Combined Effects of ESRα DNA Methylation and Progesterone on Glucose Metabolic Disorders: The Henan Rural Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Laboratory Measurements
2.4. Methylation Analysis
2.5. Ascertainment of Cases
2.6. Statistical Analysis
3. Results
3.1. Basic Characteristics
3.2. Independent Effects of ESRα Methylation and Progesterone on IFG and T2DM
3.3. Associations of ESRα Methylation (CpG 1) and Progesterone with Glucose Homeostasis Markers
3.4. Combined Effects of ESRα Methylation (CpG 1) and Progesterone on IFG and T2DM
3.5. Stratification Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
The Chinese Clinical Trial Registration
Abbreviations
| T2DM | type 2 diabetes mellitus |
| IFG | impaired fasting glucose |
| ESRα | estrogen receptor α |
| IR | insulin resistance |
| PIK3 | phosphatidylinositol-3-kinase |
| AKT | protein kinase B |
| GLUT4 | glucose transporter 4 |
| CpG | cytosine-phosphoguanine |
| HOMA | homeostasis model assessment |
| INS | insulin |
| HbA1c | glycosylated hemoglobin A1c |
| NGT | normal glucose tolerance |
| BMI | body mass index |
| TC | total cholesterol |
| TG | triglyceride |
| HDL-C | high-density lipoprotein cholesterol |
| LDL-C | low-density lipoprotein cholesterol |
| FPG | fasting plasma glucose |
| HPLC | high performance liquid chromatography |
| LC-MS/MS | liquid chromatography-tandem mass spectrometry |
| ADA | American Diabetes Association |
| WHO | World Health Organization |
| SD | standard deviations |
| IQR | interquartile ranges |
| T | tertiles |
| OR | odds ratios |
| CI | confidence intervals |
| E2 | estradiol |
| IRS | insulin receptor substrate |
References
- Magliano, D.J.; Boyko, E.J. IDF Diabetes Atlas 10th Edition Scientific Committee; IDF Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Liu, X.; Li, Y.; Li, L.; Zhang, L.; Ren, Y.; Zhou, H.; Cui, L.; Mao, Z.; Hu, D.; Wang, C. Prevalence, awareness, treatment, control of type 2 diabetes mellitus and risk factors in Chinese rural population: The RuralDiab study. Sci. Rep. 2016, 6, 31426. [Google Scholar] [CrossRef] [PubMed]
- Richter, B.; Hemmingsen, B.; Metzendorf, M.-I.; Takwoingi, Y. Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia. Cochrane Database Syst. Rev. 2018, 2018, CD012661. [Google Scholar] [CrossRef] [PubMed]
- Heianza, Y.; Hara, S.; Arase, Y.; Saito, K.; Fujiwara, K.; Tsuji, H.; Kodama, S.; Hsieh, S.D.; Mori, Y.; Shimano, H.; et al. HbA1c 5·7–6·4% and impaired fasting plasma glucose for diagnosis of prediabetes and risk of progression to diabetes in Japan (TOPICS 3): A longitudinal cohort study. Lancet 2011, 378, 147–155. [Google Scholar] [CrossRef]
- Tabák, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimäki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef] [PubMed]
- Ko, G.T.C.; Chan, J.; Cockram, C.S. Change of glycaemic status in Chinese subjects with impaired fasting glycaemia. Diabet. Med. 2001, 18, 745–748. [Google Scholar] [CrossRef]
- Abdulai, T.; Li, Y.; Zhang, H.; Tu, R.; Liu, X.; Zhang, L.; Dong, X.; Li, R.; Wang, Y.; Wang, C. Prevalence of impaired fasting glucose, type 2 diabetes and associated risk factors in undiagnosed Chinese rural population: The Henan Rural Cohort Study. BMJ Open 2019, 9, e029628. [Google Scholar] [CrossRef]
- Knowler, W.C.; Fowler, S.E.; Hamman, R.F.; Christophi, C.A.; Hoffman, H.J.; Brenneman, A.T.; Brown-Friday, J.O.; Goldberg, R.; Venditti, E.; Nathan, D.M. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009, 374, 1677–1686. [Google Scholar]
- Dayeh, T.; Tuomi, T.; Almgren, P.; Perfilyev, A.; Jansson, P.-A.; De Mello, V.D.; Pihlajamäki, J.; Vaag, A.; Groop, L.; Nilsson, E.; et al. DNA methylation of loci within ABCG1 and PHOSPHO1 in blood DNA is associated with future type 2 diabetes risk. Epigenetics 2016, 11, 482–488. [Google Scholar] [CrossRef]
- Chambers, J.C.; Loh, M.; Lehne, B.; Drong, A.; Kriebel, J.; Motta, V.; Wahl, S.; Elliott, H.R.; Rota, F.; Scott, W.R.; et al. Epigenome-wide association of DNA methylation markers in peripheral blood from Indian Asians and Europeans with incident type 2 diabetes: A nested case-control study. Lancet Diabetes Endocrinol. 2015, 3, 526–534. [Google Scholar] [CrossRef]
- Hidalgo, B.; Irvin, M.R.; Sha, J.; Zhi, D.; Aslibekyan, S.; Absher, D.; Tiwari, H.K.; Kabagambe, E.K.; Ordovas, J.M.; Arnett, D.K. Epigenome-Wide Association Study of Fasting Measures of Glucose, Insulin, and HOMA-IR in the Genetics of Lipid Lowering Drugs and Diet Network Study. Diabetes 2014, 63, 801–807. [Google Scholar] [CrossRef]
- Louet, J.-F.; Lemay, C.; Mauvais-Jarvis, F. Antidiabetic actions of estrogen: Insight from human and genetic mouse models. Curr. Atheroscler. Rep. 2004, 6, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, C.; Collison, M. Sex steroids and insulin resistance. Clin. Sci. 2002, 102, 151–166. [Google Scholar] [CrossRef]
- Nadal, A.; Magdalena, P.A.; Soriano, S.; Quesada, I.; Ropero, A.B. The pancreatic β-cell as a target of estrogens and xenoestrogens: Implications for blood glucose homeostasis and diabetes. Mol. Cell. Endocrinol. 2009, 304, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Foryst-Ludwig, A.; Kintscher, U. Metabolic impact of estrogen signalling through ERalpha and ERbeta. J. Steroid Biochem. Mol. Biol. 2010, 122, 74–81. [Google Scholar] [CrossRef]
- Meyer, M.R.; Clegg, D.J.; Prossnitz, E.R.; Barton, M. Obesity, insulin resistance and diabetes: Sex differences and role of oestrogen receptors. Acta Physiol. 2011, 203, 259–269. [Google Scholar] [CrossRef]
- Ohlsson, C.; Hellberg, N.; Parini, P.; Vidal, O.; Bohlooly, M.; Rudling, M.; Lindberg, M.K.; Warner, M.; Angelin, B.; Gustafsson, J. Obesity and Disturbed Lipoprotein Profile in Estrogen Receptor-α-Deficient Male Mice. Biochem. Biophys. Res. Commun. 2000, 278, 640–645. [Google Scholar] [CrossRef]
- Heine, P.A.; Taylor, J.A.; Iwamoto, G.A.; Lubahn, D.B.; Cooke, P.S. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc. Natl. Acad. Sci. USA 2000, 97, 12729–12734. [Google Scholar] [CrossRef]
- Ribas, V.; Nguyen, M.T.; Henstridge, D.C.; Nguyen, A.K.; Beaven, S.W.; Watt, M.J.; Hevener, A.L. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERalpha-deficient mice. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E304–E319. [Google Scholar] [CrossRef]
- Bryzgalova, G.; Gao, H.; Ahren, B.; Zierath, J.R.; Galuska, D.; Steiler, T.L.; Dahlman-Wright, K.; Nilssosn, S.; Gustafsson, J.A.; Efendic, S.; et al. Evidence that oestrogen receptor-α plays an important role in the regulation of glucose homeostasis in mice: Insulin sensitivity in the liver. Diabetologia 2006, 49, 588–597. [Google Scholar] [CrossRef]
- Smith, E.P.; Boyd, J.; Frank, G.R.; Takahashi, H.; Cohen, R.M.; Specker, B.; Williams, T.C.; Lubahn, D.B.; Korach, K.S. Estrogen Resistance Caused by a Mutation in the Estrogen-Receptor Gene in a Man. N. Engl. J. Med. 1994, 331, 1056–1061. [Google Scholar] [CrossRef]
- Yoshida, T.; Eguchi, H.; Nakachi, K.; Tanimoto, K.; Higashi, Y.; Suemasu, K.; Iino, Y.; Morishita, Y.; Hayashi, S.-I. Distinct mechanisms of loss of estrogen receptor alpha gene expression in human breast cancer: Methylation of the gene and alteration of trans-acting factors. Carcinogenesis 2000, 21, 2193–2201. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, L.; Jin, F.; Ma, W.; Ren, J.; Wen, X.; He, M.; Sun, M.; Tang, H.; Wei, M. Silencing of estrogen receptor alpha (ERalpha) gene by promoter hypermethylation is a frequent event in Chinese women with sporadic breast cancer. Breast Cancer Res. Treat. 2009, 117, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Giacinti, L.; Claudio, P.P.; Lopez, M.; Giordano, A. Epigenetic Information and Estrogen Receptor Alpha Expression in Breast Cancer. Oncol. 2006, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- White, A.J.; Chen, J.; Teitelbaum, S.L.; McCullough, L.E.; Xu, X.; Cho, Y.H.; Conway, K.; Beyea, J.; Stellman, S.D.; Steck, S.E.; et al. Sources of polycyclic aromatic hydrocarbons are associated with gene-specific promoter methylation in women with breast cancer. Env. Res. 2016, 145, 93–100. [Google Scholar] [CrossRef]
- Dai, B.; Geng, L.; Yu, Y.; Sui, C.; Xie, F.; Shen, W.; Zheng, T.; Yang, J. Methylation patterns of estrogen receptor α promoter correlate with estrogen receptor α expression and clinicopathological factors in hepatocellular carcinoma. Exp. Biol. Med. 2014, 239, 883–890. [Google Scholar] [CrossRef]
- Joensuu, E.I.; Abdel-Rahman, W.M.; Ollikainen, M.; Ruosaari, S.; Knuutila, S.; Peltomäki, P. Epigenetic Signatures of Familial Cancer Are Characteristic of Tumor Type and Family Category. Cancer Res. 2008, 68, 4597–4605. [Google Scholar] [CrossRef]
- Evaluating Progestogens for Preventing Preterm Birth International Collaborative (EPPPIC). Meta-analysis of individual participant data from randomised controlled trials. Lancet 2021, 397, 1183–1194. [Google Scholar] [CrossRef]
- Cai, W.; Zhu, Y.; Furuya, K.; Li, Z.; Sokabe, M.; Chen, L. Two different molecular mechanisms underlying progesterone neuroprotection against ischemic brain damage. Neuropharmacology 2008, 55, 127–138. [Google Scholar] [CrossRef]
- Szekeres-Bartho, J.; Barakonyi, A.; Par, G.; Polgar, B.; Palkovics, T.; Szereday, L. Progesterone as an immunomodulatory molecule. Int. Immunopharmacol. 2001, 1, 1037–1048. [Google Scholar] [CrossRef]
- Picard, F.; Wanatabe, M.; Schoonjans, K.; Lydon, J.; O’Malley, B.W.; Auwerx, J. Progesterone receptor knockout mice have an improved glucose homeostasis secondary to β-cell proliferation. Proc. Natl. Acad. Sci. USA 2002, 99, 15644–15648. [Google Scholar] [CrossRef]
- Nunes, V.A.; Portioli-Sanches, E.P.; Rosim, M.P.; Araujo, M.; Praxedes-Garcia, P.; Valle, M.M.R.; Roma, L.P.; Hahn, C.; Gurgul-Convey, E.; Lenzen, S.; et al. Progesterone induces apoptosis of insulin-secreting cells: Insights into the molecular mechanism. J. Endocrinol. 2014, 221, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.; Peitzsch, M.; Brunssen, C.; Mittag, J.; Jannasch, A.; Frenzel, A.; Brown, N.; Weldon, S.M.; Eisenhofer, G.; Bornstein, S.R.; et al. Elevated Steroid Hormone Production in the db/db Mouse Model of Obesity and Type 2 Diabetes. Horm. Metab. Res. 2016, 49, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Lau, L.H.Y.; Nano, J.; Cecil, A.; Schederecker, F.; Rathmann, W.; Prehn, C.; Zeller, T.; Lechner, A.; Adamski, J.; Peters, A.; et al. Cross-sectional and prospective relationships of endogenous progestogens and estrogens with glucose metabolism in men and women: A KORA F4/FF4 Study. BMJ Open Diabetes Res. Care 2021, 9, e001951. [Google Scholar] [CrossRef]
- Xu, L.; Lu, L.; Tong, A.; Chen, S.; Li, W.; Zhang, H.; Ping, F.; Li, Y. New insights on hyperglycemia in 17-hydroxylase/17,20-lyase deficiency. Front. Endocrinol. 2022, 13, 917420. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Hori, S.; Sugiyama, M.; Fujisawa, E.; Nakano, T.; Tsuneki, H.; Nagira, K.; Saito, S.; Sasaoka, T. Progesterone inhibits glucose uptake by affecting diverse steps of insulin signaling in 3T3-L1 adipocytes. Am. J. Physiol. Metab. 2010, 298, E881–E888. [Google Scholar] [CrossRef]
- Campello, R.S.; Fátima, L.A.; Barreto-Andrade, J.N.; Lucas, T.F.; Mori, R.C.; Porto, C.S.; Machado, U.F. Estradiol-induced regulation of GLUT4 in 3T3-L1 cells: Involvement of ESR1 and AKT activation. J. Mol. Endocrinol. 2017, 59, 257–268. [Google Scholar] [CrossRef]
- Campello, R.S.; Alves-Wagner, A.B.; Lucas, T.F.; Mori, R.C.; Furuya, D.T.; Porto, C.S.; Machado, U.F. Estrogen receptor 1 agonist PPT stimulates Slc2a4 gene expression and improves insulin-induced glucose uptake in adipocytes. Curr. Top. Med. Chem. 2012, 12, 2059–2069. [Google Scholar] [CrossRef]
- Nagira, K.; Sasaoka, T.; Wada, T.; Fukui, K.; Ikubo, M.; Hori, S.; Tsuneki, H.; Saito, S.; Kobayashi, M. Altered Subcellular Distribution of Estrogen Receptor α Is Implicated in Estradiol-Induced Dual Regulation of Insulin Signaling in 3T3-L1 Adipocytes. Endocrinology 2006, 147, 1020–1028. [Google Scholar] [CrossRef]
- Muraki, K.; Okuya, S.; Tanizawa, Y. Estrogen Receptor α Regulates Insulin Sensitivity through IRS-1 Tyrosine Phosphorylation in Mature 3T3-L1 Adipocytes. Endocr. J. 2006, 53, 841–851. [Google Scholar]
- Katzenellenbogen, B.S. Estrogen Receptors: Bioactivities and Interactions with Cell Signaling Pathways1. Biol. Reprod. 1996, 54, 287–293. [Google Scholar] [CrossRef]
- Kraus, W.L.; Weis, K.E.; Katzenellenbogen, B.S. Inhibitory cross-talk between steroid hormone receptors: Differential targeting of estrogen receptor in the repression of its transcriptional activity by agonist- and antagonist-occupied progestin receptors. Mol. Cell. Biol. 1995, 15, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Liu, X.; Liu, X.; Tian, Z.; Zhang, H.; Qian, X.; Luo, Z.; Wei, D.; Jin, S.; Wang, C.; et al. The effect of progesterone and pregnenolone on diabetes status in Chinese rural population: A dose–response analysis from Henan Rural Cohort. Eur. J. Endocrinol. 2019, 181, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Mao, Z.; Li, Y.; Wu, W.; Zhang, X.; Huo, W.; Yu, S.; Shen, L.; Li, L.; Tu, R.; et al. Cohort Profile: The Henan Rural Cohort: A prospective study of chronic non-communicable diseases. Int. J. Epidemiol. 2019, 48, 1756–1756j. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, J.; Liu, X.; Luo, Z.; Wang, Y.; Dong, X.; Wei, D.; Li, R.; Wang, Y.; Huo, W.; et al. Association of serum testosterone with different classes of glucose metabolism and the mediation effect of obesity: The Henan Rural Cohort Study. Diabetes/Metab. Res. Rev. 2019, 35, e3133. [Google Scholar] [CrossRef]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and Abuse of HOMA Modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef]
- Joseph, J.J.; Tcheugui, J.B.E.; Effoe, V.S.; Hsueh, W.A.; Allison, M.A.; Golden, S.H. Renin-Angiotensin-Aldosterone System, Glucose Metabolism and Incident Type 2 Diabetes Mellitus: MESA. J. Am. Hear. Assoc. 2018, 7, e009890. [Google Scholar] [CrossRef]
- Wang, L.; Mao, Z.; Liu, X.; Wei, D.; Liu, P.; Nie, L.; Fan, K.; Kang, N.; Song, Y.; Xu, Q.; et al. Combined effects of progesterone and SOCS3 DNA methylation on T2DM: A case–control study. Clin. Epigenet. 2021, 13, 181. [Google Scholar] [CrossRef]
- Liu, X.; Huo, W.; Zhang, R.; Wei, D.; Tu, R.; Luo, Z.; Wang, Y.; Dong, X.; Qiao, D.; Liu, P.; et al. Androgen receptor DNA methylation is an independent determinant of glucose metabolic disorders in women; testosterone plays a moderating effect. J. Diabetes 2020, 13, 282–291. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Wei, D.; Kang, N.; Nie, L.; Liu, P.; Fan, K.; Zhang, L.; Hou, J.; Li, X.; et al. The mediation role of SOCS3 methylation in the effect of serum testosterone on type 2 diabetes. J. Diabetes 2021, 13, 701–712. [Google Scholar] [CrossRef]
- Lu, M.; Xueying, Q.; Hexiang, P.; Wenjing, G.; Hägg, S.; Weihua, C.; Chunxiao, L.; Canqing, Y.; Jun, L.; Zengchang, P.; et al. Genome-wide associations between alcohol consumption and blood DNA methylation: Evidence from twin study. Epigenomics 2021, 13, 939–951. [Google Scholar] [CrossRef]
- Zhu, X.; Li, J.; Deng, S.; Yu, K.; Liu, X.; Deng, Q.; Sun, H.; Zhang, X.; He, M.; Guo, H.; et al. Genome-Wide Analysis of DNA Methylation and Cigarette Smoking in a Chinese Population. Environ. Health Perspect. 2016, 124, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Globalization of diabetes: The role of diet, lifestyle, and genes. Diabetes Care 2011, 34, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Dahlman, I.; Vaxillaire, M.; Nilsson, M.; Lecoeur, C.; Gu, H.F.; Cavalcanti-Proença, C.; Efendic, S.; Östenson, C.G.; Brismar, K.; Charpentier, G.; et al. Estrogen receptor alpha gene variants associate with type 2 diabetes and fasting plasma glucose. Pharm. Genom. 2008, 18, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Ropero, A.B.; Alonso-Magdalena, P.; Quesada, I.; Nadal, A. The role of estrogen receptors in the control of energy and glucose homeostasis. Steroids 2008, 73, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Le May, C.; Chu, K.; Hu, M.; Ortega, C.S.; Simpson, E.R.; Korach, K.S.; Tsai, M.-J.; Mauvais-Jarvis, F. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc. Natl. Acad. Sci. USA 2006, 103, 9232–9237. [Google Scholar] [CrossRef]
- Brismar, K.; Nilsson, S.E. Interrelations and associations of serum levels of steroids and pituitary hormones with markers of insulin resistance, inflammatory activity, and renal function in men and women aged >70 years in an 8-year longitudinal study of opposite-sex twins. Gend. Med. 2009, 6, 123–136. [Google Scholar] [CrossRef]
- Nilsson, S.E.; Fransson, E.; Brismar, K. Relationship between serum progesterone concentrations and cardiovascular disease, diabetes, and mortality in elderly Swedish men and women: An 8-Year prospective study. Gend. Med. 2009, 6, 433–443. [Google Scholar] [CrossRef]
- Wang, X.; Xian, T.; Jia, X.; Zhang, L.; Liu, L.; Man, F.; Zhang, X.; Zhang, J.; Pan, Q.; Guo, L. A cross-sectional study on the associations of insulin resistance with sex hormone, abnormal lipid metabolism in T2DM and IGT patients. Medicine 2017, 96, e7378. [Google Scholar] [CrossRef]
- Chiang, S.-H.; Baumann, C.A.; Kanzaki, M.; Thurmond, D.C.; Watson, R.T.; Neudauer, C.L.; Macara, I.G.; Pessin, J.E.; Saltiel, A.R. Insulin-stimulated GLUT4 translocation requires the CAP-dependent activation of TC10. Nature 2001, 410, 944–948. [Google Scholar] [CrossRef]
- Straub, S.G.; Sharp, G.W.G.; Meglasson, M.D.; De Souza, C.J. Progesterone Inhibits Insulin Secretion by a Membrane Delimited, Non-genomic Action. Biosci. Rep. 2001, 21, 653–666. [Google Scholar] [CrossRef]
- Selcer, K.W.; Leavitt, W.W. Progesterone down-regulation of nuclear estrogen receptor: A fundamental mechanism in birds and mammals. Gen. Comp. Endocrinol. 1988, 72, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Mesiano, S.; Chan, E.-C.; Fitter, J.T.; Kwek, K.; Yeo, G.; Smith, R. Progesterone Withdrawal and Estrogen Activation in Human Parturition Are Coordinated by Progesterone Receptor A Expression in the Myometrium. J. Clin. Endocrinol. Metab. 2002, 87, 2924–2930. [Google Scholar] [CrossRef] [PubMed]
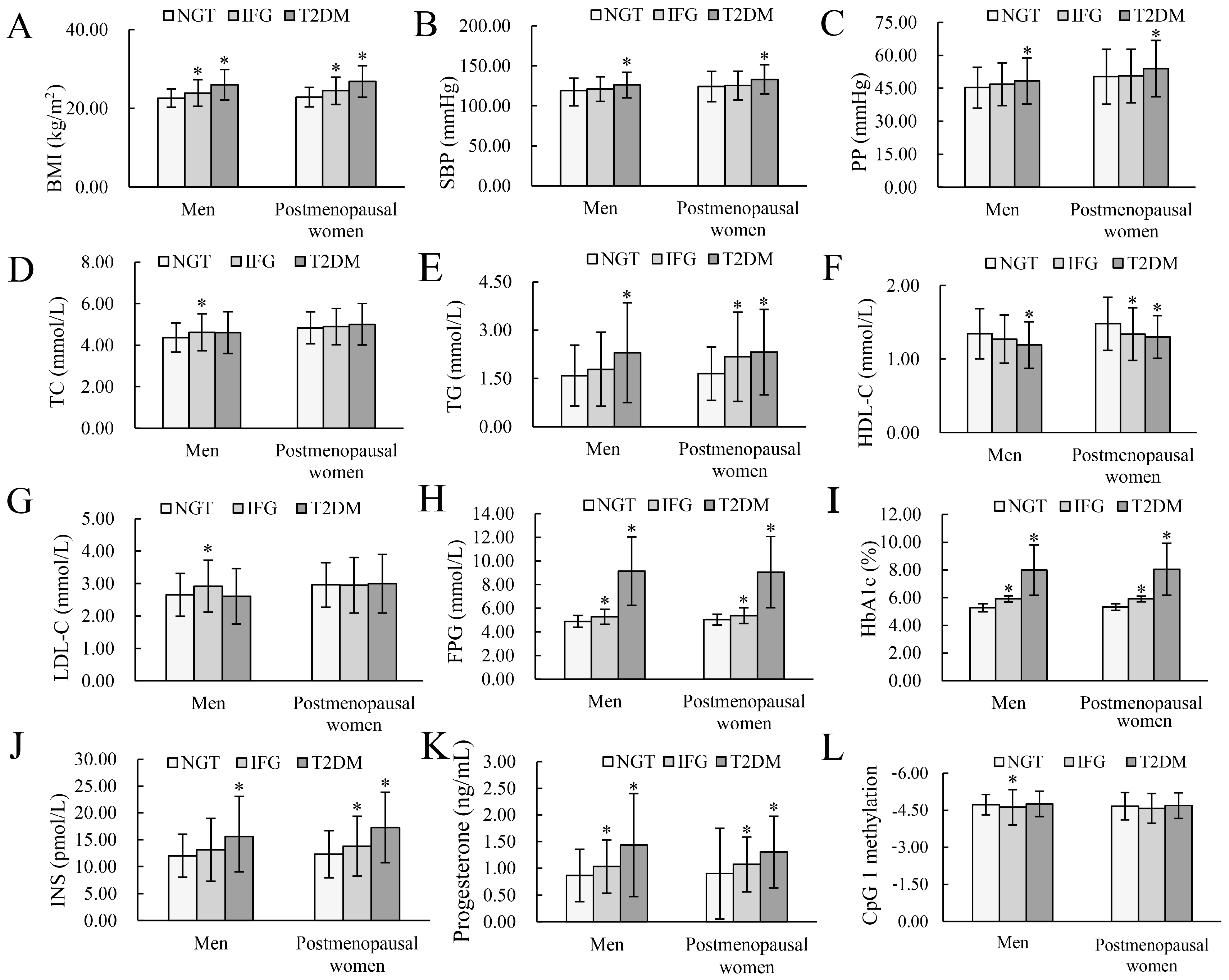


| Variables | Men | Postmenopausal Women | ||||
|---|---|---|---|---|---|---|
| NGT | IFG | T2DM | NGT | IFG | T2DM | |
| Subjects, n | 160 | 124 | 147 | 178 | 125 | 167 |
| Age (years) | 61.00 (54.00, 65.00) | 61.00 (54.00, 66.00) | 61.00 (54.00, 65.00) | 63.00 (57.75, 66.00) | 63.00 (58.00, 66.00) | 63.00 (58.00, 66.00) |
| BMI (kg/m2) | 22.23 (20.75, 24.26) | 23.93 (3.39) * | 25.86 (23.57, 29.15) * | 22.56 (20.77, 25.02) | 24.47 (3.49) * | 27.16 (23.86, 29.07) * |
| Smoking status, n (%) | ||||||
| Never smoking | 52 (32.50) | 49 (39.52) | 52 (35.37) * | 177 (99.44) | 125 (100.00) | 167 (100.00) |
| Give up smoking | 22 (13.75) | 20 (16.13) | 37 (25.17) * | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Smoking now | 86 (53.75) | 55 (44.35) | 58 (39.46) * | 1 (0.56) | 0 (0.00) | 0 (0.00) |
| Alcohol intake, n (%) | ||||||
| Never drinking | 87 (54.38) | 83 (66.94) | 65 (44.22) | 174 (97.75) | 122 (97.60) | 163 (97.60) |
| To give up drinking | 24 (15.00) | 15 (12.10) | 21 (14.28) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Drinking now | 49 (30.62) | 26 (20.97) | 61 (41.50) | 4 (2.25) | 3 (2.40) | 4 (2.40) |
| Physical activity, n (%) | ||||||
| Low | 50 (31.25) | 37 (29.84) | 45 (30.61) | 39 (21.91) | 28 (22.40) | 47 (28.14) |
| Mediate | 58 (36.25) | 40 (32.26) | 52 (35.37) | 95 (53.37) | 65 (52.00) | 86 (51.50) |
| High | 52 (32.50) | 47 (37.90) | 50 (34.01) | 44 (24.72) | 32 (25.60) | 34 (20.36) |
| Marital status, n (%) | ||||||
| Married/cohabiting | 143 (89.38) | 112 (90.32) | 133 (90.48) | 151 (84.83) | 111 (88.80) | 150 (89.82) |
| Widowed/divorced/separation/single | 17 (10.62) | 12(9.68) | 14 (9.52) | 27 (15.17) | 14 (11.20) | 17 (10.18) |
| Level of education, n (%) | ||||||
| Illiteracy | 30 (18.75) | 16 (12.90) | 16 (10.88) | 73 (41.01) | 44 (35.20) | 62 (37.12) |
| Primary school | 41 (25.62) | 36 (29.03) | 39 (26.53) | 52 (29.21) | 39 (31.20) | 51 (30.54) |
| Junior secondary and above | 89 (55.63) | 72 (58.06) | 92 (62.59) | 53 (29.78) | 42 (33.60) | 54 (32.34) |
| Per capita monthly income, n (%) | ||||||
| <500, RMB | 59 (36.87) | 55 (44.36) | 64 (43.54) | 74 (41.57) | 49 (39.20) | 74 (44.31) |
| 500~, RMB | 51 (31.88) | 36 (29.03) | 38 (25.85) | 60 (33.71) | 41 (32.80) | 49 (29.34) |
| 1000~, RMB and above | 50 (31.25) | 33 (26.61) | 45 (30.61) | 44 (24.72) | 35 (28.00) | 44 (26.35) |
| Family history of T2DM, n (%) | 3 (1.88) | 2 (1.61) | 11 (7.48) * | 1 (0.56) | 0 (0.00) | 10 (5.99) * |
| SBP (mmHg) | 116.00 (108.00, 127.75) | 120.94 (15.31) | 124.00 (112.00, 138.00) * | 120.50 (111.75, 135.25) | 123.00 (114.00, 135.00) | 132.00 (120.00, 143.50) * |
| PP (mmHg) | 43.67 (39.67, 51.50) | 45.00 (41.33, 51.17) | 47.00 (41.67, 54.33) * | 50.28 (12.51) | 50.60 (12.20) | 53.96 (12.83) * |
| TC (mmol/L) | 4.37 (0.71) | 4.62 (0.89) * | 4.47 (3.99, 5.12) | 4.84 (0.77) | 4.90 (0.87) | 5.01 (1.00) |
| TG (mmol/L) | 1.32 (0.92, 2.04) | 1.46 (0.98, 2.25) | 1.72 (1.20, 2.94) * | 1.43 (1.02, 2.10) | 1.84 (1.28, 2.72) * | 1.97 (1.42, 2.84) * |
| HDL−C (mmol/L) | 1.29 (1.10, 1.58) | 1.24 (1.02, 1.49) | 1.15 (0.99, 1.43) * | 1.48 (0.36) | 1.28 (1.14, 1.55) * | 1.30 (0.29) * |
| LDL−C (mmol/L) | 2.65 (0.66) | 2.92 (0.80) * | 2.61 (0.85) | 2.96 (0.69) | 2.95 (0.86) | 2.89 (2.24, 3.66) |
| FPG (mmol/L) | 4.89 (0.50) | 5.21(4.83, 5.56) * | 8.25 (7.27, 10.33) * | 5.04 (0.46) | 5.32 (4.94, 5.81) * | 7.96 (7.24, 9.89) * |
| HbA1c (%) | 5.30 (5.10, 5.50) | 5.90 (5.70, 6.00) * | 7.50 (6.70, 9.10) * | 5.40 (5.20, 5.50) | 5.90 (5.80, 6.00) * | 7.50 (6.70, 9.00) * |
| INS (pmol/L) | 11.64 (9.42, 14.21) | 12.30 (9.28, 16.12) | 14.11 (10.42, 18.32) * | 12.30 (4.38) | 12.95 (10.28, 17.28) * | 15.79 (12.66, 21.91) * |
| Progesterone (ng/mL) | 0.80 (0.52, 1.05) | 0.94 (0.69, 1.36) * | 1.30 (0.82, 1.74) * | 0.80 (0.56, 1.05) | 1.03 (0.72, 1.30) * | 1.15 (0.92, 1.64) * |
| CpG 1 methylation | −4.72 (0.41) | −4.54 (−4.98, −4.24) * | −4.73 (−5.05, −4.43) | −4.68 (−4.97, −4.34) | −4.58 (0.60) | −4.67 (−4.96, −4.35) |
| CpG 2 methylation | −4.47 (0.31) | −4.44 (−4.66, −4.26) | −4.50 (0.28) | −4.46 (−4.65, −4.21) | −4.43 (0.29) | −4.50 (−4.66, −4.25) |
| CpG 3 methylation | −4.53 (−4.96, −4.19) | −4.45 (−5.01, −3.98) | −4.49 (−4.92, −4.09) | −4.42 (−4.87, −4.04) | −4.68 (0.85) | −4.52 (0.60) |
| Variables | Men | Postmenopausal Women | ||
|---|---|---|---|---|
| Crude Model | Adjusted Model a | Crude Model | Adjusted Model a | |
| IFG | ||||
| ESRα methylation (CpG 1) | ||||
| Dichotomies | 2.13 (1.32, 3.43) * | 1.77 (1.05, 3.00) * | 1.70 (1.07, 2.70) * | 1.82 (1.09, 3.04) * |
| T1 | Reference | Reference | Reference | Reference |
| T2 | 1.10 (0.61, 1.97) | 0.99 (0.52, 1.89) | 1.09 (0.61, 1.93) | 1.23 (0.66, 2.29) |
| T3 | 2.42 (1.35, 4.35) * | 2.02 (1.06, 3.84) * | 1.84 (1.05, 3.24) * | 1.98 (1.06, 3.72) * |
| P-trend | 0.003 | 0.030 | 0.033 | 0.033 |
| Progesterone | ||||
| Dichotomies | 2.00 (1.24, 3.22) * | 2.03 (1.18, 3.49) * | 2.25 (1.41, 3.60) * | 2.13 (1.27, 3.56) * |
| T1 | Reference | Reference | Reference | Reference |
| T2 | 1.25 (0.70, 2.24) | 1.17 (0.62, 2.21) | 1.37 (0.76, 2.46) | 1.14 (0.61, 2.14) |
| T3 | 2.13 (1.19, 3.81) * | 2.26 (1.15, 4.47) * | 3.19 (1.79, 5.71) * | 2.65 (1.42, 4.95) * |
| P-trend | 0.011 | 0.020 | <0.001 | 0.002 |
| T2DM | ||||
| ESRα methylation (CpG 1) | ||||
| Dichotomies | 1.10 (0.70, 1.71) | 1.15 (0.64, 2.05) | 1.04 (0.68, 1.58) | 1.00 (0.57, 1.73) |
| T1 | Reference | Reference | Reference | Reference |
| T2 | 1.02 (0.59, 1.76) | 0.83 (0.41, 1.69) | 1.37 (0.81, 2.30) | 1.42 (0.73, 2.76) |
| T3 | 1.29 (0.74, 2.23) | 1.56 (0.77, 3.18) | 0.97 (0.57, 1.62) | 0.89 (0.45, 1.77) |
| P-trend | 0.365 | 0.224 | 0.895 | 0.745 |
| Progesterone | ||||
| Dichotomies | 4.05 (2.52, 6.51) * | 3.00 (1.63, 5.52) * | 4.45 (2.83, 6.99) * | 3.30 (1.85, 5.90) * |
| T1 | Reference | Reference | Reference | Reference |
| T2 | 1.65 (0.92, 2.96) | 1.52 (0.74, 3.10) * | 2.69 (1.55, 4.68) * | 2.87 (1.43, 5.77) * |
| T3 | 8.29 (4.43, 15.53) * | 6.40 (2.83, 14.45) * | 6.75 (3.79, 12.03) * | 5.28 (2.52, 11.08) * |
| P-trend | <0.001 | <0.001 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, B.; Wang, L.; Wei, D.; Huo, W.; Jing, T.; Wang, C.; Mao, Z. Combined Effects of ESRα DNA Methylation and Progesterone on Glucose Metabolic Disorders: The Henan Rural Cohort Study. Nutrients 2023, 15, 1659. https://doi.org/10.3390/nu15071659
Feng B, Wang L, Wei D, Huo W, Jing T, Wang C, Mao Z. Combined Effects of ESRα DNA Methylation and Progesterone on Glucose Metabolic Disorders: The Henan Rural Cohort Study. Nutrients. 2023; 15(7):1659. https://doi.org/10.3390/nu15071659
Chicago/Turabian StyleFeng, Bo, Lulu Wang, Dandan Wei, Wenqian Huo, Tao Jing, Chongjian Wang, and Zhenxing Mao. 2023. "Combined Effects of ESRα DNA Methylation and Progesterone on Glucose Metabolic Disorders: The Henan Rural Cohort Study" Nutrients 15, no. 7: 1659. https://doi.org/10.3390/nu15071659
APA StyleFeng, B., Wang, L., Wei, D., Huo, W., Jing, T., Wang, C., & Mao, Z. (2023). Combined Effects of ESRα DNA Methylation and Progesterone on Glucose Metabolic Disorders: The Henan Rural Cohort Study. Nutrients, 15(7), 1659. https://doi.org/10.3390/nu15071659









