Barley Leaf Ameliorates Citrobacter-rodentium-Induced Colitis through Arginine Enrichment
Highlights
- Arginine is an effective metabolite of barley leaf, exerting anti-colitis effects.
- Arginine ameliorates CR-induced colitis in a dose-dependent manner.
- The main finding highlighted the role of the gut microbiota in the amelioration of CR-induced colitis by arginine.
- The main finding demonstrates the potential application of arginine and barley leaf for prevention and improvement in IBD patients.
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. CR Infection
2.3. Determination of CR Load
2.4. Disease Activity Index (DAI)
2.5. Histological Staining
2.6. RT-qPCR
2.7. 16S rRNA Gene Sequencing
2.8. Non-Targeted Metabolomics
2.9. Arginine and Polyamine Determination
2.10. Statistics
3. Results
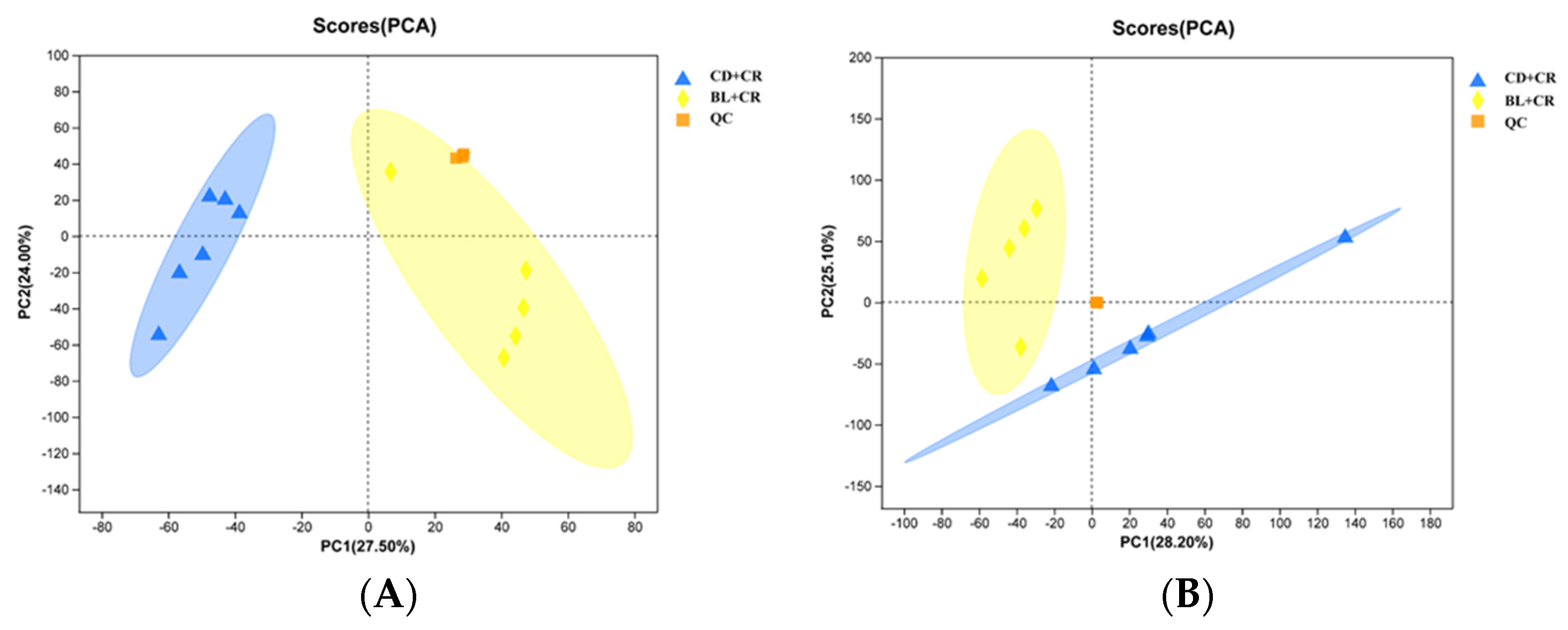
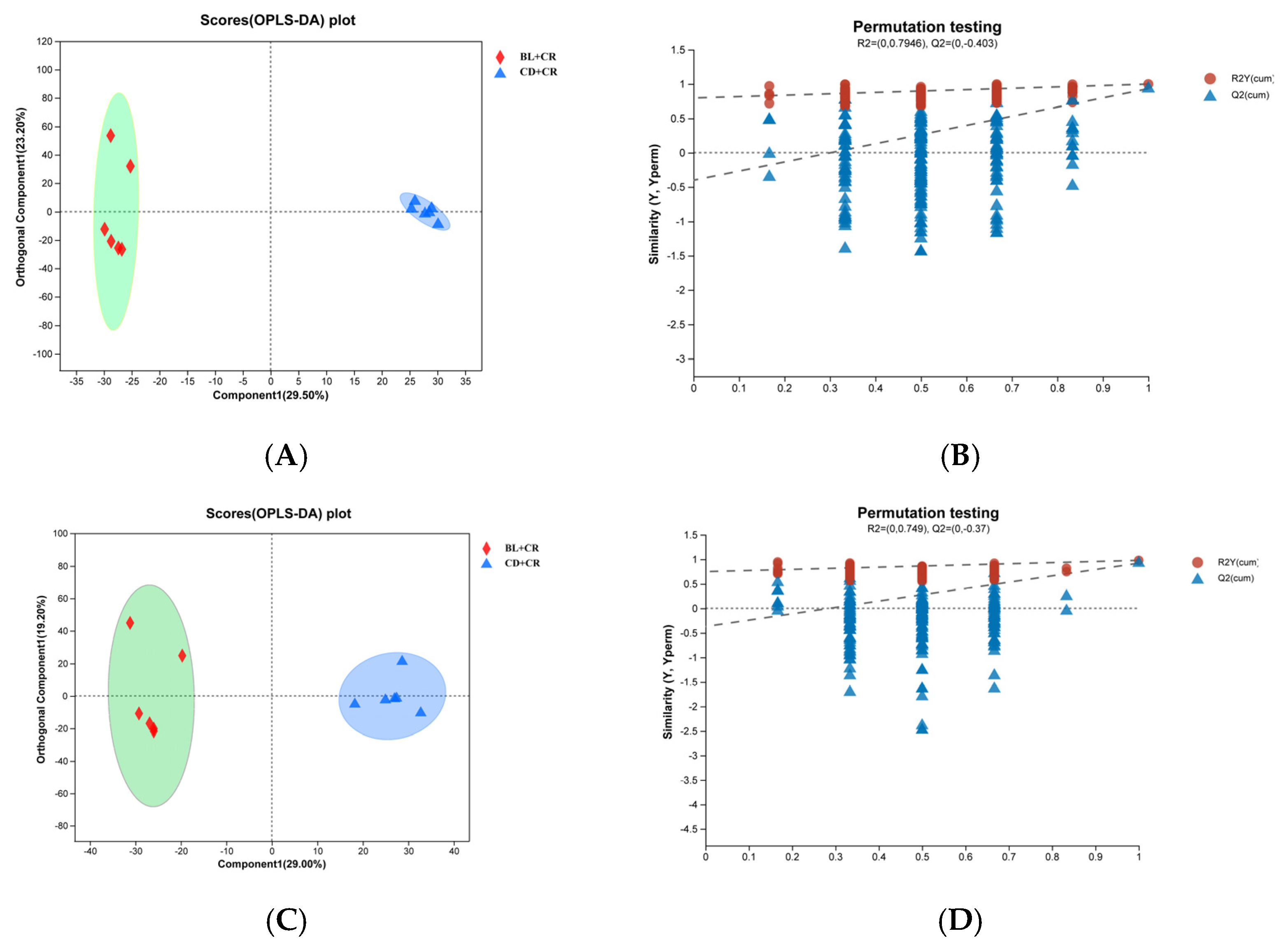
3.1. Effect of BL on Colonic Tissue Metabolites
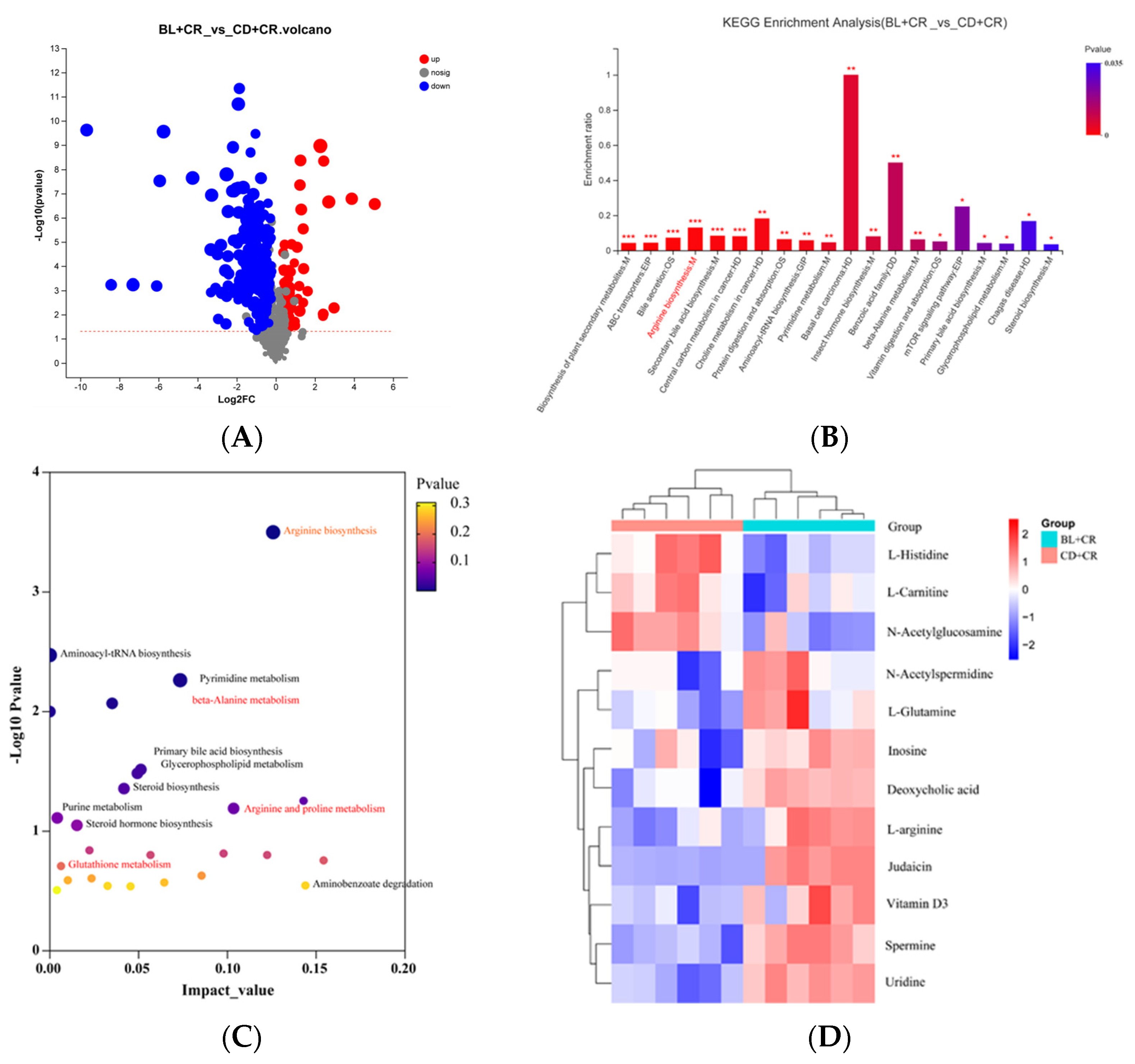
3.2. KEGG Functional Enrichment Analysis of Differential Metabolites for BL Intervention
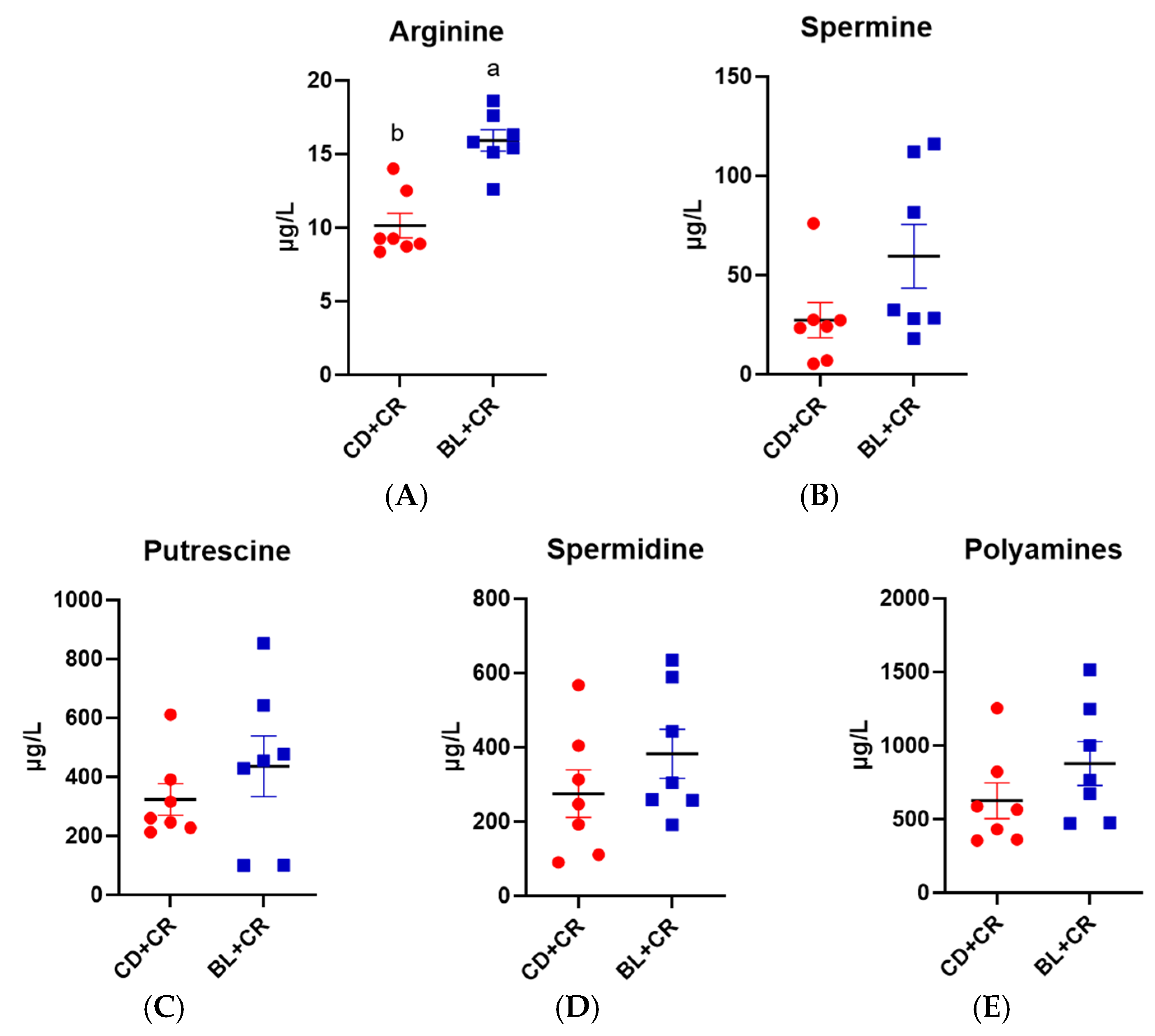
3.3. BL Intervention Enriches Arginine Production in Mice
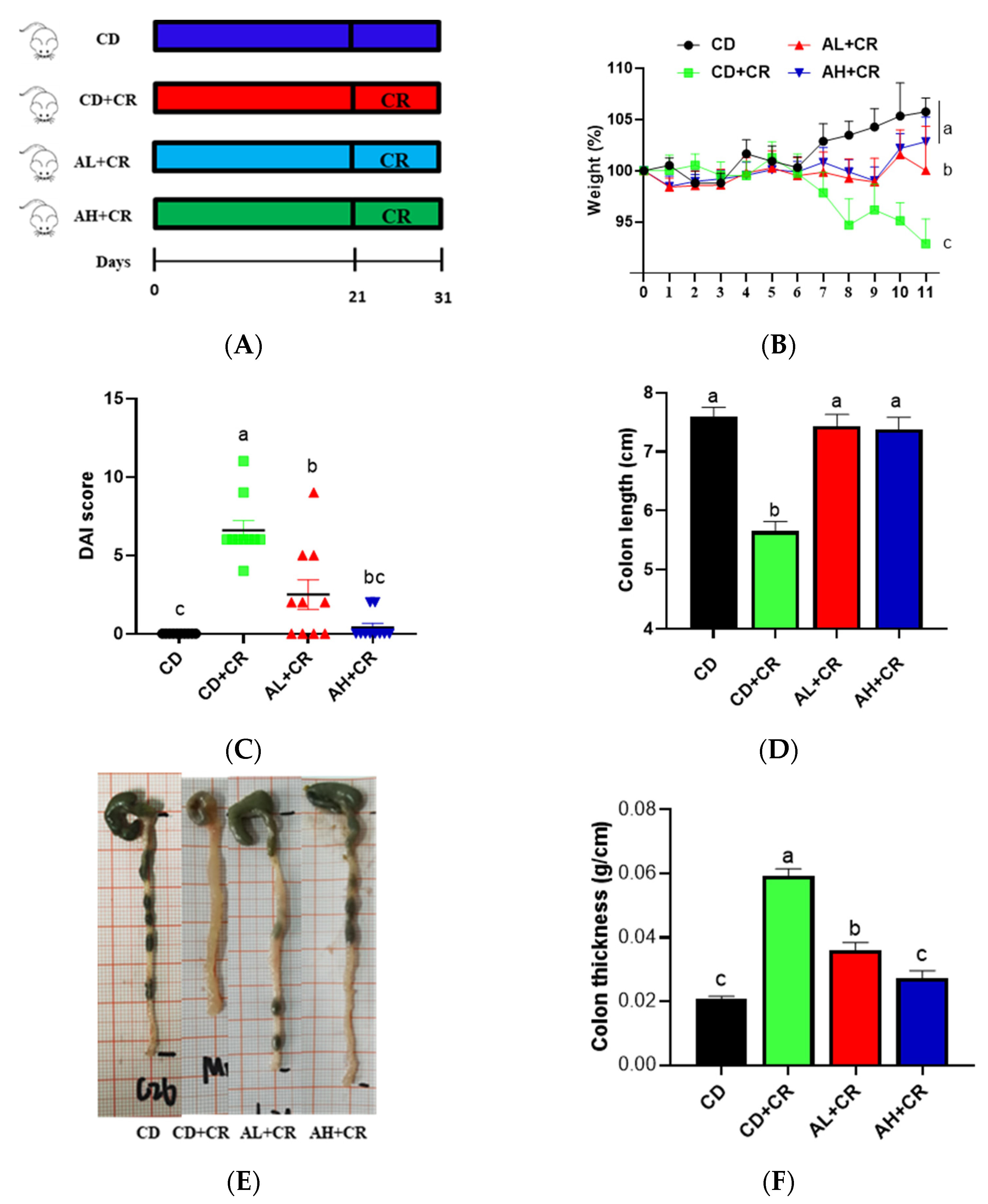
3.4. Arginine Improves CR-Induced Colitis
3.5. Arginine Ameliorates Histopathological Damage Caused by CR
3.6. Arginine Inhibits the Proliferation of CR and the Expression of Virulence Factors
3.7. Arginine Ameliorates CR-Induced Gut Microbiota Disorders
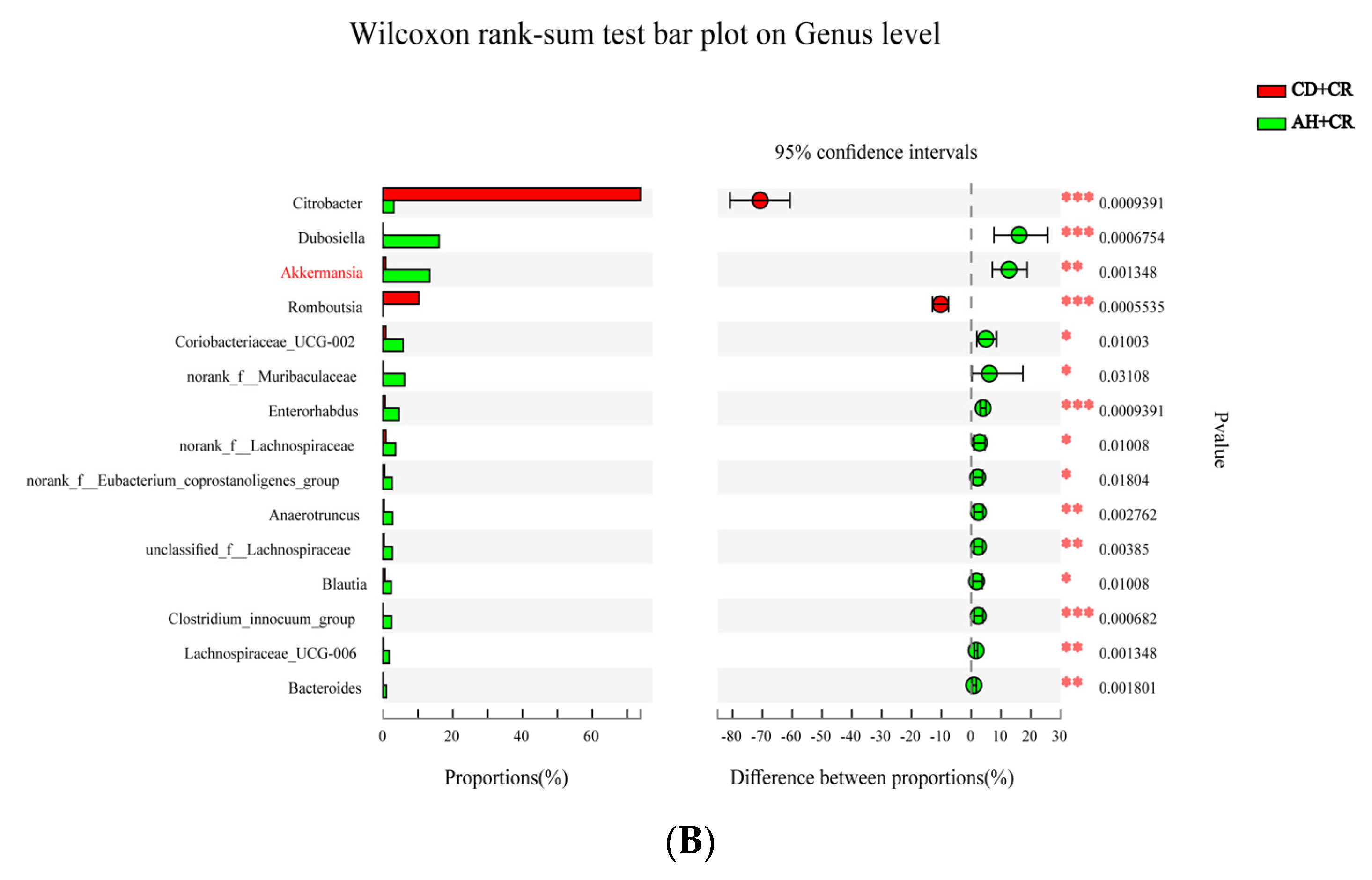
3.8. Effect of Arginine on the Gut Microbiota’s Composition and Structure
3.9. Gut Microbiota Function Prediction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Verstockt, B.; Bressler, B.; Martinez-Lozano, H.; McGovern, D.; Silverberg, M.S. Time to Revisit Disease Classification in Inflammatory Bowel Disease: Is the Current Classification of Inflammatory Bowel Disease Good Enough for Optimal Clinical Management? Gastroenterology 2022, 162, 1370–1382. [Google Scholar] [CrossRef]
- D’Arcangelo, G.; Aloi, M. Inflammatory Bowel Disease-Unclassified in Children: Diagnosis and Pharmacological Management. Paediatr. Drugs 2017, 19, 113–120. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Tsou, A.M.; Goettel, J.A.; Bao, B.; Biswas, A.; Kang, Y.H.; Redhu, N.S.; Peng, K.; Putzel, G.G.; Saltzman, J.; Kelly, R.; et al. Utilizing a reductionist model to study host-microbe interactions in intestinal inflammation. Microbiome 2021, 9, 215. [Google Scholar] [CrossRef]
- Llewellyn, S.R.; Britton, G.J.; Contijoch, E.J.; Vennaro, O.H.; Mortha, A.; Colombel, J.F.; Grinspan, A.; Clemente, J.C.; Merad, M.; Faith, J.J. Interactions Between Diet and the Intestinal Microbiota Alter Intestinal Permeability and Colitis Severity in Mice. Gastroenterology 2018, 154, 1037–1046.e1032. [Google Scholar] [CrossRef]
- Tian, M.; Li, D.; Ma, C.; Feng, Y.; Hu, X.; Chen, F. Barley Leaf Insoluble Dietary Fiber Alleviated Dextran Sulfate Sodium-Induced Mice Colitis by Modulating Gut Microbiota. Nutrients 2021, 13, 846. [Google Scholar] [CrossRef]
- Ishida, T.; Matsui, H.; Matsuda, Y.; Hosomi, R.; Shimono, T.; Kanda, S.; Nishiyama, T.; Fukunaga, K.; Yoshida, M. Oyster (Crassostrea gigas) Extract Attenuates Dextran Sulfate Sodium-Induced Acute Experimental Colitis by Improving Gut Microbiota and Short-Chain Fatty Acids Compositions in Mice. Foods 2022, 11, 373. [Google Scholar] [CrossRef]
- Sun, D.; Wang, C.; Sun, L.; Hu, L.; Fang, Z.; Deng, Q.; Zhao, J.; Gooneratne, R. Preliminary Report on Intestinal Flora Disorder, Faecal Short-Chain Fatty Acid Level Decline and Intestinal Mucosal Tissue Weakening Caused by Litchi Extract to Induce Systemic Inflammation in HFA Mice. Nutrients 2022, 14, 776. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef]
- Anitha, M.; Vijay-Kumar, M.; Sitaraman, S.V.; Gewirtz, A.T.; Srinivasan, S. Gut microbial products regulate murine gastrointestinal motility via Toll-like receptor 4 signaling. Gastroenterology 2012, 143, 1006–1016.e4. [Google Scholar] [CrossRef]
- Wu, G.; Collins, J.K.; Perkins-Veazie, P.; Siddiq, M.; Dolan, K.D.; Kelly, K.A.; Heaps, C.L.; Meininger, C.J. Dietary supplementation with watermelon pomace juice enhances arginine availability and ameliorates the metabolic syndrome in Zucker diabetic fatty rats. J. Nutr. 2007, 137, 2680–2685. [Google Scholar] [CrossRef]
- Jobgen, W.; Meininger, C.J.; Jobgen, S.C.; Li, P.; Lee, M.-J.; Smith, S.B.; Spencer, T.E.; Fried, S.K.; Wu, G. Dietary L-arginine supplementation reduces white fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats. J. Nutr. 2009, 139, 230–237. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, J.; Chen, B.; Zhao, T.; Gu, Z. Superabsorbent poly(acrylic acid) and antioxidant poly(ester amide) hybrid hydrogel for enhanced wound healing. Regen. Biomater. 2021, 8, rbaa059. [Google Scholar] [CrossRef]
- Yu, Y.M.; Ryan, C.M.; Castillo, L.; Lu, X.M.; Beaumier, L.; Tompkins, R.G.; Young, V.R. Arginine and ornithine kinetics in severely burned patients: Increased rate of arginine disposal. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E509–E517. [Google Scholar] [CrossRef]
- Rhoads, J.M.; Chen, W.; Gookin, J.; Wu, G.Y.; Fu, Q.; Blikslager, A.T.; Rippe, R.A.; Argenzio, R.A.; Cance, W.G.; Weaver, E.M.; et al. Arginine stimulates intestinal cell migration through a focal adhesion kinase dependent mechanism. Gut 2004, 53, 514–522. [Google Scholar] [CrossRef]
- Rhoads, J.M.; Liu, Y.; Niu, X.; Surendran, S.; Wu, G. Arginine stimulates cdx2-transformed intestinal epithelial cell migration via a mechanism requiring both nitric oxide and phosphorylation of p70 S6 kinase. J. Nutr. 2008, 138, 1652–1657. [Google Scholar] [CrossRef]
- Zhang, B.; Gan, L.; Shahid, M.S.; Lv, Z.; Fan, H.; Liu, D.; Guo, Y. In vivo and in vitro protective effect of arginine against intestinal inflammatory response induced by Clostridium perfringens in broiler chickens. J. Anim. Sci. Biotechnol. 2019, 10, 73. [Google Scholar] [CrossRef]
- Shah, P.S.; Shah, V.S.; Kelly, L.E. Arginine supplementation for prevention of necrotising enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2017, 4, Cd004339. [Google Scholar] [CrossRef]
- Feng, Y.; Li, D.; Ma, C.; Tian, M.; Hu, X.; Chen, F. Barley Leaf Ameliorates Citrobacter rodentium-Induced Colitis through Preventive Effects. Nutrients 2022, 14, 3833. [Google Scholar] [CrossRef]
- Crepin, V.F.; Collins, J.W.; Habibzay, M.; Frankel, G. Citrobacter rodentium mouse model of bacterial infection. Nat. Protoc. 2016, 11, 1851–1876. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, D.; Jin, Y.; Jia, H.; Yang, Y.; Kim, I.H.; Dai, Z.; Zhang, J.; Ren, F.; Wu, Z. Glycine Attenuates Citrobacter rodentium-Induced Colitis by Regulating ATF6-Mediated Endoplasmic Reticulum Stress in Mice. Mol. Nutr. Food Res. 2021, 65, e2001065. [Google Scholar] [CrossRef]
- Gruenheid, S.; DeVinney, R.; Bladt, F.; Goosney, D.; Gelkop, S.; Gish, G.D.; Pawson, T.; Finlay, B.B. Enteropathogenic E. coli Tir binds Nck to initiate actin pedestal formation in host cells. Nat. Cell Biol. 2001, 3, 856–859. [Google Scholar] [CrossRef]
- Nieto-Pelegrin, E.; Kenny, B.; Martinez-Quiles, N. Nck adaptors, besides promoting N-WASP mediated actin-nucleation activity at pedestals, influence the cellular levels of enteropathogenic Escherichia coli Tir effector. Cell Adh. Migr. 2014, 8, 404–417. [Google Scholar] [CrossRef]
- Lin, R.; Piao, M.; Song, Y. Dietary Quercetin Increases Colonic Microbial Diversity and Attenuates Colitis Severity in Citrobacter rodentium-Infected Mice. Front. Microbiol. 2019, 10, 1092. [Google Scholar] [CrossRef]
- Weiss, T.S.; Herfarth, H.; Obermeier, F.; Ouart, J.; Vogl, D.; Scholmerich, J.; Jauch, K.W.; Rogler, G. Intracellular polyamine levels of intestinal epithelial cells in inflammatory bowel disease. Inflamm. Bowel Dis. 2004, 10, 529–535. [Google Scholar] [CrossRef]
- Thompson, P.A.; Wertheim, B.C.; Zell, J.A.; Chen, W.P.; McLaren, C.E.; LaFleur, B.J.; Meyskens, F.L.; Gerner, E.W. Levels of rectal mucosal polyamines and prostaglandin E2 predict ability of DFMO and sulindac to prevent colorectal adenoma. Gastroenterology 2010, 139, 797–805.e1. [Google Scholar] [CrossRef]
- Carriche, G.M.; Almeida, L.; Stuve, P.; Velasquez, L.; Dhillon-LaBrooy, A.; Roy, U.; Lindenberg, M.; Strowig, T.; Plaza-Sirvent, C.; Schmitz, I.; et al. Regulating T-cell differentiation through the polyamine spermidine. J. Allergy Clin. Immunol. 2021, 147, 335–348.e311. [Google Scholar] [CrossRef]
- Liu, R.; Li, X.; Ma, H.; Yang, Q.; Shang, Q.; Song, L.; Zheng, Z.; Zhang, S.; Pan, Y.; Huang, P.; et al. Spermidine endows macrophages anti-inflammatory properties by inducing mitochondrial superoxide-dependent AMPK activation, Hif-1α upregulation and autophagy. Free Radic. Biol. Med. 2020, 161, 339–350. [Google Scholar] [CrossRef]
- Singh, K.; Al-Greene, N.T.; Verriere, T.G.; Coburn, L.A.; Asim, M.; Barry, D.P.; Allaman, M.M.; Hardbower, D.M.; Delgado, A.G.; Piazuelo, M.B.; et al. The L-Arginine Transporter Solute Carrier Family 7 Member 2 Mediates the Immunopathogenesis of Attaching and Effacing Bacteria. PLoS Pathog. 2016, 12, e1005984. [Google Scholar] [CrossRef]
- Ohta, F.; Takagi, T.; Sato, H.; Ignarro, L.J. Low-dose L-arginine administration increases microperfusion of hindlimb muscle without affecting blood pressure in rats. Proc. Natl. Acad. Sci. USA 2007, 104, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Coburn, L.A.; Horst, S.N.; Allaman, M.M.; Brown, C.T.; Williams, C.S.; Hodges, M.E.; Druce, J.P.; Beaulieu, D.B.; Schwartz, D.A.; Wilson, K.T. L-Arginine Availability and Metabolism Is Altered in Ulcerative Colitis. Inflamm. Bowel Dis. 2016, 22, 1847–1858. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Guo, Y.C.; Zhou, H.F.; Yue, T.T.; Wang, F.X.; Sun, F.; Wang, W.Z. Arginine metabolism regulates the pathogenesis of inflammatory bowel disease. Nutr. Rev. 2023, 81, 578–586. [Google Scholar] [CrossRef]
- McNeal, C.J.; Meininger, C.J.; Reddy, D.; Wilborn, C.D.; Wu, G. Safety and Effectiveness of Arginine in Adults. J. Nutr. 2016, 146, 2587S–2593S. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.K.; Daniell, S.; Frankel, G.; Knutton, S. Enteropathogenic Escherichia coli translocate Tir and form an intimin-Tir intimate attachment to red blood cell membranes. Microbiology 2002, 148, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, A.G.; Ellermann, M.; Abbott, W.; Sperandio, V. Diet-derived galacturonic acid regulates virulence and intestinal colonization in enterohaemorrhagic Escherichia coli and Citrobacter rodentium. Nat. Microbiol. 2020, 5, 368–378. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, C.; Li, Y.; Han, X.; Luo, X.; Chen, L.; Zhang, T.; Wang, N.; Wang, W. Alginate Oligosaccharides Ameliorate DSS-Induced Colitis through Modulation of AMPK/NF-κB Pathway and Intestinal Microbiota. Nutrients 2022, 14, 2864. [Google Scholar] [CrossRef]
- Shi, M.; Yue, Y.; Ma, C.; Dong, L.; Chen, F. Pasteurized Akkermansia muciniphila Ameliorate the LPS-Induced Intestinal Barrier Dysfunction via Modulating AMPK and NF-κB through TLR2 in Caco-2 Cells. Nutrients 2022, 14, 764. [Google Scholar] [CrossRef]
- Kobayashi, N.; Abe, K.; Akagi, S.; Kitamura, M.; Shiraishi, Y.; Yamaguchi, A.; Yutani, M.; Amatsu, S.; Matsumura, T.; Nomura, N.; et al. Membrane Vesicles Derived From Clostridium botulinum and Related Clostridial Species Induce Innate Immune Responses via MyD88/TRIF Signaling in vitro. Front. Microbiol. 2022, 13, 720308. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, J.; Wu, H.; Yu, D.; Fang, X. Akkermansia muciniphila Aspartic Protease Amuc_1434* Inhibits Human Colorectal Cancer LS174T Cell Viability via TRAIL-Mediated Apoptosis Pathway. Int. J. Mol. Sci. 2020, 21, 3385. [Google Scholar] [CrossRef]
- Bian, X.; Wu, W.; Yang, L.; Lv, L.; Wang, Q.; Li, Y.; Ye, J.; Fang, D.; Wu, J.; Jiang, X.; et al. Administration of Akkermansia muciniphila Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. Front. Microbiol. 2019, 10, 2259. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiao, Y.; Xu, X.; Guo, L.; Yu, Y.; Li, N.; Xu, C. Characteristics of Fecal Microbiota and Machine Learning Strategy for Fecal Invasive Biomarkers in Pediatric Inflammatory Bowel Disease. Front. Cell. Infect. Microbiol. 2021, 11, 711884. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Hylemon, P.B.; Ridlon, J.M.; Heuman, D.M.; Daita, K.; White, M.B.; Monteith, P.; Noble, N.A.; Sikaroodi, M.; Gillevet, P.M. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am. J. Physiology. Gastrointest. Liver Physiol. 2012, 303, G675–G685. [Google Scholar] [CrossRef]
- Tannock, G.W.; Lawley, B.; Munro, K.; Lay, C.; Taylor, C.; Daynes, C.; Baladjay, L.; McLeod, R.; Thompson-Fawcett, M. Comprehensive analysis of the bacterial content of stool from patients with chronic pouchitis, normal pouches, or familial adenomatous polyposis pouches. Inflamm. Bowel Dis. 2012, 18, 925–934. [Google Scholar] [CrossRef]
- Ma, S.; Qin, J.; Hao, Y.; Fu, L. Association of gut microbiota composition and function with an aged rat model of senile osteoporosis using 16S rRNA and metagenomic sequencing analysis. Aging 2020, 12, 10795–10808. [Google Scholar] [CrossRef]
- Phannasorn, W.; Pharapirom, A.; Thiennimitr, P.; Guo, H.; Ketnawa, S.; Wongpoomchai, R. Enriched Riceberry Bran Oil Exerts Chemopreventive Properties through Anti-Inflammation and Alteration of Gut Microbiota in Carcinogen-Induced Liver and Colon Carcinogenesis in Rats. Cancers 2022, 14, 4358. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Ye, D.; Yang, H.; Song, J.; Sun, X.; Mao, Y.; He, Z. Two-Sample Mendelian Randomization Analysis Investigates Causal Associations Between Gut Microbial Genera and Inflammatory Bowel Disease, and Specificity Causal Associations in Ulcerative Colitis or Crohn’s Disease. Front. Immunol. 2022, 13, 921546. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Li, D.; Ma, C.; Hu, X.; Chen, F. Barley Leaf Ameliorates Citrobacter-rodentium-Induced Colitis through Arginine Enrichment. Nutrients 2023, 15, 1890. https://doi.org/10.3390/nu15081890
Feng Y, Li D, Ma C, Hu X, Chen F. Barley Leaf Ameliorates Citrobacter-rodentium-Induced Colitis through Arginine Enrichment. Nutrients. 2023; 15(8):1890. https://doi.org/10.3390/nu15081890
Chicago/Turabian StyleFeng, Yu, Daotong Li, Chen Ma, Xiaosong Hu, and Fang Chen. 2023. "Barley Leaf Ameliorates Citrobacter-rodentium-Induced Colitis through Arginine Enrichment" Nutrients 15, no. 8: 1890. https://doi.org/10.3390/nu15081890
APA StyleFeng, Y., Li, D., Ma, C., Hu, X., & Chen, F. (2023). Barley Leaf Ameliorates Citrobacter-rodentium-Induced Colitis through Arginine Enrichment. Nutrients, 15(8), 1890. https://doi.org/10.3390/nu15081890





