Proanthocyanidins Inhibit Osteoblast Apoptosis via the PI3K/AKT/Bcl-xL Pathway in the Treatment of Steroid-Induced Osteonecrosis of the Femoral Head in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice, Reagents, and Antibodies
2.2. Rat Model Establishment and Treatment
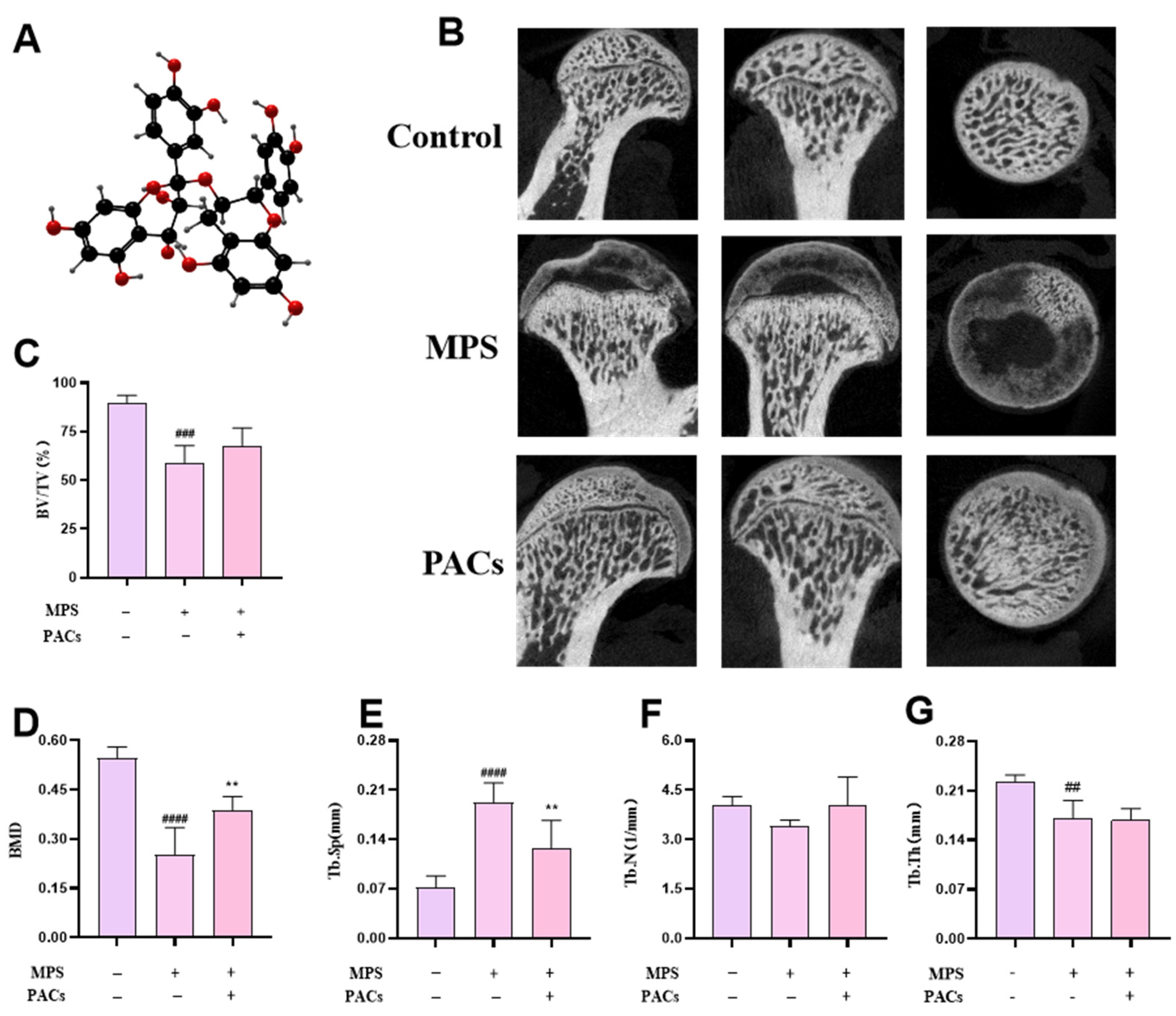
2.3. MicroCT to Detect Bone Density and Bone Microstructure of the Femur
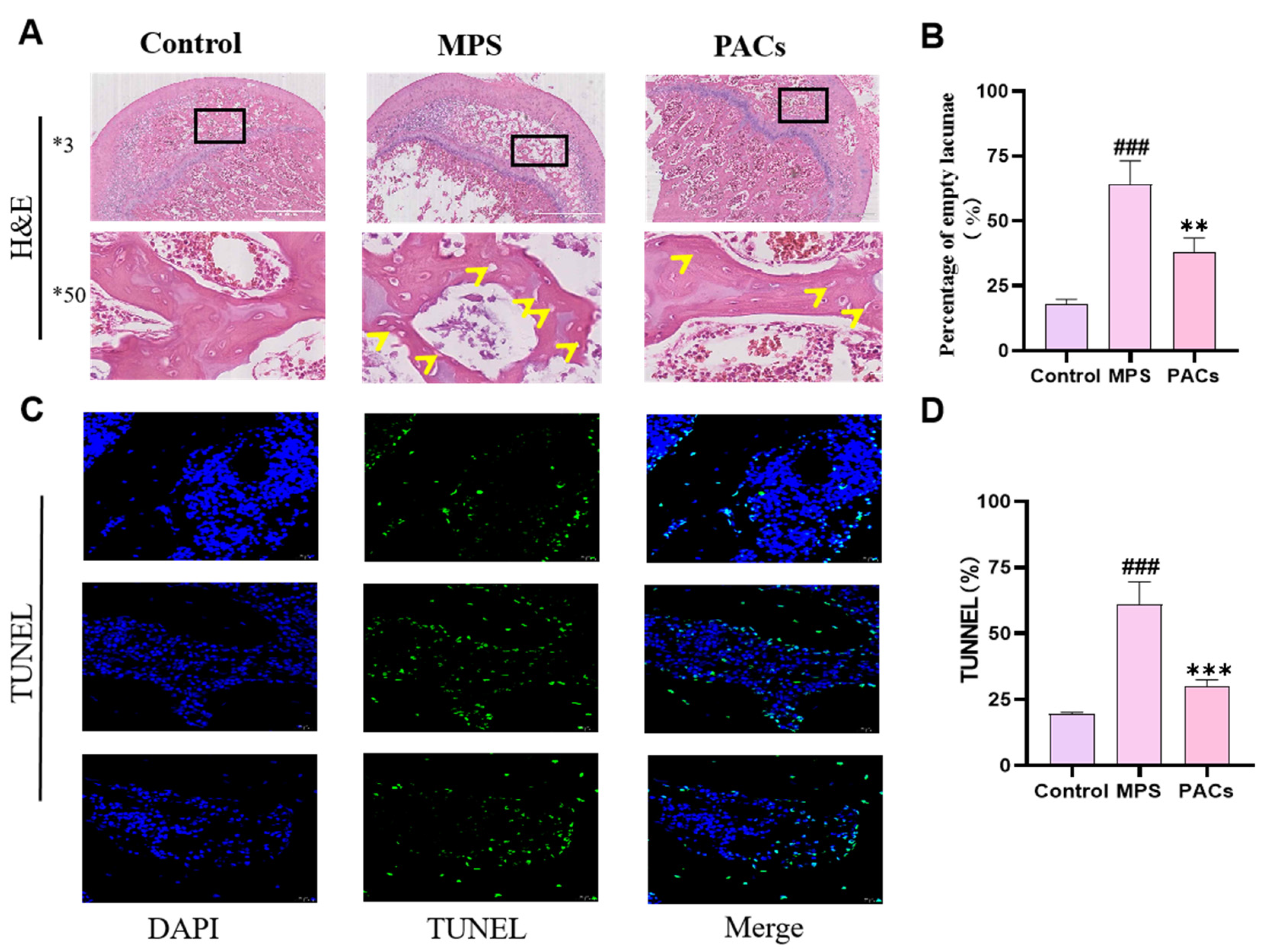
2.4. Hematoxylin and Eosin (H&E) and TdT-Mediated dUTP Nick End Labeling (TUNEL) Staining
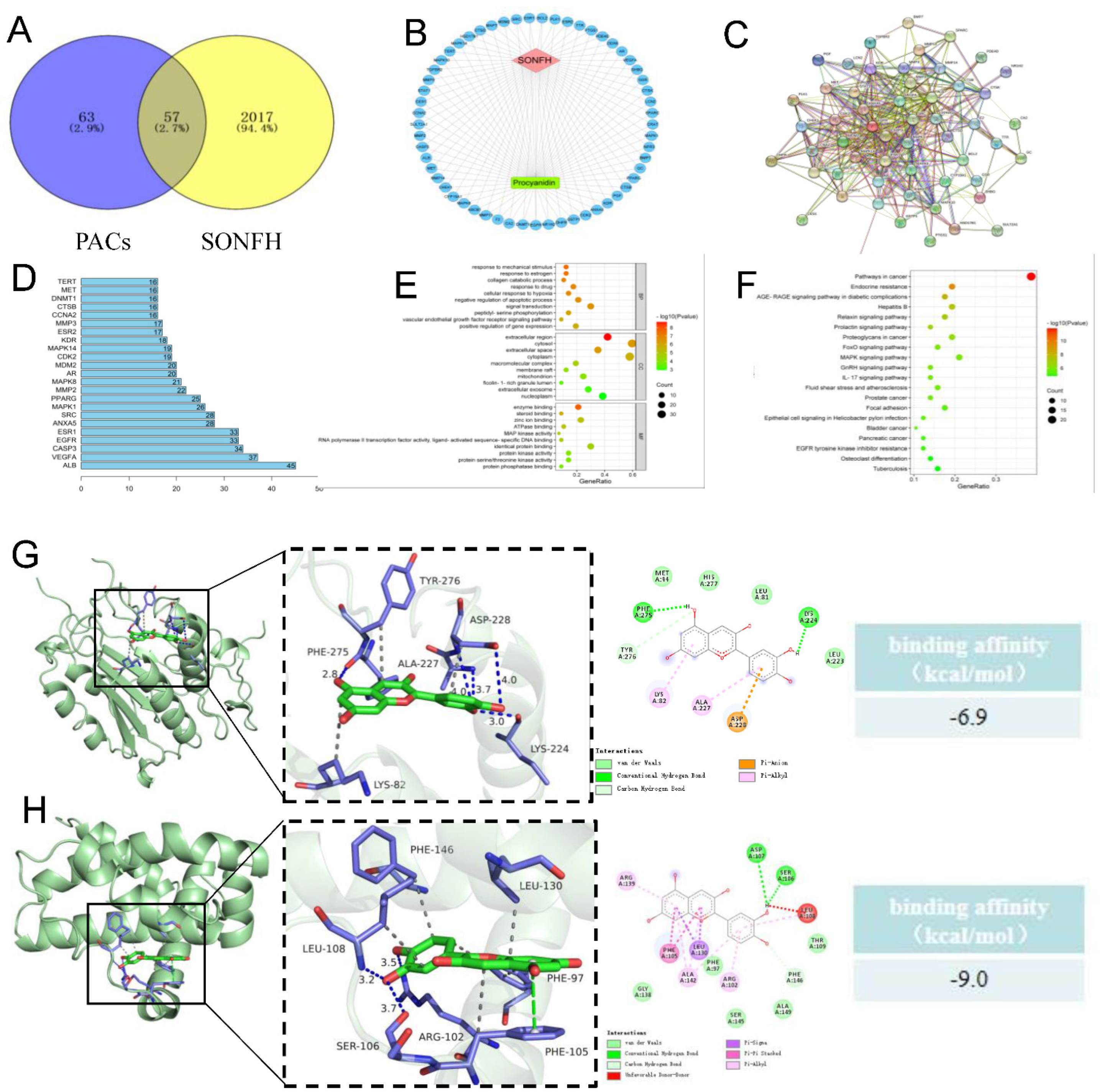
2.5. Network Pharmacology Analysis
2.6. Cell Culture and Grouping
2.6.1. Cell Culture
2.6.2. Cell Grouping
- Control group: DMEM containing 4% FBS was used for cell culture.
- Steroid group: DMEM containing 4% FBS and 100 μMDEX was used for cell culture.
- Different concentrations of proanthocyanidins group: MG63 cells were cultured with DMEM (4% FBS and 100 μMDEX) containing different concentrations of PACs prepared in advance and divided into 10 μM/mL group, 20 μM/mL group, and 40 μM/mL group according to different concentrations of PACs.
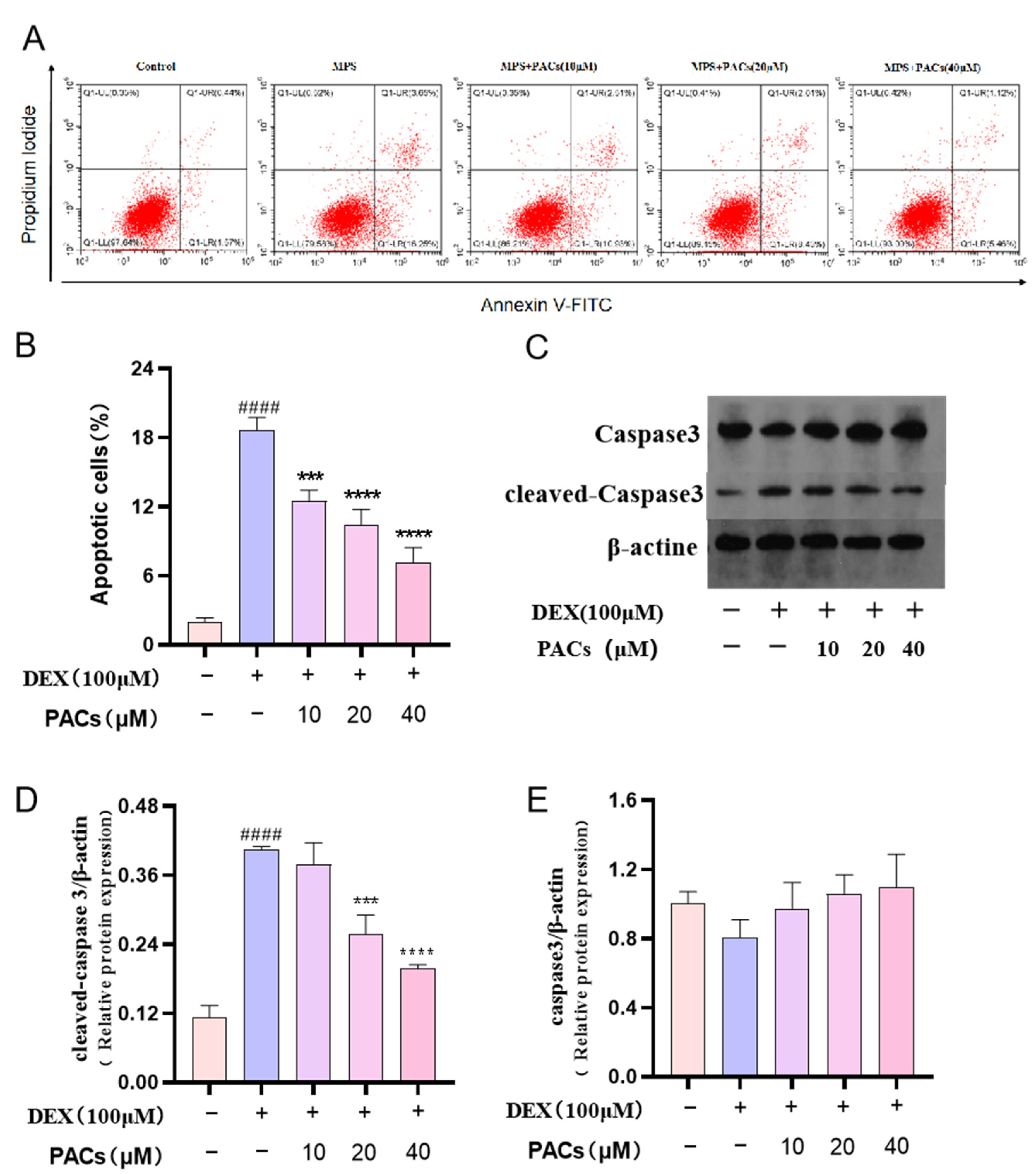
2.7. Cell Apoptosis Assay
2.8. Western Blot Analyses
2.9. Statistical Analysis
3. Results
3.1. Proanthocyanidins Treat Early DEX-Induced Bone Loss by Inhibiting Osteoblast Apoptosis In Vivo
3.2. Network Pharmacological Analysis of Proanthocyanidins and Steroid-Induced Femoral Head Necrosis
3.3. Proanthocyanidins Promote the Proliferation of MG-63 Human Osteogenic Sarcoma Cells and Inhibit DEX-Induced Apoptosis
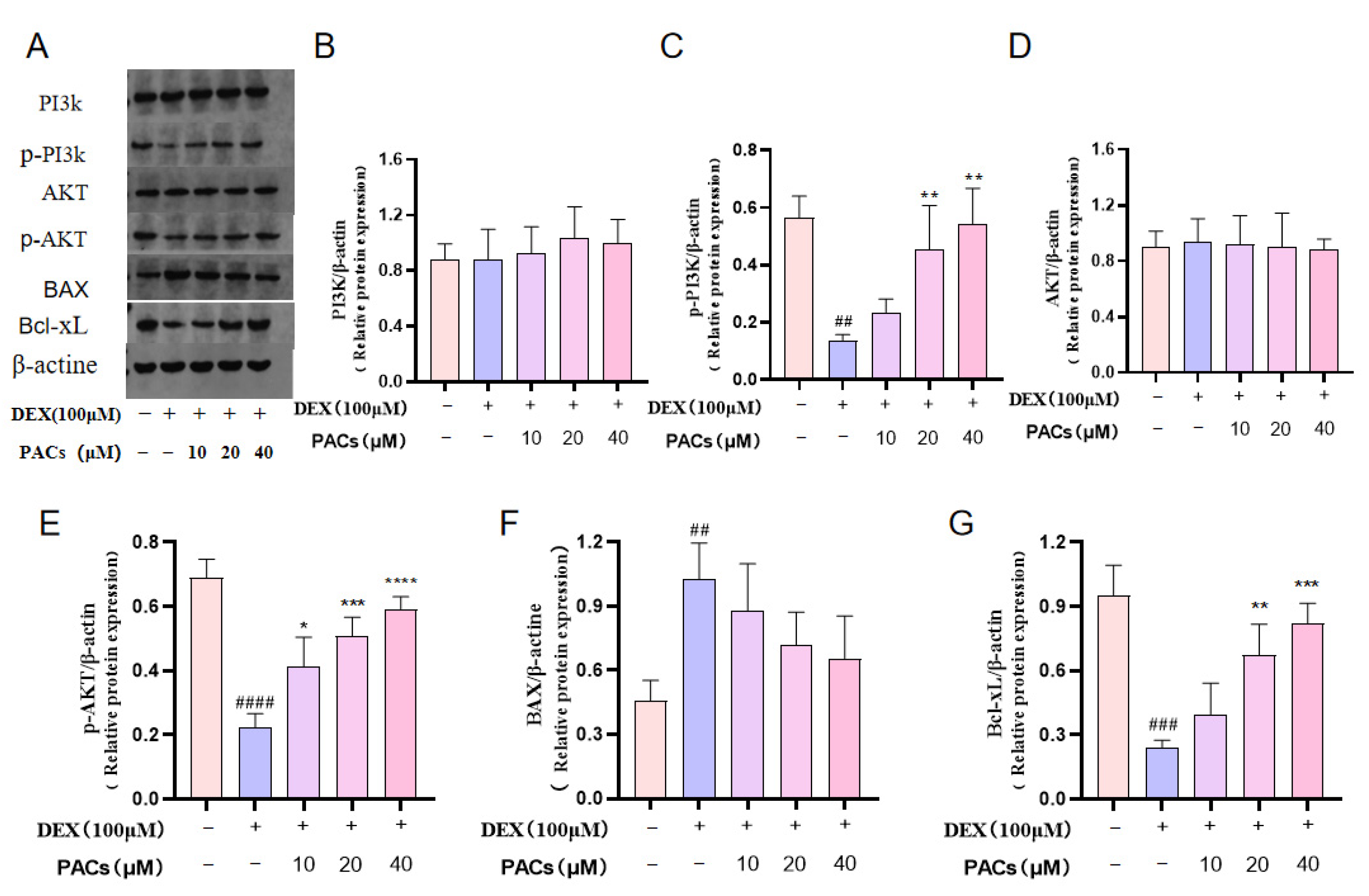
3.4. Proanthocyanidins Rescue DEX-Induced Apoptosis in Osteoblasts via the PI3KAKT Axis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barcellini, W.; Fattizzo, B. How I treat warm autoimmune hemolytic anemia. Blood 2021, 137, 1283–1294. [Google Scholar] [CrossRef] [PubMed]
- Natale, P.; Palmer, S.C.; Ruospo, M.; Saglimbene, V.M.; Craig, J.C.; Vecchio, M.; Samuels, J.A.; Molony, D.A.; Schena, F.P.; Strippoli, G.F. Immunosuppressive agents for treating IgA nephropathy. Cochrane Database Syst. Rev. 2020, 3, D3965. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Li, W.; Zeng, P.; Guo, H.; Huang, Z.; Fu, F.; Gao, H.; Wang, R.; Chen, W. Epidemiological Study Based on China Osteonecrosis of the Femoral Head Database. Orthop. Surg. 2021, 13, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Ando, W.; Fukushima, W.; Sakai, T.; Hamada, H.; Takao, M.; Ito, K.; Sugano, N. Epidemiological study of osteonecrosis of the femoral head using the national registry of designated intractable diseases in Japan. Mod. Rheumatol. 2022, 32, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Nugent, M.; Young, S.W.; Frampton, C.M.; Hooper, G.J. The lifetime risk of revision following total hip arthroplasty. Bone Joint J. 2021, 103, 479–485. [Google Scholar] [CrossRef]
- Kwak, S.C.; Cheon, Y.H.; Lee, C.H.; Jun, H.Y.; Yoon, K.H.; Lee, M.S.; Kim, J.Y. Grape Seed Proanthocyanidin Extract Prevents Bone Loss via Regulation of Osteoclast Differentiation, Apoptosis, and Proliferation. Nutrients 2020, 12, 3164. [Google Scholar] [CrossRef]
- Martins, T.F.; Palomino, O.M.; Álvarez-Cilleros, D.; Martín, M.A.; Ramos, S.; Goya, L. Cocoa Flavanols Protect Human Endothelial Cells from Oxidative Stress. Plant Foods Hum. Nutr. 2020, 75, 161–168. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, X.; Yang, W.; Gui, S. Procyanidin B2 Suppresses Lipopolysaccharides-Induced Inflammation and Apoptosis in Human Type II Alveolar Epithelial Cells and Lung Fibroblasts. J. Interferon Cytokine Res. 2020, 40, 54–63. [Google Scholar] [CrossRef]
- Wu, X.; Yu, H.; Zhou, H.; Li, Z.; Huang, H.; Xiao, F.; Xu, S.; Yang, Y. Proanthocyanidin B2 inhibits proliferation and induces apoptosis of osteosarcoma cells by suppressing the PI3K/AKT pathway. J. Cell. Mol. Med. 2020, 24, 11960–11971. [Google Scholar] [CrossRef]
- Song, Q.; Shi, Z.; Bi, W.; Liu, R.; Zhang, C.; Wang, K.; Dang, X. Beneficial effect of grape seed proanthocyanidin extract in rabbits with steroid-induced osteonecrosis via protecting against oxidative stress and apoptosis. J. Orthop. Sci. 2015, 20, 196–204. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network pharmacology. Nat. Biotechnol. 2007, 25, 1110–1111. [Google Scholar] [CrossRef]
- Sun, F.; Zhou, J.L.; Wei, S.X.; Jiang, Z.W.; Peng, H. Glucocorticoids induce osteonecrosis of the femoral head in rats via PI3K/AKT/FOXO1 signaling pathway. PeerJ 2022, 10, e13319. [Google Scholar] [CrossRef] [PubMed]
- Nogales, C.; Mamdouh, Z.M.; List, M.; Kiel, C.; Casas, A.I.; Schmidt, H. Network pharmacology: Curing causal mechanisms instead of treating symptoms. Trends Pharmacol. Sci. 2022, 43, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Jin, H.; Zhang, X.; Li, W.; Wang, D.; Tong, P.; Liu, Y.; Tan, Z. Aucubin prevents steroid-induced osteoblast apoptosis by enhancing autophagy via AMPK activation. J. Cell. Mol. Med. 2021, 25, 10175–10184. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Liu, H.; Lei, W. MiR-596 inhibits osteoblastic differentiation and cell proliferation by targeting Smad3 in steroid-induced osteonecrosis of femoral head. J. Orthop. Surg. Res. 2020, 15, 173. [Google Scholar] [CrossRef]
- Fan, Z.Q.; Bai, S.C.; Xu, Q.; Li, Z.J.; Cui, W.H.; Li, H.; Li, X.H.; Zhang, H.F. Oxidative Stress Induced Osteocyte Apoptosis in Steroid-Induced Femoral Head Necrosis. Orthop. Surg. 2021, 13, 2145–2152. [Google Scholar] [CrossRef]
- Kong, X.Y.; Wang, R.T.; Tian, N.; Li, L.; Lin, N.; Chen, W.H. Effect of Huogu II Formula (II) with medicinal guide Radix Achyranthis Bidentatae on bone marrow stem cells directional homing to necrosis area after osteonecrosis of the femoral head in rabbit. Chin. J. Integr. Med. 2012, 18, 761–768. [Google Scholar] [CrossRef]
- Abrantes, A.A.; Rafacho, A.; Rivero, E.R.; Mariano, F.V.; Siqueira, F.M.; Gondak, R.O. Tissue integrity, costs and time associated with different agents for histological bone preparation. Microsc. Res. Tech. 2017, 80, 344–349. [Google Scholar] [CrossRef]
- Bateman, A.; Martin, M.; Orchard, S.; Magrane, M.; Agivetova, R.; Ahmad, S.; Alpi, E.; Bowler-Barnett, E.H.; Britto, R.; Bursteinas, B.; et al. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar]
- Tokar, T.; Pastrello, C.; Rossos, A.; Abovsky, M.; Hauschild, A.C.; Tsay, M.; Lu, R.; Jurisica, I. mirDIP 4.1-integrative database of human microRNA target predictions. Nucleic Acids Res. 2018, 46, D360–D370. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Dennis, G.J.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003, 4, P3. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, W.; Yang, N.; Xue, Y.; Wang, T.; Wang, H.; Zheng, K.; Wang, Y.; Zhu, F.; Yang, H.; et al. Activation of cannabinoid receptor 2 alleviates glucocorticoid-induced osteonecrosis of femoral head with osteogenesis and maintenance of blood supply. Cell Death Dis. 2021, 12, 1035. [Google Scholar] [CrossRef]
- Ainiwan, M.; Wang, Q.; Yesitayi, G.; Ma, X. Identification of FERMT1 and SGCD as key marker in acute aortic dissection from the perspective of predictive, preventive, and personalized medicine. EPMA J. 2022, 13, 597–614. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Sun, Z.; Zeng, Y.; Luo, M.; Yang, J. Molecular Mechanism and Health Role of Functional Ingredients in Blueberry for Chronic Disease in Human Beings. Int. J. Mol. Sci. 2018, 19, 2785. [Google Scholar] [CrossRef]
- Panahande, S.B.; Maghbooli, Z.; Hossein-Nezhad, A.; Qorbani, M.; Moeini-Nodeh, S.; Haghi-Aminjan, H.; Hosseini, S. Effects of French maritime pine bark extract (Oligopin®) supplementation on bone remodeling markers in postmenopausal osteopenic women: A randomized clinical trial. Phytother. Res. 2019, 33, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Zhu, B.R.; Jia, Q.; Li, Y.M.; Wang, T.; Wang, H.Y. Cinnamtannin D1 Protects Pancreatic β-Cells from Glucolipotoxicity-Induced Apoptosis by Enhancement of Autophagy In Vitro and In Vivo. J. Agric. Food Chem. 2020, 68, 12617–12630. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ge, Z.; Fan, L.; Wang, K. Protective effects of molecular hydrogen on steroid-induced osteonecrosis in rabbits via reducing oxidative stress and apoptosis. BMC Musculoskelet. Disord. 2017, 18, 58. [Google Scholar] [CrossRef]
- Xu, K.; Lu, C.; Ren, X.; Wang, J.; Xu, P.; Zhang, Y. Overexpression of HIF-1α enhances the protective effect of mitophagy on steroid-induced osteocytes apoptosis. Environ. Toxicol. 2021, 36, 2123–2137. [Google Scholar] [CrossRef]
- Chen, L.; Hu, S.L.; Xie, J.; Yan, D.Y.; Weng, S.J.; Tang, J.H.; Wang, B.Z.; Xie, Z.J.; Wu, Z.Y.; Yang, L. Proanthocyanidins-Mediated Nrf2 Activation Ameliorates Glucocorticoid-Induced Oxidative Stress and Mitochondrial Dysfunction in Osteoblasts. Oxid. Med. Cell. Longev. 2020, 2020, 9102012. [Google Scholar] [CrossRef]
- Zhan, J.; Yan, Z.; Zhao, M.; Qi, W.; Lin, J.; Lin, Z.; Huang, Y.; Pan, X.; Xue, X. Allicin inhibits osteoblast apoptosis and steroid-induced necrosis of femoral head progression by activating the PI3K/AKT pathway. Food Funct. 2020, 11, 7830–7841. [Google Scholar] [CrossRef]
- Zhao, L.; Li, H.; Huang, X.; Liu, T.; Xin, Y.; Xiao, Z.; Zhao, W.; Miao, S.; Chen, J.; Li, Z.; et al. The endocytic pathway of Pt nanoclusters and their induced apoptosis of A549 and A549/Cis cells through c-Myc/p53 and Bcl-2/caspase-3 signaling pathways. Biomed. Pharmacother. 2021, 144, 112360. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Guan, X.; Chao, R.; Huang, C.; Li, D.; Yang, P.; Liu, S.; Hasegawa, T.; Guo, J.; Li, M. Diallyl Disulfide Induces Apoptosis and Autophagy in Human Osteosarcoma MG-63 Cells through the PI3K/Akt/mTOR Pathway. Molecules 2019, 24, 2665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jin, A.; Yan, D. MicroRNA-206 contributes to the progression of steroid-induced avascular necrosis of the femoral head by inducing osteoblast apoptosis by suppressing programmed cell death 4. Mol. Med. Rep. 2018, 17, 801–808. [Google Scholar] [CrossRef]
- Tsukasaki, M.; Takayanagi, H. Osteoimmunology: Evolving concepts in bone-immune interactions in health and disease. Nat. Rev. Immunol. 2019, 19, 626–642. [Google Scholar] [CrossRef]
- Duan, L.; Zuo, J.; Zhang, F.; Li, B.; Xu, Z.; Zhang, H.; Yang, B.; Song, W.; Jiang, J. Magnetic Targeting of HU-MSCs in the Treatment of Glucocorticoid-Associated Osteonecrosis of the Femoral Head Through Akt/Bcl2/Bad/Caspase-3 Pathway. Int. J. Nanomed. 2020, 15, 3605–3620. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, Z.; Sun, F.; Jin, J. Overexpression of CRABP2 inhibits dexamethasone-induced apoptosis in human osteoblast cells. J. Orthop. Surg. Res. 2021, 16, 272. [Google Scholar] [CrossRef]
- Deng, S.; Dai, G.; Chen, S.; Nie, Z.; Zhou, J.; Fang, H.; Peng, H. Dexamethasone induces osteoblast apoptosis through ROS-PI3K/AKT/GSK3β signaling pathway. Biomed. Pharmacother. 2019, 110, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; You, J.M.; Dong, X.J.; Wu, Y. Administration of mircoRNA-135b-reinforced exosomes derived from MSCs ameliorates glucocorticoid-induced osteonecrosis of femoral head (ONFH) in rats. J. Cell. Mol. Med. 2020, 24, 13973–13983. [Google Scholar] [CrossRef]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef] [PubMed]
- Ruvolo, P.P.; Deng, X.; May, W.S. Phosphorylation of Bcl2 and regulation of apoptosis. Leukemia 2001, 15, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, M.; Nkwocha, J.; Hawkins, E.; Pei, X.; Parker, R.E.; Kmieciak, M.; Leverson, J.D.; Sampath, D.; Ferreira-Gonzalez, A.; Grant, S. Cotargeting BCL-2 and PI3K Induces BAX-Dependent Mitochondrial Apoptosis in AML Cells. Cancer Res. 2018, 78, 3075–3086. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Zhang, Y.; Hao, Y.; Xu, P.; Wang, X.; Zhu, B.; Lu, C.; Xu, K. Proanthocyanidins Inhibit Osteoblast Apoptosis via the PI3K/AKT/Bcl-xL Pathway in the Treatment of Steroid-Induced Osteonecrosis of the Femoral Head in Rats. Nutrients 2023, 15, 1936. https://doi.org/10.3390/nu15081936
Li H, Zhang Y, Hao Y, Xu P, Wang X, Zhu B, Lu C, Xu K. Proanthocyanidins Inhibit Osteoblast Apoptosis via the PI3K/AKT/Bcl-xL Pathway in the Treatment of Steroid-Induced Osteonecrosis of the Femoral Head in Rats. Nutrients. 2023; 15(8):1936. https://doi.org/10.3390/nu15081936
Chicago/Turabian StyleLi, Hui, Yufei Zhang, Yangquan Hao, Peng Xu, Xingyu Wang, Bin Zhu, Chao Lu, and Ke Xu. 2023. "Proanthocyanidins Inhibit Osteoblast Apoptosis via the PI3K/AKT/Bcl-xL Pathway in the Treatment of Steroid-Induced Osteonecrosis of the Femoral Head in Rats" Nutrients 15, no. 8: 1936. https://doi.org/10.3390/nu15081936
APA StyleLi, H., Zhang, Y., Hao, Y., Xu, P., Wang, X., Zhu, B., Lu, C., & Xu, K. (2023). Proanthocyanidins Inhibit Osteoblast Apoptosis via the PI3K/AKT/Bcl-xL Pathway in the Treatment of Steroid-Induced Osteonecrosis of the Femoral Head in Rats. Nutrients, 15(8), 1936. https://doi.org/10.3390/nu15081936





