $ | 2.1 ± 0.8 $ |
| Glycated hemoglobin (%) | |||
| Baseline | 5.2 ± 0.3 | 5.3 ± 0.4 | 5.3 ± 0.3 |
| Follow-up | 5.1 ± 0.2 | 5.0 ± 0.3 $ | 5.1 ± 0.2 $ |
| Total cholesterol (mg/dL) | |||
| Baseline | 198 ± 32 | 202 ± 42 | 205 ± 39 |
| Follow-up | 196 ± 28 | 200 ± 37 | 198 ± 26 |
| HDL-cholesterol (mg/dL) | |||
| Baseline | 50 ± 8 | 52 ± 10 | 49 ± 10 |
| Follow-up | 52 ± 8 *# | 62 ± 11 $ | 59 ± 10 $ |
| LDL-cholesterol (mg/dL) | |||
| Baseline | 116 ± 28 | 120 ± 34 | 123 ± 32 |
| Follow-up | 114 ± 30 | 114 ± 24 | 112 ± 26 |
| Triglycerides (mg/dL) | |||
| Baseline | 144 ± 46 | 139 ± 36 | 147 ± 42 |
| Follow-up | 136 ± 40 *# | 112 ± 27 $ | 115 ± 30 $ |
| hsCRP (mg/L) | |||
| Baselin | 3.5 ± 1.1 | 3.0 ± 0.9 | 2.9 ± 1.2 |
| Follow-up | 2.8 ± 0.8 *#$ | 1.4 ± 0.7 $ | 1.2 ± 0.6 $ |
| Fibrinogen (mg/dL) | |||
| Baseline | 375 ± 82 | 385 ± 90 | 400 ± 105 |
| Follow-up | 360 ± 92 *# | 312 ± 86 $ | 308 ± 82 $ |
| Homocysteine (μmol/L) | |||
| Baseline | 30.7 ± 11.6 | 28.5 ± 10.6 | 32.4 ± 14.0 |
| Follow-up | 22.1 ± 8.1 *#$ | 11.8 ± 5.5 $ | 10.4 ± 4.3 $ |
| Uric acid (mg/dL) | |||
| Baseline | 5.0 ± 1.2 | 5.2 ± 2.0 | 4.8 ± 1.8 |
| Follow-up | 4.6 ± 1.4 *# | 3.4 ± 1.0 $ | 3.1 ± 1.2 $ |
| UACR (mg/g) | |||
| Baseline | 34.0 ± 11.5 | 36.8 ± 13.4 | 32.9 ± 12.5 |
| Follow-up | 30.6 ± 12.3 *# | 12.5 ± 8.9 $ | 13.2 ± 7.1 $ |
| Variable | Group A | Group B | Group C |
|---|---|---|---|
| Δ Total prolactin | −65 ± 12 | −80 ± 8 * | −79 ± 8 * |
| Δ Monomeric prolactin | −69 ± 10 | −84 ± 7 * | −84 ± 6 * |
| Δ Macroprolactin | −10 ± 8 | −11 ± 8 | −6 ± 7 |
| Δ25-hydroxyvitamin D | 1 ± 5 | 4 ± 8 | 1 ± 4 |
| Δ Estradiol | 62 ± 28 | 71 ± 26 | 70 ± 24 |
| Δ Glucose | −3 ± 8 | −5 ± 8 | −7 ± 10 |
| Δ HOMA1-IR | −14 ± 15 | −35 ± 20 * | −40 ± 22 * |
| Δ Glycated hemoglobin | −2 ± 6 | −6 ± 10 | −4 ± 6 |
| Δ Total cholesterol | −1 ± 10 | −1 ± 12 | −3 ± 16 |
| Δ HDL-cholesterol | 4 ± 8 | 19 ± 10 * | 20 ± 12 * |
| Δ LDL-cholesterol | −2 ± 10 | −5 ± 11 | −9 ± 18 |
| Δ Triglycerides | −8 ± 12 | −19 ± 20 * | −22 ± 18 * |
| Δ hsCRP | −20 ± 15 | −53 ± 18 * | −59 ± 22 * |
| Δ Fibrinogen | −4 ± 11 | −19 ± 15 * | −23 ± 16 * |
| Δ Homocysteine | −28 ± 20 | −59 ± 18 * | −68 ± 16 * |
| Δ Uric acid | −8 ± 15 | −35 ± 15 * | −35 ± 17 * |
| Δ UACR | −10 ± 20 | −66 ± 15 * | −60 ± 18 * |
| Δ BMI | −1 ± 2 | −2 ± 2 | −2 ± 3 |
| Variable | Adjusted Partial R2 for Prolactin | Adjusted Partial R2 for 25-Hydroxyvitamin D |
|---|---|---|
| HOMA1-IR | 0.321 *** | 0.278 *** |
| Glycated hemoglobin | 0.141 * | 0.008 * |
| HDL-cholesterol | 0.255 *** | 0.134 ** |
| Triglycerides | 0.243 *** | 0.197 *** |
| hsCRP | 0.314 *** | 0.328 *** |
| Fibrinogen | 0.247 *** | 0.295 *** |
| Homocysteine | 0.312 *** | 0.245 *** |
| Uric acid | 0.265 ** | 0.301 *** |
| UACR | 0.284 *** | 0.253 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krysiak, R.; Basiak, M.; Machnik, G.; Szkróbka, W.; Okopień, B. Vitamin D Status Determines Cardiometabolic Effects of Cabergoline in Women with Elevated Prolactin Levels: A Pilot Study. Nutrients 2023, 15, 2303. https://doi.org/10.3390/nu15102303
Krysiak R, Basiak M, Machnik G, Szkróbka W, Okopień B. Vitamin D Status Determines Cardiometabolic Effects of Cabergoline in Women with Elevated Prolactin Levels: A Pilot Study. Nutrients. 2023; 15(10):2303. https://doi.org/10.3390/nu15102303
Chicago/Turabian StyleKrysiak, Robert, Marcin Basiak, Grzegorz Machnik, Witold Szkróbka, and Bogusław Okopień. 2023. "Vitamin D Status Determines Cardiometabolic Effects of Cabergoline in Women with Elevated Prolactin Levels: A Pilot Study" Nutrients 15, no. 10: 2303. https://doi.org/10.3390/nu15102303
APA StyleKrysiak, R., Basiak, M., Machnik, G., Szkróbka, W., & Okopień, B. (2023). Vitamin D Status Determines Cardiometabolic Effects of Cabergoline in Women with Elevated Prolactin Levels: A Pilot Study. Nutrients, 15(10), 2303. https://doi.org/10.3390/nu15102303





