A Risk Profile for Disordered Eating Behaviors in Adolescents with Type 1 Diabetes: A Latent Class Analysis Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Procedures
2.2. DEPS-R Questionnaire
2.3. Dietary Intake Assessment
2.4. Family SocioEconomic Status
2.5. Nutritional Habits and Glycemic Control Variables
2.6. Other Variables
2.7. Statistical Analysis
3. Results
3.1. Descriptive Analyses
3.2. Latent Class Analysis
3.3. Differences in Youth’s Age, Parental SES, and Diabetes-Related Variables between Classes
3.4. Differences in Youth’s Gender, Carbohydrate Counting, and Modality of Insulin Therapy between Classes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colton, P.A.; Olmsted, M.P.; Daneman, D.; Rydall, A.C.; Rodin, G.M. Five-Year Prevalence and Persistence of Disturbed Eating Behavior and Eating Disorders in Girls With Type 1 Diabetes. Diabetes Care 2007, 30, 2861–2862. [Google Scholar] [CrossRef]
- Markowitz, J.T.; Butler, D.A.; Volkening, L.K.; Antisdel, J.E.; Anderson, B.J.; Laffel, L.M. Brief Screening Tool for Disordered Eating in Diabetes. Diabetes Care 2010, 33, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Affenito, S.G.; Backstrand, J.R.; Welch, G.W.; Lammi-Keefe, C.J.; Rodriguez, N.R.; Adams, C.H. Subclinical and Clinical Eating Disorders in IDDM Negatively Affect Metabolic Control. Diabetes Care 1997, 20, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Troncone, A.; Affuso, G.; Cascella, C.; Chianese, A.; Pizzini, B.; Zanfardino, A.; Iafusco, D.; Diabetes Study Group of Italian Society of Paediatric Endocrinology and Diabetology. Prevalence of disordered eating behaviors in adolescents with type 1 diabetes: Results of multicenter Italian nationwide study. Int. J. Eat. Disord. 2022, 55, 1108–1119. [Google Scholar] [CrossRef]
- Elhabashy, S.A.; Abd ElMalak, M.W.; Elrassas, H.H.; Thabet, R.A. Disordered eating and behaviors among young Egyptians with type 1 diabetes: Risk factors and comorbidities. J. Pediatr. Endocrinol. Metab. 2022, 35, 1385–1393. [Google Scholar] [CrossRef]
- Altınok, Y.A.; Özgür, S.; Meseri, R.; Özen, S.; Darcan, Ş.; Gökşen, D. Reliability and Validity of the Diabetes Eating Problem Survey in Turkish Children and Adolescents with Type 1 Diabetes Mellitus. J. Clin. Res. Pediatr. Endocrinol. 2017, 9, 323–328. [Google Scholar] [CrossRef]
- Wisting, L.; Wonderlich, J.; Skrivarhaug, T.; Dahl-Jørgensen, K.; Rø, Ø. Psychometric properties and factor structure of the diabetes eating problem survey-revised (DEPS-R) among adult males and females with type 1 diabetes. J. Eat Disord. 2019, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Zhong, Q.; Guo, J.; Luo, J.; Dixon, J.; Whittemore, R. Instrument Context Relevance Evaluation, Translation, and Psychometric Testing of the Diabetes Eating Problem Survey-Revised (DEPS-R) among People with Type 1 Diabetes in China. Int. J. Environ. Res. Public Health 2021, 18, 3450. [Google Scholar] [CrossRef] [PubMed]
- Karastogiannidou, C.; Giannoulaki, P.; Samaras, I.; Kotzakioulafi, E.; Didangelos, T.; Bocsan, I.C.; Vassilopoulou, E. The Diabetes Eating Problem Survey-Revised (DEPS-R) in a Greek Adult Population with Type 1 Diabetes Mellitus: Model Comparison Supporting a Single Factor Structure. Nutrients 2021, 13, 2375. [Google Scholar] [CrossRef]
- Tate, A.E.; Liu, S.; Zhang, R.; Yilmaz, Z.; Larsen, J.T.; Petersen, L.V.; Bulik, C.M.; Svensson, A.M.; Gudbjörnsdottir, S.; Larsson, H.; et al. Association and Familial Coaggregation of Type 1 Diabetes and Eating Disorders: A Register-Based Cohort Study in Denmark and Sweden. Diabetes Care 2021, 44, 1143–1150. [Google Scholar] [CrossRef]
- Bächle, C.; Stahl-Pehe, A.; Rosenbauer, J. Disordered eating and insulin restriction in youths receiving intensified insulin treatment: Results from a nationwide population-based study. Int. J. Eat. Disord. 2015, 49, 191–196. [Google Scholar] [CrossRef]
- Nip, A.S.; Reboussin, B.A.; Dabelea, D.; Bellatorre, A.; Mayer-Davis, E.J.; Kahkoska, A.R.; Lawrence, J.M.; Peterson, C.M.; Dolan, L.; Pihoker, C. Disordered Eating Behaviors in Youth and Young Adults With Type 1 or Type 2 Diabetes Receiving Insulin Therapy: The SEARCH for Diabetes in Youth Study. Diabetes Care 2019, 42, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Rodin, G.M.; Olmsted, M.P.; Devenyi, R.G.; Rydall, A.C.; Daneman, D. Disordered Eating Behavior and Microvascular Complications in Young Women with Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1997, 336, 1849–1854. [Google Scholar] [CrossRef]
- Cherubini, V.; Skrami, E.; Iannilli, A.; Cesaretti, A.; Paparusso, A.M.; Alessandrelli, M.C.; Carle, F.; Ferrito, L.; Gesuita, R. Disordered eating behaviors in adolescents with type 1 diabetes: A cross-sectional population-based study in Italy. Int. J. Eat. Disord. 2018, 51, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.M.; Fischer, S.; Young-Hyman, D. Topical Review: A Comprehensive Risk Model for Disordered Eating in Youth With Type 1 Diabetes. J. Pediatr. Psychol. 2014, 40, 385–390. [Google Scholar] [CrossRef]
- Nguyen-Michel, S.T.; Unger, J.B.; Spruijt-Metz, D. Dietary correlates of emotional eating in adolescence. Appetite 2007, 49, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.L.; Byrne, S.M.; La Puma, M.; McLean, N.; Davis, E.A. The onset and course of binge eating in 8- to 13-year-old healthy weight, overweight and obese children. Eat. Behav. 2008, 9, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Rovner, A.J.; Nansel, T.R. Are Children With Type 1 Diabetes Consuming a Healthful Diet? Diabetes Educ. 2009, 35, 97–107. [Google Scholar] [CrossRef]
- Seckold, R.; Howley, P.; King, B.R.; Bell, K.; Smith, A.; Smart, C.E. Dietary intake and eating patterns of young children with type 1 diabetes achieving glycemic targets. BMJ Open Diabetes Res. Care 2019, 7, e000663. [Google Scholar] [CrossRef]
- Cherubini, V.; Marino, M.; Marigliano, M.; Maffeis, C.; Zanfardino, A.; Rabbone, I.; Giorda, S.; Schiaffini, R.; Lorubbio, A.; Rollato, S.; et al. Rethinking carbohydrate intake and time in range in children and adolescents with type 1 diabetes. Nutrients 2021, 13, 3869. [Google Scholar] [CrossRef]
- Mayer-Davis, E.J.; Nichols, M.; Liese, A.D.; Bell, R.A.; Dabelea, D.M.; Johansen, J.M.; Pihoker, C.; Rodriguez, B.L.; Thomas, J.; Williams, D. Dietary Intake among Youth with Diabetes: The SEARCH for Diabetes in Youth Study. J. Am. Diet. Assoc. 2006, 106, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Maffeis, C.; Morandi, A.; Ventura, E.; Sabbion, A.; Contreas, G.; Tomasselli, F.; Tommasi, M.; Fasan, I.; Costantini, S.; Pinelli, L. Diet, physical, and biochemical characteristics of children and adolescents with type 1 diabetes: Relationship between dietary fat and glucose control. Pediatr. Diabetes 2011, 13, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Kroke, A.; Manz, F.; Kersting, M.; Remer, T.; Sichert-Hellert, W.; Alexy, U.; Lentze, M.J. The DONALD Study. Eur. J. Nutr. 2004, 43, 45–54. [Google Scholar] [CrossRef] [PubMed]
- ISTAT Classificazione Delle Professioni. 2013. Available online: https://www.istat.it/it/archivio/18132 (accessed on 28 March 2023).
- Davison, G.M.; Fowler, L.A.; Ramel, M.; Stein, R.I.; Conlon, R.P.; Saelens, B.E.; Welch, R.R.; Perri, M.G.; Epstein, L.H.; Wilfley, D.E. Racial and socioeconomic disparities in the efficacy of a family-based treatment programme for paediatric obesity. Pediatr. Obes. 2021, 16, e12792. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Eckert, A.J.; Tell, S.; Krnic, N.; Deja, G.; Rasmussen, V.F.; Coelho, R.; Todorovic, S.; Jefferies, C.A.; Sherif, E.; et al. The Association between Treatment Modality, Lipid Profile, Metabolic Control in Children with Type 1 Diabetes and Celiac Disease—Data from the International Sweet Registry. Nutrients 2021, 13, 4473. [Google Scholar] [CrossRef]
- Lazarsfeld, P.; Henry, N. Latent Structure Analysis; Houghton-Mifflin: Boston, MA, USA, 1968. [Google Scholar]
- Lanza, S.T.; Collins, L.M.; Lemmon, D.R.; Schafer, J.L. PROC LCA: A SAS Procedure for Latent Class Analysis. Struct. Equ. Model. Multidiscip. J. 2007, 14, 671–694. [Google Scholar] [CrossRef]
- Muthén, L.; Muthén, B. Mplus User’s Guide; Muthén & Muthén: Los Angeles, CA, USA, 1998; Volume 4. [Google Scholar]
- Vermunt, J.K. 7. Multilevel Latent Class Models. Sociol. Methodol. 2003, 33, 213–239. [Google Scholar] [CrossRef]
- Velasco, E.R.; Pals, R.A.S.; Skinner, T.C.; Grabowski, D. Pre-empting the challenges faced in adolescence: A systematic literature review of effects of psychosocial interventions for preteens with type 1 diabetes. Endocrinol. Diabetes Metab. 2020, 3, e00120. [Google Scholar] [CrossRef]
- Olmsted, M.P.; Colton, P.A.; Daneman, D.; Rydall, A.C.; Rodin, G.M. Prediction of the Onset of Disturbed Eating Behavior in Adolescent Girls With Type 1 Diabetes. Diabetes Care 2008, 31, 1978–1982. [Google Scholar] [CrossRef]
- Kim, J.E. Illness Experiences of Adolescents with Type 1 Diabetes. J. Diabetes Res. 2022, 2022, 3117253. [Google Scholar] [CrossRef]
- Saßmann, H.; Albrecht, C.; Busse-Widmann, P.; Hevelke, L.K.; Kranz, J.; Markowitz, J.T.; Marshall, L.F.; Meurs, S.; de Soye, I.H.; Lange, K. Psychometric properties of the German version of the Diabetes Eating Problem Survey-Revised: Additional benefit of disease-specific screening in adolescents with Type 1 diabetes. Diabet. Med. 2015, 32, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.; Karwautz, A. Eating disorders in adolescents with type 1 diabetes mellitus. Curr. Opin. Psychiatry 2020, 33, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Conviser, J.H.; Fisher, S.D.; McColley, S.A. Are children with chronic illnesses requiring dietary therapy at risk for disordered eating or eating disorders? A systematic review. Int. J. Eat. Disord. 2018, 51, 187–213. [Google Scholar] [CrossRef]
- Hamiel, U. Eating disorders in adolescents with type 1 diabetes: Challenges in diagnosis and treatment. World J. Diabetes 2015, 6, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.E.; LeMay-Russell, S.; Tanofsky-Kraff, M. Loss-of-Control Eating and Obesity Among Children and Adolescents. Curr. Obes. Rep. 2019, 8, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Scheuing, N.; Bartus, B.; Berger, G.; Haberland, H.; Icks, A.; Knauth, B.; Nellen-Hellmuth, N.; Rosenbauer, J.; Teufel, M.; Holl, R.W.; et al. Clinical Characteristics and Outcome of 467 Patients With a Clinically Recognized Eating Disorder Identified Among 52,215 Patients With Type 1 Diabetes: A Multicenter German/Austrian Study. Diabetes Care 2014, 37, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Maffeis, C.; Tomasselli, F.; Tommasi, M.; Bresadola, I.; Trandev, T.; Fornari, E.; Marigliano, M.; Morandi, A.; Olivieri, F.; Piona, C. Nutrition habits of children and adolescents with type 1 diabetes changed in a 10 years span. Pediatr. Diabetes 2020, 21, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Danne, T.; Phillip, M.; Buckingham, B.A.; Jarosz-Chobot, P.; Saboo, B.; Urakami, T.; Battelino, T.; Hanas, R.; Codner, E. ISPAD Clinical Practice Consensus Guidelines 2018: Insulin treatment in children and adolescents with diabetes. Pediatr. Diabetes 2018, 19, 115–135. [Google Scholar] [CrossRef]
- Smart, C.E.; Annan, F.; Higgins, L.A.; Jelleryd, E.; Lopez, M.; Acerini, C.L. ISPAD Clinical Practice Consensus Guidelines 2018: Nutritional management in children and adolescents with diabetes. Pediatr. Diabetes 2018, 19, 136–154. [Google Scholar] [CrossRef]
- Tse, J.; Nansel, T.R.; Haynie, D.L.; Mehta, S.N.; Laffel, L.M. Disordered Eating Behaviors Are Associated with Poorer Diet Quality in Adolescents with Type 1 Diabetes. J. Acad. Nutr. Diet. 2012, 112, 1810–1814. [Google Scholar] [CrossRef]
- Eisenberg Colman, M.H.; Quick, V.M.; Lipsky, L.M.; Dempster, K.W.; Liu, A.; Laffel, L.M.; Mehta, S.N.; Nansel, T.R. Disordered Eating Behaviors Are Not Increased by an Intervention to Improve Diet Quality but Are Associated With Poorer Glycemic Control Among Youth With Type 1 Diabetes. Diabetes Care 2018, 41, 869–875. [Google Scholar] [CrossRef]


| Children’s Variables | % (n) | Mean (SD) | Range |
|---|---|---|---|
| Ethnicity Italian African-American | 98.0 (145) 2.0 (3) | ||
| Age | 12.1 (3.34) | 3.3–17.8 | |
| Gender Female Male | 48.0 (71) 52.0 (77) | ||
| Diabetes Duration (years) | 7.2 (3.4) | 1.0–16.3 | |
| Insulin Therapy Modality MDI CSII | 54.7 (81) 45.3 (67) | ||
| Weekly Hours of Physical Exercise 0 1 2 3 More than 3 | 15.0 (22) 4.1 (6) 23.1 (34) 17.7 (26) 40.1 (60) | ||
| Carbohydrate Counting Yes No | 46.6 (69) 53.4 (79) | ||
| Daily Insulin Dose | 37.8 (19.2) | 4.0–98.0 | |
| CGM data over 15 days Mean % Time < 54 | 0.6 (0.9) | 0–7 | |
| Mean % Time 54–70 | 2.8 (2.9) | 0–16 | |
| Mean % Time 70–180 | 57.9 (17.1) | 15–96 | |
| Mean % Time 180–250 | 38.9 (18.3) | 2–85 | |
| Mean % Time > 250 | 14.4 (12.3) | 0–51 |
| Macronutrients | Mean % (SD) | Range % |
|---|---|---|
| Proteins | 16.78 (3.29) | 7.42–26.83 |
| Saturated Fatty Acids | 9.25 (2.53) | 3.00–19.98 |
| Monounsaturated Fatty Acids | 16.44 (4.66) | 4.52–31.81 |
| Polyunsaturated Fatty Acids | 9.91 (5.06) | 1.82–31.45 |
| Total Carbohydrates | 45.05 (5.62) | 29.64–56.87 |
| Sugars | 10.97 (3.83) | 1.88–23.00 |
| Parent’s Variables | % (n) | Mean (SD) | Range |
|---|---|---|---|
| Ethnicity Italian African-American | 98.0 (145) 2.0 (3) | ||
| Age Mothers Fathers | 44.5 (6.2) 47.8 (6.3) | 27.9–64.1 34.5–66.6 | |
| Parent Mothers Fathers | 50.8 (148) 49.2 (143) | ||
| Barratt Education Score Mothers Fathers | 13.9 (4.9) 12.6 (5.3) | 6–21 6–21 | |
| Barratt Occupation Score Mothers Fathers | 21.3 (13.6) 25.2 (12.0) | 5–45 5–45 | |
| Barratt Family SES Score | 36.3 (13.6) | 11–66 | |
| Family Annual Income <EUR 29,300 >EUR 29,300 | 37.8 (56) 62.2 (92) | ||
| Type 1 Diabetes Familiarity Yes No | 6.8 (10) 93.2 (138) | ||
| Type 2 Diabetes Familiarity Yes No | 11.5 (17) 88.5 (131) |
| LCA Models | Classes n (%) | SSA-BIC | Adjusted LMR-LRT | Entropy | |
|---|---|---|---|---|---|
| Class 1 | Class 2 | ||||
| One-Class solution | 148 (100) | 12,746.036 | - | - | |
| Two-Class solution | 114 (77) | 34 (23) | 12,645.413 | 124.050 | 0.856 |
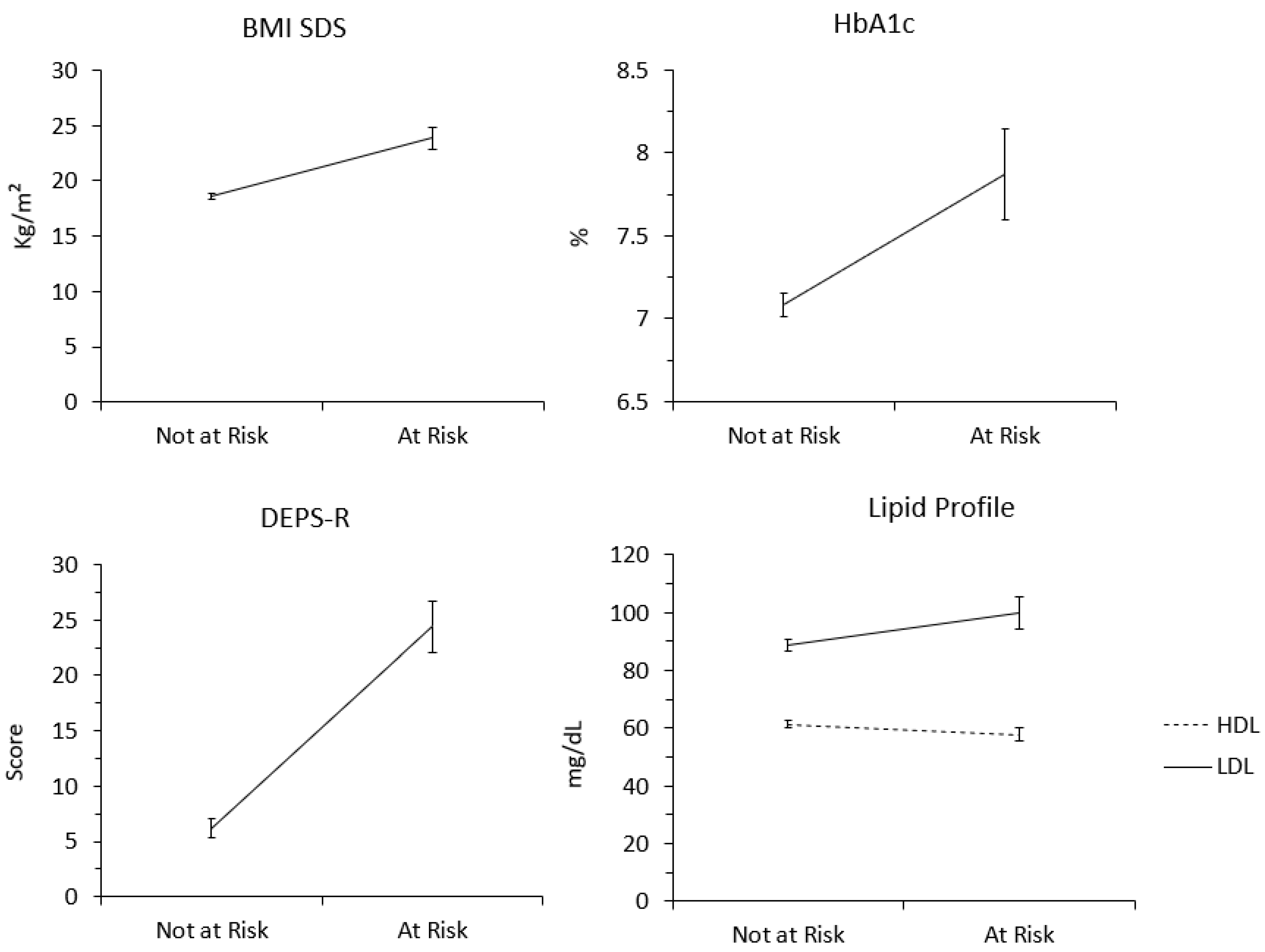
| Variables | Class 1 Mean (SD) | Class 2 Mean (SD) | |||
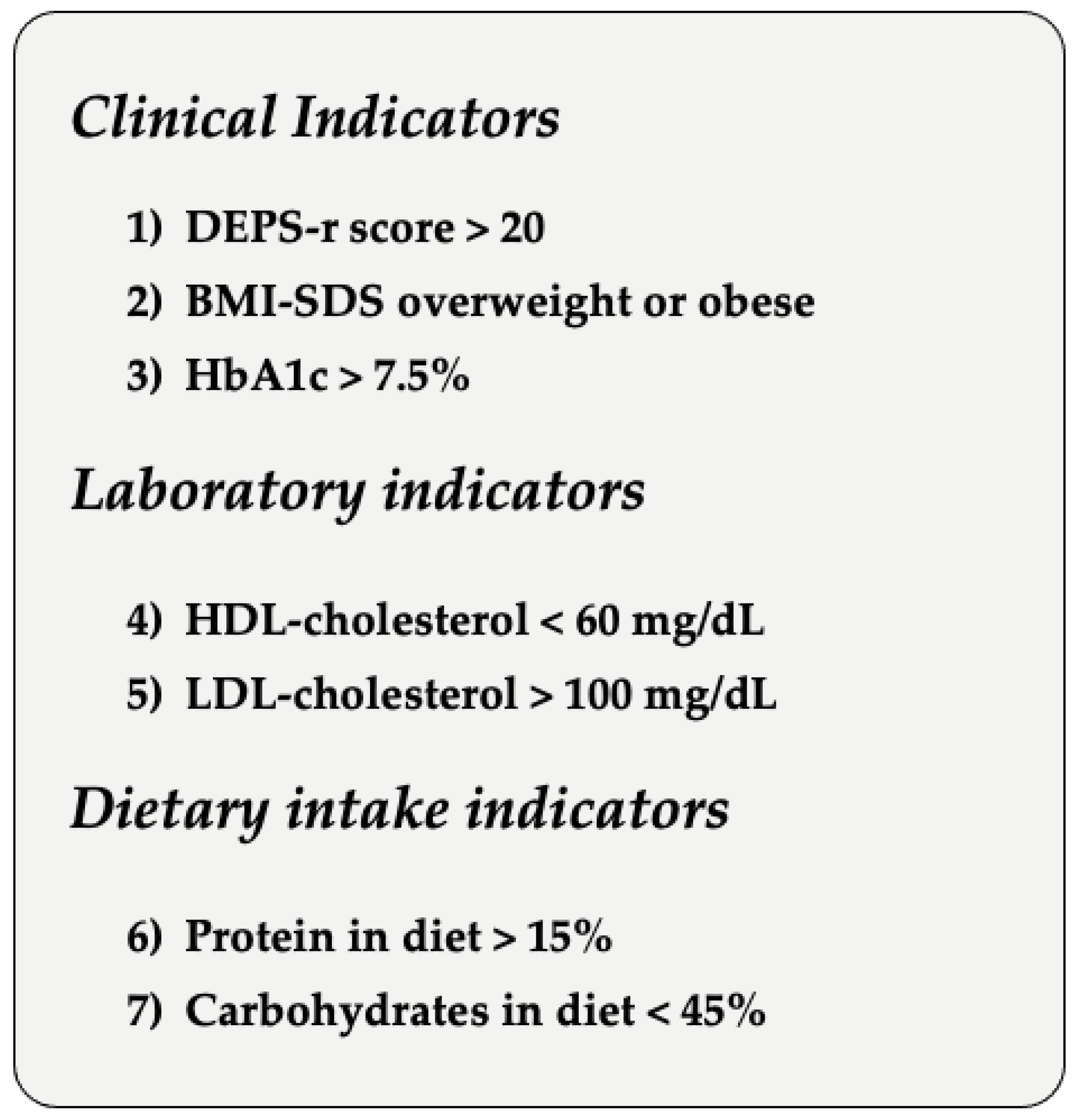
| BMI-SDS | 18.579 (0.242) | 23.858 (0.950) | |||
| HbA1c | 7.083 (0.071) | 7.867 (0.274) | |||
| HDL | 61.280 (1.221) | 57.845 (2.378) | |||
| LDL | 88.808 (1.952) | 99.848 (5.382) | |||
| Protein | 16.146 (0.269) | 18.157 (0.606) | |||
| FSA | 9.114 (0.200) | 8.898 (0.608) | |||
| PUFA | 10.914 (0.442) | 8.462 (0.762) | |||
| MUFA | 15.938 (0.371) | 17.860 (1.103) | |||
| Carbohydrates | 45.293 (0.464) | 43.993 (1.457) | |||
| Sugar | 10.748 (0.334) | 10.605 (0.813) | |||
| DEPS-R Score | 6.176 (0.836) | 24.424 (2.325) | |||
| Mean Values ‘Not At—Risk’ Class and ‘At—Risk’ Class | Sum of Squares | df | Mean Square | F | p | |
|---|---|---|---|---|---|---|
| Age (years) | 11.37–14.65 | 281.735 | 1 | 281.735 | 30.222 | 0.000 |
| Diabetes Duration | 6.74–8.70 | 99.831 | 1 | 99.831 | 9.283 | 0.003 |
| Daily Insulin Doses | 34.82–47.57 | 4255.898 | 1 | 4255.898 | 12.405 | 0.001 |
| Number of Daily Insulin Injections | 4.41–3.89 | 6.686 | 1 | 6.686 | 1.457 | 0.230 |
| Number of Omitted Insulin Doses | 0.13–0.22 | 0.186 | 1 | 0.186 | 0.797 | 0.373 |
| Severe Hypoglycemic Episodes | 0.03–0.12 | 0.218 | 1 | 0.218 | 2.207 | 0.140 |
| DKA Episodes with Hospitalization | 0.02–0.03 | 0.004 | 1 | 0.004 | 0.183 | 0.669 |
| Parental SES | 37.53–32.09 | 775.735 | 1 | 775.735 | 4.266 | 0.041 |
| CGM data over 15 days Mean % Time < 54 | ||||||
| % Time < 54 mg/dL | 0.69–0.31 | 3.661 | 1 | 3.661 | 4.014 | 0.047 |
| % Time 54–70 mg/dL | 3.07–1.93 | 33.958 | 1 | 33.958 | 4.214 | 0.042 |
| % Time 70–180 mg/dL | 58.8–55.16 | 346.657 | 1 | 346.657 | 1.184 | 0.278 |
| % Time 180–250 mg/dL | 37.85–42.46 | 558.348 | 1 | 558.348 | 1.677 | 0.197 |
| % Time > 250 mg/dL | 13.27–18.11 | 613.233 | 1 | 613.233 | 4.106 | 0.045 |
| Variables | Pearson Chi-Square Value | Likelihood Ratio | Linear-by-Linear Association | N of Valid Cases | df | p |
|---|---|---|---|---|---|---|
| Gender | 0.15 | 0.15 | 0.15 | 148 | 1 | 0.903 |
| Therapy Modality | 0.57 | 0.57 | 0.57 | 148 | 1 | 0.811 |
| Carbohydrates Counting | 2.28 | 2.31 | 2.26 | 148 | 1 | 0.131 |
| T1D Familiarity | 1.76 | 1.56 | 1.75 | 148 | 1 | 0.185 |
| T2D Familiarity | 0.003 | 0.003 | 0.003 | 148 | 1 | 0.954 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boccolini, G.; Marino, M.; Tiberi, V.; Iannilli, A.; Landi, G.; Grandi, S.; Tossani, E.; ISPED Study Group; Cherubini, V. A Risk Profile for Disordered Eating Behaviors in Adolescents with Type 1 Diabetes: A Latent Class Analysis Study. Nutrients 2023, 15, 1721. https://doi.org/10.3390/nu15071721
Boccolini G, Marino M, Tiberi V, Iannilli A, Landi G, Grandi S, Tossani E, ISPED Study Group, Cherubini V. A Risk Profile for Disordered Eating Behaviors in Adolescents with Type 1 Diabetes: A Latent Class Analysis Study. Nutrients. 2023; 15(7):1721. https://doi.org/10.3390/nu15071721
Chicago/Turabian StyleBoccolini, Giada, Monica Marino, Valentina Tiberi, Antonio Iannilli, Giulia Landi, Silvana Grandi, Eliana Tossani, ISPED Study Group, and Valentino Cherubini. 2023. "A Risk Profile for Disordered Eating Behaviors in Adolescents with Type 1 Diabetes: A Latent Class Analysis Study" Nutrients 15, no. 7: 1721. https://doi.org/10.3390/nu15071721
APA StyleBoccolini, G., Marino, M., Tiberi, V., Iannilli, A., Landi, G., Grandi, S., Tossani, E., ISPED Study Group, & Cherubini, V. (2023). A Risk Profile for Disordered Eating Behaviors in Adolescents with Type 1 Diabetes: A Latent Class Analysis Study. Nutrients, 15(7), 1721. https://doi.org/10.3390/nu15071721









