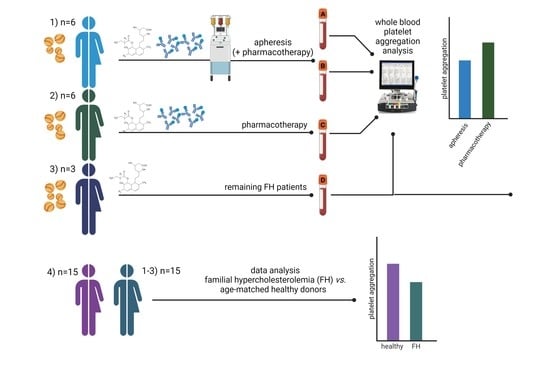
The Effect of 4-Methylcatechol on Platelets in Familial Hypercholesterolemic Patients Treated with Lipid Apheresis and/or Proprotein Convertase Subtilisin Kexin 9 Monoclonal Antibodies
Abstract
1. Introduction
2. Materials and Methods
2.1. Donors
2.2. Blood and Urine Collection
2.3. Lipid Apheresis
2.4. Chemicals
2.5. Platelet Aggregation Experiments
2.6. Measurement of Biochemical Parameters
2.7. Statistical Analysis
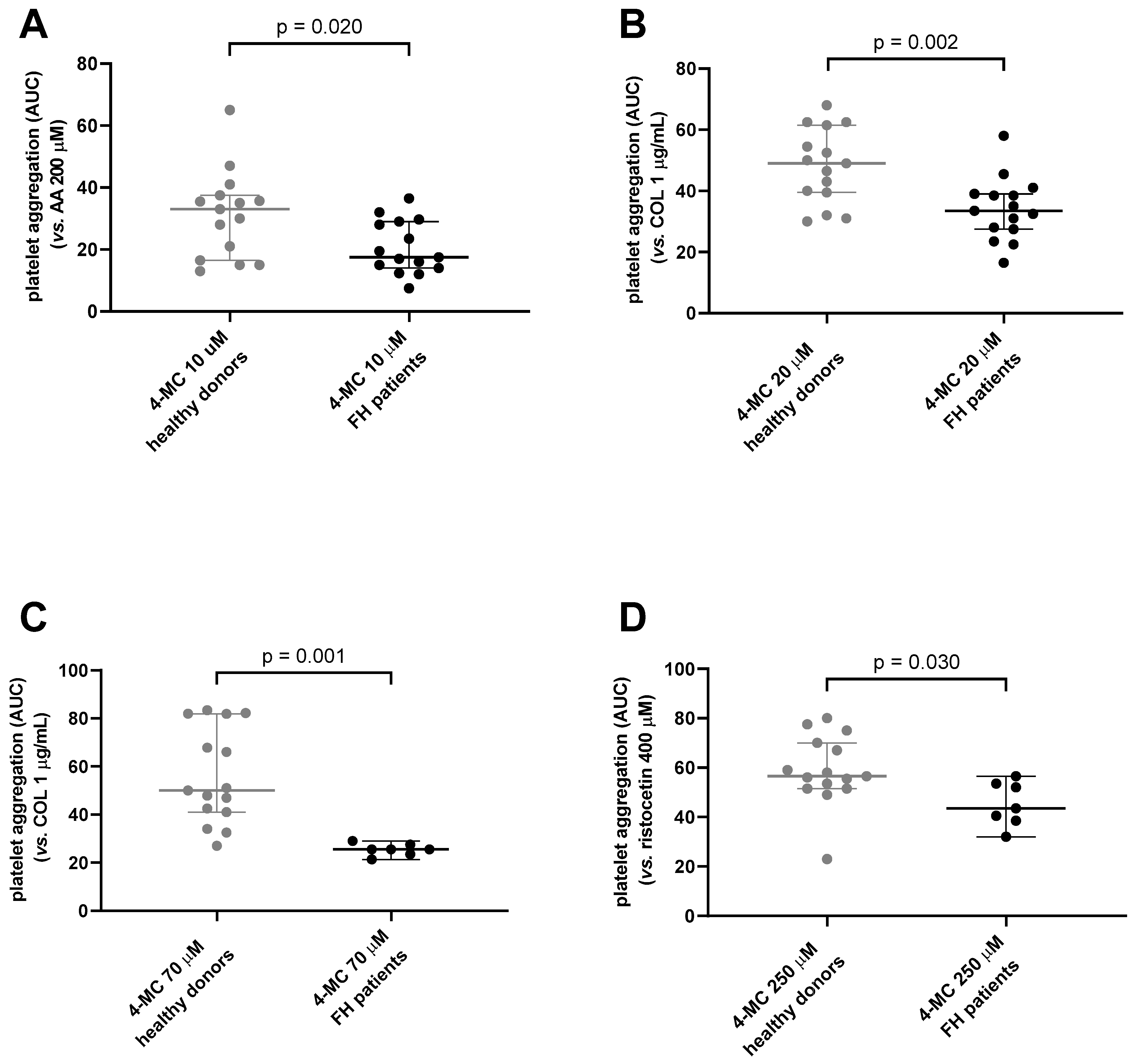
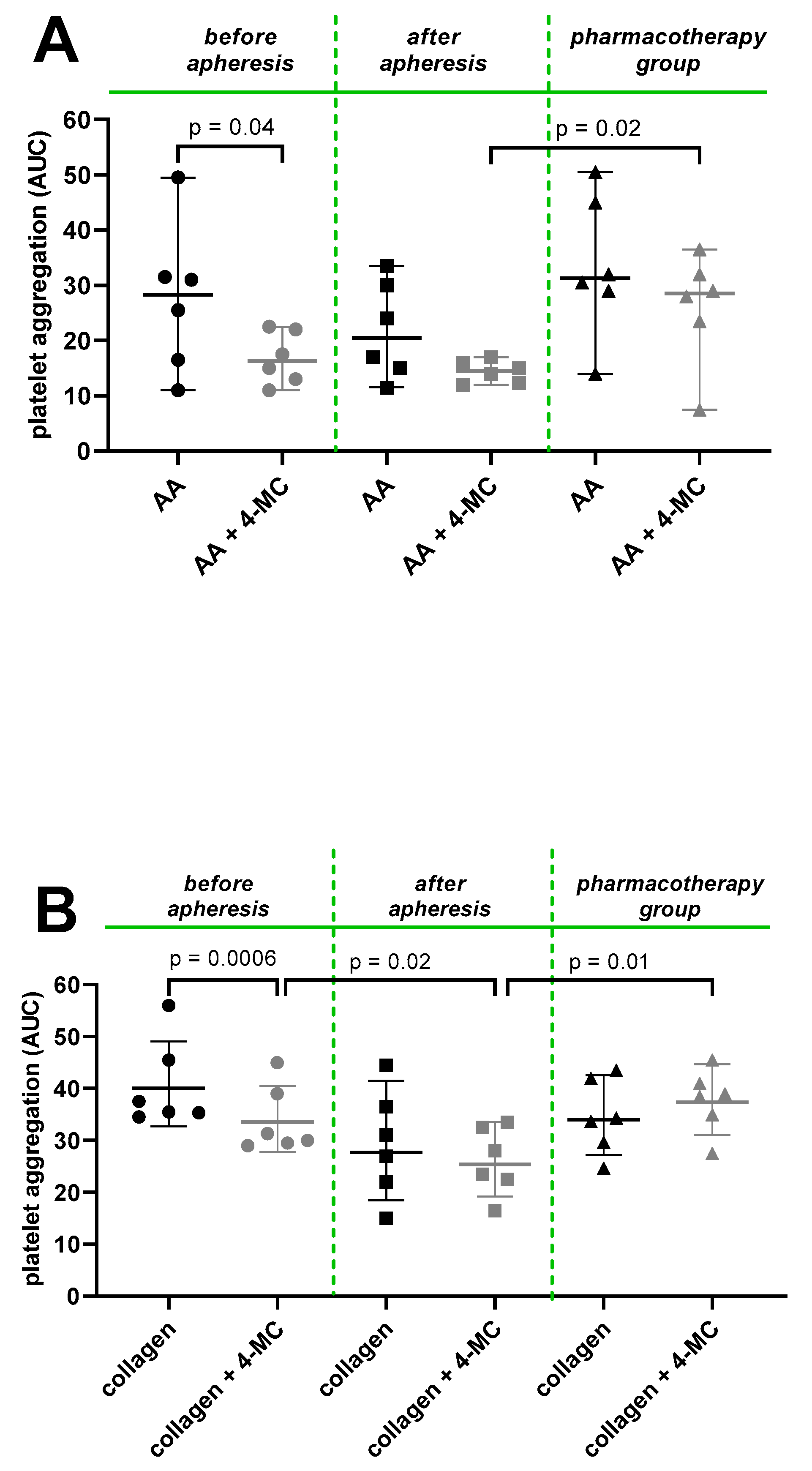
3. Results and Discussion
4. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pejic, R.N. Familial hypercholesterolemia. Ochsner J. 2014, 14, 669–672. [Google Scholar]
- Siegel-Axel, D.; Daub, K.; Seizer, P.; Lindemann, S.; Gawaz, M. Platelet lipoprotein interplay: Trigger of foam cell formation and driver of atherosclerosis. Cardiovasc. Res. 2008, 78, 8–17. [Google Scholar] [CrossRef]
- Yeung, J.; Li, W.; Holinstat, M. Platelet Signaling and Disease: Targeted Therapy for Thrombosis and Other Related Diseases. Pharm. Rev. 2018, 70, 526–548. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Hu, L.; Zhang, J.; Yang, W.; Liu, X.; Jia, D.; Yao, Z.; Chang, L.; Pan, G.; Zhong, H.; et al. PCSK9 (Proprotein Convertase Subtilisin/Kexin 9) Enhances Platelet Activation, Thrombosis, and Myocardial Infarct Expansion by Binding to Platelet CD36. Circulation 2021, 143, 45–61. [Google Scholar] [CrossRef]
- Raal, F.J.; Hovingh, G.K.; Catapano, A.L. Familial hypercholesterolemia treatments: Guidelines and new therapies. Atherosclerosis 2018, 277, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Alsayed, N.; Almahmeed, W.; Alnouri, F.; Al-Waili, K.; Sabbour, H.; Sulaiman, K.; Zubaid, M.; Ray, K.K.; Al-Rasadi, K. Consensus clinical recommendations for the management of plasma lipid disorders in the Middle East: 2021 update. Atherosclerosis 2022, 343, 28–50. [Google Scholar] [CrossRef]
- Warden, B.A.; Fazio, S.; Shapiro, M.D. Familial Hypercholesterolemia: Genes and Beyond. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.Com, Inc.: South Dartmouth, MA, USA, 2021. [Google Scholar]
- Kastelein, J.J.; Ginsberg, H.N.; Langslet, G.; Hovingh, G.K.; Ceska, R.; Dufour, R.; Blom, D.; Civeira, F.; Krempf, M.; Lorenzato, C.; et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur. Heart J. 2015, 36, 2996–3003. [Google Scholar] [CrossRef]
- Sinzinger, H.; Pirich, C.; Bednar, J.; O’Grady, J. Ex-vivo and in-vivo platelet function in patients with severe hypercholesterolemia undergoing LDL-apheresis. Thromb. Res. 1996, 82, 291–301. [Google Scholar] [CrossRef]
- Otto, C.; Baumann, M.; Schreiner, T.; Bartsch, G.; Borberg, H.; Schwandt, P.; Schmid-Schönbein, H. Standardized ultrasound as a new method to induce platelet aggregation: Evaluation, influence of lipoproteins and of glycoprotein IIb/IIIa antagonist tirofiban. Eur. J. Ultrasound. 2001, 14, 157–166. [Google Scholar] [CrossRef]
- Pares, M.N.; D’Amico, E.A.; Kutner, J.M.; Chamone Dde, A.; Bydlowski, S.P. Platelet aggregation and lipoprotein levels in a patient with familial hypercholesterolemia after selective LDL-apheresis. Sao Paulo Med. J. 1997, 115, 1448–1451. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guasch-Ferré, M.; Chung, W.; Ruiz-Canela, M.; Toledo, E.; Corella, D.; Bhupathiraju, S.N.; Tobias, D.K.; Tabung, F.K.; Hu, J.; et al. The Mediterranean diet, plasma metabolome, and cardiovascular disease risk. Eur. Heart J. 2020, 41, 2645–2656. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean diet, its components, and cardiovascular disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Franke, A.A.; Lai, J.F.; Halm, B.M. Absorption, distribution, metabolism, and excretion of isoflavonoids after soy intake. Arch. Biochem. Biophys. 2014, 559, 24–28. [Google Scholar] [CrossRef]
- Hanske, L.; Engst, W.; Loh, G.; Sczesny, S.; Blaut, M.; Braune, A. Contribution of gut bacteria to the metabolism of cyanidin 3-glucoside in human microbiota-associated rats. Br. J. Nutr. 2013, 109, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Roowi, S.; Stalmach, A.; Mullen, W.; Lean, M.E.; Edwards, C.A.; Crozier, A. Green tea flavan-3-ols: Colonic degradation and urinary excretion of catabolites by humans. J. Agric. Food Chem. 2010, 58, 1296–1304. [Google Scholar] [CrossRef]
- Sánchez-Patán, F.; Monagas, M.; Moreno-Arribas, M.V.; Bartolomé, B. Determination of microbial phenolic acids in human faeces by UPLC-ESI-TQ MS. J. Agric. Food Chem. 2011, 59, 2241–2247. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Williamson, G.; Clifford, M.N. Colonic metabolites of berry polyphenols: The missing link to biological activity? Br. J. Nutr. 2010, 104 (Suppl. 3), 48–66. [Google Scholar] [CrossRef]
- Applová, L.; Karlíčková, J.; Warncke, P.; Macáková, K.; Hrubša, M.; Macháček, M.; Tvrdý, V.; Fischer, D.; Mladěnka, P. 4-Methylcatechol, a Flavonoid Metabolite with Potent Antiplatelet Effects. Mol. Nutr. Food Res. 2019, 63, e1900261. [Google Scholar] [CrossRef] [PubMed]
- Hrubša, M.; Konečný, L.; Paclíková, M.; Parvin, M.S.; Skořepa, P.; Musil, F.; Karlíčková, J.; Javorská, L.; Matoušová, K.; Krčmová, L.K.; et al. The Antiplatelet Effect of 4-Methylcatechol in a Real Population Sample and Determination of the Mechanism of Action. Nutrients 2022, 14, 4798. [Google Scholar] [CrossRef]
- Oudot, C.; Gomes, A.; Nicolas, V.; Le Gall, M.; Chaffey, P.; Broussard, C.; Calamita, G.; Mastrodonato, M.; Gena, P.; Perfettini, J.L.; et al. CSRP3 mediates polyphenols-induced cardioprotection in hypertension. J. Nutr. Biochem. 2019, 66, 29–42. [Google Scholar] [CrossRef]
- Pourová, J.; Najmanová, I.; Vopršalová, M.; Migkos, T.; Pilařová, V.; Applová, L.; Nováková, L.; Mladěnka, P. Two flavonoid metabolites, 3,4-dihydroxyphenylacetic acid and 4-methylcatechol, relax arteries ex vivo and decrease blood pressure in vivo. Vasc. Pharmacol. 2018, 111, 36–43. [Google Scholar] [CrossRef]
- Najmanová, I.; Pourová, J.; Mladěnka, P. A Mixture of Phenolic Metabolites of Quercetin Can Decrease Elevated Blood Pressure of Spontaneously Hypertensive Rats Even in Low Doses. Nutrients 2020, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Nitta, A.; Ito, M.; Fukumitsu, H.; Ohmiya, M.; Ito, H.; Sometani, A.; Nomoto, H.; Furukawa, Y.; Furukawa, S. 4-methylcatechol increases brain-derived neurotrophic factor content and mRNA expression in cultured brain cells and in rat brain in vivo. J. Pharm. Exp. 1999, 291, 1276–1283. [Google Scholar]
- Hsieh, Y.L.; Lin, W.M.; Lue, J.H.; Chang, M.F.; Hsieh, S.T. Effects of 4-methylcatechol on skin reinnervation: Promotion of cutaneous nerve regeneration after crush injury. J. Neuropathol. Exp. Neurol. 2009, 68, 1269–1281. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, K.; Ishikawa, K.; Yasuda, S.; Kishishita, Y.; Kim, H.K.; Kakeda, T.; Yamamoto, M.; Norii, T.; Ishikawa, T. Intracerebroventricular 4-methylcatechol (4-MC) ameliorates chronic pain associated with depression-like behavior via induction of brain-derived neurotrophic factor (BDNF). Cell Mol. Neurobiol. 2012, 32, 971–977. [Google Scholar] [CrossRef]
- Sun, M.K.; Alkon, D.L. Effects of 4-methylcatechol on spatial memory and depression. Neuroreport 2008, 19, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Payton, F.; Bose, R.; Alworth, W.L.; Kumar, A.P.; Ghosh, R. 4-Methylcatechol-induced oxidative stress induces intrinsic apoptotic pathway in metastatic melanoma cells. Biochem. Pharm. 2011, 81, 1211–1218. [Google Scholar] [CrossRef]
- Bláha, V.; Bláha, M.; Lánská, M.; Havel, E.; Vyroubal, P.; Zadák, Z.; Žák, P.; Sobotka, L. LDL-apheresis in the treatment familial hypercholesterolemia. Vnitř. Lék. 2014, 60, 970–976. [Google Scholar]
- Nordestgaard, B.G.; Chapman, M.J.; Humphries, S.E.; Ginsberg, H.N.; Masana, L.; Descamps, O.S.; Wiklund, O.; Hegele, R.A.; Raal, F.J.; Defesche, J.C.; et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: Guidance for clinicians to prevent coronary heart disease: Consensus statement of the European Atherosclerosis Society. Eur. Heart J. 2013, 34, 3478–3490a. [Google Scholar] [CrossRef]
- Carazo, A.; Hrubša, M.; Konečný, L.; Skořepa, P.; Paclíková, M.; Musil, F.; Karlíčková, J.; Javorská, L.; Matoušová, K.; Krčmová, L.K.; et al. Sex-Related Differences in Platelet Aggregation: A Literature Review Supplemented with Local Data from a Group of Generally Healthy Individuals. In Seminars in Thrombosis and Hemostasis; Thieme Medical Publishers, Inc.: New York, NY, USA, 2022. [Google Scholar] [CrossRef]
- Blaha, V.; Blaha, M.; Solichová, D.; Krčmová, L.K.; Lánská, M.; Havel, E.; Vyroubal, P.; Zadák, Z.; Žák, P.; Sobotka, L. Antioxidant defense system in familial hypercholesterolemia and the effects of lipoprotein apheresis. Atheroscler. Suppl. 2017, 30, 159–165. [Google Scholar] [CrossRef]
- Borberg, H.; Tauchert, M. Rheohaemapheresis of ophthalmological diseases and diseases of the microcirculation. Transfus. Apher. Sci. 2006, 34, 41–49. [Google Scholar] [CrossRef]
- Krcmova, L.; Solichova, D.; Melichar, B.; Kasparova, M.; Plisek, J.; Sobotka, L.; Solich, P. Determination of neopterin, kynurenine, tryptophan and creatinine in human serum by high throuput HPLC. Talanta 2011, 85, 1466–1471. [Google Scholar] [CrossRef]
- Gaziano, J.M.; Brotons, C.; Coppolecchia, R.; Cricelli, C.; Darius, H.; Gorelick, B.P.; Howard, G.; Pearson, A.T.; Rothwell, M.P.; Ruilope, M.L.; et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): A randomised, double-blind, placebo-controlled trial. Lancet 2018, 392, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.K.; FitzGerald, G.A. Dose-related kinetics of aspirin. Presystemic acetylation of platelet cyclooxygenase. N. Engl. J. Med. 1984, 311, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Sirok, D.; Pátfalusi, M.; Szeleczky, G.; Somorjai, G.; Greskovits, D.; Monostory, K. Robust and sensitive LC/MS-MS method for simultaneous detection of acetylsalicylic acid and salicylic acid in human plasma. Microchem. J. 2018, 136, 200–208. [Google Scholar] [CrossRef]
- Friend, M.; Vucenik, I.; Miller, M. Research pointers: Platelet responsiveness to aspirin in patients with hyperlipidaemia. BMJ 2003, 326, 82–83. [Google Scholar] [CrossRef] [PubMed]
- Tremoli, E.; Folco, G.; Agradi, E.; Galli, C. Platelet thromboxanes and serum-cholesterol. Lancet 1979, 1, 107–108. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, J.L.; Harker, L.A. Platelet and fibrinogen survival in coronary atherosclerosis. Response to medical and surgical therapy. Am. J. Cardiol. 1977, 39, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Stalker, T.J.; Newman, D.K.; Ma, P.; Wannemacher, K.M.; Brass, L.F. Platelet signaling. In Handbook of Experimental Pharmacology; Springer: Heidelberg, Germany, 2012; Volume 210, pp. 59–85. [Google Scholar] [CrossRef]

| Patient No. (Sex) | Therapy at the Time of Analysis | Phenotype | Gene | Exone Location No. | |
|---|---|---|---|---|---|
| pharmacotherapy group | 1 (M) | PCSK9Ab + S + E | HeFH | UN | E4–E8 |
| 2 (M) | PCSK9Ab + S + E | HeFH | LDL-R | E9 | |
| 3 (F) | PCSK9Ab + S + E | HeFH | LDL-R | E8 | |
| 4 (M) | PCSK9Ab + S + E | HeFH | LDL-R | E7 | |
| 5 (M) | PCSK9Ab + S + E | HeFH | LDL-R | UN | |
| 6 (M) | PCSK9Ab + S + E | HeFH | LDL-R | UN | |
| 7 (M) | S + E | HeFH | LDL-R | E12 | |
| apheresis group | 8 (F) | Lipid apheresis + PCSK9Ab + S + E | HoFH | LDL-R | E8 |
| 9 (F) | Lipid apheresis + PCSK9Ab + S + E | HeFH | UN | UN | |
| 10 (M) | Lipid apheresis + PCSK9Ab + S + E | HeFH | UN | E9–E14 | |
| 11 (F) | Lipid apheresis + S + E | HeFH | UN | UN | |
| 12 (M) | Lipid apheresis + S + Fi | HeFH | UN | UN | |
| 13 (M) | Lipid apheresis + PCSK9Ab + S + E + L | HoFH | LDL-R | E12; E16 | |
| 14 (F) | Lipid apheresis + PCSK9Ab + S + E | HoFH | LDL-R | UN | |
| 15 (F) | Lipid apheresis + PCSK9Ab + S + E + L | HoFH | LDL-R | E10; E12 |
| Healthy Donors | FH Patients | p-Value | ||
|---|---|---|---|---|
| age | 40–77 | 59 ± 6 | 52 ± 11 | 0.127 |
| BMI | 18.5–30+ | 29.27 ± 4.49 | 26.42 ± 3.29 | 0.128 |
| smokers—N (%) | Yes | 6 (40%) | 1 (7%) | 0.031 |
| COVID-19 a—N (%) | Yes | 7 (47%) | 5 (33%) | 0.456 |
| enteric-coated ASA—N (%) | Yes | 0 (0%) | 2 (13%) | 0.143 |
| conventional ASA—N (%) | Yes | 0 (0%) | 3 (20%) | 0.068 |
| clopidogrel + ASA combination—N (%) | Yes | 0 (0%) | 2 (13%) | 0.143 |
| biochemical parameters | TC (mmol/L) | 5.53 ± 0.78 | 4.30 ± 1.48 | 0.010 |
| LDL-C (mmol/L) | 3.46 ± 0.73 | 2.52 ± 1.38 | 0.006 | |
| glucose (mmol/L) | 5.45 ± 0.58 | 6.10 ± 2.05 | 0.336 | |
| HDL-C (mmol/L) | 1.52 ± 0.43 | 1.18 ± 0.33 | 0.027 | |
| TG (mmol/L) | 1.37 ± 0.45 | 1.81 ± 1.12 | 0.250 | |
| creatinine in serum (µmol/L) | 80.54 ± 12.77 | 76.37 ± 17.22 | 0.314 | |
| creatinine in urine (mmol/L) | 9.99 ± 4.17 | 10.86 ± 6.93 | 0.921 |
| Apheresis Group | Pharmacotherapy Group | p-Value | ||
|---|---|---|---|---|
| age | 40–77 | 49 ± 13 | 51 ± 6 | 0.585 |
| BMI | 18.5–30+ | 28.00 ± 4.72 | 32.32 ± 4.08 | 0.138 |
| smokers—N (%) | Yes | 0 (0%) | 0 (0%) | - |
| COVID-19 a—N (%) | Yes | 2 (33%) | 3 (50%) | 0.558 |
| enteric-coated ASA—N (%) | Yes | 1 (16%) | 1 (16%) | 0.999 |
| conventional ASA—N (%) | Yes | 2 (33%) | 1 (16%) | 0.505 |
| clopidogrel + ASA combination—N (%) | Yes | 1 (16%) | 0 (0%) | 0.296 |
| cardiovascular diseases—N (%) | arterial hypertension | 2 (33%) | 0 (0%) | 0.121 |
| ACB | 1 (16%) | 2 (33%) | 0.505 | |
| AS of carotid arteries | 2 (33%) | 2 (33%) | 0.999 | |
| CAD | 3 (50%) | 3 (50%) | 0.999 | |
| PAD | 1 (16%) | 0 (0%) | 0.296 | |
| AS and calcifications/AS and defect/just AS of aortic valve | 3 (50%) | 1 (16%) | 0.221 | |
| stroke | 1 (16%) | 0 (0%) | 0.296 | |
| moderate stenosis of left ACC | 1 (16%) | 0 (0%) | 0.296 | |
| bilateral AS and calcifications of ACC | 1 (16%) | 0 (0%) | 0.296 | |
| haemodynamically non-significant AO stenosis | 1 (16%) | 0 (0%) | 0.296 | |
| other diseases—N (%) | familial hypercholesterolemia | 6 (100%) | 6 (100%) | |
| diabetes mellitus type 1 | 1 (16%) | 1 (16%) | 0.999 | |
| hypothyreosis | 3 (50%) | 0 (0%) | 0.045 | |
| anaemia | 1 (16%) | 0 (0%) | 0.296 | |
| allergy | 1 (16%) | 0 (0%) | 0.296 | |
| glaucoma | 1 (16%) | 0 (0%) | 0.296 | |
| asthma | 1 (16%) | 0 (0%) | 0.296 |
| Patients, N (%) | Apheresis Group | Pharmacotherapy Group | p-Value | |
|---|---|---|---|---|
| biochemical parameters | TC (mmol/L) | A 4.73 ± 1.93 B 1.82 ± 0.55 | C 3.81 ± 1.12 | A vs. B 0.010 A vs. C 0.378 B vs. C 0.005 |
| LDL-C (mmol/L) | A 2.92 ± 1.89 B 0.67 ± 0.49 | C 2.22 ± 0.93 | A vs. B 0.019 A vs. C 0.479 B vs. C 0.008 | |
| glucose (mmol/L) | A 5.52 ± 0.85 B 6.58 ± 1.84 | C 6.03 ± 0.98 | A vs. B 0.215 A vs. C 0.394 B vs. C 0.585 | |
| HDL-C (mmol/L) | A 1.34 ± 0.38 B 1.03 ± 0.30 | C 1.08 ± 0.20 | A vs. B 0.005 A vs. C 0.155 B vs. C 0.944 | |
| TG (mmol/L) | A 1.36 ± 0.76 B 0.65 ± 0.39 | C 1.52 ± 0.49 | A vs. B 0.011 A vs. C 0.707 B vs. C 0.011 | |
| creatinine in serum (µmol/L) | A 66.16 ± 32.82 B 64.26 ± 30.77 | C 77.14 ± 8.69 | A vs. B 0.434 A vs. C 0.880 B vs. C 0.687 | |
| creatinine in urine (mmol/L) | A 7.35 ± 5.81 | C 14.98 ± 6.80 | A vs. C 0.085 |
| Final Concentration | Units | ||
|---|---|---|---|
| inducers | collagen | 1 | µg/mL |
| AA | 200 | µM | |
| ristocetin | 400 | µM | |
| inhibitor | 4-MC | 10 A, 20 C, 70 C and 250 R | µM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konečný, L.; Hrubša, M.; Karlíčková, J.; Carazo, A.; Javorská, L.; Matoušová, K.; Krčmová, L.K.; Šmahelová, A.; Blaha, V.; Bláha, M.; et al. The Effect of 4-Methylcatechol on Platelets in Familial Hypercholesterolemic Patients Treated with Lipid Apheresis and/or Proprotein Convertase Subtilisin Kexin 9 Monoclonal Antibodies. Nutrients 2023, 15, 1842. https://doi.org/10.3390/nu15081842
Konečný L, Hrubša M, Karlíčková J, Carazo A, Javorská L, Matoušová K, Krčmová LK, Šmahelová A, Blaha V, Bláha M, et al. The Effect of 4-Methylcatechol on Platelets in Familial Hypercholesterolemic Patients Treated with Lipid Apheresis and/or Proprotein Convertase Subtilisin Kexin 9 Monoclonal Antibodies. Nutrients. 2023; 15(8):1842. https://doi.org/10.3390/nu15081842
Chicago/Turabian StyleKonečný, Lukáš, Marcel Hrubša, Jana Karlíčková, Alejandro Carazo, Lenka Javorská, Kateřina Matoušová, Lenka Kujovská Krčmová, Alena Šmahelová, Vladimír Blaha, Milan Bláha, and et al. 2023. "The Effect of 4-Methylcatechol on Platelets in Familial Hypercholesterolemic Patients Treated with Lipid Apheresis and/or Proprotein Convertase Subtilisin Kexin 9 Monoclonal Antibodies" Nutrients 15, no. 8: 1842. https://doi.org/10.3390/nu15081842
APA StyleKonečný, L., Hrubša, M., Karlíčková, J., Carazo, A., Javorská, L., Matoušová, K., Krčmová, L. K., Šmahelová, A., Blaha, V., Bláha, M., & Mladěnka, P. (2023). The Effect of 4-Methylcatechol on Platelets in Familial Hypercholesterolemic Patients Treated with Lipid Apheresis and/or Proprotein Convertase Subtilisin Kexin 9 Monoclonal Antibodies. Nutrients, 15(8), 1842. https://doi.org/10.3390/nu15081842