Renal-Protective Roles of Lipoic Acid in Kidney Disease
Abstract
1. Introduction
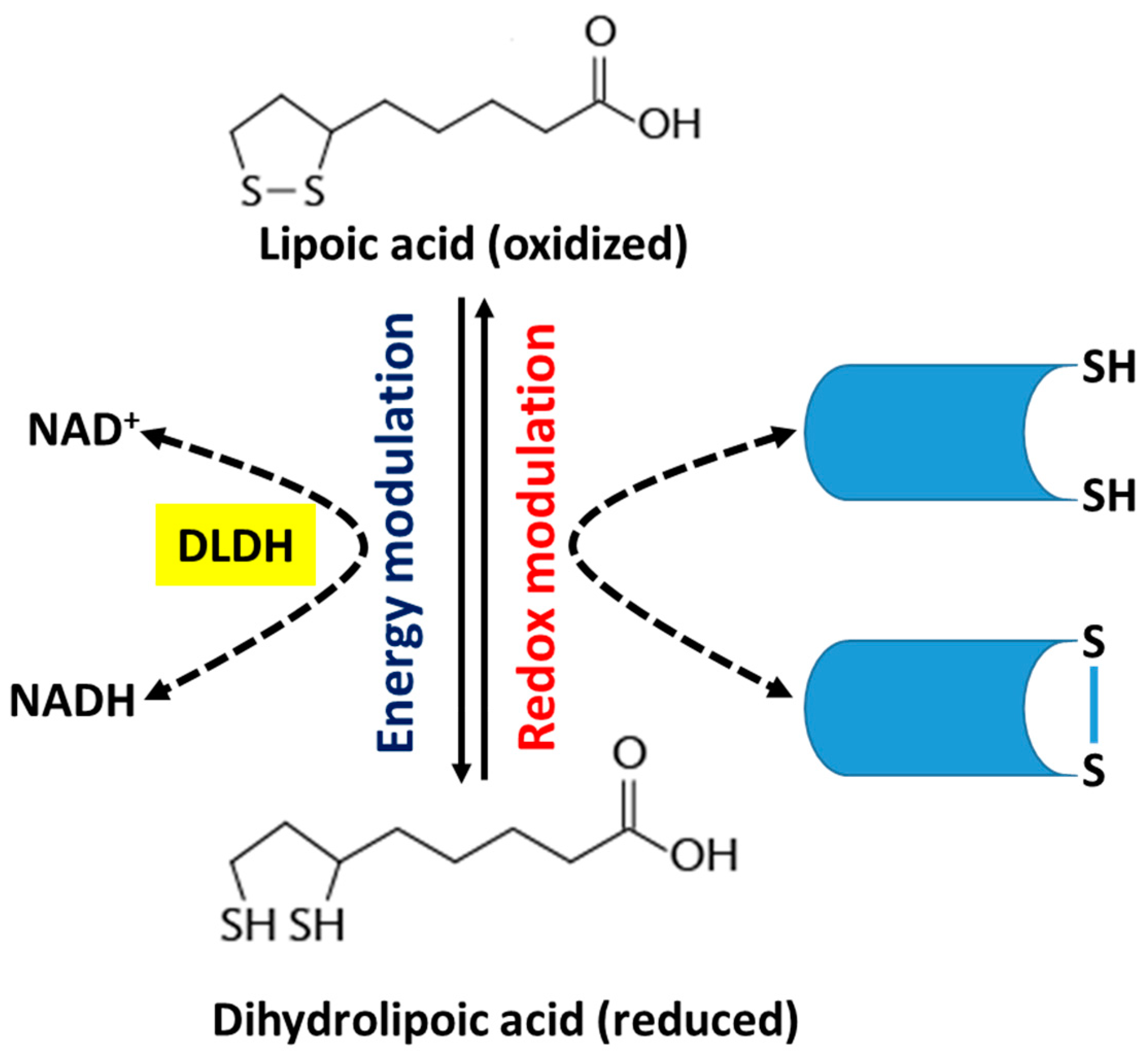
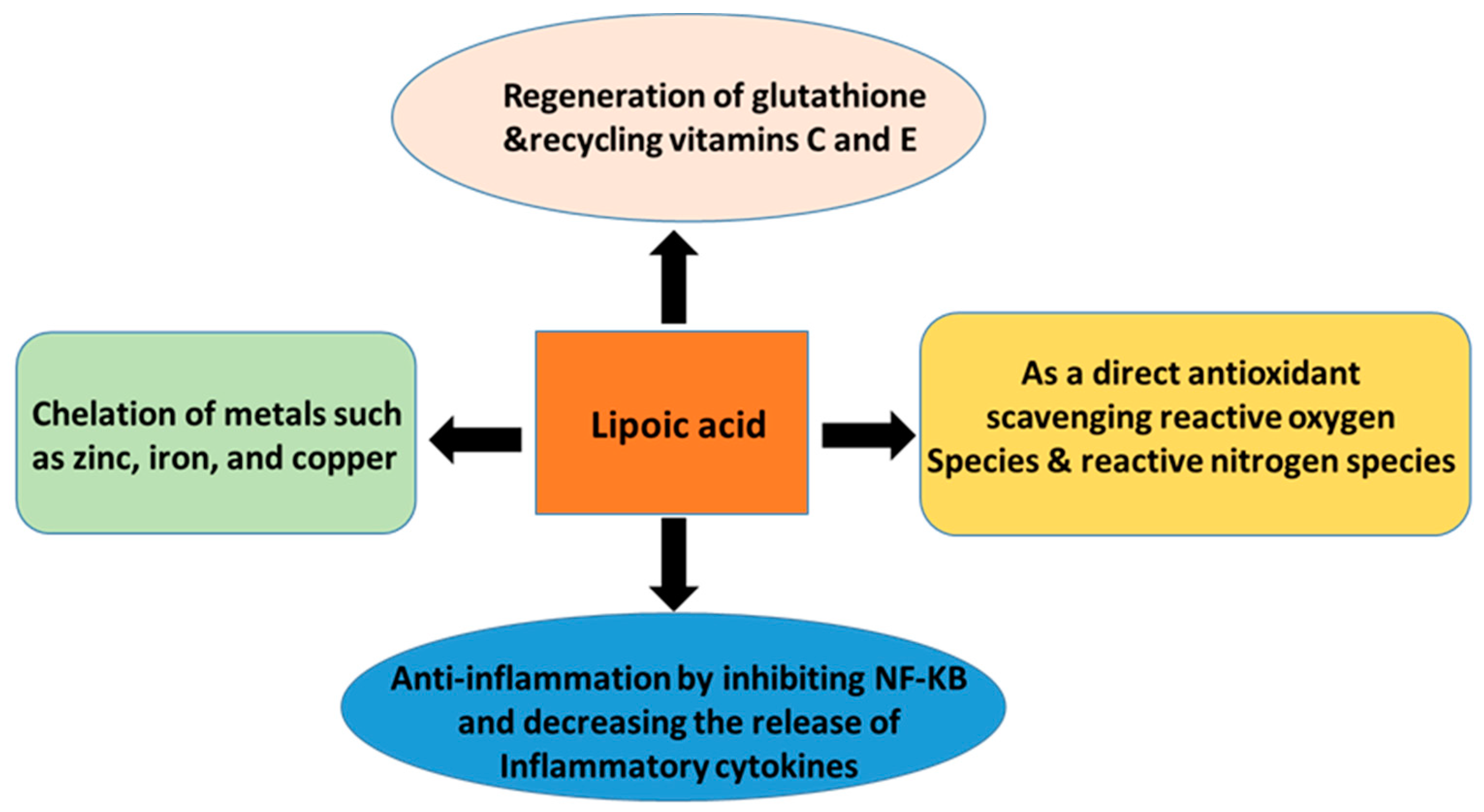
2. Alpha-Lipoic Acid
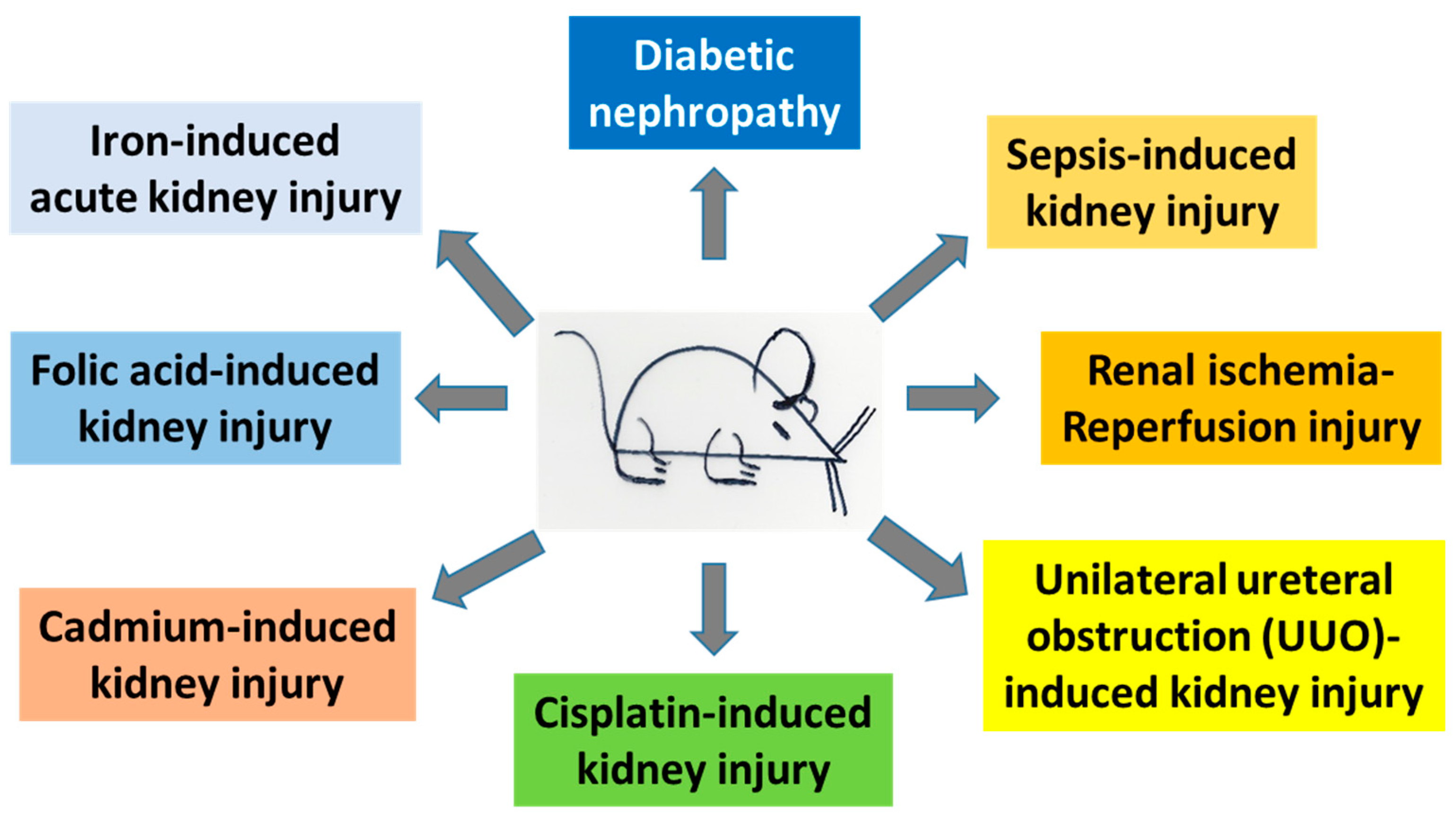
3. Protective Roles of α-Lipoic Acid (ALA) in Kidney Injury
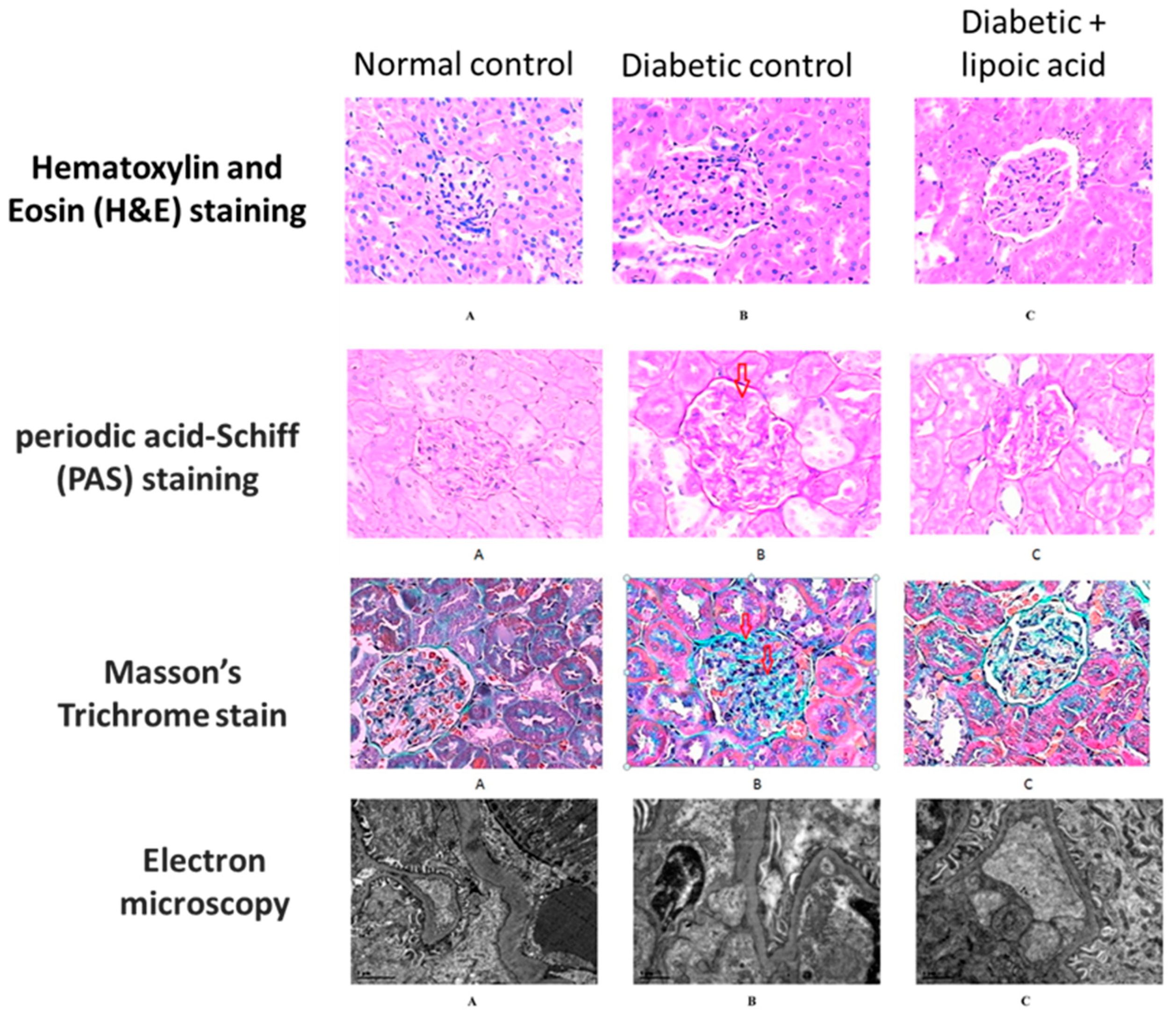
3.1. Diabetic Nephropathy
3.2. Sepsis-Induced Kidney Injury
3.3. Renal Ischemic Reperfusion
3.4. Unilateral Ureteral Obstruction (UUO)-Induced Kidney Injury
3.5. Cisplatin-Induced Nephrotoxicity
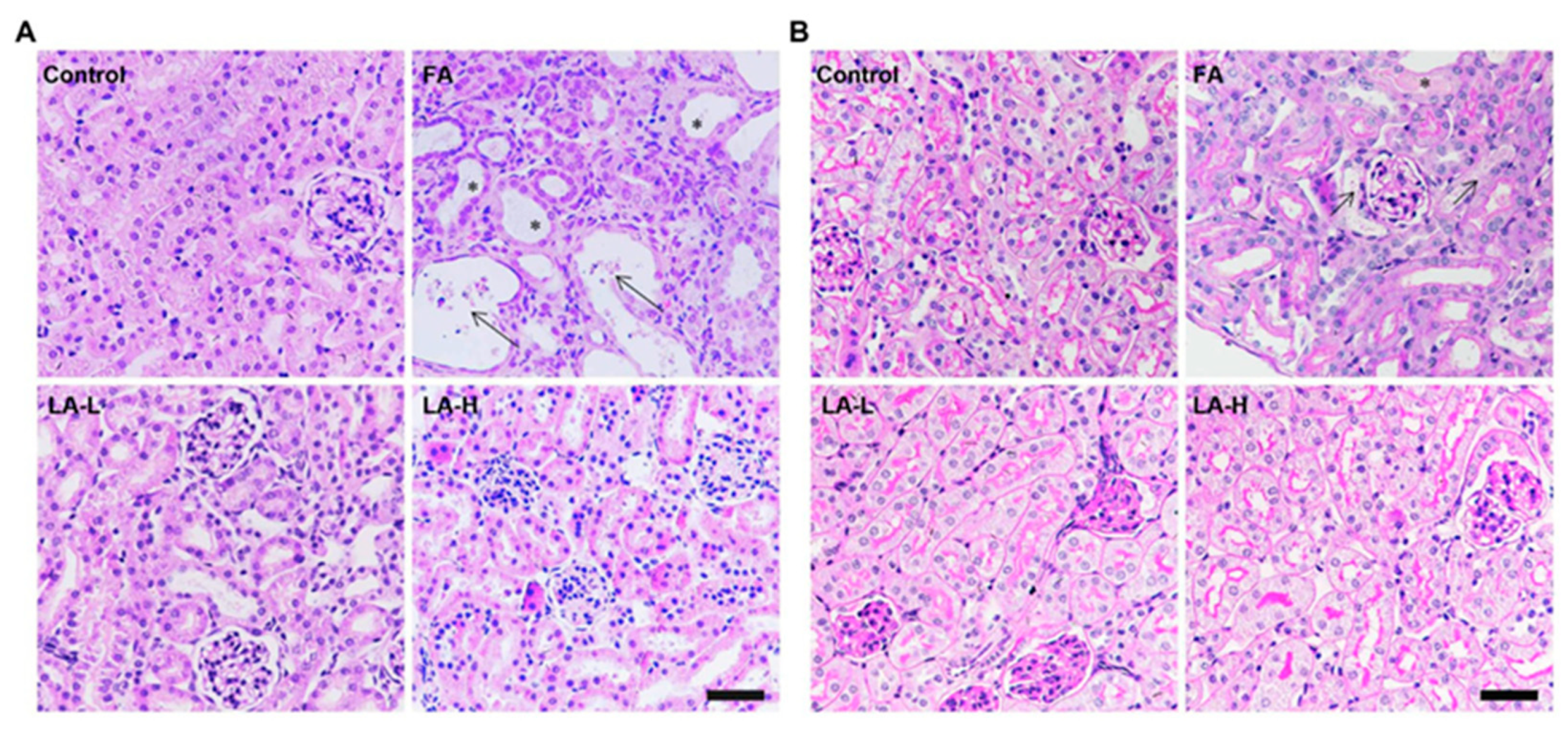
3.6. Folic Acid-Induced Nephrotoxicity
3.7. Cadmium-Induced Nephrotoxicity
3.8. Iron-Induced Acute Kidney Injury
4. Miscellaneous
5. Summary
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rennke, H.G.; Denker, B.M. Renal Pathology: The Essentials, 5th ed.; Wolters Kluwer: New York, NY, USA, 2020. [Google Scholar]
- Koeppen, B.M.; Stanton, B.A. Renal Physiology, 5th ed.; Elsevier: Philadelphia, PA, USA, 2013. [Google Scholar]
- Dipiro, J.T.; Talbet, R.L.; Yee, G.C.; Matzke, G.R.; Wells, B.G.; Posey, L.M. Pharmacotherapy: A Pathophysiological Approach, 9th ed.; McGraw-Hill Education: New York, NY, USA, 2014. [Google Scholar]
- Lieberman, M.; Marks, A.D. Marks’ Basic Medical Biochemistry: A Clinical Approach, 4th ed.; Wolters Kluwer/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Ammirati, A.L. Chronic kidney disease. Rev. Assoc. Med. Bras. (1992) 2020, 66 (Suppl. S1), s03–s09. [Google Scholar] [CrossRef] [PubMed]
- Turgut, F.; Awad, A.S.; Abdel-Rahman, E.M. Acute kidney injury: Medical causes and pathogenesis. J. Clin. Med. 2023, 12, 375. [Google Scholar] [CrossRef] [PubMed]
- Duann, P.; Lin, P.H. Mitochondria damage and kidney disease. Adv. Exp. Med. Biol. 2017, 982, 529–551. [Google Scholar] [PubMed]
- Sanz, A.B.; Sanchez-Nino, M.D.; Ramos, A.M.; Ortiz, A. Regulated cell death pathways in kidney disease. Nat. Rev. Nephrol. 2023, 1–19. [Google Scholar] [CrossRef]
- Levey, A.S.; Becker, C.; Inker, L.A. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: A systematic review. JAMA 2015, 313, 837–846. [Google Scholar] [CrossRef]
- Mima, A. A narrative review of diabetic kidney disease: Previous and current evidence-based therapeutic approaches. Adv. Ther. 2022, 39, 3488–3500. [Google Scholar] [CrossRef]
- Mima, A. Mitochondria-targeted drugs for diabetic kidney disease. Heliyon 2022, 8, e08878. [Google Scholar] [CrossRef]
- Bhatia, D.; Capili, A.; Choi, M.E. Mitochondrial dysfunction in kidney injury, inflammation, and disease: Potential therapeutic approaches. Kidney Res. Clin. Pract. 2020, 39, 244–258. [Google Scholar] [CrossRef]
- Bhatia, D.; Choi, M.E. Autophagy in kidney disease: Advances and therapeutic potential. Prog. Mol. Biol. Transl. Sci. 2020, 172, 107–133. [Google Scholar]
- Chen, J.H.; Wu, C.H.; Chiang, C.K. Therapeutic approaches targeting proteostasis in kidney disease and fibrosis. Int. J. Mol. Sci. 2021, 22, 8674. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Anton, M.I.; Floria, M.; Seritean Isac, P.N.; Hurjui, L.L.; Tarniceriu, C.C.; Costea, C.F.; Ciocoiu, M.; Rezus, C. Oxidative stress and nrf2/keap1/are pathway in diabetic kidney disease (dkd): New perspectives. Biomolecules 2022, 12, 1227. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S. Iron homeostasis pathways as therapeutic targets in acute kidney injury. Nephron 2018, 140, 156–159. [Google Scholar] [CrossRef]
- Pieretti, J.C.; Junho, C.V.C.; Carneiro-Ramos, M.S.; Seabra, A.B. H2S- and NO-releasing gasotransmitter platform: A crosstalk signaling pathway in the treatment of acute kidney injury. Pharmacol. Res. 2020, 161, 105121. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Lima Sampaio, T.; Khan, H.; Jeandet, P.; Kupeli Akkol, E.; Bahadar, H.; Costa Martins, A.M. Plants with therapeutic potential for ischemic acute kidney injury: A systematic review. Evid. Based Complement. Altern. Med. 2022, 2022, 6807700. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wu, W.; Liao, J.; Zhang, X.; Shen, M.; Li, X.; Lin, Q.; Cao, C. Molecular mechanisms underlying the renal protective effects of coenzyme q10 in acute kidney injury. Cell. Mol. Biol. Lett. 2022, 27, 57. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Han, J.; Fang, J.; Li, R. The beneficial effects of mesenchymal stem cells in acute kidney injury: A narrative review. Curr. Stem Cell Res. Ther. 2023. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Krishan, P. Dietary restriction regimens for fighting kidney disease: Insights from rodent studies. Exp. Gerontol. 2019, 128, 110738. [Google Scholar] [CrossRef]
- Li, G.; Gao, L.; Jia, J.; Gong, X.; Zang, B.; Chen, W. α-Lipoic acid prolongs survival and attenuates acute kidney injury in a rat model of sepsis. Clin. Exp. Pharmacol. Physiol. 2014, 41, 459–468. [Google Scholar] [CrossRef]
- Yan, L.J.; Thangthaeng, N.; Sumien, N.; Forster, M.J. Serum dihydrolipoamide dehydrogenase is a labile enzyme. J. Biochem. Pharmacol. Res. 2013, 1, 30–42. [Google Scholar]
- Yang, X.; Song, J.; Yan, L.J. Chronic inhibition of mitochondrial dihydrolipoamide dehydrogenase (dldh) as an approach to managing diabetic oxidative stress. Antioxidants 2019, 8, 32. [Google Scholar] [CrossRef]
- Zaazaa, A.M.; Motelp, B.; Aniss, N.N. Potential protective role of rutin and alpha-lipoic acid against cisplatin-induced nephrotoxicity in rats. Pak. J. Biol. Sci. 2019, 22, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Cadenas, E. Lipoic acid: Energy metabolism and redox regulation of transcription and cell signaling. J. Clin. Biochem. Nutr. 2011, 48, 26–32. [Google Scholar] [CrossRef]
- Solmonson, A.; DeBerardinis, R.J. Lipoic acid metabolism and mitochondrial redox regulation. J. Biol. Chem. 2018, 293, 7522–7530. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Ghibu, S.; Muresan, A.; Vergely, C. Alpha-lipoic acid: Molecular mechanisms and therapeutic potential in diabetes. Can. J. Physiol. Pharmacol. 2015, 93, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, E.A.; Nabil Ahmed, A.; Hashem, K.S.; Allah, A.G. Therapeutic effects of the combination of alpha-lipoic acid (ala) and coenzyme q10 (coq10) on cisplatin-induced nephrotoxicity. Int. J. Inflam. 2020, 2020, 5369797. [Google Scholar] [CrossRef]
- Tong, Z.J.; Kuo, C.W.; Yen, P.C.; Lin, C.C.; Tsai, M.T.; Lu, S.H.; Chang, Y.P.; Liu, W.S.; Tsou, H.H.; Cheng, H.W.; et al. Acrolein plays a culprit role in the pathogenesis of diabetic nephropathy in vitro and in vivo. Eur. J. Endocrinol. 2022, 187, 579–592. [Google Scholar] [CrossRef]
- Gao, W.W.; Chun, S.Y.; Kim, B.S.; Ha, Y.S.; Lee, J.N.; Lee, E.H.; Kim, I.Y.; You, S.; Kwon, T.G. Locally transplanted human urine-induced nephron progenitor cells contribute to renal repair in mice kidney with diabetic nephropathy. Biochem. Biophys. Res. Commun. 2022, 629, 128–134. [Google Scholar] [CrossRef]
- Wu, J.; Li, R.; Li, W.; Ren, M.; Thangthaeng, N.; Sumien, N.; Liu, R.; Yang, S.; Simpkins, J.W.; Forster, M.J.; et al. Administration of 5-methoxyindole-2-carboxylic acid that potentially targets mitochondrial dihydrolipoamide dehydrogenase confers cerebral preconditioning against ischemic stroke injury. Free Radic. Biol. Med. 2017, 113, 244–254. [Google Scholar] [CrossRef]
- Yan, L.J.; Thangthaeng, N.; Forster, M.J. Changes in dihydrolipoamide dehydrogenase expression and activity during postnatal development and aging in the rat brain. Mech. Ageing Dev. 2008, 129, 282–290. [Google Scholar] [CrossRef]
- Yan, L.J.; Yang, S.H.; Shu, H.; Prokai, L.; Forster, M.J. Histochemical staining and quantification of dihydrolipoamide dehydrogenase diaphorase activity using blue native page. Electrophoresis 2007, 28, 1036–1045. [Google Scholar] [CrossRef]
- Zhang, J.; McCullough, P.A. Lipoic acid in the prevention of acute kidney injury. Nephron 2016, 134, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Oktan, M.A.; Heybeli, C.; Ural, C.; Kocak, A.; Bilici, G.; Cavdar, Z.; Ozbal, S.; Arslan, S.; Yilmaz, O.; Cavdar, C. Alpha-lipoic acid alleviates colistin nephrotoxicity in rats. Hum. Exp. Toxicol. 2021, 40, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Busse, L.W. Novel therapies for acute kidney injury. Kidney Int. Rep. 2017, 2, 785–799. [Google Scholar] [CrossRef] [PubMed]
- Putra, I.; Fakhrudin, N.; Nurrochmad, A.; Wahyuono, S. A review of medicinal plants with renoprotective activity in diabetic nephropathy animal models. Life 2023, 13, 560. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Kitada, M.; Koya, D. NAD+ homeostasis in diabetic kidney disease. Front. Med. 2021, 8, 703076. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.A.; Draves, S.O.; Rosca, M. Mitochondria in diabetic kidney disease. Cells 2021, 10, 2945. [Google Scholar] [CrossRef]
- Yan, L.J. Nadh/NAD+ redox imbalance and diabetic kidney disease. Biomolecules 2021, 11, 730. [Google Scholar] [CrossRef]
- Song, Y.; Yu, H.; Sun, Q.; Pei, F.; Xia, Q.; Gao, Z.; Li, X. Grape seed proanthocyanidin extract targets p66shc to regulate mitochondrial biogenesis and dynamics in diabetic kidney disease. Front. Pharmacol. 2022, 13, 1035755. [Google Scholar] [CrossRef]
- Cleveland, K.H.; Schnellmann, R.G. Pharmacological targeting of mitochondria in diabetic kidney disease. Pharmacol. Rev. 2023, 75, 250–262. [Google Scholar] [CrossRef]
- Mohandes, S.; Doke, T.; Hu, H.; Mukhi, D.; Dhillon, P.; Susztak, K. Molecular pathways that drive diabetic kidney disease. J. Clin. Invest. 2023, 133, e165654. [Google Scholar] [CrossRef]
- Obrosova, I.G.; Fathallah, L.; Liu, E.; Nourooz-Zadeh, J. Early oxidative stress in the diabetic kidney: Effect of dl-alpha-lipoic acid. Free Radic. Biol. Med. 2003, 34, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Pop, C.; Stefan, M.G.; Muntean, D.M.; Stoicescu, L.; Gal, A.F.; Kiss, B.; Morgovan, C.; Loghin, F.; Rochette, L.; Lauzier, B.; et al. Protective effects of a discontinuous treatment with alpha-lipoic acid in obesity-related heart failure with preserved ejection fraction, in rats. Antioxidants 2020, 9, 1073. [Google Scholar] [CrossRef]
- Yan, L.J.; Lodge, J.K.; Traber, M.G.; Matsugo, S.; Packer, L. Comparison between copper-mediated and hypochlorite-mediated modifications of human low density lipoproteins evaluated by protein carbonyl formation. J. Lipid Res. 1997, 38, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.J.; Lodge, J.K.; Traber, M.G.; Packer, L. Apolipoprotein b carbonyl formation is enhanced by lipid peroxidation during copper-mediated oxidation of human low-density lipoproteins. Arch. Biochem. Biophys. 1997, 339, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, C.G.; Fang, C.Q.; Gao, J.; Liu, Y.Z.; Chen, Y.; Chen, Y.N.; Xu, Z.G. The protective effect of alpha-lipoic acid on mitochondria in the kidney of diabetic rats. Int. J. Clin. Exp. Med. 2013, 6, 90–97. [Google Scholar] [PubMed]
- Golbidi, S.; Badran, M.; Laher, I. Diabetes and alpha lipoic acid. Front. Pharmacol. 2011, 2, 69. [Google Scholar] [CrossRef]
- Zhang, H.F.; Liu, H.M.; Xiang, J.Y.; Zhou, X.C.; Wang, D.; Chen, R.Y.; Tan, W.L.; Liang, L.Q.; Liu, L.L.; Shi, M.J.; et al. Alpha lipoamide inhibits diabetic kidney fibrosis via improving mitochondrial function and regulating rxralpha expression and activation. Acta Pharmacol. Sin. 2022, 1–15. [Google Scholar] [CrossRef]
- Yan, L.J. The nicotinamide/streptozotocin rodent model of type 2 diabetes: Renal pathophysiology and redox imbalance features. Biomolecules 2022, 12, 1225. [Google Scholar] [CrossRef]
- Dugbartey, G.J.; Alornyo, K.K.; Diaba, D.E.; Adams, I. Activation of renal cse/H2S pathway by alpha-lipoic acid protects against histological and functional changes in the diabetic kidney. Biomed. Pharm. 2022, 153, 113386. [Google Scholar] [CrossRef]
- Jiang, Z.; Tan, Z.; Meng, F.; Li, X. Curative effects of valsartan alone or combined with alpha-lipoic acid on inflammatory cytokines and renal function in early-stage diabetic kidney disease. J. Coll. Physicians Surg. Pak. 2019, 29, 1009–1011. [Google Scholar] [CrossRef]
- Feng, B.; Yan, X.F.; Xue, J.L.; Xu, L.; Wang, H. The protective effects of alpha-lipoic acid on kidneys in type 2 diabetic goto-kakisaki rats via reducing oxidative stress. Int. J. Mol. Sci. 2013, 14, 6746–6756. [Google Scholar] [CrossRef] [PubMed]
- Louhelainen, M.; Merasto, S.; Finckenberg, P.; Lapatto, R.; Cheng, Z.J.; Mervaala, E.M. Lipoic acid supplementation prevents cyclosporine-induced hypertension and nephrotoxicity in spontaneously hypertensive rats. J. Hypertens 2006, 24, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. The effects and mechanisms of mitochondrial nutrient alpha-lipoic acid on improving age-associated mitochondrial and cognitive dysfunction: An overview. Neurochem. Res. 2008, 33, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Granata, S.; Dalla Gassa, A.; Tomei, P.; Lupo, A.; Zaza, G. Mitochondria: A new therapeutic target in chronic kidney disease. Nutr. Metab. 2015, 12, 49. [Google Scholar] [CrossRef]
- Bhatti, F.; Mankhey, R.W.; Asico, L.; Quinn, M.T.; Welch, W.J.; Maric, C. Mechanisms of antioxidant and pro-oxidant effects of alpha-lipoic acid in the diabetic and nondiabetic kidney. Kidney Int. 2005, 67, 1371–1380. [Google Scholar] [CrossRef]
- Halabi, Z.; El Helou, C.; Al Balushi, H.; Gittinger, M.; Steck, A.R.; Kaakour, A.; Abu-Alfa, A.; El Zahran, T. Alpha lipoic acid toxicity: The first reported mortality in an adult patient after multiorgan failure. J. Emerg. Med. 2023, 64, 190–194. [Google Scholar] [CrossRef]
- Lo, S.M.; Dal Lin, F.T.; Soares, M.F.; Hauser, A.B.; Pecoits-Filho, R.; Nakao, L.S. Lipoic acid does not improve renal function markers in 5/6 nephrectomy model: Possible role of Nrf2 inactivation. Ren. Fail. 2016, 38, 558–563. [Google Scholar] [CrossRef]
- Ghibu, S.; Craciun, C.E.; Rusu, R.; Morgovan, C.; Mogosan, C.; Rochette, L.; Gal, A.F.; Dronca, M. Impact of alpha-lipoic acid chronic discontinuous treatment in cardiometabolic disorders and oxidative stress induced by fructose intake in rats. Antioxidants 2019, 8, 636. [Google Scholar] [CrossRef]
- Prabhakar, S.; Starnes, J.; Shi, S.; Lonis, B.; Tran, R. Diabetic nephropathy is associated with oxidative stress and decreased renal nitric oxide production. J. Am. Soc. Nephrol. 2007, 18, 2945–2952. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Cha, D.R.; Kim, B.; An, E.J.; Lee, S.R.; Cha, J.J.; Kang, Y.S.; Ghee, J.Y.; Han, J.Y.; Bae, Y.S. Lps-induced acute kidney injury is mediated by nox4-sh3yl1. Cell Rep. 2020, 33, 108245. [Google Scholar] [CrossRef]
- Doi, K.; Leelahavanichkul, A.; Yuen, P.S.; Star, R.A. Animal models of sepsis and sepsis-induced kidney injury. J. Clin. Investig. 2009, 119, 2868–2878. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.; Martens, M.D.; Takahara, S.; Silver, H.L.; Maayah, Z.H.; Ussher, J.R.; Ferdaoussi, M.; Dyck, J.R.B. Exogenous ketone ester administration attenuates systemic inflammation and reduces organ damage in a lipopolysaccharide model of sepsis. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166507. [Google Scholar] [CrossRef] [PubMed]
- Wanner, G.A.; Keel, M.; Steckholzer, U.; Beier, W.; Stocker, R.; Ertel, W. Relationship between procalcitonin plasma levels and severity of injury, sepsis, organ failure, and mortality in injured patients. Crit. Care Med. 2000, 28, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Branco, R.G.; Garcia, P.C.; Garcia, J.P.; Piva, J.P.; Ross-Russell, R. Interactions of sepsis, organ dysfunction, and outcome of critically ill children. Am. J. Respir. Crit. Care Med. 2005, 172, 1606, author reply 1606–1607. [Google Scholar] [CrossRef]
- Chang, Y.M.; Chou, Y.T.; Kan, W.C.; Shiao, C.C. Sepsis and acute kidney injury: A review focusing on the bidirectional interplay. Int. J. Mol. Sci. 2022, 23, 9159. [Google Scholar] [CrossRef] [PubMed]
- Petronilho, F.; Florentino, D.; Danielski, L.G.; Vieira, L.C.; Martins, M.M.; Vieira, A.; Bonfante, S.; Goldim, M.P.; Vuolo, F. Alpha-lipoic acid attenuates oxidative damage in organs after sepsis. Inflammation 2016, 39, 357–365. [Google Scholar] [CrossRef]
- Jia, J.; Gong, X.; Zhao, Y.; Yang, Z.; Ji, K.; Luan, T.; Zang, B.; Li, G. Autophagy enhancing contributes to the organ protective effect of alpha-lipoic acid in septic rats. Front. Immunol. 2019, 10, 1491. [Google Scholar] [CrossRef]
- Li, G.; Fu, J.; Zhao, Y.; Ji, K.; Luan, T.; Zang, B. Alpha-lipoic acid exerts anti-inflammatory effects on lipopolysaccharide-stimulated rat mesangial cells via inhibition of nuclear factor kappa b (nf-kappab) signaling pathway. Inflammation 2015, 38, 510–519. [Google Scholar] [CrossRef]
- Suh, S.H.; Lee, K.E.; Kim, I.J.; Kim, O.; Kim, C.S.; Choi, J.S.; Choi, H.I.; Bae, E.H.; Ma, S.K.; Lee, J.U.; et al. Alpha-lipoic acid attenuates lipopolysaccharide-induced kidney injury. Clin. Exp. Nephrol. 2015, 19, 82–91. [Google Scholar] [CrossRef]
- Amini, N.; Maleki, M.; Badavi, M. Nephroprotective activity of naringin against chemical-induced toxicity and renal ischemia/reperfusion injury: A review. Avicenna J. Phytomed. 2022, 12, 357–370. [Google Scholar]
- Ding, Y.; Zhang, Y.; Zhang, W.; Shang, J.; Xie, Z.; Chen, C. Effects of lipoic acid on ischemia-reperfusion injury. Oxid. Med. Cell. Longev. 2021, 2021, 5093216. [Google Scholar] [CrossRef] [PubMed]
- Malek, M.; Nematbakhsh, M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J. Renal Inj. Prev. 2015, 4, 20–27. [Google Scholar] [PubMed]
- Yan, Y.; Bai, J.; Zhou, X.; Tang, J.; Jiang, C.; Tolbert, E.; Bayliss, G.; Gong, R.; Zhao, T.C.; Zhuang, S. P2X7 receptor inhibition protects against ischemic acute kidney injury in mice. Am. J. Physiol. Cell Physiol. 2015, 308, C463–C472. [Google Scholar] [CrossRef]
- Bae, E.H.; Lee, K.S.; Lee, J.; Ma, S.K.; Kim, N.H.; Choi, K.C.; Frokiaer, J.; Nielsen, S.; Kim, S.Y.; Kim, S.Z.; et al. Effects of alpha-lipoic acid on ischemia-reperfusion-induced renal dysfunction in rats. Am. J. Physiol. Renal Physiol. 2008, 294, F272–F280. [Google Scholar] [CrossRef] [PubMed]
- Sehirli, O.; Sener, E.; Cetinel, S.; Yuksel, M.; Gedik, N.; Sener, G. Alpha-lipoic acid protects against renal ischaemia-reperfusion injury in rats. Clin. Exp. Pharmacol. Physiol. 2008, 35, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.A.; Mubarak, H.A.; Sayed, M.M. Ameliorative role of alpha-lipoic acid in renal cortical structural damage, induced by limb ischemia-reperfusion injury in the rat. Ultrastruct. Pathol. 2022, 46, 110–121. [Google Scholar] [CrossRef]
- Farag, M.M.; Ahmed, S.M.; Elhadidy, W.F.; Rashad, R.M. Superior protective effects of febuxostat plus alpha-lipoic acid on renal ischemia/reperfusion-induced hepatorenal injury in rats. Saudi J. Kidney Dis. Transpl. 2019, 30, 1364–1374. [Google Scholar] [CrossRef]
- Takaoka, M.; Ohkita, M.; Kobayashi, Y.; Yuba, M.; Matsumura, Y. Protective effect of alpha-lipoic acid against ischaemic acute renal failure in rats. Clin. Exp. Pharmacol. Physiol. 2002, 29, 189–194. [Google Scholar] [CrossRef]
- Mao, Y.; Yu, J.; Da, J.; Yu, F.; Zha, Y. Acteoside alleviates uuo-induced inflammation and fibrosis by regulating the hmgn1/tlr4/trem1 signaling pathway. PeerJ 2023, 11, e14765. [Google Scholar] [CrossRef]
- Sun, D.; Guo, J.; Liang, W.; Chen, Y.; Chen, X.; Wang, L. Anlotinib alleviates renal fibrosis via inhibition of the erk and akt signaling pathways. Oxid. Med. Cell. Longev. 2023, 2023, 1686804. [Google Scholar] [CrossRef]
- Wu, R.; Li, J.; Tu, G.; Su, Y.; Zhang, X.; Luo, Z.; Rong, R.; Zhang, Y. Comprehensive molecular and cellular characterization of acute kidney injury progression to renal fibrosis. Front. Immunol. 2021, 12, 699192. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.X.; Lin, W.; Yang, K.; Wei, L.J.; Chen, J.L.; Liu, X.Y.; Zhong, K.; Chen, X.; Pei, M.; Yang, H.T. Transcriptome-based network analysis reveals hirudin potentiates anti-renal fibrosis efficacy in uuo rats. Front. Pharmacol. 2021, 12, 741801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hao, J.; Ma, X.; Zhao, Q.; Gao, X.; Wang, X.; Xu, Q. Huoxue jiedu huayu recipe ameliorates mesangial cell pyroptosis in contralateral kidney of uuo rats. Evid. Based Complement. Altern. Med. 2020, 2020, 2530431. [Google Scholar] [CrossRef] [PubMed]
- Wongmekiat, O.; Leelarungrayub, D.; Thamprasert, K. Alpha-lipoic acid attenuates renal injury in rats with obstructive nephropathy. Biomed Res. Int. 2013, 2013, 138719. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Y.; Zhao, D.; Ding, G.; Huang, S.; Zhang, A.; Jia, Z. Rotenone remarkably attenuates oxidative stress, inflammation, and fibrosis in chronic obstructive uropathy. Mediat. Inflamm. 2014, 2014, 670106. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Z.; Liu, B.; Gao, Y.; Nie, J.; Wen, S.; Lai, X.; Liang, H. Identification of circular rna expression profiles in renal fibrosis induced by obstructive injury. Ren. Fail. 2021, 43, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Guo, Y.; Zhang, Y.; Zhang, X.; Zhu, L.; Yan, T. Salidroside ameliorates renal interstitial fibrosis by inhibiting the TLR4/NF-kappab and MAPK signaling pathways. Int. J. Mol. Sci. 2019, 20, 1103. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, L.; Qi, H.; Wang, J.; Wang, Y.; Jiang, W.; Xu, L.; Liu, N.; Zhuang, S. Nintedanib, a triple tyrosine kinase inhibitor, attenuates renal fibrosis in chronic kidney disease. Clin. Sci. 2017, 131, 2125–2143. [Google Scholar] [CrossRef]
- Miguel, V.; Ramos, R.; Garcia-Bermejo, L.; Rodriguez-Puyol, D.; Lamas, S. The program of renal fibrogenesis is controlled by micrornas regulating oxidative metabolism. Redox Biol. 2021, 40, 101851. [Google Scholar] [CrossRef]
- Dendooven, A.; Ishola, D.A., Jr.; Nguyen, T.Q.; Van der Giezen, D.M.; Kok, R.J.; Goldschmeding, R.; Joles, J.A. Oxidative stress in obstructive nephropathy. Int. J. Exp. Pathol. 2011, 92, 202–210. [Google Scholar] [CrossRef]
- Grande, M.T.; Perez-Barriocanal, F.; Lopez-Novoa, J.M. Role of inflammation in tubulo-interstitial damage associated to obstructive nephropathy. J. Inflamm. 2010, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.S.; Kim, J.H.; Jang, H.N.; Lee, T.W.; Jung, M.H.; Kim, T.H.; Chang, S.H.; Park, D.J. Alpha-lipoic acid ameliorates the epithelial mesenchymal transition induced by unilateral ureteral obstruction in mice. Sci. Rep. 2017, 7, 46065. [Google Scholar] [CrossRef] [PubMed]
- Iskander, A.; Yan, L.J. Cisplatin-induced kidney toxicity: Potential roles of major NAD+-dependent enzymes and plant-derived natural products. Biomolecules 2022, 12, 1078. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lee, B.; Kim, Y.R.; Kim, M.A.; Ryu, N.; Jung, D.J.; Kim, U.K.; Baek, J.I.; Lee, K.Y. Evaluating protective and therapeutic effects of alpha-lipoic acid on cisplatin-induced ototoxicity. Cell Death Dis. 2018, 9, 827. [Google Scholar] [CrossRef]
- Kang, K.P.; Kim, D.H.; Jung, Y.J.; Lee, A.S.; Lee, S.; Lee, S.Y.; Jang, K.Y.; Sung, M.J.; Park, S.K.; Kim, W. Alpha-lipoic acid attenuates cisplatin-induced acute kidney injury in mice by suppressing renal inflammation. Nephrol. Dial. Transplant. 2009, 24, 3012–3020. [Google Scholar] [CrossRef]
- El-Beshbishy, H.A.; Bahashwan, S.A.; Aly, H.A.; Fakher, H.A. Abrogation of cisplatin-induced nephrotoxicity in mice by alpha lipoic acid through ameliorating oxidative stress and enhancing gene expression of antioxidant enzymes. Eur. J. Pharmacol. 2011, 668, 278–284. [Google Scholar] [CrossRef]
- Bae, E.H.; Lee, J.; Ma, S.K.; Kim, I.J.; Frokiaer, J.; Nielsen, S.; Kim, S.Y.; Kim, S.W. Alpha-lipoic acid prevents cisplatin-induced acute kidney injury in rats. Nephrol. Dial. Transplant. 2009, 24, 2692–2700. [Google Scholar] [CrossRef]
- Hussein, A.; Ahmed, A.A.; Shouman, S.A.; Sharawy, S. Ameliorating effect of dl-α-lipoic acid against cisplatin-induced nephrotoxicity and cardiotoxicity in experimental animals. Drug Discov. Ther. 2012, 6, 147–156. [Google Scholar] [CrossRef]
- Pianta, T.J.; Succar, L.; Davidson, T.; Buckley, N.A.; Endre, Z.H. Monitoring treatment of acute kidney injury with damage biomarkers. Toxicol. Lett. 2017, 268, 63–70. [Google Scholar] [CrossRef]
- Yan, L.J.; Allen, D.C. Cadmium-induced kidney injury: Oxidative damage as a unifying mechanism. Biomolecules 2021, 11, 1575. [Google Scholar] [CrossRef]
- Li, X.; Zou, Y.; Fu, Y.Y.; Xing, J.; Wang, K.Y.; Wan, P.Z.; Zhai, X.Y. A-lipoic acid alleviates folic acid-induced renal damage through inhibition of ferroptosis. Front. Physiol. 2021, 12, 680544. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.J. Folic acid-induced animal model of kidney disease. Anim. Model. Exp. Med. 2021, 4, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Almeer, R.S.; AlBasher, G.I.; Alarifi, S.; Alkahtani, S.; Ali, D.; Abdel Moneim, A.E. Royal jelly attenuates cadmium-induced nephrotoxicity in male mice. Sci. Rep. 2019, 9, 5825. [Google Scholar] [CrossRef]
- Chen, C.; Han, X.; Wang, G.; Liu, D.; Bao, L.; Jiao, C.; Luan, J.; Hou, Y.; Xu, Y.; Wang, H.; et al. Nrf2 deficiency aggravates the kidney injury induced by subacute cadmium exposure in mice. Arch. Toxicol. 2021, 95, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Almeer, R.S.; Kassab, R.B.; AlBasher, G.I.; Alarifi, S.; Alkahtani, S.; Ali, D.; Abdel Moneim, A.E. Royal jelly mitigates cadmium-induced neuronal damage in mouse cortex. Mol. Biol. Rep. 2019, 46, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, G.; Long, M.; Zou, H.; Cui, H. Alpha lipoic acid attenuates cadmium-induced nephrotoxicity via the mitochondrial apoptotic pathways in rat. J. Inorg. Biochem. 2018, 184, 19–26. [Google Scholar] [CrossRef]
- Luo, T.; Liu, G.; Long, M.; Yang, J.; Song, R.; Wang, Y.; Yuan, Y.; Bian, J.; Liu, X.; Gu, J.; et al. Treatment of cadmium-induced renal oxidative damage in rats by administration of alpha-lipoic acid. Environ. Sci. Pollut. Res. Int. 2017, 24, 1832–1844. [Google Scholar] [CrossRef]
- Veljkovic, A.R.; Nikolic, R.S.; Kocic, G.M.; Pavlovic, D.D.; Cvetkovic, T.P.; Sokolovic, D.T.; Jevtovic, T.M.; Basic, J.T.; Laketic, D.M.; Marinkovic, M.R.; et al. Protective effects of glutathione and lipoic acid against cadmium-induced oxidative stress in rat’s kidney. Ren. Fail. 2012, 34, 1281–1287. [Google Scholar] [CrossRef]
- Fukuda, A.; Osawa, T.; Oda, H.; Tanaka, T.; Toyokuni, S.; Uchida, K. Oxidative stress response in iron-induced acute nephrotoxicity: Enhanced expression of heat shock protein 90. Biochem. Biophys. Res. Commun. 1996, 219, 76–81. [Google Scholar] [CrossRef]
- Leaf, D.E.; Swinkels, D.W. Catalytic iron and acute kidney injury. Am. J. Physiol. Renal Physiol. 2016, 311, F871–F876. [Google Scholar] [CrossRef]
- Amarapurkar, P.; Roberts, L.; Navarrete, J.; El Rassi, F. Sickle cell disease and kidney. Adv. Chronic Kidney Dis. 2022, 29, 141–148e1. [Google Scholar] [CrossRef] [PubMed]
- Chawla, L.S.; Beers-Mulroy, B.; Tidmarsh, G.F. Therapeutic opportunities for hepcidin in acute care medicine. Crit. Care Clin. 2019, 35, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Martines, A.M.; Masereeuw, R.; Tjalsma, H.; Hoenderop, J.G.; Wetzels, J.F.; Swinkels, D.W. Iron metabolism in the pathogenesis of iron-induced kidney injury. Nat. Rev. Nephrol. 2013, 9, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Gwozdzinski, K.; Pieniazek, A.; Gwozdzinski, L. Reactive oxygen species and their involvement in red blood cell damage in chronic kidney disease. Oxid. Med. Cell. Longev. 2021, 2021, 6639199. [Google Scholar] [CrossRef] [PubMed]
- Walker, V.J.; Agarwal, A. Targeting iron homeostasis in acute kidney injury. Semin. Nephrol. 2016, 36, 62–70. [Google Scholar] [CrossRef]
- Chaudhary, K.; Chilakala, A.; Ananth, S.; Mandala, A.; Veeranan-Karmegam, R.; Powell, F.L.; Ganapathy, V.; Gnana-Prakasam, J.P. Renal iron accelerates the progression of diabetic nephropathy in the hfe gene knockout mouse model of iron overload. Am. J. Physiol. Renal Physiol. 2019, 317, F512–F517. [Google Scholar] [CrossRef]
- Gao, W.; Li, X.; Gao, Z.; Li, H. Iron increases diabetes-induced kidney injury and oxidative stress in rats. Biol. Trace Elem. Res. 2014, 160, 368–375. [Google Scholar] [CrossRef]
- Guan, P.; Sun, Z.M.; Luo, L.F.; Zhao, Y.S.; Yang, S.C.; Yu, F.Y.; Wang, N.; Ji, E.S. Hydrogen gas alleviates chronic intermittent hypoxia-induced renal injury through reducing iron overload. Molecules 2019, 24, 1184. [Google Scholar] [CrossRef]
- Annamalai, C.; Ganesh, R.N.; Viswanathan, P. Ferrotoxicity and its amelioration by endogenous vitamin d in experimental acute kidney injury. Exp. Biol. Med. 2020, 245, 1474–1489. [Google Scholar] [CrossRef]
- Li, Y.H.; Chien, S.P.; Chu, P.Y.; Liu, M.Y. Prophylactic and therapeutic effects of a subcutaneous injection of sesame oil against iron-induced acute renal injury in mice. JPEN J. Parenter. Enteral. Nutr. 2012, 36, 344–348. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, S.; Chopra, K. Reversal of iron-induced nephrotoxicity in rats by molsidomine, a nitric oxide donor. Food Chem. Toxicol. 2008, 46, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Cavdar, Z.; Oktan, M.A.; Ural, C.; Calisir, M.; Kocak, A.; Heybeli, C.; Yildiz, S.; Arici, A.; Ellidokuz, H.; Celik, A.; et al. Renoprotective effects of alpha lipoic acid on iron overload-induced kidney injury in rats by suppressing nadph oxidase 4 and p38 mapk signaling. Biol. Trace Elem. Res. 2020, 193, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Cavdar, Z.; Oktan, M.A.; Ural, C.; Kocak, A.; Calisir, M.; Heybeli, C.; Yildiz, S.; Ozbal, S.; Arslan, S.; Ergur, B.U.; et al. Alpha lipoic acid attenuates iron induced oxidative acute kidney injury in rats. Biotech. Histochem. 2021, 96, 409–417. [Google Scholar] [CrossRef]
- Di Stefano, A.; Sozio, P.; Cocco, A.; Iannitelli, A.; Santucci, E.; Costa, M.; Pecci, L.; Nasuti, C.; Cantalamessa, F.; Pinnen, F. L-dopa- and dopamine-(r)-alpha-lipoic acid conjugates as multifunctional codrugs with antioxidant properties. J. Med. Chem. 2006, 49, 1486–1493. [Google Scholar] [CrossRef]
- Persson, H.L.; Svensson, A.I.; Brunk, U.T. Alpha-lipoic acid and alpha-lipoamide prevent oxidant-induced lysosomal rupture and apoptosis. Redox Rep. 2001, 6, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Camiolo, G.; Tibullo, D.; Giallongo, C.; Romano, A.; Parrinello, N.L.; Musumeci, G.; Di Rosa, M.; Vicario, N.; Brundo, M.V.; Amenta, F.; et al. Alpha-lipoic acid reduces iron-induced toxicity and oxidative stress in a model of iron overload. Int. J. Mol. Sci. 2019, 20, 609. [Google Scholar] [CrossRef]
- Sharifi-Zahabi, E.; Abdollahzad, H.; Mostafa Nachvak, S.; Moloudi, J.; Golpayegani, M.R.; Asiaei, S.; Rezavand, L.; Iraji, Z.; Jamshidi, K. Effects of alpha lipoic acid on iron overload, lipid profile and oxidative stress indices in beta-thalassemia major patients: A cross-over randomised controlled clinical trial. Int. J. Clin. Pract. 2021, 75, e14062. [Google Scholar] [CrossRef] [PubMed]
- Chang-Panesso, M. Acute kidney injury and aging. Pediatr. Nephrol. 2021, 36, 2997–3006. [Google Scholar] [CrossRef]
- Zhou, X.J.; Rakheja, D.; Yu, X.; Saxena, R.; Vaziri, N.D.; Silva, F.G. The aging kidney. Kidney Int. 2008, 74, 710–720. [Google Scholar] [CrossRef]
- Kotob, M.; Hussein, A.; Abd-Elkareem, M. Histopathological changes of kidney tissue during aging. SVU-Int. J. Vet. Sci. 2021, 4, 54–65. [Google Scholar] [CrossRef]
- Khafaga, A.F.; Elewa, Y.H.A.; Atta, M.S.; Noreldin, A.E. Aging-related functional and structural changes in renal tissues: Lesson from a camel model. Microsc. Microanal. 2021, 27, 566–578. [Google Scholar] [CrossRef]
- Arivazhagan, P.; Ramanathan, K.; Panneerselvam, C. Effect of dl-alpha-lipoic acid on glutathione metabolic enzymes in aged rats. Exp. Gerontol. 2001, 37, 81–87. [Google Scholar] [CrossRef]
- Arivazhagan, P.; Ramanathan, K.; Panneerselvam, C. Effect of dl-alpha-lipoic acid on mitochondrial enzymes in aged rats. Chem. Biol. Interact. 2001, 138, 189–198. [Google Scholar] [CrossRef]
- Arivazhagan, P.; Ramanathan, K.; Panneerselvam, C. Effect of dl-alpha-lipoic acid on the status of lipid peroxidation and antioxidants in mitochondria of aged rats. J. Nutr. Biochem. 2001, 12, 2–6. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Yang, L.; Zu, Y.; Lu, Q. Effects of rosmarinic acid on liver and kidney antioxidant enzymes, lipid peroxidation and tissue ultrastructure in aging mice. Food Funct. 2015, 6, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Yerramilli, M.; Obare, E.; Yerramilli, M.; Panickar, K.S.; Bobe, G.; Jewell, D.E. Nutritional interventions that slow the age-associated decline in renal function in a canine geriatric model for elderly humans. J. Nutr. Health Aging 2016, 20, 1010–1023. [Google Scholar] [CrossRef]
- Zhang, C.J.; Li, H.; Xiong, Y.Z.; Chang, Y.; Yang, F.; Ma, X.L.; Wang, X.T.; Shimosawa, T.; Ji, E.S.; Xu, Q.Y. Chronic intermittent hypoxia induces renal fibrosis through mr activation. Exp. Gerontol. 2022, 163, 111780. [Google Scholar] [CrossRef]
- Su, Y.; Li, C.; Liu, W.; Liu, Y.; Li, L.; Chen, Q. Comprehensive analysis of differentially expressed mirnas in mice with kidney injury induced by chronic intermittent hypoxia. Front. Genet. 2022, 13, 918728. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Hong, Z.; Chen, Z.; Chen, Y.; Liu, D. Circrna expression profiles and functional analysis in a mouse model of chronic intermittent hypoxia-induced renal injury: New insight into pathogenesis. PeerJ 2023, 11, e14957. [Google Scholar] [CrossRef] [PubMed]
- Badran, M.; Abuyassin, B.; Golbidi, S.; Ayas, N.; Laher, I. Alpha lipoic acid improves endothelial function and oxidative stress in mice exposed to chronic intermittent hypoxia. Oxid. Med. Cell. Longev. 2019, 2019, 4093018. [Google Scholar] [CrossRef]
- Abuyassin, B.; Badran, M.; Ayas, N.T.; Laher, I. The antioxidant alpha-lipoic acid attenuates intermittent hypoxia-related renal injury in a mouse model of sleep apnea. Sleep 2019, 42, zsz066. [Google Scholar] [CrossRef] [PubMed]
- Mennuni, S.; Rubattu, S.; Pierelli, G.; Tocci, G.; Fofi, C.; Volpe, M. Hypertension and kidneys: Unraveling complex molecular mechanisms underlying hypertensive renal damage. J. Hum. Hypertens. 2014, 28, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Zhou, Y.; Lu, S.; Lu, F.; Hu, Y. Pathogenesis of higher blood pressure and worse renal function in salt-sensitive hypertension. Kidney Blood Press. Res. 2021, 46, 236–244. [Google Scholar] [CrossRef]
- Ameer, O.Z. Hypertension in chronic kidney disease: What lies behind the scene. Front. Pharmacol. 2022, 13, 949260. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, I.; Tomassoni, D.; Roy, P.; Di Cesare Mannelli, L.; Amenta, F.; Tayebati, S.K. Antioxidant properties of alpha-lipoic (thioctic) acid treatment on renal and heart parenchyma in a rat model of hypertension. Antioxidants 2021, 10, 1006. [Google Scholar] [CrossRef] [PubMed]
- Diwan, V.; Brown, L.; Gobe, G.C. Adenine-induced chronic kidney disease in rats. Nephrology 2018, 23, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zheng, C.; Zhu, L.; Song, Z.; Dai, L.; Hu, Q.; Wang, L.; Chen, Y.; Xiong, J. Contribution of tfeb-mediated autophagy to tubulointerstitial fibrosis in mice with adenine-induced chronic kidney disease. Biomed. Pharm. 2021, 133, 110949. [Google Scholar] [CrossRef]
- Ho, H.J.; Shirakawa, H. Oxidative stress and mitochondrial dysfunction in chronic kidney disease. Cells 2022, 12, 88. [Google Scholar] [CrossRef]
- Ali, B.H.; Inuwa, I.; Al Za’abi, M.; Al Bahlani, S.; Al Issaei, H.; Ramkumar, A.; Madanagopal, T.; Nemmar, A.; Malheiros, D.M.; Zatz, R. Renal and myocardial histopathology and morphometry in rats with adenine—induced chronic renal failure: Influence of gum acacia. Cell. Physiol. Biochem. 2014, 34, 818–828. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, B.; Huang, S.; Wang, F.; Zheng, L.; Lu, J.; Zeng, Y.; Chen, J.; Li, S. Metabolomics analysis reveals the protection mechanism of huangqi-danshen decoction on adenine-induced chronic kidney disease in rats. Front. Pharmacol. 2019, 10, 992. [Google Scholar] [CrossRef]
- Kumaran, M.; Lingaraju, M.C.; Srivastava, V.; Mathesh, K.; Manickam, K.; Parida, S.; Singh, T.U.; Kumar, D. Pre-084 ameliorated kidney injury by reducing endoplasmic reticulum stress in the rat model of adenine-induced chronic kidney disease. Mol. Biol. Rep. 2023, 50, 3681–3691. [Google Scholar] [CrossRef] [PubMed]
- Natesan, V.; Kim, S.J. Diabetic nephropathy—A review of risk factors, progression, mechanism, and dietary management. Biomol. Ther. 2021, 29, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R. Pathogenesis of diabetic nephropathy. In Chronic Kidney Disease and Type 2 Diabetes; American Diabetes Association: Arlington, VA, USA, 2021; pp. 2–7. [Google Scholar]
- Alkhatib, L.; Velez Diaz, L.A.; Varma, S.; Chowdhary, A.; Bapat, P.; Pan, H.; Kukreja, G.; Palabindela, P.; Selvam, S.A.; Kalra, K. Lifestyle modifications and nutritional and therapeutic interventions in delaying the progression of chronic kidney disease: A review. Cureus 2023, 15, e34572. [Google Scholar] [CrossRef]
- Floege, J.; Daha, M.R. Iga nephropathy: New insights into the role of complement. Kidney Int. 2018, 94, 16–18. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, L.; Li, K.; Yang, J. The role of NLRP3 inflammasome in iga nephropathy. Medicina 2022, 59, 82. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Hua, K.F.; Yang, S.R.; Tsai, Y.S.; Yang, S.M.; Hsieh, C.Y.; Wu, C.C.; Chang, J.F.; Arbiser, J.L.; Chang, C.T.; et al. Tris dba ameliorates iga nephropathy by blunting the activating signal of NLRP3 inflammasome through sirt1- and sirt3-mediated autophagy induction. J. Cell. Mol. Med. 2020, 24, 13609–13622. [Google Scholar] [CrossRef]
- Selvaskandan, H.; Gonzalez-Martin, G.; Barratt, J.; Cheung, C.K. Iga nephropathy: An overview of drug treatments in clinical trials. Expert Opin. Investig. Drugs 2022, 31, 1321–1338. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Zhou, Y.; Xu, X.; Huang, K.; Chen, P.; Wu, Y.; Jin, B.; Hu, Q.; Chen, G.; Zhao, S. Current knowledge of targeted-release budesonide in immunoglobulin a nephropathy: A comprehensive review. Front. Immunol. 2022, 13, 926517. [Google Scholar] [CrossRef]
- Taherkhani, A.; Farrokhi Yekta, R.; Mohseni, M.; Saidijam, M.; Arefi Oskouie, A. Chronic kidney disease: A review of proteomic and metabolomic approaches to membranous glomerulonephritis, focal segmental glomerulosclerosis, and iga nephropathy biomarkers. Proteome Sci. 2019, 17, 7. [Google Scholar] [CrossRef]
- Cao, W.; Xu, J.; Zhou, Z.M.; Wang, G.B.; Hou, F.F.; Nie, J. Advanced oxidation protein products activate intrarenal renin-angiotensin system via a CD36-mediated, redox-dependent pathway. Antioxid. Redox Signal. 2013, 18, 19–35. [Google Scholar] [CrossRef]
- Tian, J.; Chen, J.H.; Li, Q.; He, Q.; Lin, W.Q. Lipid peroxidation in iga nephropathy and the effect of lipo-prostaglandin e1. J. Nephrol. 2005, 18, 243–248. [Google Scholar] [PubMed]
- Parinyasiri, U.; Ong-Ajyooth, L.; Parichatikanond, P.; Ong-Ajyooth, S.; Liammongkolkul, S.; Kanyog, S. Effect of fish oil on oxidative stress, lipid profile and renal function in iga nephropathy. J. Med. Assoc. Thai. 2004, 87, 143–149. [Google Scholar] [PubMed]
- Chaudhary, A.; Mishra, A.; Sethi, S. Oxidized omega-3 fatty acids inhibit pro-inflammatory responses in glomerular endothelial cells. Nephron Exp. Nephrol. 2004, 97, e136–e145. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, J.; Kobayashi, K.; Maeshima, Y.; Yamasaki, Y.; Yasuda, T.; Matsuura, E.; Makino, H. Clinical significance of serum oxidized low-density lipoprotein/beta2-glycoprotein i complexes in patients with chronic renal diseases. Nephron Clin. Pract. 2004, 98, c15–c24. [Google Scholar] [CrossRef] [PubMed]
- Wehbi, B.; Pascal, V.; Zawil, L.; Cogne, M.; Aldigier, J.C. History of iga nephropathy mouse models. J. Clin. Med. 2021, 10, 3142. [Google Scholar] [CrossRef]
- Monteiro, R.C.; Suzuki, Y. Are there animal models of iga nephropathy? Semin. Immunopathol. 2021, 43, 639–648. [Google Scholar] [CrossRef]
- Mao, Z.M.; Shen, S.M.; Wan, Y.G.; Sun, W.; Chen, H.L.; Huang, M.M.; Yang, J.J.; Wu, W.; Tang, H.T.; Tang, R.M. Huangkui capsule attenuates renal fibrosis in diabetic nephropathy rats through regulating oxidative stress and p38mapk/akt pathways, compared to alpha-lipoic acid. J. Ethnopharmacol. 2015, 173, 256–265. [Google Scholar] [CrossRef]
- Ensergueix, G.; Pallet, N.; Joly, D.; Levi, C.; Chauvet, S.; Trivin, C.; Augusto, J.F.; Boudet, R.; Aboudagga, H.; Touchard, G.; et al. Ifosfamide nephrotoxicity in adult patients. Clin. Kidney J. 2020, 13, 660–665. [Google Scholar] [CrossRef]
- Boskabadi, J.; Yousefi-Mazhin, E.; Salehifar, E. Ifosfamide-induced acute kidney injury in a patient with leiomyosarcoma: A case report. Cancer Rep. 2022, 5, e1666. [Google Scholar] [CrossRef]
- Arjmand, A.; Mashhadi, M.; Kaveh, A.; Kamranfar, F.; Seydi, E.; Pourahmad, J. Mitochondrial transplantation therapy against ifosfamide induced toxicity on rat renal proximal tubular cells. Drug Res. 2023, 73, 113–120. [Google Scholar] [CrossRef]
- El-Sisi Ael, D.; El-Syaad, M.E.; El-Desoky, K.I.; Moussa, E.A. Protective effects of alpha lipoic acid versus n-acetylcysteine on ifosfamide-induced nephrotoxicity. Toxicol. Ind. Health 2015, 31, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, G.M.; Al-Ayed, M.S.; Abdelhalim, M.A.; Al-Harbi, L.N.; Yahya, M.A. Effects of antioxidant combinations on the renal toxicity induced rats by gold nanoparticles. Molecules 2023, 28, 1879. [Google Scholar] [CrossRef] [PubMed]
- Castellani, C.; Radu, C.M.; Morillas-Becerril, L.; Barison, I.; Menato, F.; Do Nascimento, T.M.; Fedrigo, M.; Giarraputo, A.; Virzi, G.M.; Simioni, P.; et al. Poly(lipoic acid)-based nanoparticles as a new therapeutic tool for delivering active molecules. Nanomedicine 2022, 45, 102593. [Google Scholar] [CrossRef] [PubMed]
- Tillman, L.; Tabish, T.A.; Kamaly, N.; Moss, P.; El-Briri, A.; Thiemermann, C.; Pranjol, M.Z.I.; Yaqoob, M.M. Advancements in nanomedicines for the detection and treatment of diabetic kidney disease. Biomater. Biosyst. 2022, 6, 100047. [Google Scholar] [CrossRef]
- Peres, R.A.S.; Silva-Aguiar, R.P.; Teixeira, D.E.; Peruchetti, D.B.; Alves, S.A.S.; Leal, A.B.C.; Castro, G.F.; Ribeiro, N.B.S.; Guimaraes, F.V.; Pinheiro, A.A.S.; et al. Gold nanoparticles reduce tubule-interstitial injury and proteinuria in a murine model of subclinical acute kidney injury. Biochim. Biophys. Acta Gen. Subj. 2023, 1867, 130314. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, H.; Zhang, L.; Yao, P.; Wang, S.; Yang, Q. Nanosystems for oxidative stress regulation in the anti-inflammatory therapy of acute kidney injury. Front. Bioeng. Biotechnol. 2023, 11, 1120148. [Google Scholar] [CrossRef]
- Awadalla, A.; Hamam, E.T.; El-Senduny, F.F.; Omar, N.M.; Mahdi, M.R.; Barakat, N.; Ammar, O.A.; Hussein, A.M.; Shokeir, A.A.; Khirallah, S.M. Zinc oxide nanoparticles and spironolactone-enhanced Nrf2/HO-1 pathway and inhibited wnt/beta-catenin pathway in adenine-induced nephrotoxicity in rats. Redox Rep. 2022, 27, 249–258. [Google Scholar] [CrossRef]
- Ortega, M.T.; Riviere, J.E.; Choi, K.; Monteiro-Riviere, N.A. Biocorona formation on gold nanoparticles modulates human proximal tubule kidney cell uptake, cytotoxicity and gene expression. Toxicol. Vitro 2017, 42, 150–160. [Google Scholar] [CrossRef]
- Abdelmoneim, D.; Porter, G.; Duncan, W.; Lim, K.; Easingwood, R.; Woodfield, T.; Coates, D. Three-dimensional evaluation of the cytotoxicity and antibacterial properties of alpha lipoic acid-capped silver nanoparticle constructs for oral applications. Nanomaterials 2023, 13, 705. [Google Scholar] [CrossRef]

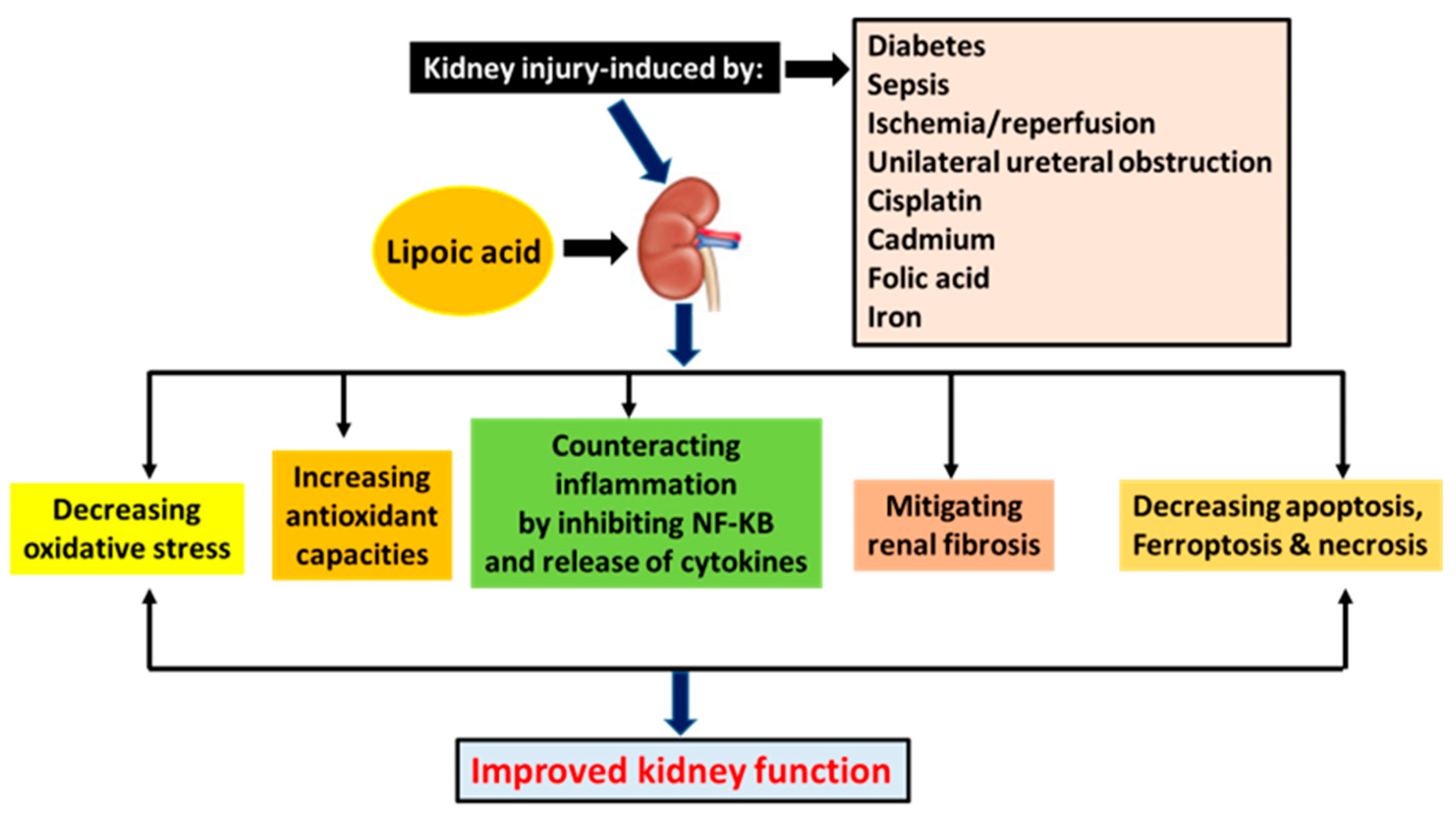
| Model of Kidney Injury | Mechanism | Reference |
|---|---|---|
| Diabetic kidney injury | Activating Nrf2, decreasing oxidative stress, enhancing mitochondrial function, and inhibiting fibrosis | [7,29,50,51] |
| Sepsis-induced kidney injury | Enhancing autophagy, inhibiting NF-KB, attenuating mitochondrial oxidative stress, inhibiting inflammatory cytokine release | [22,71,72,73] |
| Ischemia/reperfusion injury | Counteracting oxidative stress, downregulating Na-K-ATPase and NOS, mitigating neutrophil infiltration, inhibiting inflammation, and suppressing endothelin-1 upregulation | [75,78,79,80,81,82] |
| UUO-induced kidney injury | Attenuating oxidative stress, decreasing nitric oxide production, decreasing transforming factor-1 expression, and ameliorating mesenchymal transition | [88,96] |
| Cisplatin-induced kidney injury | Increasing glomerular filtration, lowering plasma creatinine levels, increasing creatinine clearance, and attenuating oxidative damage | [98,99,101,103] |
| FA-induced kidney injury | Blocking p53 from causing ferroptosis | [105] |
| Cadmium-induced kidney injury | Chelating cadmium, decreasing oxidative stress, elevating glutathione content, and decreasing apoptosis | [108,110,111,112] |
| Iron-induced kidney injury | Attenuating oxidative damage, inhibiting p38 MAPK, inhibiting NADPH oxidase4, and chelating iron | [126,127,128,129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamt, S.F.; Liu, J.; Yan, L.-J. Renal-Protective Roles of Lipoic Acid in Kidney Disease. Nutrients 2023, 15, 1732. https://doi.org/10.3390/nu15071732
Kamt SF, Liu J, Yan L-J. Renal-Protective Roles of Lipoic Acid in Kidney Disease. Nutrients. 2023; 15(7):1732. https://doi.org/10.3390/nu15071732
Chicago/Turabian StyleKamt, Sulin F., Jiankang Liu, and Liang-Jun Yan. 2023. "Renal-Protective Roles of Lipoic Acid in Kidney Disease" Nutrients 15, no. 7: 1732. https://doi.org/10.3390/nu15071732
APA StyleKamt, S. F., Liu, J., & Yan, L.-J. (2023). Renal-Protective Roles of Lipoic Acid in Kidney Disease. Nutrients, 15(7), 1732. https://doi.org/10.3390/nu15071732








