Association of Body Iron Metabolism with Type 2 Diabetes Mellitus in Chinese Women of Childbearing Age: Results from the China Adult Chronic Disease and Nutrition Surveillance (2015)
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Data Collection and Variable Classifications
2.3. Laboratory Measurements
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Characteristics of the Iron Status
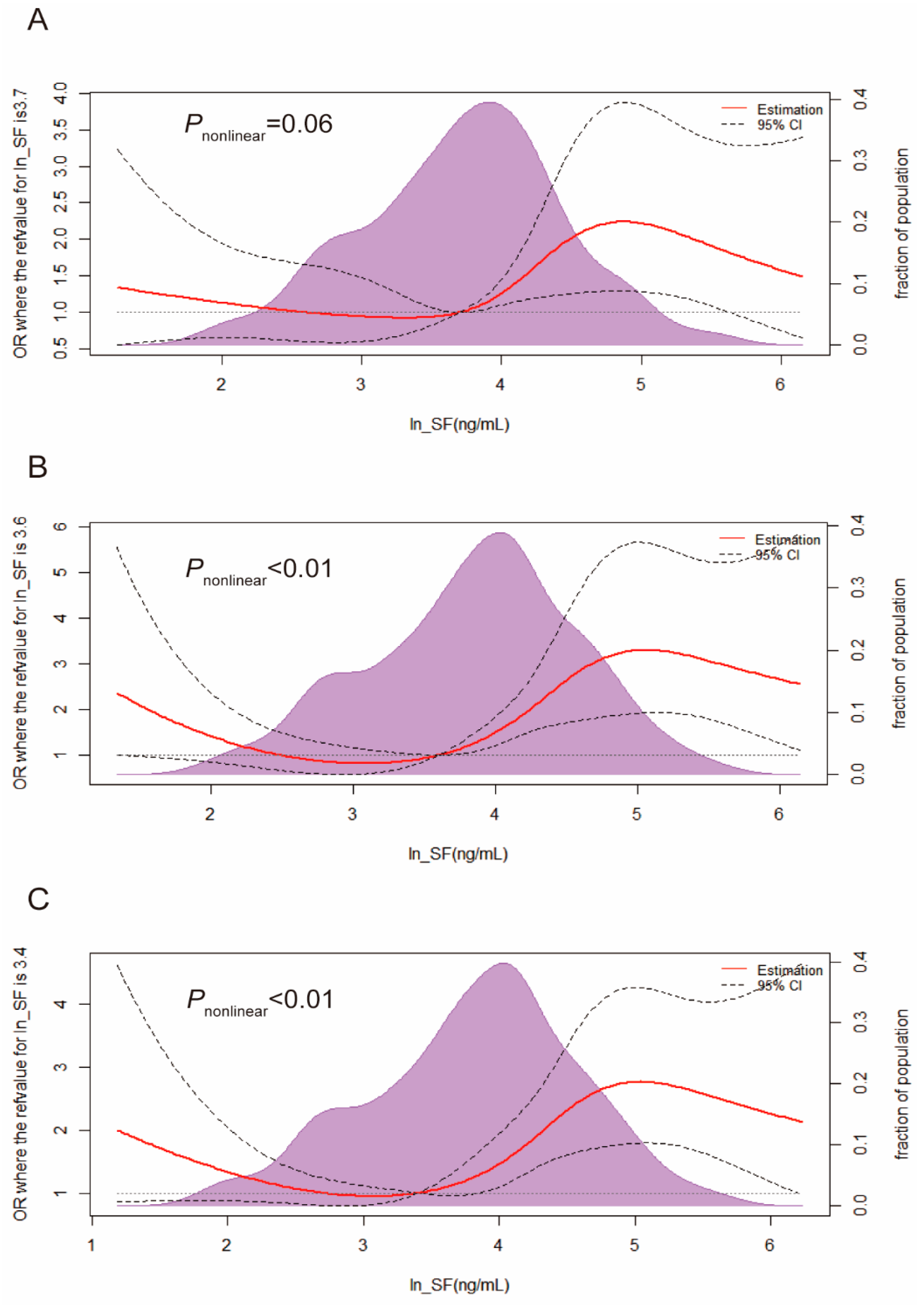
3.3. Odds Ratios for IGM, T2DM, and Hyperglycemia in Quartiles of Markers of Iron Storage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Simcox, J.A.; McClain, D.A. Iron and diabetes risk. Cell Metab. 2013, 17, 329–341. [Google Scholar] [CrossRef]
- Wilson, J.G.; Lindquist, J.H.; Grambow, S.C.; Crook, E.D.; Maher, J.F. Potential role of increased iron stores in diabetes. Am. J. Med. Sci. 2003, 325, 332–339. [Google Scholar] [CrossRef]
- Pitchika, A.; Schipf, S.; Nauck, M.; Dörr, M.; Lerch, M.M.; Felix, S.B.; Markus, M.R.P.; Völzke, H.; Ittermann, T. Associations of iron markers with type 2 diabetes mellitus and metabolic syndrome: Results from the prospective SHIP study. Diabetes Res. Clin. Pract. 2020, 163, 108149. [Google Scholar] [CrossRef] [PubMed]
- Yeap, B.B.; Divitini, M.L.; Gunton, J.E.; Olynyk, J.K.; Beilby, J.P.; McQuillan, B.; Hung, J.; Knuiman, M.W. Higher ferritin levels, but not serum iron or transferrin saturation, are associated with Type 2 diabetes mellitus in adult men and women free of genetic haemochromatosis. Clin. Endocrinol. 2015, 82, 525–532. [Google Scholar] [CrossRef]
- Jehn, M.L.; Guallar, E.; Clark, J.M.; Couper, D.; Duncan, B.B.; Ballantyne, C.M.; Hoogeveen, R.C.; Harris, Z.L.; Pankow, J.S. A prospective study of plasma ferritin level and incident diabetes: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Epidemiol. 2007, 165, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Kim, H.K.; Bae, S.J.; Park, J.Y.; Lee, K.U. Association of elevated serum ferritin concentration with insulin resistance and impaired glucose metabolism in Korean men and women. Metabolism 2011, 60, 414–420. [Google Scholar] [CrossRef]
- Montonen, J.; Boeing, H.; Steffen, A.; Lehmann, R.; Fritsche, A.; Joost, H.G.; Schulze, M.B.; Pischon, T. Body iron stores and risk of type 2 diabetes: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. Diabetologia 2012, 55, 2613–2621. [Google Scholar] [CrossRef]
- Podmore, C.; Meidtner, K.; Schulze, M.B.; Scott, R.A.; Ramond, A.; Butterworth, A.S.; Di Angelantonio, E.; Danesh, J.; Arriola, L.; Barricarte, A.; et al. Association of Multiple Biomarkers of Iron Metabolism and Type 2 Diabetes: The EPIC-InterAct Study. Diabetes Care 2016, 39, 572–581. [Google Scholar] [CrossRef]
- Jung, C.H.; Lee, M.J.; Hwang, J.Y.; Jang, J.E.; Leem, J.; Park, J.Y.; Lee, J.; Kim, H.K.; Lee, W.J. Elevated serum ferritin level is associated with the incident type 2 diabetes in healthy Korean men: A 4 year longitudinal study. PLoS ONE 2013, 8, e75250. [Google Scholar]
- Sun, L.; Zong, G.; Pan, A.; Ye, X.; Li, H.; Yu, Z.; Zhao, Y.; Zou, S.; Yu, D.; Jin, Q.; et al. Elevated plasma ferritin is associated with increased incidence of type 2 diabetes in middle-aged and elderly Chinese adults. J. Nutr. 2013, 143, 1459–1465. [Google Scholar] [CrossRef]
- Ponka, P.; Beaumont, C.; Richardson, D.R. Function and regulation of transferrin and ferritin. Semin. Hematol. 1998, 35, 35–54. [Google Scholar] [PubMed]
- Fumeron, F.; Péan, F.; Driss, F.; Balkau, B.; Tichet, J.; Marre, M.; Grandchamp, B. Ferritin and transferrin are both predictive of the onset of hyperglycemia in men and women over 3 years: The data from an epidemiological study on the Insulin Resistance Syndrome (DESIR) study. Diabetes Care 2006, 29, 2090–2094. [Google Scholar] [CrossRef] [PubMed]
- Rajpathak, S.N.; Wylie-Rosett, J.; Gunter, M.J.; Negassa, A.; Kabat, G.C.; Rohan, T.E.; Crandall, J. Biomarkers of body iron stores and risk of developing type 2 diabetes. Diabetes Obes. Metab. 2009, 11, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Ellervik, C.; Mandrup-Poulsen, T.; Andersen, H.U.; Tybjærg-Hansen, A.; Frandsen, M.; Birgens, H.; Nordestgaard, B.G. Elevated Transferrin Saturation and Risk of Diabetes: Three population-based studies. Diabetes Care 2011, 34, 2256–2258. [Google Scholar] [CrossRef]
- Kim, J.D.; Lim, D.M.; Park, K.Y.; Park, S.E.; Rhee, E.J.; Park, C.Y.; Lee, W.Y.; Oh, K.W. Serum Transferrin Predicts New-Onset Type 2 Diabetes in Koreans: A 4-Year Retrospective Longitudinal Study. Endocrinol. Metab. 2020, 35, 610–617. [Google Scholar] [CrossRef]
- Park, R.J.; Moon, J.D. Low transferrin saturation is associated with impaired fasting glucose and insulin resistance in the South Korean adults: The 2010 Korean National Health and Nutrition Examination Survey. Diabet. Med. 2015, 32, 673–678. [Google Scholar] [CrossRef]
- He, J.; Fang, A.; Yu, S.; Shen, X.; Li, K. Dietary Nonheme, Heme, and Total Iron Intake and the Risk of Diabetes in Adults: Results from the China Health and Nutrition Survey. Diabetes Care 2020, 43, 776–784. [Google Scholar] [CrossRef]
- WHO Guidelines Approved by the Guidelines Review Committee. In Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation; World Health Organization. Copyright ©; World Health Organization: Geneva, Switzerland, 2011.
- Boshuizen, M.; Binnekade, J.M.; Nota, B.; van de Groep, K.; Cremer, O.L.; Tuinman, P.R.; Horn, J.; Schultz, M.J.; van Bruggen, R.; Juffermans, N.P.; et al. Iron metabolism in critically ill patients developing anemia of inflammation: A case control study. Ann. Intensive Care 2018, 8, 56. [Google Scholar] [CrossRef]
- Cook, J.D.; Flowers, C.H.; Skikne, B.S. The quantitative assessment of body iron. Blood 2003, 101, 3359–3364. [Google Scholar] [CrossRef]
- Beutler, E.; Felitti, V.; Ho, N.J.; Gelbart, T. Relationship of body iron stores to levels of serum ferritin, serum iron, unsaturated iron binding capacity and transferrin saturation in patients with iron storage disease. Acta Haematol. 2002, 107, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Nanri, A.; Kuwahara, K.; Matsushita, Y.; Nakagawa, T.; Konishi, M.; Honda, T.; Yamamoto, S.; Hayashi, T.; Noda, M.; et al. Circulating ferritin concentrations and risk of type 2 diabetes in Japanese individuals. J. Diabetes Investig. 2017, 8, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Koh, W.P.; Yuan, J.M.; Pan, A. Plasma ferritin, C-reactive protein, and risk of incident type 2 diabetes in Singapore Chinese men and women. Diabetes Res. Clin. Pract. 2017, 128, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Orban, E.; Schwab, S.; Thorand, B.; Huth, C. Association of iron indices and type 2 diabetes: A meta-analysis of observational studies. Diabetes Metab. Res. Rev. 2014, 30, 372–394. [Google Scholar] [CrossRef]
- Gao, H.; Yang, J.; Pan, W.; Yang, M. Iron Overload and the Risk of Diabetes in the General Population: Results of the Chinese Health and Nutrition Survey Cohort Study. Diabetes Metab. J. 2022, 46, 307–318. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Haffner, S.M. The metabolic syndrome: Inflammation, diabetes mellitus, and cardiovascular disease. Am. J. Cardiol. 2006, 97, 3a–11a. [Google Scholar] [CrossRef]
- Sharifi, F.; Nasab, N.M.; Zadeh, H.J. Elevated serum ferritin concentrations in prediabetic subjects. Diab. Vasc. Dis. Res. 2008, 5, 15–18. [Google Scholar] [CrossRef]
- Huth, C.; Beuerle, S.; Zierer, A.; Heier, M.; Herder, C.; Kaiser, T.; Koenig, W.; Kronenberg, F.; Oexle, K.; Rathmann, W.; et al. Biomarkers of iron metabolism are independently associated with impaired glucose metabolism and type 2 diabetes: The KORA F4 study. Eur. J. Endocrinol. 2015, 173, 643–653. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Harding, A.H.; Allison, M.; Sandhu, M.S.; Welch, A.; Luben, R.; Bingham, S.; Khaw, K.T.; Wareham, N.J. Elevated serum ferritin levels predict new-onset type 2 diabetes: Results from the EPIC-Norfolk prospective study. Diabetologia 2007, 50, 949–956. [Google Scholar] [CrossRef]
- Cooksey, R.C.; Jouihan, H.A.; Ajioka, R.S.; Hazel, M.W.; Jones, D.L.; Kushner, J.P.; McClain, D.A. Oxidative stress, beta-cell apoptosis, and decreased insulin secretory capacity in mouse models of hemochromatosis. Endocrinology 2004, 145, 5305–5312. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E. Insulin resistance, iron, and the liver. Lancet 2000, 355, 2181–2182. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Choi, J.S.; Lee, H.J.; Kim, W.H.; Park, S.I.; Song, J. Effect of excess iron on oxidative stress and gluconeogenesis through hepcidin during mitochondrial dysfunction. J. Nutr. Biochem. 2015, 26, 1414–1423. [Google Scholar] [CrossRef]
- Huang, J.; Jones, D.; Luo, B.; Sanderson, M.; Soto, J.; Abel, E.D.; Cooksey, R.C.; McClain, D.A. Iron overload and diabetes risk: A shift from glucose to Fatty Acid oxidation and increased hepatic glucose production in a mouse model of hereditary hemochromatosis. Diabetes 2011, 60, 80–87. [Google Scholar] [CrossRef]
- Green, A.; Basile, R.; Rumberger, J.M. Transferrin and iron induce insulin resistance of glucose transport in adipocytes. Metabolism 2006, 55, 1042–1045. [Google Scholar] [CrossRef] [PubMed]
- Speeckaert, M.M.; Speeckaert, R.; Delanghe, J.R. Biological and clinical aspects of soluble transferrin receptor. Crit. Rev. Clin. Lab. Sci. 2010, 47, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Bird, R.; Clague, A.; Carter, A. Clinical utility of serum soluble transferrin receptor levels and comparison with bone marrow iron stores as an index for iron-deficient erythropoiesis in a heterogeneous group of patients. Pathology 2007, 39, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cao, J.C.; Arija, V.; Aranda, N.; Basora, J.; Diez-Espino, J.; Estruch, R.; Fitó, M.; Corella, D.; Salas-Salvadó, J. Soluble transferrin receptor and risk of type 2 diabetes in the obese and nonobese. Eur. J. Clin. Invest. 2017, 47, 221–230. [Google Scholar] [CrossRef]
- Clairmont, K.B.; Czech, M.P. Insulin injection increases the levels of serum receptors for transferrin and insulin-like growth factor-II/mannose-6-phosphate in intact rats. Endocrinology 1990, 127, 1568–1573. [Google Scholar] [CrossRef]
- Vargas, L.; Kawada, M.E.; Bazaes, S.; Karplus, P.A.; Faerman, C.H. Insulin antagonism: A novel role for human serum transferrin. Horm. Metab. Res. 1998, 30, 113–117. [Google Scholar] [CrossRef]
- Rumberger, J.M.; Peters, T., Jr.; Burrington, C.; Green, A. Transferrin and iron contribute to the lipolytic effect of serum in isolated adipocytes. Diabetes 2004, 53, 2535–2541. [Google Scholar] [CrossRef] [PubMed]
- Arner, P. Insulin resistance in type 2 diabetes: Role of fatty acids. Diabetes Metab. Res. Rev. 2002, 18 (Suppl. S2), S5–S9. [Google Scholar] [CrossRef] [PubMed]
- Mainous, A.G., 3rd; King, D.E.; Pearson, W.S.; Garr, D.R. Is an elevated serum transferrin saturation associated with the development of diabetes? J. Fam. Pract. 2002, 51, 933–936. [Google Scholar] [PubMed]
- Freixenet, N.; Vilardell, C.; Llauradó, G.; Giménez-Palop, O.; Berlanga, E.; Gutiérrez, C.; Caixàs, A.; Vendrell, J.; González-Clemente, J.M. Men with hyperferritinemia and diabetes in the Mediterranean area do not have a higher iron overload than those without diabetes. Diabetes Res. Clin. Pract. 2011, 91, e33–e36. [Google Scholar] [CrossRef]
- Gabrielsen, J.S.; Gao, Y.; Simcox, J.A.; Huang, J.; Thorup, D.; Jones, D.; Cooksey, R.C.; Gabrielsen, D.; Adams, T.D.; Hunt, S.C.; et al. Adipocyte iron regulates adiponectin and insulin sensitivity. J. Clin. Investig. 2012, 122, 3529–3540. [Google Scholar] [CrossRef]
| Characteristics | Glucose Metabolism Status | p-Value | ||
|---|---|---|---|---|
| NGM (n = 381) | IGM (n = 353) | T2DM (n = 411) | ||
| Age (years) | 32.6 (25.8–40.4) * | 39.4 (34.0–42.8) | 39.9 (34.6–43.1) | <0.001 |
| Nationality (%) | 0.198 | |||
| Han | 315 (82.7) | 305 (86.4) | 357 (86.9) | |
| Ethnic minority | 66 (17.3) | 48 (13.6) | 54 (13.1) | |
| Education (%) | <0.001 | |||
| Primary | 117 (30.7) | 156 (44.2) | 187 (45.5) | |
| Medium | 220 (57.7) * | 172 (48.7) | 176 (42.8) | |
| Advanced | 44 (11.5) | 25 (7.1) | 48 (11.7) | |
| Smoking (%) | 2 (0.5) | 1 (0.3) | 4 (1) | 0.459 |
| Alcohol drinking (%) | 72 (18.9) | 72 (20.4) | 75 (18.2) | 0.746 |
| Physically active (%) | 48 (12.6) | 43 (12.2) | 75 (18.2) | 0.026 |
| District | 0.025 | |||
| Eastern | 128 (33.6) | 98 (27.8) | 144 (35) | |
| Center | 124 (32.5) | 107 (30.3) | 140 (34.1) | |
| Western | 129 (33.9) | 148 (41.9) * | 127 (30.9) | |
| City (%) | 171 (44.9) | 203 (57.5) | 243 (59.1) | <0.001 |
| BMI (kg/m2) | 23.63 (20.96–26.15) * | 24.87 (22.49–27.69) | 24.99 (22.22–27.94) | <0.001 |
| WC (cm) | 78.5 (71.5–85.4) * | 81.5 (75.3–88.4) | 83.0 (76.4–90.3) | <0.001 |
| Heart rate | 77 (71–84) * | 80 (74–90) | 80 (74–87) | <0.001 |
| Systolic pressure (mmHg) | 118 (109–127) * | 125 (116–137) | 126 (117–138) | <0.001 |
| Diastolic pressure (mmHg) | 72 (66–79) * | 77 (70–84) | 78 (71–86) | <0.001 |
| FBG (mmol/L) | 5.07 (4.65–6.01) * | 6.34 (6.2–6.57) * | 8.04 (7.18–10.35) * | <0.001 |
| HbA1c (%) | 4.8 (4.4–5.1) * | 4.9 (4.5–5.4) * | 6.5 (5.4–7.7) * | <0.001 |
| TC (mmol/L) | 4.25 (3.8–4.86) * | 4.61 (4.1–5.23) | 4.7 (4.12–5.43) | <0.001 |
| TG (mmol/L) | 0.86 (0.62–1.23) * | 1.26 (0.88–1.96) * | 1.54 (0.97–2.29) * | <0.001 |
| HDL-C (mmol/L) | 1.31 (1.12–1.52) * | 1.2 (1.05–1.42) | 1.19 (1–1.41) | <0.001 |
| LDL-C (mmol/L) | 2.50 (2.14–3.09) * | 2.83 (2.33–3.43) | 2.94 (2.44–3.61) | <0.001 |
| UA (μmol/L) | 255.4 (212.6–303.25) * | 268 (223.45–319.3) | 272 (222.3–318.9) | 0.018 |
| hsCRP (mg/L) | 0.46 (0.19–1.08) * | 0.75 (0.31–1.67) * | 1.01 (0.36–2.12) * | <0.001 |
| AAG (g/L) | 0.51 (0.43–0.62) * | 0.58 (0.47–0.67) | 0.58 (0.48–0.7) | <0.001 |
| Hb (mmol/L) | 142.07 (132.12–151.22) | 142.74 (132.12–151.85) | 144.66 (132.72–153.67) | 0.223 |
| Characteristics | Glucose Metabolism Status | p-Value | ||
|---|---|---|---|---|
| NGM (n = 381) | IGM (n = 353) | T2DM (n = 411) | ||
| SF (ng/mL) | 41.1 (19.5–74.0) * | 55.2 (25.2–103.5) * | 72.9 (32.7–138.9) * | <0.001 |
| TRF (g/L) | 2.60 (2.33–2.87) | 2.67 (2.36–2.96) | 2.65 (2.33–2.91) | 0.182 |
| sTfR (mg/L) | 2.20 (1.73–2.74) * | 2.63 (2.14–3.32) | 2.54 (2.14–3.27) | <0.001 |
| SI (μmol/L) | 22.4 (17.7–28.1) a | 21.5 (16.6–26.1) | 20.9 (16.8–25.6) | 0.004 |
| TBI (mg/kg) | 34.1 (31.1–36.3) | 34.7 (31.3–36.8) | 35.5 (32.4–37.7) * | <0.001 |
| TSAT (%) | 35.6 (25.9–44.4) a | 32.7 (23.7–41.5) | 32.7 (24.5–39.5) | 0.026 |
| sTfR-F index (units) | 1.4 (1.0–1.9) a | 1.5 (1.1–2.2) | 1.4 (1.1–2.0) | 0.002 |
| SF # | TRSF # | sTfR # | SI # | TBI # | TSAT # | sTfR-F Index # | |
|---|---|---|---|---|---|---|---|
| SF # | 1 | −0.49 ‡ | −0.31 ‡ | 0.40 ‡ | 0.93 ‡ | 0.54 ‡ | −0.72 ‡ |
| TRSF # | −0.49 ‡ | 1 | 0.39 ‡ | −0.15 ‡ | −0.54 ‡ | −0.51 ‡ | 0.52 ‡ |
| STFR # | −0.31 ‡ | 0.39 ‡ | 1 | −0.32 ‡ | −0.63 ‡ | −0.43 ‡ | 0.87 ‡ |
| SI # | 0.40 ‡ | −0.15 ‡ | −0.32 ‡ | 1 | 0.46 ‡ | 0.93 ‡ | −0.45 ‡ |
| TBI # | 0.93 ‡ | −0.54 ‡ | −0.63 ‡ | 0.46 ‡ | 1 | 0.61 ‡ | −0.92 ‡ |
| TSAT # | 0.54 ‡ | −0.51 ‡ | −0.43 ‡ | 0.93 ‡ | 0.61 ‡ | 1 | −0.58 ‡ |
| sTfR-F index # | −0.72 ‡ | 0.52 ‡ | 0.87 ‡ | −0.45 ‡ | −0.92 ‡ | −0.59 ‡ | 1 |
| AAG # | 0.12 ‡ | 0.19 ‡ | 0.13 ‡ | 0.003 | 0.05 | −0.07 † | 0.03 |
| hsCRP # | 0.22 ‡ | 0.01 | 0.05 | −0.008 | 0.16 ‡ | −0.01 | −0.07 † |
| FBG # | 0.13 ‡ | 0.02 | 0.13 ‡ | 0.03 | 0.06 | 0.02 | 0.02 |
| HbA1c # | 0.05 | 0.08 ‡ | 0.02 | 0.04 | 0.03 | 0.005 | −0.01 |
| UA # | 0.26 ‡ | −0.09 ‡ | −0.01 | 0.08 † | 0.22 ‡ | 0.10 ‡ | −0.14 ‡ |
| BMI # | 0.10 ‡ | 0.16 ‡ | 0.008 | 0.05 | 0.08 ‡ | −0.02 | −0.04 |
| WC # | 0.12 ‡ | 0.17 ‡ | 0.05 | 0.07 † | 0.09 ‡ | −0.001 | −0.03 |
| TC/HDL # | 0.24 ‡ | 0.02 | −0.02 | 0.05 | 0.20 ‡ | 0.04 | −0.13 ‡ |
| TG # | 0.18 ‡ | 0.08 † | 0.06 | 0.05 | 0.13 ‡ | 0.02 | −0.05 |
| SBP # | 0.06 † | 0.11 ‡ | 0.09 ‡ | 0.04 | 0.02 | −0.01 | 0.03 |
| DBP # | 0.03 | 0.17 ‡ | 0.07 † | 0.06 | 0.003 | −0.02 | 0.03 |
| Iron Markers | ORs (95% CI) for IGM (n = 353) | ORs (95% CI) for T2DM (n = 411) | ORs (95% CI) for Hyperglycemia (n = 764) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Quartile 2 | Quartile 3 | Quartile 4 | Quartile 2 | Quartile 3 | Quartile 4 | Quartile 2 | Quartile 3 | Quartile 4 | |
| SF (ng/mL) | 20.9–46.4 | 46.4–87.1 | >87.1 | 22.9–53.9 | 53.9–101.35 | >101.35 | 23.7–54.7 | 54.7–102.5 | >102.5 |
| Crude Model | 1.05 (0.69–1.58) | 1.32 (0.87–1.99) | 2.09 (1.38–3.17) | 0.88 (0.59–1.32) | 1.66 (1.12–2.47) | 3.60 (2.37–5.48) | 1.00 (0.72–1.40) | 1.56 (1.11–2.20) | 3.13 (2.14–4.58) |
| Model 1 | 0.97 (0.61–1.60) | 1.29 (0.79–2.11) | 1.93 (1.17–3.20) | 0.76 (0.47–1.24) | 1.62 (0.99–2.64) | 2.39 (1.40–4.06) | 0.91 (0.61–1.34) | 1.54 (1.02–2.32) | 2.29 (1.45–3.61) |
| transferrin (g/L) | 2.34–2.63 | 2.63–2.92 | >2.92 | 2.33–2.63 | 2.63–2.89 | >2.89 | 2.34–2.64 | 2.64–2.91 | >2.91 |
| Crude Model | 0.911 (0.60–1.38) | 1.12 (0.74–1.68) | 1.39 (0.92–2.09) | 0.70 (0.47–1.04) | 1.13 (0.76–1.68) | 1.09 (0.73–1.61) | 0.79 (0.56–1.12) | 1.16 (0.81–1.65) | 1.21 (0.86–1.72) |
| Model 1 | 0.69 (0.43–1.10) | 0.67 (0.42–1.08) | 0.84 (0.52–1.36) | 0.53 (0.33–0.85) | 0.84 (0.52–1.36) | 0.66 (0.40–1.08) | 0.66 (0.44–0.98) | 0.87 (0.58–1.31) | 0.76 (0.50–1.16) |
| sTfR (mg/L) | 1.93–2.38 | 2.38–3.05 | >3.05 | 1.93–2.39 | 2.39–3.04 | >3.04 | 1.98–2.44 | 2.44–3.11 | >3.11 |
| Crude Model | 2.37 (1.53–3.67) | 3.62 (2.34–5.61) | 4.48 (2.87–6.98) | 2.08 (1.38–3.13) | 3.60 (2.38–5.46) | 3.84 (2.53–5.83) | 2.09 (1.49–2.92) | 3.32 (2.33–4.74) | 3.89 (2.70–5.60) |
| Model 1 | 1.80 (1.10–2.95) | 2.75 (1.68–4.48) | 3.08 (1.84–5.14) | 1.51 (0.93–2.45) | 2.35 (1.44–3.84) | 2.47 (1.50–4.07) | 1.65 (1.12–2.43) | 2.42 (1.61–3.62) | 2.76 (1.80–4.25) |
| SI (μmol/L) | 17.2–21.9 | 21.9–27.3 | >27.3 | 17.1–21.6 | 21.6–26.8 | >26.8 | 17.0–21.5 | 21.5–26.7 | >26.7 |
| Crude Model | 0.81 (0.54–1.22) | 0.96 (0.64–1.44) | 0.67 (0.44–1.01) | 0.94 (0.63–1.40) | 0.96 (0.65–1.43) | 0.57 (0.38–0.84) | 0.91 (0.64–1.30) | 1.02 (0.71–1.45) | 0.61 (0.43–0.87) |
| Model 1 | 0.82 (0.51–1.32) | 1.13 (0.70–1.83) | 0.64 (0.39–1.05) | 0.97 (0.60–1.56) | 0.89 (0.55–1.44) | 0.58 (0.35–0.95) | 0.86 (0.57–1.30) | 0.98 (0.64–1.48) | 0.58 (0.38–0.88) |
| TBI (mg/kg) | 31.2–34.3 | 34.3–36.6 | >36.6 | 31.5–34.9 | 34.9–37.2 | >37.2 | 31.5–34.8 | 34.8–37.1 | >37.1 |
| Crude Model | 0.95 (0.63–1.43) | 1.11 (0.74–1.66) | 1.31 (0.86–1.98) | 0.97 (0.65–1.44) | 1.47 (0.99–2.18) | 2.36 (1.57–3.53) | 0.91 (0.65–1.28) | 1.18 (0.84–1.67) | 1.84 (1.28–2.64) |
| Model 1 | 0.81 (0.50–1.31) | 1.08 (0.66–1.75) | 1.32 (0.80–2.19) | 0.92 (0.57–1.49) | 1.50 (0.91–2.48) | 1.87 (1.12–3.14) | 0.84 (0.56–1.25) | 1.11 (0.73–1.69) | 1.53 (0.98–2.37) |
| TSAT (%) | 25.0–34.3 | 34.3–42.9 | >42.9 | 25.3–33.7 | 33.7–42.1 | >42.1 | 24.8–33.5 | 33.5–41.8 | >41.8 |
| Crude Model | 0.85 (0.56–1.28) | 0.78 (0.52–1.17) | 0.63(0.42–0.95) | 0.98 (0.66–1.46) | 0.88 (0.59–1.30) | 0.57 (0.39–0.85) | 0.93 (0.65–1.33) | 0.85 (0.60–1.22) | 0.61 (0.43–0.86) |
| Model 1 | 0.93 (0.57–1.49) | 1.10 (0.68–1.78) | 0.77 (0.47–1.25) | 1.21 (0.75–1.96) | 1.02 (0.63–1.66) | 0.74 (0.45–1.20) | 0.97 (0.64–1.47) | 1.04 (0.68–1.57) | 0.72 (0.48–1.09) |
| sTfR-F index | 1.08–1.44 | 1.44–2.05 | >2.05 | 1.06–1.38 | 1.38–1.91 | >1.91 | 1.08–1.42 | 1.42–2.01 | >2.01 |
| Crude Model | 1.60 (1.05–2.42) | 1.45 (0.96–2.19) | 1.96 (1.30–2.97) | 1.51 (1.02–2.24) | 1.10 (0.74–1.63) | 1.33 (0.90–1.98) | 1.52 (1.07–2.15) | 1.19 (0.84–1.67) | 1.60 (1.13–2.26) |
| Model 1 | 1.31 (0.82–2.09) | 1.07 (0.66–1.73) | 1.85 (1.12–3.05) | 1.15 (0.72–1.83) | 0.73 (0.45–1.17) | 1.16 (0.70–1.92) | 1.23 (0.83–1.83) | 0.88 (0.59–1.31) | 1.51 (0.99–2.32) |
| Study (First Author, Year) | Study Design | Country/ Ethnicity | Age (Years) | N (T2DM/ Control) | Sex (% Female) | T2DM Diagnosis and Criteria | Iron Biomarkers | Main Findings |
|---|---|---|---|---|---|---|---|---|
| Forouhi 2007 [31] | Case-control | U.K. | 40–74 | 360/758 | 42% | Self-report or HbA1c level > 7% | Ferritin | Ferritin was an independent predictor of the development of T2DM. |
| Rajpathak 2009 [14] | Nested case-control | USA | ≥25 | 280/280 | 63.6% | Standardized criteria of the American Diabetes Association and the World Health Organization | Ferritin, sTfR | Elevated sTfR levels were associated with increased T2DM risk among overweight and obese individuals with impaired glucose tolerance. Ferritin levels were not statistically different. |
| Jung 2013 [10] | Cohort | Korean | 23–82 | 186/1843 | Only men | Self-report or FPG ≥ 126 mg/dl or HbA1c ≥ 6.5% | Ferritin | Elevated level of ferritin at baseline was associated with incident T2DM in an Asian population. |
| Yeap 2014 [5] | Cross-sectional | Australia | 17–97 | 263/3922 | 56% | Self-report, FPG ≥ 7 mM, or taking glucose-lowering medications | Ferritin, iron, transferrin saturation | Higher ferritin levels were independently associated with prevalent T2DM. Neither iron nor transferrin saturation were associated with T2DM risk in men or women. |
| Huth 2015 [30] | Cross-sectional | German | 25–74 | 315/2099 | 51.7% | 1999 WHO criteria | Ferritin, transferrin, sTfR, TSAT, sTfR-F index, iron | Ferritin and transferrin were positively associated with IGM and T2DM. TSAT and iron were inversely associated with T2DM. |
| Podmore 2016 [9] | Cohort | European | >40 | 11052/14061 | Not given | Self-report, linkage to primary care registers, or hospital admissions | Ferritin, TSAT, serum iron, transferrin | Higher ferritin and transferrin were associated with higher risk of T2DM in men and women. Elevated TSAT was associated with lower risk of T2DM in women, but not in men. Serum iron was not associated with T2DM. |
| Wang 2017 [24] | Case-control | Singapore, Chinese men and women | 45–74 | 485/485 | 56.3% | Self-report questionnaire | Ferritin | Elevation of ferritin was significantly associated with increased risk of T2DM. |
| Akter 2017 [23] | Cohort | Japan | 51 | 327/641 | 10% | American Diabetes Association criteria for the diagnosis of diabetes | Ferritin | Elevated serum ferritin levels were associated with a significantly increased risk of T2DM. |
| Gao 2022 [26] | Cohort | China | >19 | 75/5704 | 54.18% | Self-reported questionnaire | Ferritin | Iron overload increased the risk of T2DM and the association is sex-specific. |
| Current study | Case-control | China | 18–44 | 411(T2DM)/353 (IGM)/381 (control) | Only women of childbearing | 1999 WHO criteria | Ferritin, transferrin, sTfR, SI, TSAT, TBI, sTfR-to-lgferritin index | Ferritin and sTfR were independently positively associated with the risk of IGM, T2DM and hyperglycemia. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Shan, X.; Wang, L.; Lu, J.; Cao, Y.; Yang, L. Association of Body Iron Metabolism with Type 2 Diabetes Mellitus in Chinese Women of Childbearing Age: Results from the China Adult Chronic Disease and Nutrition Surveillance (2015). Nutrients 2023, 15, 1935. https://doi.org/10.3390/nu15081935
Feng J, Shan X, Wang L, Lu J, Cao Y, Yang L. Association of Body Iron Metabolism with Type 2 Diabetes Mellitus in Chinese Women of Childbearing Age: Results from the China Adult Chronic Disease and Nutrition Surveillance (2015). Nutrients. 2023; 15(8):1935. https://doi.org/10.3390/nu15081935
Chicago/Turabian StyleFeng, Jie, Xiaoyun Shan, Lijuan Wang, Jiaxi Lu, Yang Cao, and Lichen Yang. 2023. "Association of Body Iron Metabolism with Type 2 Diabetes Mellitus in Chinese Women of Childbearing Age: Results from the China Adult Chronic Disease and Nutrition Surveillance (2015)" Nutrients 15, no. 8: 1935. https://doi.org/10.3390/nu15081935
APA StyleFeng, J., Shan, X., Wang, L., Lu, J., Cao, Y., & Yang, L. (2023). Association of Body Iron Metabolism with Type 2 Diabetes Mellitus in Chinese Women of Childbearing Age: Results from the China Adult Chronic Disease and Nutrition Surveillance (2015). Nutrients, 15(8), 1935. https://doi.org/10.3390/nu15081935





