Nutritional Status Differs by Prescription Opioid Use among Women of Reproductive Age: NHANES 1999–2018
Abstract
1. Introduction
2. Materials and Methods
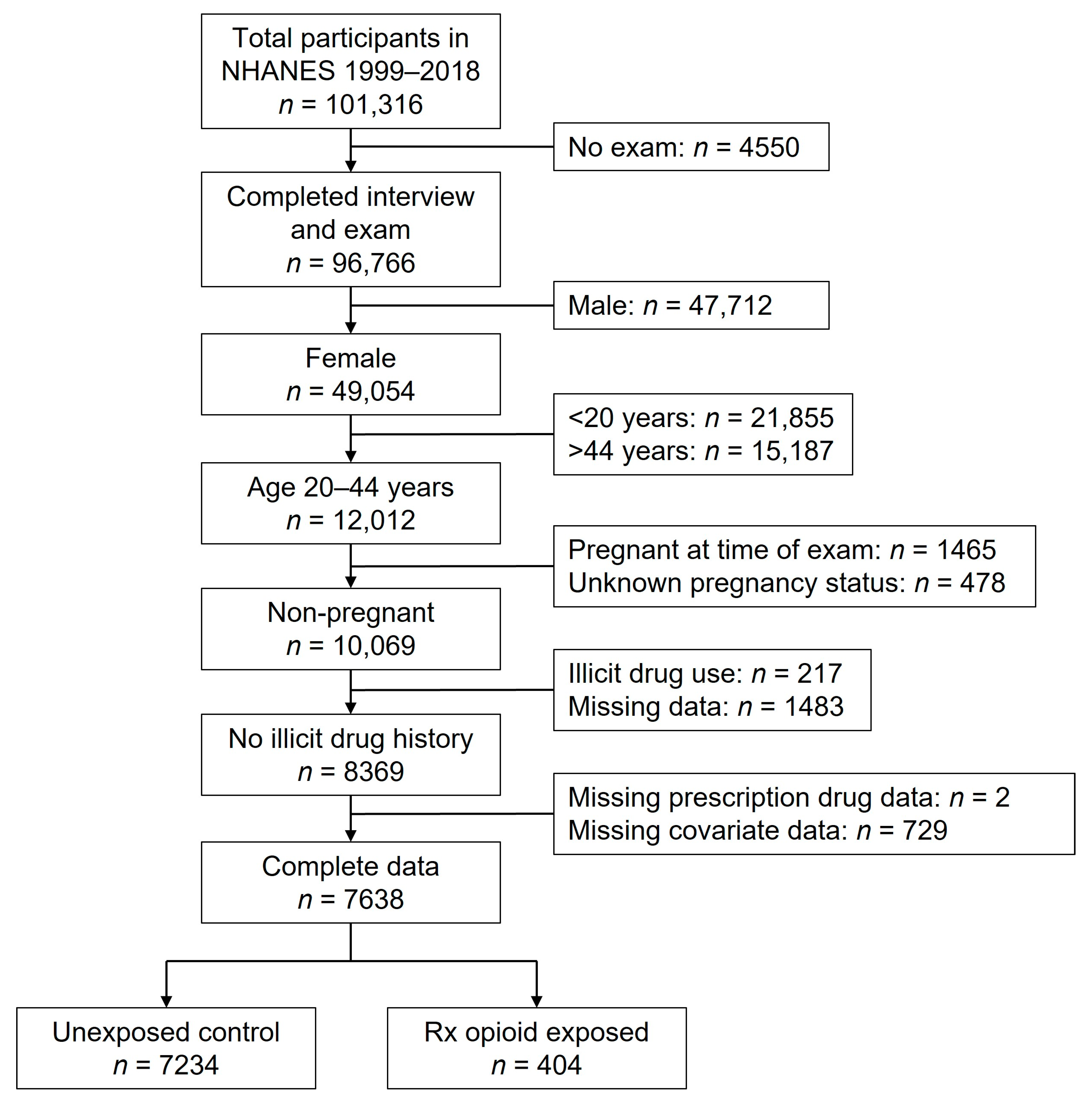
2.1. Data and Participants
2.2. Prescription Opioid Use
2.3. Nutrition and Health Status Measurements
2.4. Analysis
3. Results
3.1. Subgroup Analyses
3.1.1. Age
3.1.2. Obesity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ailes, E.C.; Dawson, A.L.; Lind, J.N.; Gilboa, S.M.; Frey, M.T.; Broussard, C.S.; Honein, M.A. Opioid Prescription Claims Among Women of Reproductive Age—United States, 2008–2012. Morb. Mortal. Wkly. Rep. 2015, 64, 37. [Google Scholar]
- Epstein, R.A.; Bobo, W.V.; Martin, P.R.; Morrow, J.A.; Wang, W.; Chandrasekhar, R.; Cooper, W.O. Increasing pregnancy-related use of prescribed opioid analgesics. Ann. Epidemiol. 2013, 23, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Patrick, S.W.; Dudley, J.; Martin, P.R.; Harrell, F.E.; Warren, M.D.; Hartmann, K.E.; Ely, E.W.; Grijalva, C.G.; Cooper, W.O. Prescription opioid epidemic and infant outcomes. Pediatrics 2015, 135, 842–850. [Google Scholar] [CrossRef]
- Desai, R.J.; Hernandez-Diaz, S.; Bateman, B.T.; Huybrechts, K.F. Increase in Prescription Opioid Use During Pregnancy Among Medicaid-Enrolled Women. Obstet. Gynecol. 2014, 123, 997–1002. [Google Scholar] [CrossRef]
- Bateman, B.T.; Hernandez-Diaz, S.; Rathmell, J.P.; Seeger, J.D.; Doherty, M.; Fischer, M.A.; Huybrechts, K.F. Patterns of Opioid Utilization in Pregnancy in a Large Cohort of Commercial Insurance Beneficiaries in the United States. Anesthesiology 2014, 120, 1216–1224. [Google Scholar] [CrossRef]
- Smith, M.V.; Costello, D.; Yonkers, K.A. Clinical Correlates of Prescription Opioid Analgesic Use in Pregnancy. Matern. Child Health J. 2015, 19, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Downs, D.S.; Pauley, A.M.; Leonard, K.S.; Satti, M.; Cumbo, N.; Teti, I.; Stephens, M.; Corr, T.; Roeser, R.; Deimling, T.; et al. Obstetric Physicians’ Beliefs and Knowledge on Guidelines and Screening Tools to Reduce Opioid Use After Childbirth. Obstet. Gynecol. 2021, 137, 325–333. [Google Scholar] [CrossRef]
- Kallen, B.; Borg, N.; Reis, M. The use of central nervous system active drugs during pregnancy. Pharmaceuticals 2013, 6, 1221–1286. [Google Scholar] [CrossRef]
- Broussard, C.S.; Rasmussen, S.A.; Reefhuis, J.; Friedman, J.M.; Jann, M.W.; Riehle-Colarusso, T.; Honein, M.A.; Study, N.B.D.P. Maternal treatment with opioid analgesics and risk for birth defects. Am. J. Obstet. Gynecol. 2011, 204, 314.e1–314.e11. [Google Scholar] [CrossRef]
- Yazdy, M.M.; Mitchell, A.A.; Tinker, S.C.; Parker, S.E.; Werler, M.M. Periconceptional Use of Opioids and the Risk of Neural Tube Defects. Obstet. Gynecol. 2013, 122, 838–844. [Google Scholar] [CrossRef]
- Desai, R.J.; Huybrechts, K.F.; Hernandez-Diaz, S.; Mogun, H.; Patorno, E.; Kaltenbach, K.; Kerzner, L.S.; Bateman, B.T. Exposure to prescription opioid analgesics in utero and risk of neonatal abstinence syndrome: Population based cohort study. BMJ 2015, 350, h2102. [Google Scholar] [CrossRef] [PubMed]
- Huybrechts, K.F.; Bateman, B.T.; Desai, R.J.; Hernandez-Diaz, S.; Rough, K.; Mogun, H.; Kerzner, L.S.; Davis, J.M.; Stover, M.; Bartels, D.; et al. Risk of neonatal drug withdrawal after intrauterine co-exposure to opioids and psychotropic medications: Cohort study. BMJ 2017, 358, j3326. [Google Scholar] [CrossRef] [PubMed]
- Young, J.K.; Giesbrecht, H.E.; Eskin, M.N.; Aliani, M.; Suh, M. Nutrition implications for fetal alcohol spectrum disorder. Adv. Nutr. 2014, 5, 675–692. [Google Scholar] [CrossRef]
- Lindsay, K.L.; Buss, C.; Wadhwa, P.D.; Entringer, S. The Interplay Between Nutrition and Stress in Pregnancy: Implications for Fetal Programming of Brain Development. Biol. Psychiatry 2019, 85, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Makrides, M.; Anderson, A.; Gibson, R.A. Early influences of nutrition on fetal growth. In Recent Advances in Growth Research: Nutritional, Molecular and Endocrine Perspectives; Nestlé Nutrition Institute Workshop Series; Nestec Ltd., Vevey/S. Karger AG.: Basel, Switzerland, 2013; Volume 71, pp. 1–9. [Google Scholar] [CrossRef]
- Abu-Saad, K.; Fraser, D. Maternal nutrition and birth outcomes. Epidemiol. Rev. 2010, 32, 5–25. [Google Scholar] [CrossRef]
- Schwarzenberg, S.J.; Georgieff, M.K.; Committee on Nutrition; Daniels, S.; Corkins, M.; Golden, N.H.; Kim, J.H.; Lindsey, C.W.; Magge, S.N. Advocacy for Improving Nutrition in the First 1000 Days to Support Childhood Development and Adult Health. Pediatrics 2018, 141, e20173716. [Google Scholar] [CrossRef]
- Marchi, J.; Berg, M.; Dencker, A.; Olander, E.K.; Begley, C. Risks associated with obesity in pregnancy, for the mother and baby: A systematic review of reviews. Obes. Rev. 2015, 16, 621–638. [Google Scholar] [CrossRef]
- Hewapathirana, N.M.; Murphy, H.R. Perinatal outcomes in type 2 diabetes. Curr. Diabetes Rep. 2014, 14, 461. [Google Scholar] [CrossRef]
- Bramham, K.; Parnell, B.; Nelson-Piercy, C.; Seed, P.T.; Poston, L.; Chappell, L.C. Chronic hypertension and pregnancy outcomes: Systematic review and meta-analysis. BMJ 2014, 348, g2301. [Google Scholar] [CrossRef]
- Tamura, T.; Picciano, M.F. Folate and human reproduction. Am. J. Clin. Nutr. 2006, 83, 993–1016. [Google Scholar] [CrossRef]
- Finer, L.B.; Zolna, M.R. Declines in Unintended Pregnancy in the United States, 2008–2011. N. Engl. J. Med. 2016, 374, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Coles, C.D.; Kable, J.A.; Keen, C.L.; Jones, K.L.; Wertelecki, W.; Granovska, I.V.; Pashtepa, A.O.; Chambers, C.D.; Cifasd. Dose and Timing of Prenatal Alcohol Exposure and Maternal Nutritional Supplements: Developmental Effects on 6-Month-Old Infants. Matern. Child Health J. 2015, 19, 2605–2614. [Google Scholar] [CrossRef] [PubMed]
- Keen, C.L.; Uriu-Adams, J.Y.; Skalny, A.; Grabeklis, A.; Grabeklis, S.; Green, K.; Yevtushok, L.; Wertelecki, W.W.; Chambers, C.D. The plausibility of maternal nutritional status being a contributing factor to the risk for fetal alcohol spectrum disorders: The potential influence of zinc status as an example. Biofactors 2010, 36, 125–135. [Google Scholar] [CrossRef] [PubMed]
- May, P.A.; Gossage, J.P.; White-Country, M.; Goodhart, K.; Decoteau, S.; Trujillo, P.M.; Kalberg, W.O.; Viljoen, D.L.; Hoyme, H.E. Alcohol consumption and other maternal risk factors for fetal alcohol syndrome among three distinct samples of women before, during, and after pregnancy: The risk is relative. Am. J. Med. Genet. Part C Semin. Med. Genet. 2004, 127, 10–20. [Google Scholar] [CrossRef]
- Tomedi, L.E.; Bogen, D.L.; Hanusa, B.H.; Wisner, K.L.; Bodnar, L.M. A pilot study of the nutritional status of opiate-using pregnant women on methadone maintenance therapy. Subst. Use Misuse 2012, 47, 286–295. [Google Scholar] [CrossRef]
- Shrestha, S.; Jimenez, E.; Garrison, L.; Pribis, P.; Raisch, D.W.; Stephen, J.M.; Bakhireva, L.N. Dietary Intake Among Opioid- and Alcohol-Using Pregnant Women. Subst. Use Misuse 2018, 53, 260–269. [Google Scholar] [CrossRef]
- National Health and Nutrition Examination Survey. Available online: https://wwwn.cdc.gov/nchs/nhanes/default.aspx (accessed on 25 June 2021).
- National Institute on Alcohol Abuse and Alcoholism. Drinking Levels Defined. Available online: https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking (accessed on 7 September 2019).
- World Health Organization. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ. Tech. Rep. Ser. 2000, 894, 1–253. [Google Scholar]
- Janssen, I.; Katzmarzyk, P.T.; Ross, R. Body mass index, waist circumference, and health risk: Evidence in support of current National Institutes of Health guidelines. Arch. Intern. Med. 2002, 162, 2074–2079. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, e13–e115. [Google Scholar] [CrossRef]
- National Cholesterol Education Program (US); Expert Panel on Detection, Evaluation, & Treatment of High Blood Cholesterol in Adults. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Cefalu, W.T.; Berg, E.G.; Saraco, M.; Petersen, M.P.; Uelmen, S.; Robinson, S.; Assoc, A.D. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef]
- CDC. Recommendations to prevent and control iron deficiency in the United States. Centers for Disease Control and Prevention. MMWR Recomm. Rep. 1998, 47, 1–29. [Google Scholar]
- Merck Manual Professional Version: Blood Tests: Normal Values. Available online: https://www.merckmanuals.com/professional/resources/normal-laboratory-values/blood-tests-normal-values (accessed on 7 November 2019).
- WHO. Serum Ferritin Concentrations for the Assessment of Iron Status and Iron Deficiency in Populations; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 95: Anemia in pregnancy. Obstet. Gynecol. 2008, 112, 201–207. [Google Scholar] [CrossRef]
- Mei, Z.; Pfeiffer, C.M.; Looker, A.C.; Flores-Ayala, R.C.; Lacher, D.A.; Mirel, L.B.; Grummer-Strawn, L.M. Serum soluble transferrin receptor concentrations in US preschool children and non-pregnant women of childbearing age from the National Health and Nutrition Examination Survey 2003–2010. Clin. Chim. Acta 2012, 413, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Cogswell, M.E.; Looker, A.C.; Pfeiffer, C.M.; Cook, J.D.; Lacher, D.A.; Beard, J.L.; Lynch, S.R.; Grummer-Strawn, L.M. Assessment of iron deficiency in US preschool children and nonpregnant females of childbearing age: National Health and Nutrition Examination Survey 2003–2006. Am. J. Clin. Nutr. 2009, 89, 1334–1342. [Google Scholar] [CrossRef]
- De Benoist, B. Conclusions of a WHO Technical Consultation on folate and vitamin B12 deficiencies. Food Nutr. Bull. 2008, 29, S238–S244. [Google Scholar] [CrossRef]
- Jacques, P.F.; Selhub, J.; Bostom, A.G.; Wilson, P.W.; Rosenberg, I.H. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N. Engl. J. Med. 1999, 340, 1449–1454. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press: Washington, DC, USA, 1998. [Google Scholar] [CrossRef]
- WHO. Serum Retinol Concentrations for Determining the Prevalence of Vitamin A Deficiency in Populations; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Schleicher, R.L.; Carroll, M.D.; Ford, E.S.; Lacher, D.A. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am. J. Clin. Nutr. 2009, 90, 1252–1263. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Stokes, A.; Berry, K.M.; Collins, J.M.; Hsiao, C.W.; Waggoner, J.R.; Johnston, S.S.; Ammann, E.M.; Scamuffa, R.F.; Lee, S.; Lundberg, D.J.; et al. The contribution of obesity to prescription opioid use in the United States. Pain 2019, 160, 2255–2262. [Google Scholar] [CrossRef]
- Cho, G.; Chang, V.W. Obesity and the Receipt of Prescription Pain Medications in the US. J. Gen. Intern. Med. 2021, 36, 2631–2638. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xu, L.; Wang, Y.; Zhang, Y.; Du, Y.; Sun, Y.; Wang, Z. Association between perinatal outcomes and maternal pre-pregnancy body mass index. Obes. Rev. 2016, 17, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Poobalan, A.S.; Aucott, L.S.; Gurung, T.; Smith, W.C.S.; Bhattacharya, S. Obesity as an independent risk factor for elective and emergency caesarean delivery in nulliparous women—systematic review and meta-analysis of cohort studies. Obes. Rev. 2009, 10, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Lutsiv, O.; Mah, J.; Beyene, J.; McDonald, S.D. The effects of morbid obesity on maternal and neonatal health outcomes: A systematic review and meta-analyses. Obes. Rev. 2015, 16, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Azuine, R.E.; Ji, Y.; Chang, H.Y.; Kim, Y.; Ji, H.; DiBari, J.; Hong, X.; Wang, G.; Singh, G.K.; Pearson, C.; et al. Prenatal Risk Factors and Perinatal and Postnatal Outcomes Associated with Maternal Opioid Exposure in an Urban, Low-Income, Multiethnic US Population. JAMA Netw. Open 2019, 2, e196405. [Google Scholar] [CrossRef]
- Chui, P.W.; Gordon, K.S.; Dziura, J.; Burg, M.M.; Brandt, C.A.; Sico, J.J.; Leapman, M.S.; Cavanagh, C.E.; Rosman, L.; Haskell, S.; et al. Association of prescription opioids and incident cardiovascular risk factors among post-9/11 Veterans. Prev. Med. 2020, 134, 106036. [Google Scholar] [CrossRef]
- Khodneva, Y.; Muntner, P.; Kertesz, S.; Kissela, B.; Safford, M.M. Prescription Opioid Use and Risk of Coronary Heart Disease, Stroke, and Cardiovascular Death Among Adults from a Prospective Cohort (REGARDS Study). Pain Med. 2016, 17, 444–455. [Google Scholar] [CrossRef]
- Baumfeld, Y.; Novack, L.; Wiznitzer, A.; Sheiner, E.; Henkin, Y.; Sherf, M.; Novack, V. Pre-Conception Dyslipidemia Is Associated with Development of Preeclampsia and Gestational Diabetes Mellitus. PLoS ONE 2015, 10, e0139164. [Google Scholar] [CrossRef]
- Smith, C.J.; Baer, R.J.; Oltman, S.P.; Breheny, P.J.; Bao, W.; Robinson, J.G.; Dagle, J.M.; Liang, L.; Feuer, S.K.; Chambers, C.D.; et al. Maternal dyslipidemia and risk for preterm birth. PLoS ONE 2018, 13, e0209579. [Google Scholar] [CrossRef] [PubMed]
- Grieger, J.A.; Bianco-Miotto, T.; Grzeskowiak, L.E.; Leemaqz, S.Y.; Poston, L.; McCowan, L.M.; Kenny, L.C.; Myers, J.E.; Walker, J.J.; Dekker, G.A.; et al. Metabolic syndrome in pregnancy and risk for adverse pregnancy outcomes: A prospective cohort of nulliparous women. PLoS Med. 2018, 15, e1002710. [Google Scholar] [CrossRef]
- Bedson, J.; Chen, Y.; Ashworth, J.; Hayward, R.A.; Dunn, K.M.; Jordan, K.P. Risk of adverse events in patients prescribed long-term opioids: A cohort study in the UK Clinical Practice Research Datalink. Eur. J. Pain 2019, 23, 908–922. [Google Scholar] [CrossRef]
- Juul, S.E.; Derman, R.J.; Auerbach, M. Perinatal Iron Deficiency: Implications for Mothers and Infants. Neonatology 2019, 115, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.L.; Layden, A.J.; Stover, P.J. Vitamin B-12 and Perinatal Health. Adv. Nutr. 2015, 6, 552–563. [Google Scholar] [CrossRef]
- Mei, Z.; Cogswell, M.E.; Looker, A.C.; Pfeiffer, C.M.; Cusick, S.E.; Lacher, D.A.; Grummer-Strawn, L.M. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1999–2006. Am. J. Clin. Nutr. 2011, 93, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Bemanian, M.; Vold, J.H.; Chowdhury, R.; Aas, C.F.; Gjestad, R.; Johansson, K.A.; Fadnes, L.T. Folate Status as a Nutritional Indicator among People with Substance Use Disorder; A Prospective Cohort Study in Norway. Int. J. Environ. Res. Public Health 2022, 19, 5754. [Google Scholar] [CrossRef] [PubMed]
- Osadnik, K.; Osadnik, T.; Lonnie, M.; Lejawa, M.; Regula, R.; Fronczek, M.; Gawlita, M.; Wadolowska, L.; Gasior, M.; Pawlas, N. Metabolically healthy obese and metabolic syndrome of the lean: The importance of diet quality. Analysis of MAGNETIC cohort. Nutr. J. 2020, 19, 19. [Google Scholar] [CrossRef]
- Slagter, S.N.; van Vliet-Ostaptchouk, J.V.; Vonk, J.M.; Boezen, H.M.; Dullaart, R.P.; Kobold, A.C.; Feskens, E.J.; van Beek, A.P.; van der Klauw, M.M.; Wolffenbuttel, B.H. Associations between smoking, components of metabolic syndrome and lipoprotein particle size. BMC Med. 2013, 11, 195. [Google Scholar] [CrossRef]
| Variable | Prescription Opioid Exposed (n = 404) | Unexposed (n = 7234) | p-Value |
|---|---|---|---|
| Mean (SE) | |||
| Age (years) | 33.9 (0.4) | 32.4 (0.1) | 0.0001 |
| Number of previous pregnancies | 2.7 (0.1) | 2.0 (0.04) | <0.0001 |
| Percent (SE) | |||
| Race/ethnicity | 0.0008 | ||
| Mexican American | 8.4 (1.2) | 9.7 (0.7) | |
| Other Hispanic | 3.0 (0.9) | 6.6 (0.5) | |
| Non-Hispanic White | 72.5 (2.6) | 63.6 (1.2) | |
| Non-Hispanic Black | 12.0 (1.7) | 12.7 (0.7) | |
| Other/Multiracial | 4.2 (1.1) | 7.3 (0.4) | |
| Education | <0.0001 | ||
| Less than high school | 17.0 (2.0) | 12.0 (0.5) | |
| High school grad or equivalent | 25.2 (2.4) | 19.9 (0.7) | |
| Some college/assoc. degree | 39.1 (2.8) | 36.1 (0.9) | |
| College grad or higher | 18.7 (2.8) | 32.1 (1.1) | |
| Marital status | <0.0001 | ||
| Married | 47.6 (3.0) | 49.4 (0.9) | |
| Living with partner | 14.8 (1.9) | 11.2 (0.5) | |
| Divorced/separated/widowed | 19.0 (2.1) | 11.2 (0.5) | |
| Never married | 18.6 (2.2) | 28.2 (0.9) | |
| Income to poverty ratio | 0.0003 | ||
| <1.30 | 36.0 (2.9) | 25.6 (0.8) | |
| 1.30–3.49 | 36.2 (3.2) | 36.8 (0.8) | |
| ≥3.50 | 27.9 (2.9) | 37.6 (1.0) | |
| Employed | 56.9 (2.7) | 73.5 (0.8) | <0.0001 |
| Health insurance (any type) | 86.3 (1.9) | 79.3 (0.7) | 0.002 |
| Alcohol use | 0.07 | ||
| Non-drinker | 28.9 (3.3) | 22.4 (0.8) | |
| Low risk | 56.3 (3.6) | 61.1 (0.9) | |
| High risk | 14.9 (1.9) | 16.5 (0.6) | |
| Current cigarette smoking | 39.5 (2.8) | 21.2 (0.7) | <0.0001 |
| Ever diagnosed with | |||
| Arthritis (any type) | 33.2 (2.5) | 8.7 (0.5) | <0.0001 |
| Asthma | 31.9 (3.0) | 16.4 (0.6) | <0.0001 |
| Cancer (any type) | 12.2 (2.0) | 3.6 (0.3) | <0.0001 |
| Chronic bronchitis | 17.7 (2.2) | 5.0 (0.4) | <0.0001 |
| Diabetes or borderline diabetes | 7.1 (1.2) | 3.6 (0.3) | 0.0001 |
| Thyroid condition | 12.7 (1.9) | 7.9 (0.4) | 0.002 |
| Measure | Rx Opioid Exposed | Unexposed Control | Model 1 a | Model 2 b | Model 3 c | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SE | n | Mean | SE | p | p | p | |
| BMI (kg/m2) | 404 | 30.6 | 0.5 | 7234 | 28.3 | 0.1 | <0.0001 | 0.0005 | 0.05 |
| Waist circumference (cm) | 397 | 98.4 | 0.2 | 7137 | 92.4 | 0.3 | <0.0001 | 0.0008 | 0.70 |
| Systolic BP (mm Hg) | 393 | 113.3 | 0.7 | 7050 | 111.7 | 0.2 | 0.04 | 0.25 | 0.67 |
| Diastolic BP (mm Hg) | 393 | 70.1 | 0.6 | 7050 | 69.0 | 0.2 | 0.06 | 0.51 | 0.85 |
| HDL (mg/dL) | 388 | 53.2 | 0.9 | 6956 | 56.4 | 0.3 | 0.0004 | 0.02 | 0.66 |
| Fasting serum LDL cholesterol (mg/dL) | 176 | 112.3 | 2.9 | 3096 | 106.6 | 0.7 | 0.06 | 0.51 | 0.87 |
| Fasting serum triglycerides (mg/dL) | 178 | 132.8 | 8.4 | 3114 | 100.0 | 1.9 | 0.0002 | 0.01 | 0.14 |
| Fasting glucose (mg/dL) | 180 | 07.2 | 1.4 | 3141 | 95.6 | 0.4 | 0.30 | 0.57 | 0.75 |
| Hemoglobin A1C (%) | 392 | 5.34 | 0.03 | 7000 | 5.27 | 0.01 | 0.049 | 0.23 | 0.69 |
| Hemoglobin (g/dL) | 393 | 13.46 | 0.07 | 7016 | 13.40 | 0.02 | 0.36 | 0.80 | 0.30 |
| Hematocrit (%) | 393 | 39.6 | 0.2 | 7016 | 39.5 | 0.1 | 0.60 | 0.78 | 0.28 |
| RBC count (million cells/µL) | 393 | 4.48 | 0.02 | 7016 | 4.48 | 0.01 | 0.87 | 0.99 | 0.16 |
| Mean cell volume (fL) | 393 | 88.8 | 0.3 | 7016 | 88.4 | 0.1 | 0.24 | 0.44 | 0.32 |
| Serum ferritin (µg/L) | 304 | 59.7 | 3.7 | 5442 | 53.6 | 0.8 | 0.11 | 0.22 | 0.39 |
| Serum iron (µg/dL) | 174 | 72.3 | 2.6 | 3065 | 82.7 | 1.0 | 0.0003 | 0.003 | 0.009 |
| Serum transferrin receptor (mg/L) | 239 | 3.75 | 0.18 | 4250 | 3.52 | 0.04 | 0.22 | 0.26 | 0.23 |
| Serum transferrin saturation (%) | 174 | 20.4 | 0.8 | 3060 | 23.4 | 0.3 | 0.0009 | 0.006 | 0.01 |
| Serum total iron binding capacity (µg/dL) | 174 | 365.8 | 6.8 | 3060 | 364.5 | 1.7 | 0.84 | 0.95 | 0.77 |
| Serum folate (nmol/L) | 390 | 36.5 | 1.1 | 6955 | 41.3 | 0.5 | <0.0001 | 0.0005 | 0.02 |
| Serum vitamin B12 (pmol/L) | 233 | 374.8 | 11.4 | 3861 | 407.4 | 24.0 | 0.23 | 0.26 | 0.60 |
| Plasma homocysteine (µmol/L) | 150 | 7.68 | 0.29 | 2377 | 6.93 | 0.06 | 0.01 | 0.048 | 0.06 |
| Serum PLP (vitamin B6) (nmol/L) | 144 | 59.3 | 8.5 | 2220 | 63.6 | 2.6 | 0.62 | 0.80 | 0.91 |
| Serum retinol (µmol/L) | 149 | 1.92 | 0.07 | 2354 | 1.84 | 0.01 | 0.20 | 0.40 | 0.24 |
| Serum vitamin C (μmol/L) | 110 | 45.4 | 2.9 | 1894 | 53.0 | 1.1 | 0.01 | 0.07 | 0.63 |
| Serum 25OH vitamin D (nmol/L) | 304 | 64.7 | 2.2 | 4925 | 64.7 | 0.7 | 0.98 | 0.58 | 0.67 |
| Measure | Cutoff | Rx Opioid | Control | Model 1 a | Model 2 b | Model 3 c | ||
|---|---|---|---|---|---|---|---|---|
| % | SE | % | SE | OR (CI) | OR (CI) | OR (CI) | ||
| Body mass index | Underweight (<18.5 kg/m2) | 3.8 | 1.4 | 2.9 | 0.2 | 1.8 (0.8–4.0) | 1.7 (0.8–3.7) | 1.6 (0.7–3.5) |
| Overweight (25–29.9 kg/m2) | 22.9 | 2.8 | 24.4 | 0.6 | 1.3 (0.9–1.9) | 1.2 (0.8–1.8) | 1.2 (0.8–1.7) | |
| Obese I (30–34.9 kg/m2) | 16.5 | 2.1 | 16.6 | 0.5 | 1.4 (0.9–2.0) | 1.2 (0.8–1.8) | 1.0 (0.7–1.5) | |
| Obese II (35–39.9 kg/m2) | 15.6 | 1.9 | 9.4 | 0.4 | 2.3 (1.7–3.3) | 2.0 (1.4–3.2) | 1.6 (1.1–2.3) | |
| Obese III (≥40.0 kg/m2) | 14.2 | 1.9 | 8.2 | 0.4 | 2.4 (1.7–3.5) | 2.2 (1.5–3.2) | 1.6 (1.1–2.5) | |
| Waist circumference | High (>88 cm) | 66.2 | 2.9 | 52.7 | 0.8 | 1.8 (1.4–2.3) | 1.5 (1.2–2.0) | 1.2 (0.8–1.8) |
| Blood pressure | High (≥120/80 mm Hg) | 29.8 | 2.2 | 24.6 | 0.6 | 1.3 (1.1–1.6) | 1.2 (0.9–1.5) | 1.0 (0.8–1.3) |
| Serum HDL cholesterol | Low (<50 mg/dL) | 43.8 | 3.1 | 35.8 | 0.8 | 1.4 (1.1–1.8) | 1.2 (0.9–1.5) | 0.8 (0.6–1.1) |
| Fasting LDL cholesterol | High (>100 mg/dL) | 59.0 | 4.4 | 54.2 | 1.2 | 1.2 (0.8–1.8) | 1.0 (0.7–1.5) | 0.9 (0.6–1.3) |
| Fasting serum triglycerides | High (≥150 mg/dL) | 30.1 | 3.9 | 14.4 | 0.8 | 2.6 (1.8–3.7) | 2.0 (1.4–2.9) | 1.5 (1.0–2.4) |
| Fasting plasma glucose | High (≥100 mg/dL) | 29.4 | 3.9 | 21.4 | 0.9 | 1.5 (1.0–2.2) | 1.5 (1.0–2.2) | 1.2 (0.8–1.9) |
| Hemoglobin A1C | High (≥5.7%) | 14.0 | 1.9 | 10.8 | 0.5 | 1.3 (1.0–1.9) | 1.3 (0.9–1.8) | 0.9 (0.6–1.4) |
| Metabolic syndrome | Meets 3 or more criteria | 35.5 | 4.2 | 18.0 | 0.7 | 2.5 (1.7–3.7) | 2.1 (1.4–3.2) | 1.6 (1.0–2.6) |
| Hemoglobin | Low (<12 g/dL) | 7.1 | 1.4 | 8.6 | 0.4 | 0.8 (0.6–1.2) | 0.8 (0.5–1.2) | 0.9 (0.5–1.3) |
| Hematocrit | Low (<36%) | 11.0 | 1.7 | 10.7 | 0.5 | 1.0 (0.7–1.5) | 1.0 (0.7–1.5) | 1.1 (0.8–1.7) |
| RBC count | Low (<4.2 × 106 cells/µL) | 22.4 | 2.4 | 19.4 | 0.7 | 1.2 (0.9–1.6) | 1.1 (0.9–1.5) | 1.3 (1.0–1.7) |
| Mean corpuscular volume | Low (<80 fL) | 7.4 | 1.3 | 7.2 | 0.3 | 1.1 (0.7–1.6) | 1.1 (0.7–1.6) | 1.1 (0.7–1.5) |
| High (>100 fL) | 2.8 | 1.0 | 0.6 | 0.1 | 4.7 (2.0–11.0) | 2.5 (1.1–5.7) | 2.4 (1.0–5.8) | |
| Serum ferritin | Low (<15 µg/L) | 12.6 | 1.2 | 15.3 | 0.5 | 0.8 (0.6–1.2) | 0.8 (0.6–1.2) | 0.9 (0.6–1.4) |
| High (>150 µg/L) | 8.5 | 2.1 | 4.7 | 0.4 | 1.8 (1.1–3.2) | 1.6 (0.9–2.8) | 1.4 (0.8–2.5) | |
| Serum iron | Low (<40 µg/dL) | 15.6 | 3.2 | 12.3 | 0.7 | 1.5 (0.8–2.6) | 1.2 (0.7–2.1) | 1.1 (0.7–2.0) |
| Serum transferrin receptor | High (>5.33 mg/L) | 11.7 | 2.0 | 8.4 | 0.5 | 1.5 (1.0–2.2) | 1.4 (0.9–2.2) | 1.5 (1.0–2.3) |
| Serum transferrin saturation | Low (<15%) | 35.3 | 3.6 | 25.2 | 1.0 | 1.6 (1.2–2.2) | 1.6 (1.1–2.3) | 1.5 (1.1–2.2) |
| Serum TIBC | High (>460 µg/dL) | 9.1 | 2.4 | 7.1 | 0.6 | 1.3 (0.7–2.3) | 1.3 (0.7–2.4) | 1.4 (0.7–2.5) |
| Serum PLP (vitamin B6) | Low (<20 µmol/L) | 23.1 | 3.7 | 13.5 | 1.0 | 1.9 (1.3–2.9) | 1.6 (1.0–2.5) | 1.2 (0.7–1.9) |
| Serum vitamin C | Low (<11.4 μmol/L) | 10.8 | 3.4 | 6.3 | 0.7 | 1.8 (0.9–3.4) | 1.3 (0.6–2.6) | 0.8 (0.4–1.6) |
| Serum 25OH Vitamin D | Low (<30 nmol/L) | 9.7 | 1.9 | 7.7 | 0.6 | 1.3 (0.9–1.9) | 1.6 (1.0–2.5) | 1.5 (1.0–2.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hohman, E.E.; Corr, T.E.; Kawasaki, S.; Savage, J.S.; Symons Downs, D. Nutritional Status Differs by Prescription Opioid Use among Women of Reproductive Age: NHANES 1999–2018. Nutrients 2023, 15, 1891. https://doi.org/10.3390/nu15081891
Hohman EE, Corr TE, Kawasaki S, Savage JS, Symons Downs D. Nutritional Status Differs by Prescription Opioid Use among Women of Reproductive Age: NHANES 1999–2018. Nutrients. 2023; 15(8):1891. https://doi.org/10.3390/nu15081891
Chicago/Turabian StyleHohman, Emily E., Tammy E. Corr, Sarah Kawasaki, Jennifer S. Savage, and Danielle Symons Downs. 2023. "Nutritional Status Differs by Prescription Opioid Use among Women of Reproductive Age: NHANES 1999–2018" Nutrients 15, no. 8: 1891. https://doi.org/10.3390/nu15081891
APA StyleHohman, E. E., Corr, T. E., Kawasaki, S., Savage, J. S., & Symons Downs, D. (2023). Nutritional Status Differs by Prescription Opioid Use among Women of Reproductive Age: NHANES 1999–2018. Nutrients, 15(8), 1891. https://doi.org/10.3390/nu15081891





