Taurine: A Source and Application for the Relief of Visual Fatigue
Abstract
1. Introduction
1.1. Retinal Stress Damage: Oxidation, Inflammation, Apoptosis
1.2. Damage to Nerve Cells
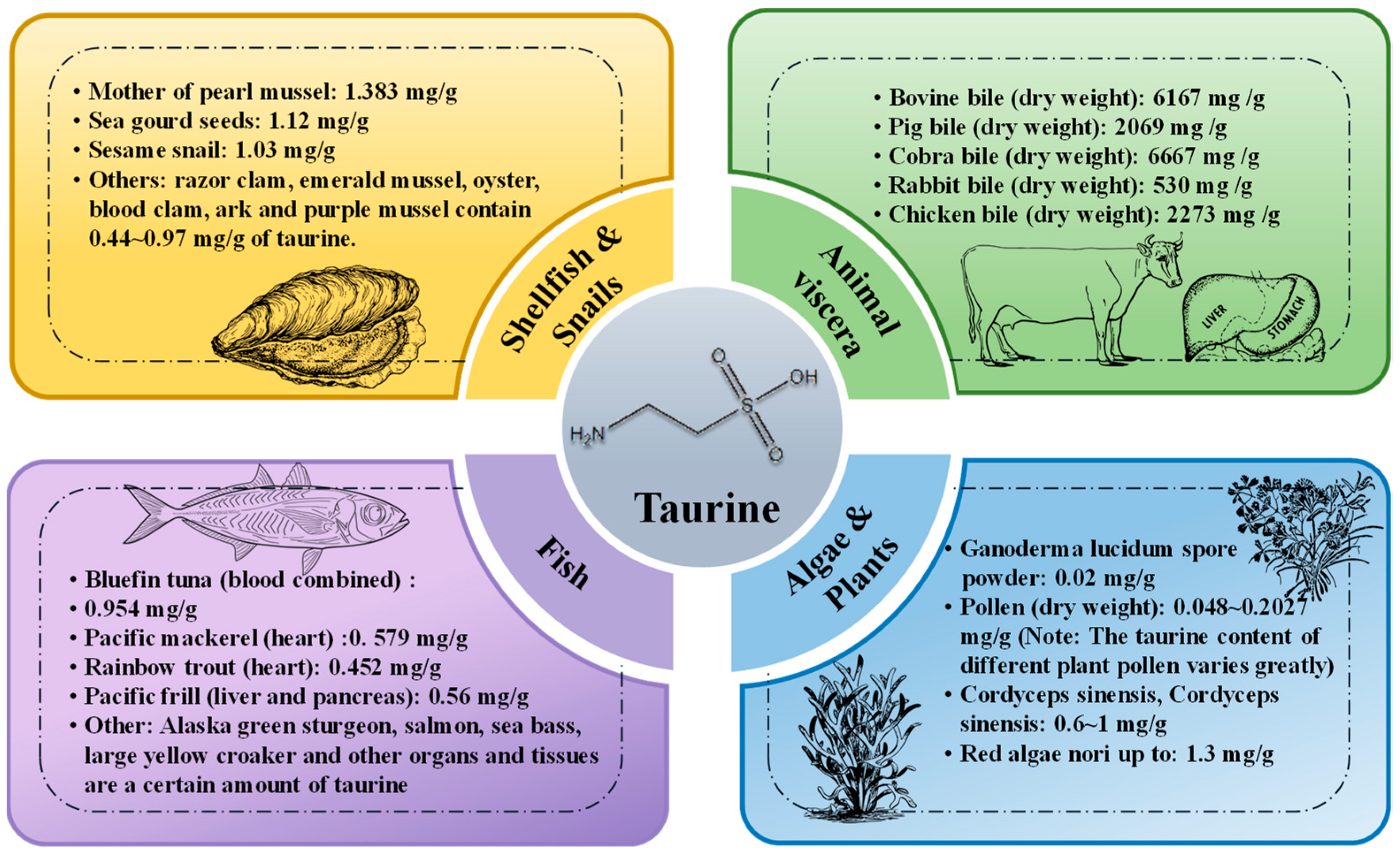
2. Sources of Taurine
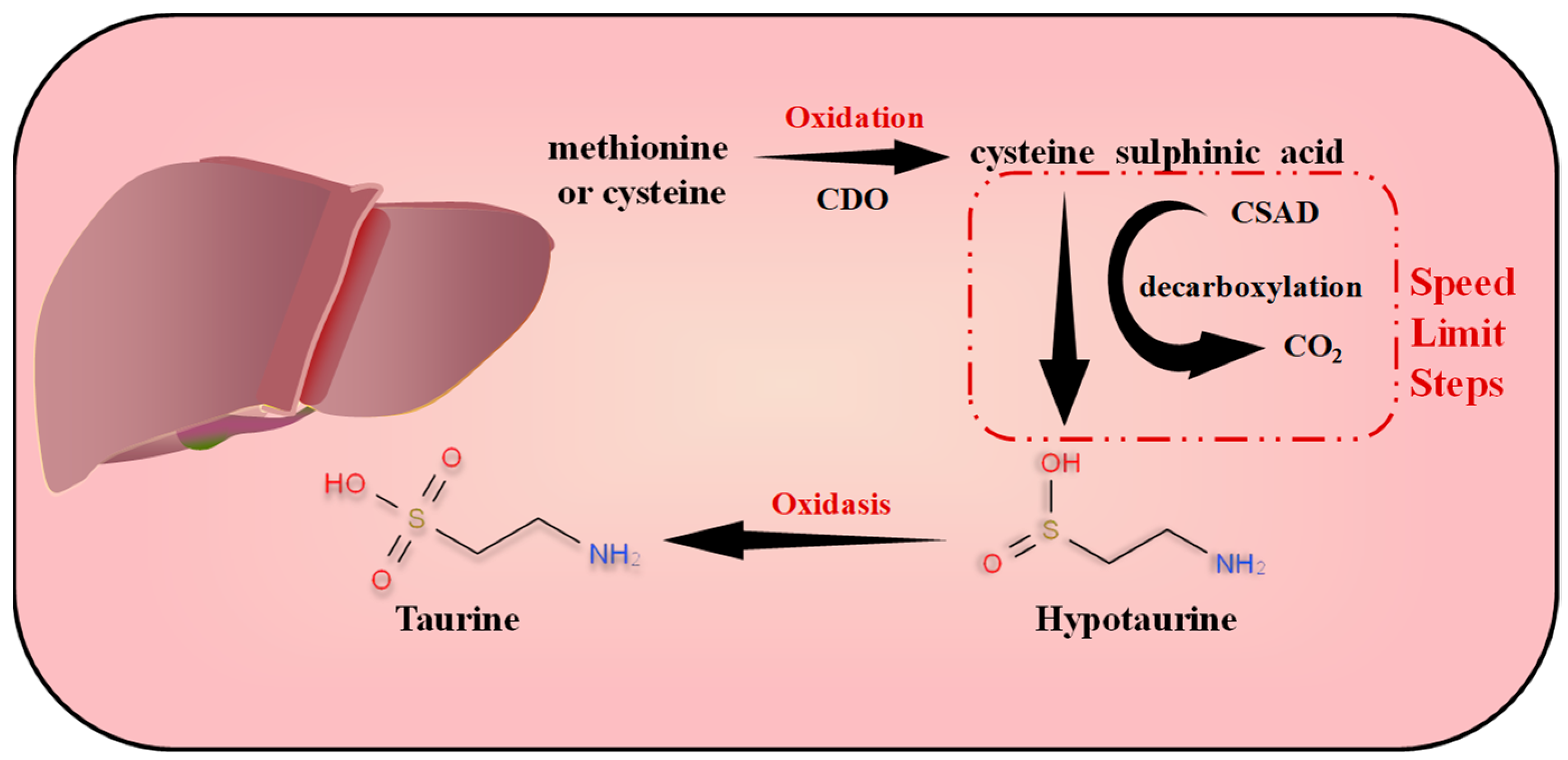
2.1. Endogenous Metabolic Synthesis of Taurine
2.2. Exogenous Distribution and Mode of Production of Taurine
2.2.1. Sources and Distribution
2.2.2. Mode of Production of Exogenous Taurine
Extraction Methods
Chemical Synthesis and Bio-Fermentation Methods
3. Research Progress on Taurine in the Relief of Visual Fatigue
3.1. Food Safety
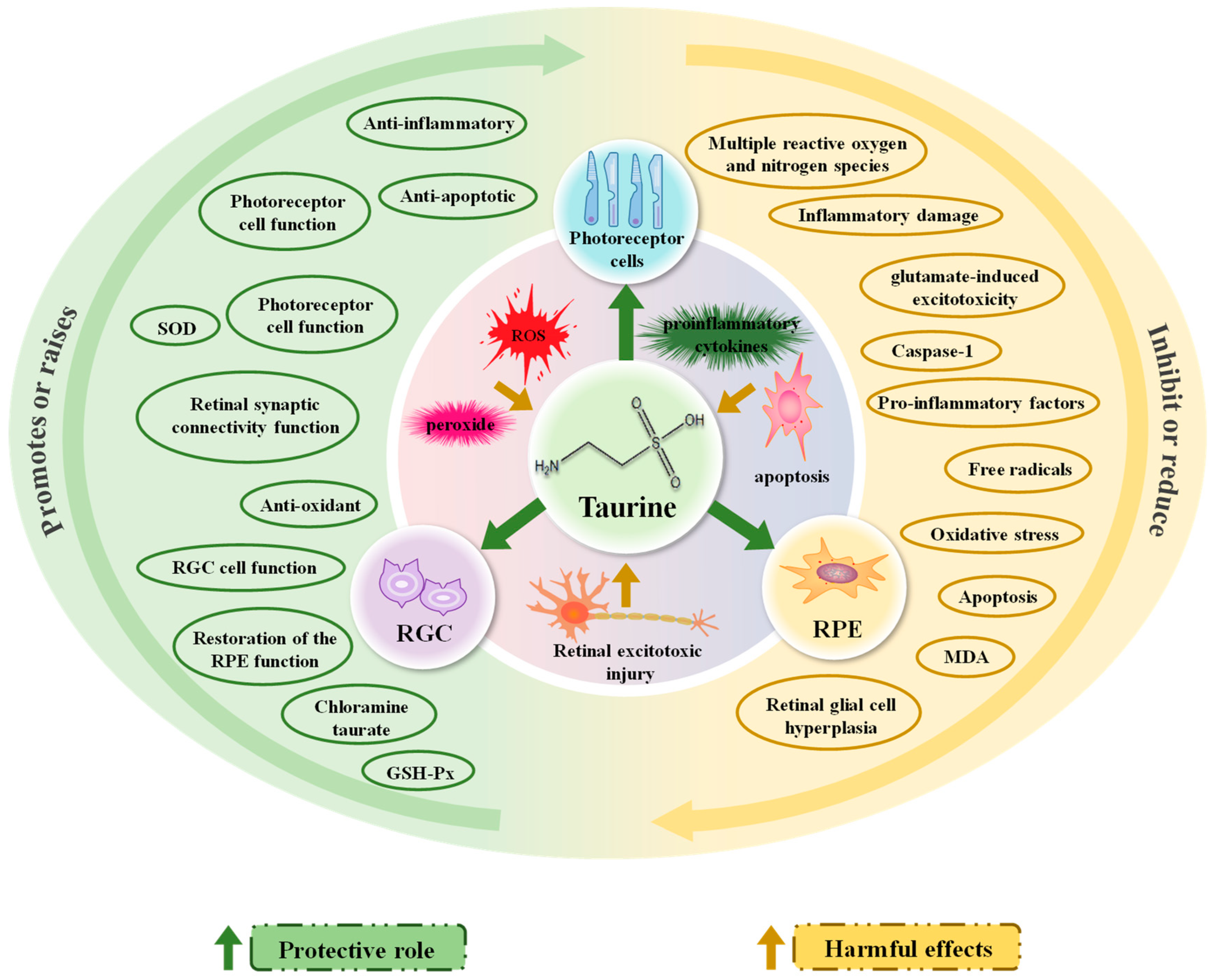
3.2. Progress in Research on the Mechanism of Action of Taurine in Relieving Visual Fatigue
3.2.1. Reducing Retinal Stress Damage: Oxidation, Inflammation, Apoptosis
3.2.2. Reducing Retinal Excitotoxic Damage and Providing Neuroprotection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le, Z.; Antonov, E.; Mao, Q.; Petrov, V.; Wang, Y.; Wang, W.; Shevkolenko, M.; Dong, W. Anti-Fatigue Glasses Based on Microprisms for Preventing Eyestrain. Sensors 2022, 22, 1933. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Hou, F.; Chen, R.; Mei, J.; Huang, P.; Chen, B.; Wang, Y. Investigation of the Relationship Between Subjective Symptoms of Visual Fatigue and Visual Functions. Front. Neurosci. 2021, 15, 686740. [Google Scholar] [CrossRef] [PubMed]
- Vilela, M.A.; Pellanda, L.C.; Fassa, A.G.; Castagno, V.D. “Prevalence of asthenopia in children: A systematic review with meta-analysis. J. Pediatr. 2015, 91, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Toda, I.; Fujishima, H.; Tsubota, K. Ocular fatigue is the major symptom of dry eye. Acta Ophthalmol. 1993, 71, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, A.L.; Wolffsohn, J.S. Digital eye strain: Prevalence, measurement and amelioration. BMJ Open Ophthalmol. 2018, 3, e000146. [Google Scholar] [CrossRef]
- Abdi, S.; Rydberg, A. Asthenopia in Schoolchildren, Orthoptic and Ophthalmological Findings and Treatment. Doc. Ophthalmol. 2005, 111, 65–72. [Google Scholar] [CrossRef]
- Portello, J.K.; Rosenfield, M.; Bababekova, Y.; Estrada, J.M.; Leon, A. Computer-related visual symptoms in office workers. Ophthalmic Physiol. Opt. 2012, 32, 375–382. [Google Scholar] [CrossRef]
- Reddy, S.C.; Low, C.K.; Lim, Y.P.; Low, L.L.; Mardina, F.; Nursaleha, M.P. Computer vision syndrome: A study of knowledge and practices in university students. Nepal. J. Ophthalmol. 2013, 5, 161–168. [Google Scholar] [CrossRef]
- Fan, B.; Zhang, C.; Chi, J.; Liang, Y.; Bao, X.; Cong, Y.; Yu, B.; Li, X.; Li, G.-Y. The Molecular Mechanism of Retina Light Injury Focusing on Damage from Short Wavelength Light. Oxidative Med. Cell Longev. 2022, 2022, 8482149. [Google Scholar] [CrossRef]
- Ayaki, M.; Kuze, M.; Kondo, M.; Tsubota, K.; Negishi, K. Association between Retinal Nerve Fiber Layer Thickness and Eye Fatigue. BioMed Res. Int. 2019, 2019, 3014567-8. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, J.; Li, D. Functions and Diseases of the Retinal Pigment Epithelium. Front. Pharmacol. 2021, 12, 727870. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, M.; Milliner, C.; Bell, B.A.; Bonilha, V.L. Oxidative stress in the retina and retinal pigment epithelium (RPE): Role of aging, and DJ-1. Redox Biol. 2020, 37, 101623. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, M.D.; Iyer, M.; Nair, A.P.; Venkatesan, D.; Mathavan, S.; Eruppakotte, N.; Kizhakkillach, S.; Chandran, M.K.; Roy, A.; Gopalakrishnan, A.V.; et al. Oxidative stress and mitochondrial transfer: A new dimension towards ocular diseases. Genes Dis. 2022, 9, 610–637. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Pastor, M.J.; Kutsyr, O.; Lax, P.; Cuenca, N. Decrease in DHA and other fatty acids correlates with photoreceptor degeneration in retinitis pigmentosa. Exp. Eye Res. 2021, 209, 108667. [Google Scholar] [CrossRef]
- Suzumura, A.; Terao, R.; Kaneko, H. Protective Effects and Molecular Signaling of n-3 Fatty Acids on Oxidative Stress and Inflammation in Retinal Diseases. Antioxidants 2020, 9, 920. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, D.H.; Kwak, H.S.; Yu, I.-S.; Um, M.Y. Protective Effect of Chrysanthemum boreale Flower Extracts against A2E-Induced Retinal Damage in ARPE-19 Cell. Antioxidants 2022, 11, 669. [Google Scholar] [CrossRef]
- Di Pierdomenico, J.; García-Ayuso, D.; Pinilla, I.; Cuenca, N.; Vidal-Sanz, M.; Agudo-Barriuso, M.; Villegas-Pérez, M.P. Early Events in Retinal Degeneration Caused by Rhodopsin Mutation or Pigment Epithelium Malfunction: Differences and Similarities. Front. Neuroanat. 2017, 11, 14. [Google Scholar] [CrossRef]
- Cuenca, N.; Fernández-Sánchez, L.; Campello, L.; Maneu, V.; De la Villa, P.; Lax, P.; Pinilla, I. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog. Retin. Eye Res. 2014, 43, 17–75. [Google Scholar] [CrossRef]
- de Hoz, R.; Rojas, B.; Ramírez, A.I.; Salazar, J.J.; Gallego, B.I.; Triviño, A.; Ramírez, J.M. Retinal Macroglial Responses in Health and Disease. BioMed Res. Int. 2016, 2016, 2954721. [Google Scholar] [CrossRef]
- Liu, B.; Hunter, D.J.; Smith, A.A.; Chen, S.; Helms, J.A. The capacity of neural crest-derived stem cells for ocular repair. Birth Defects Res. Part C Embryo Today Rev. 2014, 102, 299–308. [Google Scholar] [CrossRef]
- Peng, L.; Parpura, V.; Verkhratsky, A. EDITORIAL Neuroglia as a Central Element of Neurological Diseases: An Underappreciated Target for Therapeutic Intervention. Curr. Neuropharmacol. 2014, 12, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cheng, M.; Chintala, S.K. Kainic Acid–Mediated Upregulation of Matrix Metalloproteinase-9 Promotes Retinal Degeneration. Investig. Opthalmol. Vis. Sci. 2004, 45, 2374–2383. [Google Scholar] [CrossRef] [PubMed]
- Roche, S.L.; Ruiz-Lopez, A.M.; Moloney, J.N.; Byrne, A.M.; Cotter, T.G. Microglial-induced Müller cell gliosis is attenuated by progesterone in a mouse model of retinitis pigmentosa. Glia 2018, 66, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Di Pierdomenico, J.; Garcia-Ayuso, D.; Agudo-Barriuso, M.; Vidal-Sanz, M.; Villegas-Perez, M.P. Role of microglial cells in photoreceptor degeneration. Neural Regen. Res. 2019, 14, 1186–1190. [Google Scholar] [CrossRef]
- Kalloniatis, M.; Napper, G.A. Retinal neurochemical changes following application of glutamate as a metabolic substrate. Clin. Exp. Optom. 2002, 85, 27–36. [Google Scholar] [CrossRef]
- Barabas, P.; Kovacs, I.; Kardos, J.; Schousboe, A. Exogenous glutamate and taurine exert differential actions on light-induced release of two endogenous amino acids in isolated rat retina. J. Neurosci. Res. 2003, 73, 731–736. [Google Scholar] [CrossRef]
- Payet, O.; Maurin, L.; Bonne, C.; Muller, A. Hypoxia stimulates glutamate uptake in whole rat retinal cells in vitro. Neurosci. Lett. 2004, 356, 148–150. [Google Scholar] [CrossRef]
- Zhao, L.; Zabel, M.K.; Wang, X.; Ma, W.; Shah, P.; Fariss, R.N.; Qian, H.; Parkhurst, C.N.; Gan, W.B.; Wong, W.T. Microglial phagocytosis of living photoreceptors contributes to inherited retinal. EMBO Mol. Med. 2015, 7, 1179–1197. [Google Scholar] [CrossRef]
- Chen, F.; Mi, M.; Zhang, Q.; Wei, N.; Chen, K.; Xu, H.; Yuan, J.; Zhou, Y.; Lang, H.; Yu, X.; et al. Taurine Buffers Glutamate Homeostasis in Retinal Cells in vitro under Hypoxic Conditions. Ophthalmic Res. 2010, 44, 105–112. [Google Scholar] [CrossRef]
- Ripps, H.; Shen, W. Review: Taurine: A “very essential” amino acid. Mol. Vis. 2012, 18, 2673–2686. [Google Scholar]
- Wójcik, O.P.; Koenig, K.L.; Zeleniuch-Jacquotte, A.; Costa, M.; Chen, Y. The potential protective effects of taurine on coronary heart disease. Atherosclerosis 2010, 208, 19–25. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Scientific Opinion on the safety and efficacy of taurine as a feed additive for all animal species. EFSA J. 2012, 10, 2736. [Google Scholar]
- Froger, N.; Cadetti, L.; Lorach, H.; Martins, J.; Bemelmans, A.; Dubus, E.; Degardin, J.; Pain, D.; Forster, V.; Chicaud, L.; et al. Taurine Provides Neuroprotection against Retinal Ganglion Cell Degeneration. PLoS ONE 2012, 7, e42017. [Google Scholar] [CrossRef]
- Hayes, K.C.; Care, R.; Schmidt, S.Y. Retinal degeneration associated with taurine deficiency in the cat. Science 1976, 188, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Vacas, A.; Di Pierdomenico, J.; Valiente-Soriano, F.J.; Vidal-Sanz, M.; Picaud, S.; Villegas-Pérez, M.P.; García-Ayuso, D. Glial Cell Activation and Oxidative Stress in Retinal Degeneration Induced by β-Alanine Caused Taurine Depletion and Light Exposure. Int. J. Mol. Sci. 2021, 23, 346. [Google Scholar] [CrossRef] [PubMed]
- Hadj-Saïd, W.; Froger, N.; Ivkovic, I.; Jiménez-López, M.; Dubus, É.; Dégardin-Chicaud, J.; Simonutti, M.; Quénol, C.; Neveux, N.; Villegas-Pérez, M.P.; et al. Quantitative and Topographical Analysis of the Losses of Cone Photoreceptors and Retinal Ganglion Cells Under Taurine Depletion. Investig. Opthalmol. Vis. Sci. 2016, 57, 4692–4703. [Google Scholar] [CrossRef]
- Zeng, K.; Xu, H.; Mi, M.; Zhang, Q.; Zhang, Y.; Chen, K.; Chen, F.; Zhu, J.; Yu, X. Dietary Taurine Supplementation Prevents Glial Alterations in Retina of Diabetic Rats. Neurochem. Res. 2009, 34, 244–254. [Google Scholar] [CrossRef]
- Wu, D.; Song, L.; Zhu, C.; Zhang, X.; Guo, H.; Yang, C. Solubility of taurine and its application for the crystallization process improvement. J. Mol. Liq. 2017, 241, 326–333. [Google Scholar] [CrossRef]
- Wang, L.W. Effect of taurine supplementation on athletic performance of sports athletes. Food Res. Dev. 2022, 43, 231–232. [Google Scholar]
- Tevatia, R.; Allen, J.; Rudrappa, D.; White, D.; Clemente, T.E.; Cerutti, H.; Demirel, Y.; Blum, P. The taurine biosynthetic pathway of microalgae. Algal Res. 2015, 9, 21–26. [Google Scholar] [CrossRef]
- Vitvitsky, V.; Garg, S.K.; Banerjee, R. Taurine Biosynthesis by Neurons and Astrocytes. J. Biol. Chem. 2011, 286, 32002–32010. [Google Scholar] [CrossRef] [PubMed]
- Lund, E.K. Health benefits of seafood; Is it just the fatty acids? Food Chem. 2013, 140, 413–420. [Google Scholar] [CrossRef]
- Wang, F. Extraction, Isolation and Purification of Taurine from Zea mays. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2014. [Google Scholar]
- Xie, Z.; Yao, Y.; Liu, B.; Chen, X.; Fang, T. Research progress of taurine extraction and detection methods. Food Ind. Sci. Technol. 2019, 40, 323–331. [Google Scholar] [CrossRef]
- Huang, X.; Hou, S.; Li, G.; Cai, D.K.; Su, Z. A review of taurine as an active ingredient in Chinese medicine. Chin. Folk. Remedies 2005, 9, 64–65. [Google Scholar] [CrossRef]
- Uchida, M.; Kurushima, H.; Ishihara, K.; Murata, Y.; Touhata, K.; Ishida, N.; Niwa, K.; Araki, T. Characterization of fermented seaweed sauce prepared from nori (Pyropia yezoensis). J. Biosci. Bioeng. 2017, 123, 327–332. [Google Scholar] [CrossRef]
- Lv, R.; Chen, R.; Chen, X.; Fang, T. Research progress on extraction of natural taurine from abalone offal. Anhui Agric. Bull. 2019, 25, 14–16+83. [Google Scholar] [CrossRef]
- Zhang, M.D.; Zan, N.; Li, S.R.; Zhou, Y.J. Research progress on the processing and detection technology of taurine and its application. Agric. Prod. Process. 2019, 19, 68–72+74. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Cao, Y.; Huang, J.; Lin, H.; Zhao, T.; Liu, L.; Shen, P.; McClements, D.J.; Chen, J.; et al. Extraction and characterization of pectic polysaccharides from Choerospondias axillaris peels: Comparison of hot water and ultrasound-assisted extraction methods. Food Chem. 2023, 401, 134156. [Google Scholar] [CrossRef]
- Vinatoru, M. Ultrasonically assisted extraction (UAE) of natural products some guidelines for good practice and reporting. Ultrason. Sonochem. 2015, 25, 94–95. [Google Scholar] [CrossRef]
- Martín-García, B.; Pasini, F.; Verardo, V.; Díaz-De-Cerio, E.; Tylewicz, U.; Gómez-Caravaca, A.M.; Caboni, M.F. Optimization of Sonotrode Ultrasonic-Assisted Extraction of Proanthocyanidins from Brewers’ Spent Grains. Antioxidants 2019, 8, 282. [Google Scholar] [CrossRef]
- Ji, L.; Liu, T.; Wang, Y.; Li, X.; Li, H.; Jiang, X.; Sun, Y. Study on the extraction process of taurine in kui clams. China Agric. Sci. Technol. Her. 2017, 19, 132–138. [Google Scholar] [CrossRef]
- Jiang, X. Process study of taurine extraction from emerald mussel. Food Sci. Technol. 2006, 1, 62–64. [Google Scholar]
- Qian, A.; Yan, S.; Yu, Y.; Lin, X. Exploration of pre-treatment methods for the determination of taurine in seafood. China Agron. Bull. 2006, 5, 94–97. [Google Scholar]
- Qian, Q. Research progress on the extraction of active substances such as taurine, polysaccharides and peptides from marine organisms. Food Ind. Sci. Technol. 2013, 34, 383–387. [Google Scholar] [CrossRef]
- Ma, C.-C.; Butler, D.; Milligan, V.; Hammann, B.A.; Luo, H.; Brazdil, J.F.; Liu, D.; Chaudhari, R.V.; Subramaniam, B. Continuous Process for the Production of Taurine from Monoethanolamine. Ind. Eng. Chem. Res. 2020, 59, 13007–13015. [Google Scholar] [CrossRef]
- Bulychev, E.Y.; Rubanyak, N.Y. Commercial Synthesis of 2-Aminoethanesulfonic Acid (Taurine). Pharm. Chem. J. 2013, 46, 740–741. [Google Scholar] [CrossRef]
- Yeh, S.; Deng, J.; Liu, Q.-S.; Deng, B. Research progress of taurine nutrition and its application in cat food. Guangdong Anim. Husb. Vet. Sci. Technol. 2021, 46, 21–26+43. [Google Scholar]
- Joo, Y.-C.; Ko, Y.J.; You, S.K.; Shin, S.K.; Hyeon, J.E.; Musaad, A.S.; Han, S.O. Creating a New Pathway in Corynebacterium glutamicum for the Production of Taurine as a Food Additive. J. Agric. Food Chem. 2018, 66, 13454–13463. [Google Scholar] [CrossRef]
- Jenny. High-Voltage Pulsed Electric Field-Assisted Enzymatic Digestion of Mussel Meat for the Preparation of Taurine. Master’s Thesis, Jilin University, Changchun, China, 2020. [Google Scholar] [CrossRef]
- Liu, X.; Huang, L.; Bai, Y.; Liu, X.; Li, S. Extracting bio-zinc and taurine from Pinctada martensii meat. J. Food Sci. 2020, 85, 1125–1131. [Google Scholar] [CrossRef]
- Guo, Z.; Ge, X.; Han, L.; Yang, L.; Yu, Q. Ultrasound-assisted extraction and purification of taurine from bovine liver. J. Food Compos. Anal. 2020, 90. prepublish. [Google Scholar] [CrossRef]
- Wang, F.; Guo, X.-Y.; Zhang, D.-N.; Wu, Y.; Wu, T.; Chen, Z.-G. Ultrasound-assisted extraction and purification of taurine from the red algae Porphyra yezoensis. Ultrason. Sonochem. 2015, 24, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.-L.; Gao, R.-C.; Yang, F.-S.; Liu, W.-T.; Yang, J.-F. Study on the process of taurine preparation by enzymatic digestion of Philippine clam boiling liquid. J. Light Ind. 2018, 33, 26–33. [Google Scholar]
- Liu, Y.; Zhang, Z.; Tong, H.; Sun, K.; Song, X. Optimization of enzymatic process conditions of oyster taurine extraction by neutral protease using response surface methodology. Food Sci. 2011, 32, 25–28. [Google Scholar]
- Chen, J.; Ouyang, J.; Liu, Z.; Zhou, L.; Huang, H.; Ying, X. Research progress on the synthesis process and crystallization purification of taurine. Mod. Chem. 2021, 41, 57–62. [Google Scholar] [CrossRef]
- Scientific Committee on Food (SCF). Opinion on Caffeine, T aurine and D-Glucurono-γ-Lactone as Constituents of So-Called “Energy” Drinks; SCF: Brussels, Belgium, 1999; pp. 1–12. [Google Scholar]
- Sanz-Serrano, J.; Vettorazzi, A.; Muruzabal, D.; Azqueta, A.; de Cerain, A.L. In Vitro Genotoxicity Assessment of Functional Ingredients: Betaine, Choline, and Taurine. Foods 2021, 10, 339. [Google Scholar] [CrossRef]
- Menzie, J.; Prentice, H.; Wu, J.-Y. Neuroprotective mechanisms of taurine against ischemic stroke. Brain Sci. 2013, 3, 877–907. [Google Scholar] [CrossRef]
- Zhang, M.; Bi, L.F.; Fang, J.H.; Su, X.L.; Da, G.L.; Kuwamori, T.; Kagamimori, S. Beneficial effects of taurine on serum lipids in overweight or obese non- diabeticsubjects. Amino Acids 2004, 26, 267–271. [Google Scholar] [CrossRef]
- Murakami, S.; Kondo, Y.; Nagate, T. Effects of long-term treatment with taurine in mice fed a high-fat diet: Improvement in cholesterol metabolism and vascular lipid accumulation by taurine. Adv. Exp. Med. Biol. 2000, 483, 177–186. [Google Scholar]
- Sturman, J.A.; Messing, J.M. High Dietary Taurine Effects on Feline Tissue Taurine Concentrations and Reproductive Performance. J. Nutr. 1992, 122, 82–88. [Google Scholar] [CrossRef]
- Shao, A.; Hathcock, J.N. Risk assessment for the amino acids taurine, l-glutamine and l-arginine. Regul. Toxicol. Pharmacol. 2008, 50, 376–399. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y. Study on the safety evaluation of taurine, vitamin E and inositol complex powder. Clin. Res. Chin. Med. 2020, 12, 1–5. [Google Scholar]
- Shu, Z.; Zhou, X.; Zhao, P.; Zhao, F. Toxicological safety evaluation of Qili taurine vitamin drink. Beverage Ind. 2016, 19, 5–9. [Google Scholar]
- Zhao, K.; AI, F.; Zheng, L.; Chen, X.; Lin, J. Safety study of selenium-rich yeast and taurine formulated products. Strait J. Prev. Med. 2019, 25, 47–49. [Google Scholar]
- Geng, K.J.; Liu, X. Safety and functionality of combined tablets of milk mineral salts and taurine. J. Food Saf. 2022, 2, 86–90. [Google Scholar] [CrossRef]
- Duan, H.; Yan, W.J. Research progress on raw materials and their efficacious components for the function of relieving visual fatigue. Food Ind. Sci. Technol. 2022, 1–13. [Google Scholar] [CrossRef]
- Kim, C.; Cha, Y.-N. Taurine chloramine produced from taurine under inflammation provides anti-inflammatory and cytoprotective effects. Amino Acids 2013, 46, 89–100. [Google Scholar] [CrossRef]
- Marcinkiewicz, J.; Grabowska, A.; Bereta, J.; Stelmaszynska, T. Taurine chloramine, a product of activated neutrophils, inhibits in vitro the generation of nitric oxide and other macrophage inflammatory mediators. J. Leukoc. Biol. 1995, 58, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.; Kim, H.W. Effects and Mechanisms of Taurine as a Therapeutic Agent. Biomol. Ther. 2018, 26, 225–241. [Google Scholar] [CrossRef]
- Jong, C.J.; Azuma, J.; Schaffer, S. Mechanism underlying the antioxidant activity of taurine: Prevention of mitochondrial oxidant production. Amino Acids 2011, 42, 2223–2232. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef]
- Gaucher, D.; Arnault, E.; Husson, Z.; Froger, N.; Dubus, E.; Gondouin, P.; Dherbécourt, D.; Degardin, J.; Simonutti, M.; Fouquet, S.; et al. Taurine deficiency damages retinal neurones: Cone photoreceptors and retinal ganglion cells. Amino Acids 2012, 43, 1979–1993. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chen, K.; Wei, N.; Zhang, Q.; Liu, J.; Mi, M. Dietary taurine reduces retinal damage produced by photochemical stress via antioxidant and anti-apoptotic mechanisms in Sprague–Dawley rats. mechanisms in Sprague-Dawley rats. Br. J. Nutr. 2007, 98, 711–719. [Google Scholar] [CrossRef] [PubMed]
- García-Ayuso, D.; Di Pierdomenico, J.; Hadj-Said, W.; Marie, M.; Agudo-Barriuso, M.; Vidal-Sanz, M.; Villegas-Pérez, M.P. Taurine Depletion Causes ipRGC Loss and Increases Light-Induced Photoreceptor Degeneration. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1396–1409. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Vacas, A.; Di Pierdomenico, J.; Gallego-Ortega, A.; Valiente-Soriano, F.J.; Vidal-Sanz, M.; Picaud, S.; Villegas-Pérez, M.P.; García-Ayuso, D. Systemic taurine treatment affords functional and morphological neuroprotection of photoreceptors and restores retinal pigment epithelium function in RCS rats. Redox Biol. 2022, 57, 102506. [Google Scholar] [CrossRef] [PubMed]
- Castelli, V.; Paladini, A.; D’Angelo, M.; Allegretti, M.; Mantelli, F.; Brandolini, L.; Cocchiaro, P.; Cimini, A.; Varrassi, G. Taurine and oxidative stress in retinal health and disease. CNS Neurosci. Ther. 2021, 27, 403–412. [Google Scholar] [CrossRef]
- Agarwal, R.; Arfuzir, N.N.N.; Iezhitsa, I.; Agarwal, P.; Sidek, S.; Ismail, N.M. Taurine protects against retinal and optic nerve damage induced by endothelin-1 in rats via antioxidant effects. Neural Regen. Res. 2018, 13, 2014–2021. [Google Scholar] [CrossRef] [PubMed]
- Militante, J.; Lombardini, J.B. Age-Related Retinal Degeneration in Animal Models of Aging: Possible Involvement of Taurine Deficiency and Oxidative Stress. Neurochem. Res. 2004, 29, 151–160. [Google Scholar] [CrossRef]
- Wang, Y.; Grenell, A.; Zhong, F.; Yam, M.; Hauer, A.; Gregor, E.; Zhu, S.; Lohner, D.; Zhu, J.; Du, J. Metabolic signature of the aging eye in mice. Neurobiol. Aging 2018, 71, 223–233. [Google Scholar] [CrossRef]
- Dayang, W.; Dongbo, P. Taurine reduces blue light-induced retinal neuronal cell apoptosis in vitro. Cutan. Ocul. Toxicol. 2018, 37, 240–244. [Google Scholar] [CrossRef]
- Park, J.-S.; Park, J.-H.; Kim, K.-Y. Neuroprotective effects of myristargenol A against glutamate-induced apoptotic HT22 cell death. RSC Adv. 2019, 9, 31247–31254. [Google Scholar] [CrossRef]
- Araszkiewicz, A.; Zozulinska-Ziolkiewicz, D. Retinal neurodegeneration in the course of diabetes-pathogenesis and clinical perspective. Curr. Neuropharmacol. 2016, 14, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M. Abnormalities in Glutamate Metabolism and Excitotoxicity in the Retinal Diseases. Scientifica 2013, 2013, 528940. [Google Scholar] [CrossRef] [PubMed]
- Choi, D. Glutamate neurotoxicity and diseases of the nervous system. Neuron 1988, 1, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Agarwal, P. Rodent models of glaucoma and their applicability for drug discovery. Expert Opin. Drug Discov. 2017, 12, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Leon, R.; Wu, H.; Jin, Y.; Wei, J.; Buddhala, C.; Prentice, H.; Wu, J.Y. Protective function of taurine in glutamate-induced apoptosis in cultured neurons. J. Neurosci. Res. 2009, 87, 1185–1194. [Google Scholar] [CrossRef]
- Bulley, S.; Shen, W. Reciprocal regulation between taurine and glutamate response via Ca2+- dependent pathways in retinal third-order neurons. J. Biomed. Sci. 2010, 17 (Suppl. 1), S5. [Google Scholar] [CrossRef]
- Yu, X.; Xu, Z.; Mi, M.; Xu, H.; Zhu, J.; Wei, N.; Chen, K.; Zhang, Q.; Zeng, K.; Wang, J.; et al. Dietary taurine supplementation ameliorates diabetic retinopathy via anti-exitotoxicity of glutamate in streptozotocin-induced Sprague-Dawley rats. excitotoxicity of glutamate in streptozotocin-induced Sprague-Dawley rats. Neurochem. Res. 2007, 33, 500–507. [Google Scholar] [CrossRef]
- Oliveira, M.W.; Minotto, J.B.; de Oliveira, M.R.; Zanotto-Filho, A.; Behr, G.A.; Rocha, R.F.; Moreira, J.C.; Klamt, F. Scavenging and antioxidant potential of physiological taurine concentrations against different reactive oxygen/nitrogen species. Pharmacol. Rep. 2010, 62, 185–193. [Google Scholar] [CrossRef]
- Jafri, A.J.A.; Agarwal, R.; Iezhitsa, I.; Agarwal, P.; Ismail, N.M. Taurine protects against NMDA-induced retinal damage by reducing retinal oxidative stress. Amino Acids 2019, 51, 641–646. [Google Scholar] [CrossRef]
- Audo, I.; Mohand-Said, S.; Boulanger-Scemama, E.; Zanlonghi, X.; Condroyer, C.; Démontant, V.; Boyard, F.; Antonio, A.; Méjécase, C.; El Shamieh, S.; et al. MERTK mutation update in inherited retinal diseases. Hum. Mutat. 2018, 39, 887–913. [Google Scholar] [CrossRef]
- Edwards, R.B.; Szamier, R.B. Defective Phagocytosis of Isolated Rod Outer Segments by RCS Rat Retinal Pigment Epithelium in Culture. Science 1977, 197, 1001–1003. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Turner, J.E. Inherited retinal dystrophy in the RCS rat: Prevention of photoreceptor degeneration by pigment epithelial cell transplantation. Exp. Eye Res. 1988, 47, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Valiente-Soriano, F.J.; Salinas-Navarro, M.; Di Pierdomenico, J.; García-Ayuso, D.; Lucas-Ruiz, F.; Pinilla, I.; Cuenca, N.; Vidal-Sanz, M.; Villegas-Pérez, M.P.; Agudo-Barriuso, M. Tracing the retina to analyze the integrity and phagocytic capacity of the retinal pigment epithelium. Sci. Rep. 2020, 10, 7273. [Google Scholar] [CrossRef] [PubMed]
- García-Ayuso, D.; Di Pierdomenico, J.; Vidal-Sanz, M.; Villegas-Pérez, M.P. Retinal Ganglion Cell Death as a Late Remodeling Effect of Photoreceptor Degeneration. Int. J. Mol. Sci. 2019, 20, 4649. [Google Scholar] [CrossRef] [PubMed]
- Di Pierdomenico, J.; García-Ayuso, D.; González-Herrero, M.E.R.; García-Bernal, D.; Blanquer, M.; Bernal-Garro, J.M.; García-Hernández, A.M.; Vidal-Sanz, M.; Villegas-Pérez, M.P. Bone Marrow-Derived Mononuclear Cell Transplants Decrease Retinal Gliosis in Two Animal Models of Inherited Photoreceptor Degeneration. Int. J. Mol. Sci. 2020, 21, 7252. [Google Scholar] [CrossRef]
- Di Pierdomenico, J.; Scholz, R.; Valiente-Soriano, F.J.; Sánchez-Migallón, M.C.; Vidal-Sanz, M.; Langmann, T.; Agudo-Barriuso, M.; García-Ayuso, D.; Villegas-Pérez, M.P. Neuroprotective Effects of FGF2 and Minocycline in Two Animal Models of Inherited Retinal Degeneration. Investig. Opthalmol. Vis. Sci. 2018, 59, 4392–4403. [Google Scholar] [CrossRef]
- Dowling, J.E.; Sidman, R.L. Inherited retinal dystrophy in the rat. J. Cell Biol. 1962, 14, 73–109. [Google Scholar] [CrossRef]
- García-Ayuso, D.; Di Pierdomenico, J.; Agudo-Barriuso, M.; Vidal-Sanz, M.; Villegas-Pérez, M. Retinal remodeling following photoreceptor degeneration causes retinal ganglion cell death. Neural Regen. Res. 2018, 13, 1885–1886. [Google Scholar] [CrossRef]
- García-Ayuso, D.; Salinas-Navarro, M.; Nadal-Nicol, F.M.; Ortín-Martínez, A.; Agudo-Barriuso, M.; Vidal-Sanz, M.; Villegas-Pérez, M.P. Sectorial loss of retinal ganglion cells in inherited photoreceptor degeneration is due to RGC death. Br. J. Ophthalmol. 2014, 98, 396–401. [Google Scholar] [CrossRef]
- Villegas-P, M.P.; Lawrence, J.M.; Vidal-Sanz, M.; Lavail, M.M.; Lund, R.D. Ganglion cell loss in RCS rat retina: A result of compression of axons by contracting intraretinal vessels linked to the pigment epithelium. J. Comp. Neurol. 1998, 392, 58–77. [Google Scholar] [CrossRef]
- Rascher, K.; Servos, G.; Berthold, G.; Hartwig, H.-G.; Warskulat, U.; Heller-Stilb, B.; Häussinger, D. Light deprivation slows but does not prevent the loss of photoreceptors in taurine transporter knockout mice. Vis. Res. 2004, 44, 2091–2100. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Lai, J.; Yuan, Y.; Wang, L.; Wang, Q.; Yuan, F. Taurine Protects Retinal Cells and Improves Synaptic Connections in Early Diabetic Rats. Curr. Eye Res. 2019, 45, 52–63. [Google Scholar] [CrossRef] [PubMed]
- García-Ayuso, D.; Di Pierdomenico, J.; Valiente-Soriano, F.J.; Martínez-Vacas, A.; Agudo-Barriuso, M.; Vidal-Sanz, M.; Picaud, S.; Villegas-Pérez, M.P. β-alanine supplementation induces taurine depletion and causes alterations of the retinal nerve fiber layer and axonal transport by retinal ganglion cells. Exp. Eye Res. 2019, 188, 107781. [Google Scholar] [CrossRef] [PubMed]
- Horvath, G.-A.; Hukin, J.; Stockler-Ipsiroglu, S.; Aroichane, M. Eye findings on vigabatrin and taurine treatment in two patients with succinic semialdehyde dehydrogenase deficiency. Neuropediatrics 2016, 47, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Preising, M.N.; Görg, B.; Friedburg, C.; Qvartskhava, N.; Budde, B.S.; Bonus, M.; Toliat, M.R.; Pfleger, C.; Altmüller, J.; Herebian, D.; et al. Biallelic mutation of human SLC6A6 encoding the taurine transporter TAUT is linked to early retinal degeneration. FASEB J. 2019, 33, 11507–11527. [Google Scholar] [CrossRef]
- Jammoul, F.; Dégardin, J.; Pain, D.; Gondouin, P.; Simonutti, M.; Dubus, E.; Caplette, R.; Fouquet, S.; Craft, C.M.; Sahel, J.A.; et al. Taurine deficiency damages photoreceptors and retinal ganglion cells in vigabatrin-treated neonatal rats. Mol. Cell Neurosci. 2010, 43, 414–421. [Google Scholar] [CrossRef]
- Tao, Y.; Yang, J.; Ma, Z.; Yan, Z.; Liu, C.; Ma, J.; Wang, Y.; Yang, Z.; Huang, Y.F. The Vigabatrin Induced Retinal Toxicity is Associated with Photopic Exposure and Taurine Deficiency: An In Vivo Study. Cell Physiol. Biochem. 2016, 40, 831–846. [Google Scholar] [CrossRef]
- Lambuk, L.; Iezhitsa, I.; Agarwal, R.; Bakar, N.S.; Agarwal, P.; Ismail, N.M. Antiapoptotic effect of taurine against NMDA-induced retinal excitotoxicity in rats. Neurotoxicology 2018, 70, 62–71. [Google Scholar] [CrossRef]
| Production Methods | Scale of Production | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Extraction method | Study of extract-based ingredients suitable for functional foods | The extraction and development of natural products are very important for functional food | It is not suitable for industrial production of taurine because of its high cost and low efficiency | [47,48,49,50,51,52,53,54,55] |
| Chemical synthesis | Suitable for industrial mass production | Suitable for the production of large quantities of taurine | Chemical reagents are used and safety needs to be carefully considered | [56,57,58] |
| Bio fermentation | Suitable for industrial mass production | Suitable for producing large quantities of taurine in a safe and cost-effective manner | The type of enzymatic digestion used needs to be determined | [59] |
| No. | Withdrawal Method | Extraction Objects | Optimal Extraction Process Parameters | Achievement Rate | Ref. |
|---|---|---|---|---|---|
| 1 | Solvent extraction | Scapharca broughtonii | Material–liquid ratio 1:12 (g/mL), 75% ethanol by volume, extraction time 50 min, temperature 70 °C | 5.36 mg/g | [52] |
| 2 | Solvent extraction | Perna viridis | Extraction temperature 80 °C, ethanol concentration 60%, extraction time 60 min, material to liquid ratio 1:5 (g/mL), extraction times 3 times | 9.20 mg/g | [53] |
| 3 | Ultrasound-assisted enzymatic extraction | Mussel Meat | Extraction enzyme: papain; enzyme addition 4374 U/g, sonication time 19 min, sonication power 200 W, enzymatic digestion time 3 h | 11.21 mg/g | [60] |
| 4 | Enzymatic digestion | Mussel Meat | Extraction enzyme: papain; enzymatic digestion temperature 50 °C, enzyme addition 4000 U/g, enzymatic digestion time 3 h, enzymatic digestion pH 7.5 | 10.74 mg/g | [60] |
| 5 | UHP-assisted enzymatic extraction | Mussel Meat | Extraction enzyme: papain; pressure 200 MPa, holding time 3 min, enzymatic digestion time 3.5 h and enzyme addition 5000 U/g | 11.63 mg/g | [60] |
| 6 | High voltage pulsed electric field assisted enzymatic digestion | Mussel Meat | Extraction enzyme: papain; electric field strength 25 kV/cm, number of pulses 10, enzymatic digestion time 2.95 h | 13.77 mg/g | [60] |
| 7 | ultrasonic-assisted water extraction | aquatic shellfish (Pinctada martensii meat) | Ultrasonic power 240 W, ultrasonic time 70 min, water extraction temperature 70 °C, water extraction time 2 h, material to liquid ratio 1:4 (g/g) | 7.155 ± 0.04 mg/g | [61] |
| 8 | ultrasonic-assisted water extraction | bovine liver | Ultrasonic power of 205 W, ultrasonic time of 12.18 min, liquid to material ratio of 1:4 (mL/g) | 6.17 ± 0.17 mg/g | [62] |
| 9 | ultrasonic-assisted water extraction | red algae Porphyra yezoensis | Ultrasonic power of 300.0 W, extraction time of 38.3 min, extraction temperature of 40.5 °C | 13.0 mg/g | [63] |
| 10 | Enzymatic digestion | ruditapes philippinarum | Alkaline protease was the most suitable enzyme for the extraction; the enzymatic digestion temperature was 45 °C, the digestion time was 3.5 h and the pH was 8.0. | 3.08 mg/g | [64] |
| 11 | Enzymatic digestion | Oyster Meat | Extraction enzyme: neutral protease; 1300 U/g, pH 7.5, temperature 48 °C | 2.724 mg/g | [65] |
| No. | Animal/Cell | Experimental Models | Intervention Pathways | Dose | Periodicity | Experimental Results | Conclusions/Potential Mechanisms | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | SD rats | glial cell activation and oxidative stress induced by taurine deficiency secondary to β-alanine administration and light exposure | 3% β-alanine in drinking water (taurine depleted) | -- | 2 m | (1) TAU depletion caused a decrease in retinal thickness, shortening of photoreceptor outer segments, microglial cell activation, oxidative stress in the outer and inner nuclear layers and the ganglion cell layer and synaptic loss. (2) These events were also observed in light exposed animals, which in addition showed photoreceptor death and macroglial cell reactivity. (3) Light exposure under taurine depletion further increased glial cell reaction and oxidative stress. (4) The retinal pigment epithelial cells were Fluorogold labeled and whole-mounted and we document that taurine depletion impairs their phagocytic capacity. | TAU depletion causes cell damage to various retinal layers including retinal pigment epithelial cells, photoreceptors and retinal ganglion cells, and increases the susceptibility of the photoreceptor outer segments to light damage. | [35] |
| 2 | Albino SD rats | β-alanine supplementation induces taurine depletion | β-alanine supplementation (3%) in the drinking water | -- | 2 m | (1) β-alanine supplementation induces TAU depletion and causes alterations of the retinal nerve fiber layer and axonal transport by retinal ganglion cells. | TAU depletion causes RGC loss and axonal transport impairment | [36] |
| 3 | SD rats | retinal damage produced by photochemical stress | Blended into feed | 4 g taurine/100 g diet | 15 d | (1) Dietary TAU is effective in preventing morphological changes in the retina from photochemical damage. (2) Increased TAU content, decreased MDA content and increased SOD and GSH-Px activity in the retina. (3) Dietary TAU inhibited activator protein-1 (AP-1) (c-fos/c-jun subunits) expression, up-regulated NF-kB(p65) expression and decreased caspase-1 expression so as to reduce the apoptosis of photoreceptors in the retina. | Dietary TAU attenuates photochemical stress-induced retinal damage through antioxidant and antiap-1-NF-kB-caspase-1 apoptotic mechanisms. | [85] |
| 4 | albino SD rats | β-alanine in the drinking water to induce taurine depletion + light- induced photoreceptor degeneration | Drinking water added | -- | 2 m | (1) Light exposure did not affect the numbers of Brn3a+RGCs or m+RGCs but diminished the numbers of S- and L/M-cones and caused the appearance of rings devoid of cones, mainly in an “arciform” area in the superotemporal retina. (2) Light exposure under taurine depletion increased photoreceptor degeneration but did not seem to increase Brn3a+RGCs or m+RGCs loss. | TAU is essential for the survival of rat retinal cells, especially under light-induced photoreceptor degeneration. TAU supplementation may help prevent retinal damage. | [86] |
| 5 | RCS rats | RCS rats suffering retinal degeneration secondary to impaired retinal pigment epithelium phagocytosis caused by a MERTK mutation | Drinking water added | 2 mol/L | 24 d | (1) TAU increases taurine plasma levels and photoreceptor survival in rats. (2) Electroretinograms showed increases of 70% in the rod response, 400% in the a-wave amplitude, 30% in the b-wave amplitude and 75% in the photopic b-wave response in treated animals. Electroretinograms showed increases of 70% in the rod response, 400% in the a-wave amplitude, 30% in the b-wave amplitude and 75% in the photopic b-wave response in treated animals. (3) Animals in the TAU intervention group had a reduced number of microglia in the outer retinal layer, reduced GFAP expression in Müller cells, reduced oxidative stress in the outer and inner nuclear layers and improved maintenance of synaptic connections. (4) Increased FG phagocytosis in retinal pigment epithelial cells of animals in the taurine intervention group. | TAU reduces photoreceptor damage in dystrophic RCS rats. Increases the electrical response of the retina and these effects may be mediated through various neuroprotective mechanisms. | [87] |
| 6 | SD rats | ET-1 induced retinal and optic nerve damage | received an intravitreal injection | 320 nmol/L | 7 d | (1) TAU has a significant protective effect against ET-1-induced retinal and optic nerve damage. (2) Based on morphological observations, caspase immunostaining showed a significant reduction in the number of apoptotic retinal cells in the TAU pretreatment group. (3) Retinal oxidative stress was reduced in all TAU intervention groups. | TAU prevents ET-1-induced apoptosis in retinal cells and the protective effect on ET-1-induced retinal and optic nerve damage is associated with reduced retinal oxidative stress. | [89] |
| 7 | retinal neuronal cells | blue light-induced apoptosis in retinal neuronal cells | in vitro | -- | -- | (1) Blue light increased osmolyte transporter mRNA expression together with osmolyte uptake. (2) TAU significantly suppressed blue light-induced retinal neuronal cell apoptosis. | The compatible osmolyte taurine may have an important role in cell resistance to blue light and cell survival. | [92] |
| 8 | Cultured Neurons | Glutamate-induced Apoptosis in Cultured Neurons | in vitro | -- | -- | (1) TAU inhibits glutamate-induced down-regulation of Bcl-2 and up-regulation of Bax Glutamate-induced apoptosis is dependent on calpain activation and TAU inhibits glutamate-induced calpain activation. | The antiapoptotic function of TAU is due to its inhibition of glutamate-induced membrane de- polarization. | [98] |
| 9 | SD rats | injected with streptozotocin to establish experimental diabetic model | Feed adulteration | 1.2% taurine feed | 4–12 W | (1) Dietary TAU supplementation is effective in improving the histopathological and ultrastructural changes in diabetic retinopathy. (2) Dietary taurine supplementation can increase TAU levels and decrease glutamate and aminobutyric acid levels in the diabetic retina. (3) TAU supplementation increased glutamate transporter (GLAST) expression, decreased intermediate filament glial fibrillary acidic protein (GFAP) and N-methyl-D-aspartate receptor subunit 1 (NR1) expression in diabetic retina. | Chronic TAU supplementation improves retinopathy in diabetic rats through a pathway that counteracts the excitotoxicity of glutamate. | [100] |
| 10 | SD rats | NMDA-induced retinal damage | received an intravitreal injection | -- | 7 d | (1) Treatment with TAU, particularly pre-treatment, significantly increased retinal glutathione, SOD and catalase levels compared to NMDA-treated rats. The levels of MDA reduced significantly. (2) Reduction in retinal oxidative stress in TAU pre-treated group was associated with significantly greater fractional thickness of ganglion cell layer within inner retina and retinal cell density in inner retina. (3) TUNEL staining showed significantly reduced apoptotic cell count in TAU pre-treated group compared to NMDA group. | TAU protects against NMDA-induced retinal injury in rats by reducing retinal oxidative stress. | [102] |
| 11 | SD rats | STZ-induced diabetic rats | intraperitoneal injection or by intragastric administration | 420 mg/kg(wt)/d by intraperitoneal injection or 1 mL/100 g(wt)/d by gavage in drinking water at a concentration of 0.5 mol/L | 4 W | (1) TAU significantly prevented the reduction of photopic b-wave amplitude and retinal cone cells and ganglion cells loss and maintained the Bcl-2/Bax ratio balance in diabetic rats. TAU also prevented the upregulation of glial fibrillary acidic protein (GFAP) and reduced retinal reactive gliosis (2) TAU reduced plasma glutamate and tyrosine levels, which were elevated in diabetic rats. (3) mGluR6 levels reduction detected by western blot and immunofluorescence in diabetic retinas was inhibited and the displacement of mGluR6 in OPL into the inner nuclear layer (INL) detected by immunofluorescence was reduced by TAU treatment. | TAU may protect retinal cells from diabetic attacks by activating Tau-T, reducing retinal reactive gliosis, improving retinal synaptic connections and decreasing retinal cell apoptosis. | [115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, H.; Song, W.; Guo, J.; Yan, W. Taurine: A Source and Application for the Relief of Visual Fatigue. Nutrients 2023, 15, 1843. https://doi.org/10.3390/nu15081843
Duan H, Song W, Guo J, Yan W. Taurine: A Source and Application for the Relief of Visual Fatigue. Nutrients. 2023; 15(8):1843. https://doi.org/10.3390/nu15081843
Chicago/Turabian StyleDuan, Hao, Wei Song, Jinhong Guo, and Wenjie Yan. 2023. "Taurine: A Source and Application for the Relief of Visual Fatigue" Nutrients 15, no. 8: 1843. https://doi.org/10.3390/nu15081843
APA StyleDuan, H., Song, W., Guo, J., & Yan, W. (2023). Taurine: A Source and Application for the Relief of Visual Fatigue. Nutrients, 15(8), 1843. https://doi.org/10.3390/nu15081843