Long-Term Impact of Multiple Micronutrient Supplementation on Micronutrient Status, Hemoglobin Level, and Growth in Children 24 to 59 Months of Age: A Non-Randomized Community-Based Trial from Pakistan
Abstract
1. Introduction
2. Methodology
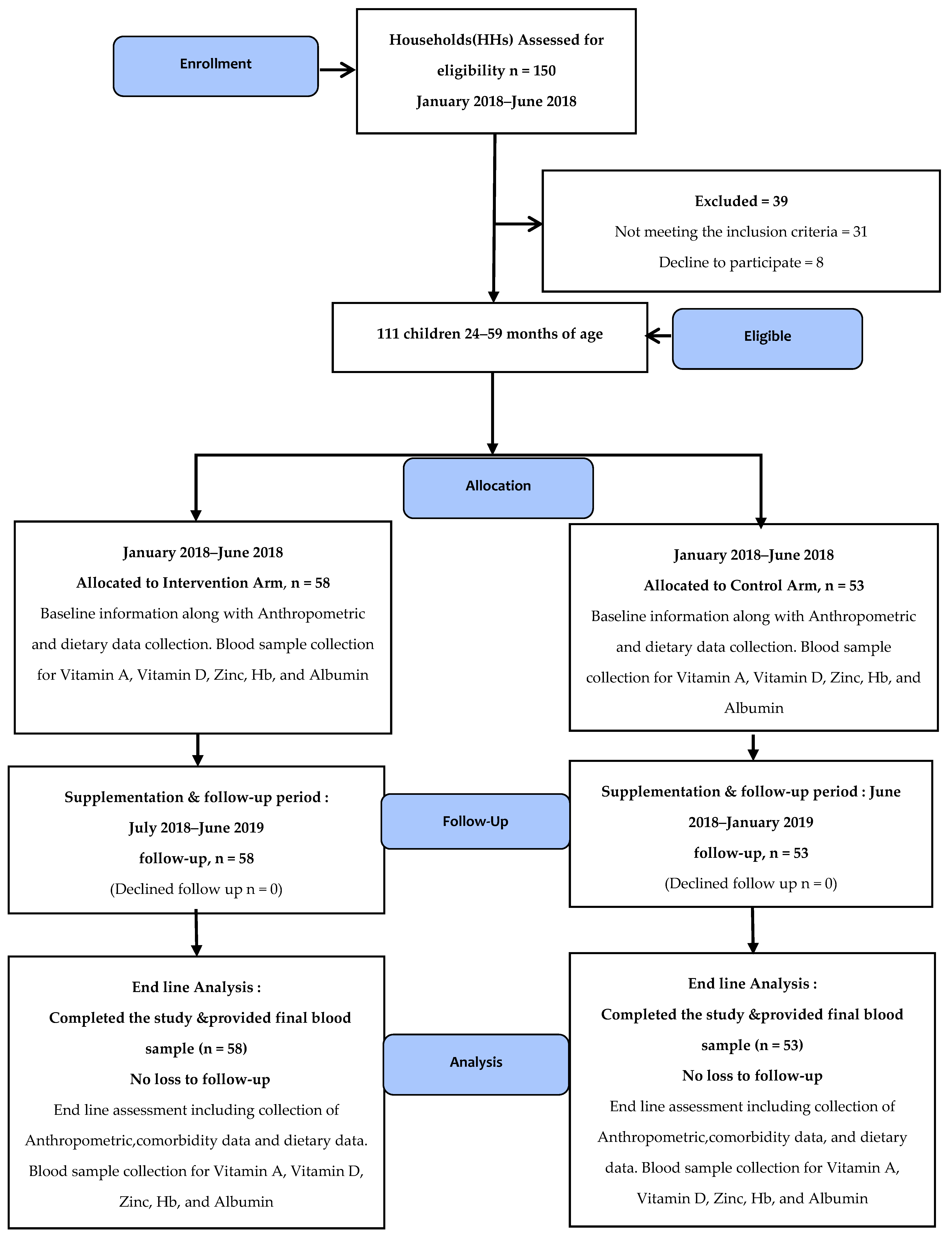
2.1. Study Design and Participants
2.2. Sample Size Estimation
2.3. Inclusion and Exclusion Criteria
2.4. MNP Intervention, Composition, Dosage, Distribution, and Utilization
2.5. Data Collection
2.6. Data Quality Control and Quality Assurance
2.7. Biochemical Analysis
2.8. Statistical Analyses
2.9. Ethical Consideration
3. Results
4. Discussion
5. Conclusions
Strengths and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Webb, P.; Stordalen, G.A.; Singh, S.; Wijesinha-Bettoni, R.; Shetty, P.; Lartey, A. Hunger and malnutrition in the 21st century. BMJ 2018, 361, k2238. [Google Scholar] [CrossRef] [PubMed]
- Boah, M.; Azupogo, F.; Amporfro, D.A.; Abada, L.A. The epidemiology of undernutrition and its determinants in children under five years in Ghana. PLoS ONE 2019, 14, e0219665. [Google Scholar] [CrossRef] [PubMed]
- Clark, H.; Coll-Seck, A.M.; Banerjee, A.; Peterson, S.; Dalglish, S.L.; Ameratunga, S.; Balabanova, D.; Bhan, M.K.; Bhutta, Z.A.; Borrazzo, J.; et al. A future for the world’s children? A WHO–UNICEF–Lancet Commission. Lancet 2020, 395, 605–658. [Google Scholar] [CrossRef] [PubMed]
- Stevens, G.A.; Beal, T.; Mbuya, M.N.N.; Luo, H.; Neufeld, L.M.; Addo, O.Y.; Adu-Afarwuah, S.; Alayón, S.; Bhutta, Z.; Brown, K.H.; et al. Micronutrient deficiencies among preschool-aged children and women of reproductive age worldwide: A pooled analysis of individual-level data from population-representative surveys. Lancet Glob. Health 2022, 10, e1590–e1599. [Google Scholar] [CrossRef] [PubMed]
- Pakistan, UNICEF. National Nutritional Survery 2018: Key Finding Reports; Nurtition wing MoNHSRacGoP: Islamabad, Pakistan, 2018. [Google Scholar]
- Black, R.E.; Allen, L.H.; Bhutta, Z.A.; Caulfield, L.E.; De Onis, M.; Ezzati, M.; Mathers, C.; Rivera, J.; Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet 2008, 371, 243–260. [Google Scholar] [CrossRef]
- Black, R.E. Micronutrients in pregnancy. Br. J. Nutr. 2001, 85, S193–S197. [Google Scholar] [CrossRef]
- Le, H.T.; Brouwer, I.D.; Verhoef, H.; Nguyen, K.C.; Kok, F.J. Anemia and intestinal parasite infection in school children in rural Vietnam. Asia Pac. J. Clin. Nutr. 2007, 16, 716–723. [Google Scholar]
- Lawless, J.W.; Latham, M.C.; Stephenson, L.S.; Kinoti, S.N.; Pertet, A.M. Iron Supplementation Improves Appetite and Growth in Anemic Kenyan Primary School Children. J. Nutr. 1994, 124, 645–654. [Google Scholar] [CrossRef]
- Black, R.E. Zinc Deficiency, Infectious Disease and Mortality in the Developing World. J. Nutr. 2003, 133, 1485S–1489S. [Google Scholar] [CrossRef]
- Thurnham, D.I. Micronutrients and immune function: Some recent developments. J. Clin. Pathol. 1997, 50, 887–891. [Google Scholar] [CrossRef]
- Miniero, R.; Talarico, V.; Galati, M.C.; Giancotti, L.; Saracco, P.; Raiola, G. Iron Deficiency and Iron Deficiency Anemia in Children. In Iron Deficiency Anemia; IntechOpen: Londong, UK, 2019; pp. 23–38. [Google Scholar]
- Xu, Y.; Shan, Y.; Lin, X.; Miao, Q.; Lou, L.; Wang, Y.; Ye, J. Global patterns in vision loss burden due to vitamin A deficiency from 1990 to 2017. Public Health Nutr. 2021, 24, 5786–5794. [Google Scholar] [CrossRef]
- Sizar, O.; Khare, S.; Goyal, A. Vitamin D Deficiency; [Updated 21 July 2021]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Maxfield, L.; Shukla, S.; Crane, J.S. Zinc Deficiency; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Zlotkin, S.H.; Schauer, C.; Christofides, A.; Sharieff, W.; Tondeur, M.C.; Hyder, S.M.Z. Micronutrient Sprinkles to Control Childhood Anaemia. PLoS Med. 2005, 2, e1. [Google Scholar] [CrossRef] [PubMed]
- Suchdev, P.; Ruth, L.; Woodruff, B.; Mbakaya, C.; Mandava, U.; Flores-Ayala, R.; Jefferds, M.; Quick, R. Selling Sprinkles micronutrient powder reduces anemia, iron deficiency, and vitamin A deficiency in young children in Western Kenya: A cluster-randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.; Das, J.K.; Khan, G.N.; Najmi, R.; Shah, M.M.; Soofi, S.B. Food supplements to reduce stunting in Pakistan: A process evaluation of community dynamics shaping uptake. BMC Public Health 2020, 20, 1046. [Google Scholar] [CrossRef] [PubMed]
- Aga Khan University WFPaDoSGoP. Effectiveness of Nutrition Supplementation within the Primary Health Care System to Prevent Stunting among Children under Five Years in Thatta and Sujawal Districts, Sindh Province: A Cluster Randomized Controlled Trial; Aga khan University: Karachi, Pakistan, 2018. [Google Scholar]
- Jack, S.J.; Ou, K.; Chea, M.; Chhin, L.; Devenish, R.; Dunbar, M.; Eang, C.; Hou, K.; Ly, S.; Khin, M.; et al. Effect of micronutrient sprinkles on reducing anemia: A cluster-randomized effectiveness trial. Arch. Pediatr. Adolesc. Med. 2012, 166, 842–850. [Google Scholar] [CrossRef]
- De Pee, S.; Baldi, G. Technical Specifications for Micronutrient Powder-Children 6–59 Months. WFP, WFP; Report No.: MIXMNP000 Contract No.: MIXMNP000. 25 May 2016. Available online: https://documents.wfp.org/stellent/groups/public/documents/manual_guide_proced/wfp284911.pdf (accessed on 30 January 2023).
- World Health Organization. Measuring Change in Nutritional Status; World Health Organization: Geneva, Switzerland, 1982. [Google Scholar]
- Gibson, R.S.; Ferguson, E.L. An Interactive 24-Hour Recall for Assessing the Adequacy of Iron and Zinc Intakes in Developing Countries; ILSI Press: Washington, DC, USA, 1999. [Google Scholar]
- International Zinc Nutrition Consultative Group; Brown, K.H.; Rivera, J.A.; Bhutta, Z.; Gibson, R.S.; King, J.C.; Lonnerdal, B.; Ruel, M.T.; Sandtrom, B.; Wasantwisut, E.; et al. International zinc nutrition consultative group (izincg) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr. Bull. 2004, 25, S99–S203. [Google Scholar]
- Badrick, T. Quality leadership and quality control. Clin. Biochem. Rev. 2003, 24 (Suppl. 2), 81–93. [Google Scholar]
- WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. 2006, 450, 76–85.
- Pakistan Dietary Guidelines for Better Nutrition; Food and Agriculture Organization of the United Nations and Ministry of Planning Development and Reform: Rome, Italy; Government of Pakistan: Islamabad, Pakistan, 2018.
- Soofi, S.; Cousens, S.; Iqbal, S.P.; Akhund, T.; Khan, J.; Ahmed, I.; Zaidi, A.K.; Bhutta, Z.A. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: A cluster-randomised trial. Lancet 2013, 382, 29–40. [Google Scholar] [CrossRef]
- Chen, K.; Li, T.-Y.; Chen, L.; Qu, P.; Liu, Y.-X. Effects of Vitamin A, Vitamin A plus Iron and Multiple Micronutrient-Fortified Seasoning Powder on Preschool Children in a Suburb of Chongqing, China. J. Nutr. Sci. Vitaminol. 2008, 54, 440–447. [Google Scholar] [CrossRef]
- Salam, R.A.; MacPhail, C.; Das, J.K.; Bhutta, Z.A. Effectiveness of Micronutrient Powders (MNP) in women and children. BMC Public Health 2013, 13, S22. [Google Scholar] [CrossRef] [PubMed]
- Untoro, J.; Karyadi, E.; Wibowo, L.; Erhardt, M.W.; Gross, R. Multiple Micronutrient Supplements Improve Micronutrient Status and Anemia But Not Growth and Morbidity of Indonesian Infants: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Nutr. 2005, 135, 639S–645S. [Google Scholar] [CrossRef] [PubMed]
- Ford, N.D.; Ruth, L.J.; Ngalombi, S.; Lubowa, A.; Halati, S.; Ahimbisibwe, M.; Baingana, R.; Whitehead, R.D.; Mapango, C.; Jefferds, M.E. An Integrated Infant and Young Child Feeding and Micronutrient Powder Intervention Does Not Affect Anemia, Iron Status, or Vitamin A Status among Children Aged 12–23 Months in Eastern Uganda. J. Nutr. 2020, 150, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Varma, J.L.; Das, S.; Sankar, R.; Mannar, M.G.V.; Levinson, F.J.; Hamer, D.H. Community-level micronutrient fortification of a food supplement in India: A controlled trial in preschool children aged 36–66 mo. Am. J. Clin. Nutr. 2007, 85, 1127–1133. [Google Scholar] [CrossRef]
- Serdula, M.K.; Lundeen, E.; Nichols, E.K.; Imanalieva, C.; Minbaev, M.; Mamyrbaeva, T.; Timmer, A.; Aburto, N.J.; Samohleb, G.; Whitehead, R.D.; et al. Effects of a large-scale micronutrient powder and young child feeding education program on the micronutrient status of children 6–24 months of age in the Kyrgyz Republic. Eur. J. Clin. Nutr. 2013, 67, 703–707. [Google Scholar] [CrossRef]
- Brett, N.R.; Lavery, P.; Agellon, S.; Vanstone, C.A.; Maguire, J.L.; Rauch, F.; Weiler, H.A. Dietary vitamin D dose-response in healthy children 2 to 8 y of age: A 12-wk randomized controlled trial using fortified foods. Am. J. Clin. Nutr. 2016, 103, 144–152. [Google Scholar] [CrossRef]
- Mongolia, W.V. Effectiveness of Home-Based Fortification of Complementary Foods with Sprinkles in an Integrated Nutrition Program to Address Rickets and Anemia; World Vision Ulannbaatar: Ulaanbaatar, Mongolia, 2005. [Google Scholar]
- Li, J.; Cao, D.; Huang, Y.; Chen, B.; Chen, Z.; Wang, R.; Dong, Q.; Wei, Q.; Liu, L. Zinc Intakes and Health Outcomes: An Umbrella Review. Front. Nutr. 2022, 9, 798078. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, N.; Bahl, R.; Taneja, S.; Strand, T.; Mølbak, K.; Ulvik, R.J.; Sommerfelt, H.; Bhan, M.K. Substantial Reduction in Severe Diarrheal Morbidity by Daily Zinc Supplementation in Young North Indian Children. Pediatrics 2002, 109, e86. [Google Scholar] [CrossRef]
- Hoang, N.T.D.; Orellana, L.; Gibson, R.S.; Le, T.D.; Worsley, A.; Sinclair, A.J.; Hoang, N.T.T.; Szymlek-Gay, E.A. Multiple micronutrient supplementation improves micronutrient status in primary school children in Hai Phong City, Vietnam: A randomised controlled trial. Sci. Rep. 2021, 11, 3728. [Google Scholar] [CrossRef]
- García-Guerra, A.; Rivera, J.A.; Neufeld, L.M.; Quezada-Sánchez, A.D.; Dominguez Islas, C.; Fernández-Gaxiola, A.C.; Bonvecchio Arenas, A. Consumption of Micronutrient Powder, Syrup or Fortified Food Significantly Improves Zinc and Iron Status in Young Mexican Children: A Cluster Randomized Trial. Nutrients 2022, 14, 2231. [Google Scholar] [CrossRef]
- Barffour, M.A.; Hinnouho, G.-M.; Kounnavong, S.; Wessells, K.R.; Ratsavong, K.; Bounheuang, B.; Chanhthavong, B.; Sitthideth, D.; Sengnam, K.; Arnold, C.D.; et al. Effects of Daily Zinc, Daily Multiple Micronutrient Powder, or Therapeutic Zinc Supplementation for Diarrhea Prevention on Physical Growth, Anemia, and Micronutrient Status in Rural Laotian Children: A Randomized Controlled Trial. J. Pediatr. 2019, 207, 80–89.e2. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Black, R.E.; Krebs, N.F.; Westcott, J.; Long, J.M.; Islam, K.M.; Peerson, J.M.; Alam Sthity, R.; Khandaker, A.M.; Hasan, M.; et al. Effects of Different Doses, Forms, and Frequencies of Zinc Supplementation on Biomarkers of Iron and Zinc Status among Young Children in Dhaka, Bangladesh. Nutrients 2022, 14, 5334. [Google Scholar] [CrossRef] [PubMed]
- Becquey, E.; Ouédraogo, C.T.; Hess, S.Y.; Rouamba, N.; Prince, L.; Ouédraogo, J.-B.; AVosti, S.; Brown, K.H. Comparison of Preventive and Therapeutic Zinc Supplementation in Young Children in Burkina Faso: A Cluster-Randomized, Community-Based Trial. J. Nutr. 2016, 146, 2058–2066. [Google Scholar] [CrossRef]
- Schlesinger, L.; Arevalo, M.; Arredondo, S.; Diaz, M.; Lönnerdal, B.; AStekel, A. Effect of a zinc-fortified formula on immunocompetence and growth of malnourished infants. Am. J. Clin. Nutr. 1992, 56, 491–498. [Google Scholar] [CrossRef]
- Kujinga, P.; Galetti, V.; Onyango, E.; Jakab, V.; Buerkli, S.; Andang’o, P.; Brouwer, I.D.; Zimmermann, M.B.; Moretti, D. Effectiveness of zinc-fortified water on zinc intake, status and morbidity in Kenyan pre-school children: A randomised controlled trial. Public Health Nutr. 2018, 21, 2855–2865. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Comprehensive Implementation Plan on Maternal, Infant, and Young Child Nutrition; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Tam, E.; Keats, E.C.; Rind, F.; Das, J.K.; Bhutta, A.Z.A. Micronutrient Supplementation and Fortification Interventions on Health and Development Outcomes among Children Under-Five in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 289. [Google Scholar] [CrossRef]
- Machado, M.; Lopes, M.; Schincaglia, R.; da Costa, P.; Coelho, A.; Hadler, M. Effect of Fortification with Multiple Micronutrient Powder on the Prevention and Treatment of Iron Deficiency and Anaemia in Brazilian Children: A Randomized Clinical Trial. Nutrients 2021, 13, 2160. [Google Scholar] [CrossRef]
- Kejo, D.; Petrucka, P.; Martin, H.; Mosha, T.C.E.; Kimanya, M.E. Efficacy of Different Doses of Multiple Micronutrient Powder on Haemoglobin Concentration in Children Aged 6–59 Months in Arusha District. Scientifica 2019, 2019, 8979456. [Google Scholar] [CrossRef]
- De-Regil, L.M.; Jefferds, M.E.D.; Peña-Rosas, J.P. Point-of-use fortification of foods with micronutrient powders containing iron in children of preschool and school-age. Cochrane Database Syst. Rev. 2017, 11, CD009666. [Google Scholar] [CrossRef]
- Black, M.M.; Fernandez-Rao, S.; Nair, K.M.; Balakrishna, N.; Tilton, N.; Radhakrishna, K.V.; Ravinder, P.; Harding, K.B.; Reinhart, G.; Yimgang, D.P.; et al. A Randomized Multiple Micronutrient Powder Point-of-Use Fortification Trial Implemented in Indian Preschools Increases Expressive Language and Reduces Anemia and Iron Deficiency. J. Nutr. 2021, 151, 2029–2042. [Google Scholar] [CrossRef]
- Locks, L.M.; Dahal, P.; Pokharel, R.; Joshi, N.; Paudyal, N.; Whitehead, R.D., Jr.; Chitekwe, S.; Mei, Z.; Lamichhane, B.; Garg, A.; et al. Changes in growth, anaemia, and iron deficiency among children aged 6–23 months in two districts in Nepal that were part of the post-pilot scale-up of an integrated infant and young child feeding and micronutrient powder intervention. Matern. Child Nutr. 2019, 15, e12693. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.S.; Sampaio, P.; Muniz, P.T.; A Cardoso, M.; For the ENFAC Working Group. Multiple micronutrients in powder delivered through primary health care reduce iron and vitamin A deficiencies in young Amazonian children. Public Health Nutr. 2016, 19, 3039–3047. [Google Scholar] [CrossRef] [PubMed]
- Albelbeisi, A.; Shariff, Z.M.; Mun, C.Y.; Rahman, H.A.; Abed, Y. Multiple micronutrient supplementation improves growth and reduces the risk of anemia among infants in Gaza Strip, Palestine: A prospective randomized community trial. Nutr. J. 2020, 19, 133. [Google Scholar] [CrossRef] [PubMed]
- Dewey, K.G.; Mridha, M.K.; Matias, S.L.; Arnold, C.D.; Cummins, J.R.; Khan, S.A.; Maalouf-Manasseh, Z.; Siddiqui, Z.; Ullah, B.; AVosti, S. Lipid-based nutrient supplementation in the first 1000 d improves child growth in Bangladesh: A cluster-randomized effectiveness trial. Am. J. Clin. Nutr. 2017, 105, 944–957. [Google Scholar] [CrossRef] [PubMed]
- Vist, G.E.; Suchdev, P.S.; De-Regil, L.M.; Walleser, S.; Peña-Rosas, J.P. Home fortification of foods with multiple micronutrient powders for health and nutrition in children under 2 years of age. Cochrane Database Syst. Rev. 2020, 2, CD008959. [Google Scholar]
- Thurnham, D.I.; Northrop-Clewes, C.A. Inflammation and biomarkers of micronutrient status. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 458–463. [Google Scholar] [CrossRef]
- Tomkins, A. Assessing Micronutrient Status in the Presence of Inflammation. J. Nutr. 2003, 133, 1649S–1655S. [Google Scholar] [CrossRef]
| Nutrients Values Per One Gram Sachet | Unit/Day | Quantity | Chemical Form | Recommended Daily Allowances for the Pakistani Population for 24 to 59 Month-Old Children |
|---|---|---|---|---|
| Retinol (VitaminA) | μg | 400 | (as dry CWS vitamin A acetate or palmitate beadlets) | 300–400 |
| Thiamin (VitaminB1) | mg | 0.5 | (as Thiamine mononitrate) | 0.5–0.6 |
| Riboflavin (Vitamin B2) | mg | 0.5 | (Riboflavin or riboflavin -5-phosphate) | 0.5–0.6 |
| Niacin (VitaminB3) | mg | 6 | (as Nicotinamide) | 6–8 |
| Pyridoxine (Vitamin B6) | mg | 0.5 | (as Pyridoxine hydrochloride) | 0.5–0.6 |
| Folate (VitaminB9) | μg | 90 | (anhydrous) | 150–200 |
| Cobalamin (Vitamin B12) | μg | 0.9 | (1% or 0.1% Cyanocobalamin on a carrier) | 0.9–1.2 |
| Ascorbate (VitaminC) | mg | 30 | (as sodium or calcium ascorbate) | 15–25 |
| Cholecalciferol (Vitamin D) | μg | 5 | (as dry CWS Cholecalciferol) | 8.3–10 |
| Tocopherol Acetate (Vitamin E) | mg aTE | 5 | (as CWS d or dl-alpha tocopheryl acetate) | 6–7 |
| Copper (Cu) | mg | 0.7 | (as Copper gluconate or sulphate) | 340–440 |
| Iodine (I) | μg | 90 | (as Potassium iodide, or potassium iodate) | 90 |
| Iron (Fe) | mg | 10 | (as coated Ferrous fumerate, NaFe EDTA*or Ferrous bisglycinate) | 20 |
| Selenium (Se) | μg | 17 | (as Sodium selenate or selenite or selenomethionine) | 20–30 |
| Zinc (Zn) | mg | 4.1 | mg (as Zinc sulphate, or gluconate) | 15 |
| Baseline Characteristics | Intervention (n = 58) | Control (n = 53) |
|---|---|---|
| Socioeconomic status of * households n (%) | ||
| Poor | 31 (53.45) | 31 (58.49) |
| Non-poor | 27 (46.55) | 22 (41.51) |
| Mother Age (y) (mean ± SD) | 30.21 (5.20) | 30.93 (6.45) |
| Mother Education n (%) | ||
| Formal education > 3 y | 14 (24.14) | 14 (26.42) |
| Informal education < 3 y | 44 (75.86) | 39 (73.58) |
| Mother Work Status n (%) | ||
| Working woman | 6 (10.34) | 5 (9.43) |
| Housewife | 52 (89.66) | 48 (90.57) |
| Father Education n (%) | ||
| Formal education | 37 (63.79) | 39 (73.58) |
| Informal education | 21 (36.21) | 14 (26.42) |
| Father Work Status | ||
| Paid work | 42 (72.41) | 38 (71.70) |
| Unemployed | 16 (27.59) | 15 (28.30) |
| Family structure n (%) | ||
| Single | 21 (36.21) | 16 (30.19) |
| Joint | 37 (63.79) | 37 (69.81) |
| Age of child in months | 37.2 (4.30) | 36.6 (5.60) |
| Gender of child, n (%) | ||
| Male | 28 (48.30) | 27 (51.0) |
| Female | 30 (51.7) | 26 (49.0) |
| Childhood specific morbidities (in the previous month) | ||
| Diarrhea n (%) | ||
| No | 52 (89.66) | 48 (90.57) |
| Yes | 6 (10.34) | 5 (9.43) |
| Acute respiratory infections n (%) | ||
| No | 48 (82.76) | 45 (84.91) |
| Yes | 10 (17.24) | 8 (15.09) |
| Vaccination status of the child n (%) | ||
| Fully Vaccinated | 46 (79.31) | 43 (81.13) |
| Incomplete vaccination | 12 (20.69) | 10 (18.87) |
| Breastfeeding in the first hour after birth n (%) | ||
| No | 19 (32.76) | 14 (26.42) |
| Yes | 39 (67.24) | 39 (73.58) |
| Exclusive Breastfeeding n (%) | ||
| No | 8 (13.79) | 6 (11.32) |
| Yes | 50 (86.21) | 47 (88.68) |
| Age of complementary feeding introduction (mean ± SD) | 7.28 (3.41) | 6.85 (2.27) |
| Characteristics | Control Baseline (N = 53) | Control End line (N = 53) | Mean Difference | p-Value | Intervention Baseline (N = 58) | Intervention End Line (N = 58) | Mean Difference | p-Value | DID | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||||||
| Growth indicators | ||||||||||
| HAZ | −0.94 (1.44) | −1.12 (1.46) | −0.18 | 0.47 | −1.29 (0.88) | −1.30 (0.86) | −0.01 | 0.93 | 0.17 | 0.51 |
| WHZ | −1.32 (1.21) | −1.31 (1.08) | −0.004 | 0.94 | −1.0 (0.88) | −0.40 (1.01) | 0.60 | <0.001 | 0.60 | <0.001 |
| WAZ | −1.37 (1.0) | −1.51 (0.98) | −0.14 | 0.35 | −1.40 (0.50) | −1.05 (0.49) | 0.34 | <0.001 | 0.50 | <0.001 |
| Micronutrients status | ||||||||||
| Plasma zinc (µg/dL) | 49.46 (16.82) | 54.17 (15.53) | 4.71 | 0.07 | 47.63 (21.48) | 90.0 (21.18) | 42.0 | <0.001 | 37.36 | <0.001 |
| Plasma vitamin A (ng/mL) | 18.29 (5.57) | 18.82 (5.05) | 0.52 | 0.43 | 17.11 (7.49) | 24.68 (7.12) | 7.5 | <0.001 | 7.03 | <0.001 |
| Plasma vitamin D (ng/mL) | 28.20 (10.74) | 29.52 (7.41) | 1.32 | 0.33 | 25.71 (7.43) | 35.15 (6.43) | 9.44 | <0.001 | 8.12 | <0.001 |
| Anemia status | ||||||||||
| Anemia Hb (g/dL) | 10.7 (1.65) | 11.0 (0.94) | 0.18 | 0.50 | 11.10 (1.65) | 13.0 (0.64) | 1.84 | <0.001 | 1.67 | <0.001 |
| Inflammatory marker | ||||||||||
| Serum albumin (g/dL) | 3.57 (0.85) | 3.41 (0.64) | −0.16 | 0.30 | 3.52 (0.95) | 4.09 (0.80) | 0.56 | 0.002 | 0.73 | 0.002 |
| Variable | Control Baseline (N = 53) | Control End Line (N = 53) | p-Value | Intervention Baseline (N = 58) | Intervention End Line (N = 58) | p-Value | Daily Dietary Recommendation PK Guidelines |
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||||
| Energy (kcal/d) | 1144.83 (90.46) | 1170.50 (85.08) | 0.11 | 1141.75 (86.70) | 1173.05 (99.85) | 0.06 | 1510 |
| Fat (g/d) | 28.49 (4.39) | 29.00 (4.57) | 0.61 | 27.8 (3.10) | 28.75 (4.66) | 0.21 | 30–35 |
| Carbohydrate (g/d) | 167.54 (35.17) | 167.20 (33.27) | 0.93 | 165.29 (16.5) | 163.55 (12.96) | 0.49 | |
| Protein (g/d) | 22.55 (4.07) | 23.16 (3.56) | 0.39 | 22.97 (2.79) | 24.22 (4.68) | 0.10 | 26 |
| Vitamin A (µg/d) | 190.79 (81.19) | 220.75 (86.64) | 0.08 | 213.84 (88.03) | 220.93 (86.69) | 0.66 | 400 |
| Vitamin D (µg/d) | 0.55 (0.63) | 0.56 (0.65) | 0.89 | 0.64 (0.63) | 0.73 (0.60) | 0.40 | 8.3 |
| Iron (mg/d) | 5.79 (2.20) | 6.48 (1.70) | 0.09 | 6.40 (1.93) | 6.36 (1.74) | 0.85 | 20 |
| Zinc (mg/d) | 5.83 (0.81) | 5.62 (0.94) | 0.19 | 5.19 (0.94) | 5.35 (1.13) | 0.42 | 15 |
| Models | Results | ||
|---|---|---|---|
| Coefficient (95% CI) | p-Value | ||
| Plasma Zinc at end line Intervention group (µg/dL) | |||
| Unadjusted | 35.8 | 28.8, 43.0 | <0.001 |
| Adjusted * | 33.42 | 23.80, 43.0 | <0.001 |
| Plasma vitamin D at end line Intervention group (ng/mL) | |||
| Unadjusted | 5.70 | 3.02, 8.35 | <0.001 |
| Adjusted ** | 4.79 | 1.63, 7.95 | 0.002 |
| Plasma vitamin A at end line Intervention group (ng/mL) | |||
| Unadjusted | 5.94 | 3.59, 8.30 | <0.001 |
| Adjusted *** | 7.57 | 5.13, 10.02 | <0.001 |
| Hemoglobin g/dL at end line Intervention group | |||
| Unadjusted | 2.04 | 1.74, 2.3 | <0.001 |
| Adjusted **** | 2.0 | 1.64, 2.40 | <0.001 |
| Children’s Characteristics | Control Baseline N = 53 | Intervention Baseline N = 58 | p-Value | Control End Line N = 53 | Intervention End Line N = 58 | p-Value |
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |||
| Nutritional status | ||||||
| Stunting (HAZ < −2SD) | 16 (30.19) | 13 (22.41) | 0.35 | 13 (24.53) | 12 (20.70) | 0.63 |
| Wasting (WHZ < −2SD) | 15 (28.30) | 8 (13.79) | 0.10 | 14 (26.42) | 4 (7.0) | 0.005 |
| Underweight (WAZ < −2SD) | 14 (26.42) | 9 (15.52) | 0.15 | 16 (30.19) | 3 (5.17) | <0.001 |
| Micronutrients status | ||||||
| Plasma zinc n(%) | 42 (79.25) | 40 (74.07) | 0.52 | 28 (55.0) | 8 (14.04) | <0.001 |
| Plasma vitamin A n(%) | 29 (55.77) | 40 (70.18) | 0.11 | 19 (40.0) | 9 (16.36) | 0.008 |
| Plasma vitamin D n(%) | 33 (63.46) | 44 (77.19) | 0.11 | 26 (52.0) | 11 (19.30) | <0.001 |
| Anemia status | ||||||
| Anemia n(%) | 29 (54.72) | 25 (43.10) | 0.22 | 25 (47.17) | 10 (17.24) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.; Ul-Haq, Z.; Fatima, S.; Ahmed, J.; Alobaid, H.M.; Fazid, S.; Muhammad, N.; Garzon, C.; Ihtesham, Y.; Habib, I.; et al. Long-Term Impact of Multiple Micronutrient Supplementation on Micronutrient Status, Hemoglobin Level, and Growth in Children 24 to 59 Months of Age: A Non-Randomized Community-Based Trial from Pakistan. Nutrients 2023, 15, 1690. https://doi.org/10.3390/nu15071690
Khan A, Ul-Haq Z, Fatima S, Ahmed J, Alobaid HM, Fazid S, Muhammad N, Garzon C, Ihtesham Y, Habib I, et al. Long-Term Impact of Multiple Micronutrient Supplementation on Micronutrient Status, Hemoglobin Level, and Growth in Children 24 to 59 Months of Age: A Non-Randomized Community-Based Trial from Pakistan. Nutrients. 2023; 15(7):1690. https://doi.org/10.3390/nu15071690
Chicago/Turabian StyleKhan, Aslam, Zia Ul-Haq, Sadia Fatima, Jawad Ahmed, Hussah M. Alobaid, Sheraz Fazid, Nawshad Muhammad, Cecilia Garzon, Yasir Ihtesham, Ijaz Habib, and et al. 2023. "Long-Term Impact of Multiple Micronutrient Supplementation on Micronutrient Status, Hemoglobin Level, and Growth in Children 24 to 59 Months of Age: A Non-Randomized Community-Based Trial from Pakistan" Nutrients 15, no. 7: 1690. https://doi.org/10.3390/nu15071690
APA StyleKhan, A., Ul-Haq, Z., Fatima, S., Ahmed, J., Alobaid, H. M., Fazid, S., Muhammad, N., Garzon, C., Ihtesham, Y., Habib, I., Tanimoune, M., Iqbal, K., Arshad, M., & Safi, S. Z. (2023). Long-Term Impact of Multiple Micronutrient Supplementation on Micronutrient Status, Hemoglobin Level, and Growth in Children 24 to 59 Months of Age: A Non-Randomized Community-Based Trial from Pakistan. Nutrients, 15(7), 1690. https://doi.org/10.3390/nu15071690








