Docosahexaenoic Acid, a Key Compound for Enhancing Sensitization to Drug in Doxorubicin-Resistant MCF-7 Cell Line
Abstract
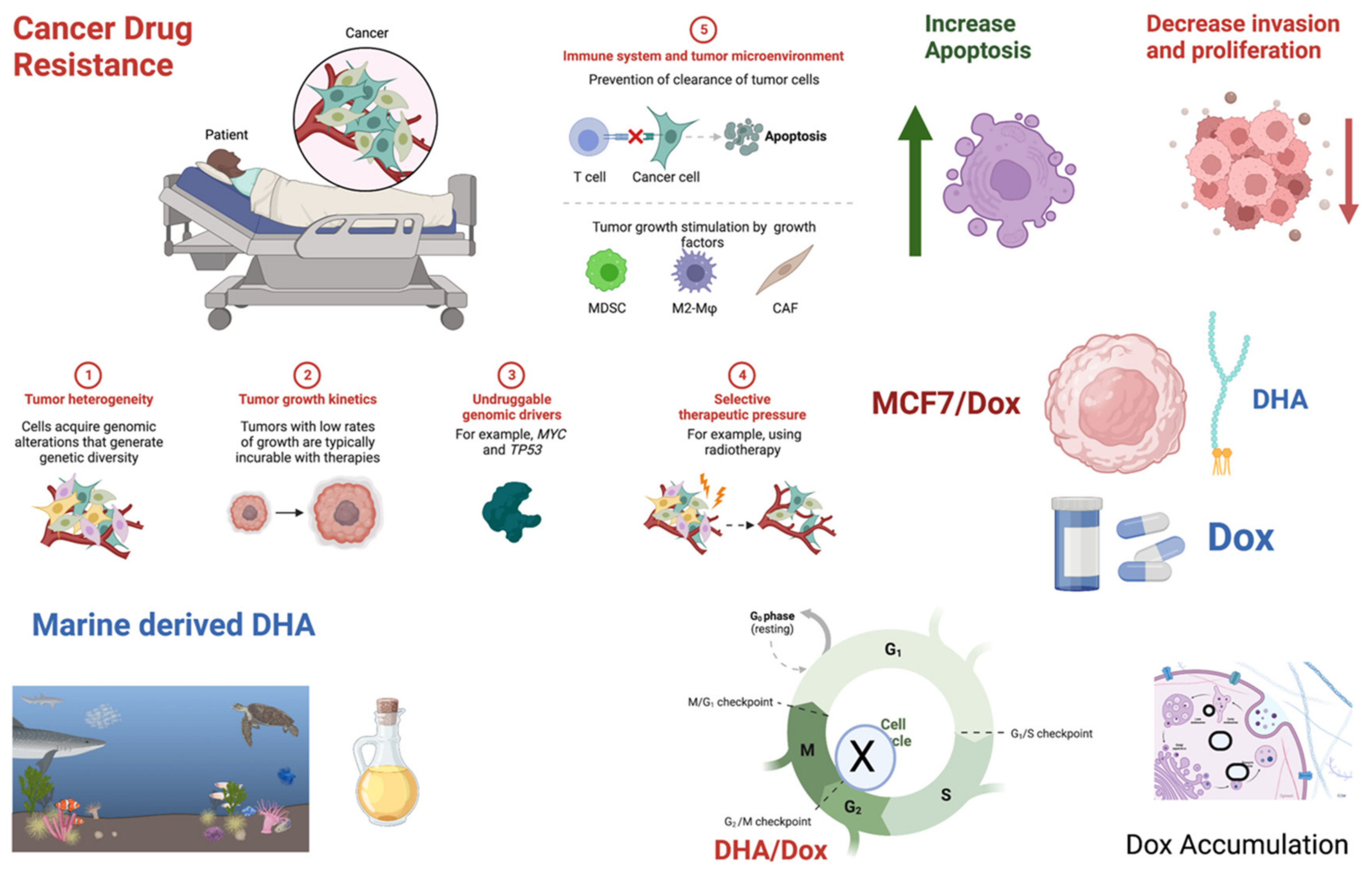
1. Introduction
2. Methods
2.1. Cell Culture
2.2. Cell Proliferation Assay
2.3. Invasion Assay
2.4. Doxorubicin Accumulation
2.5. Cell Cycle Analysis by Flow Cytometry
2.6. Annexin V Apoptosis Assay
2.7. RNA Isolation and Quantitative Real-Time RT-PCR
2.8. Lipid Peroxidation Assay
2.9. Statistical Analysis
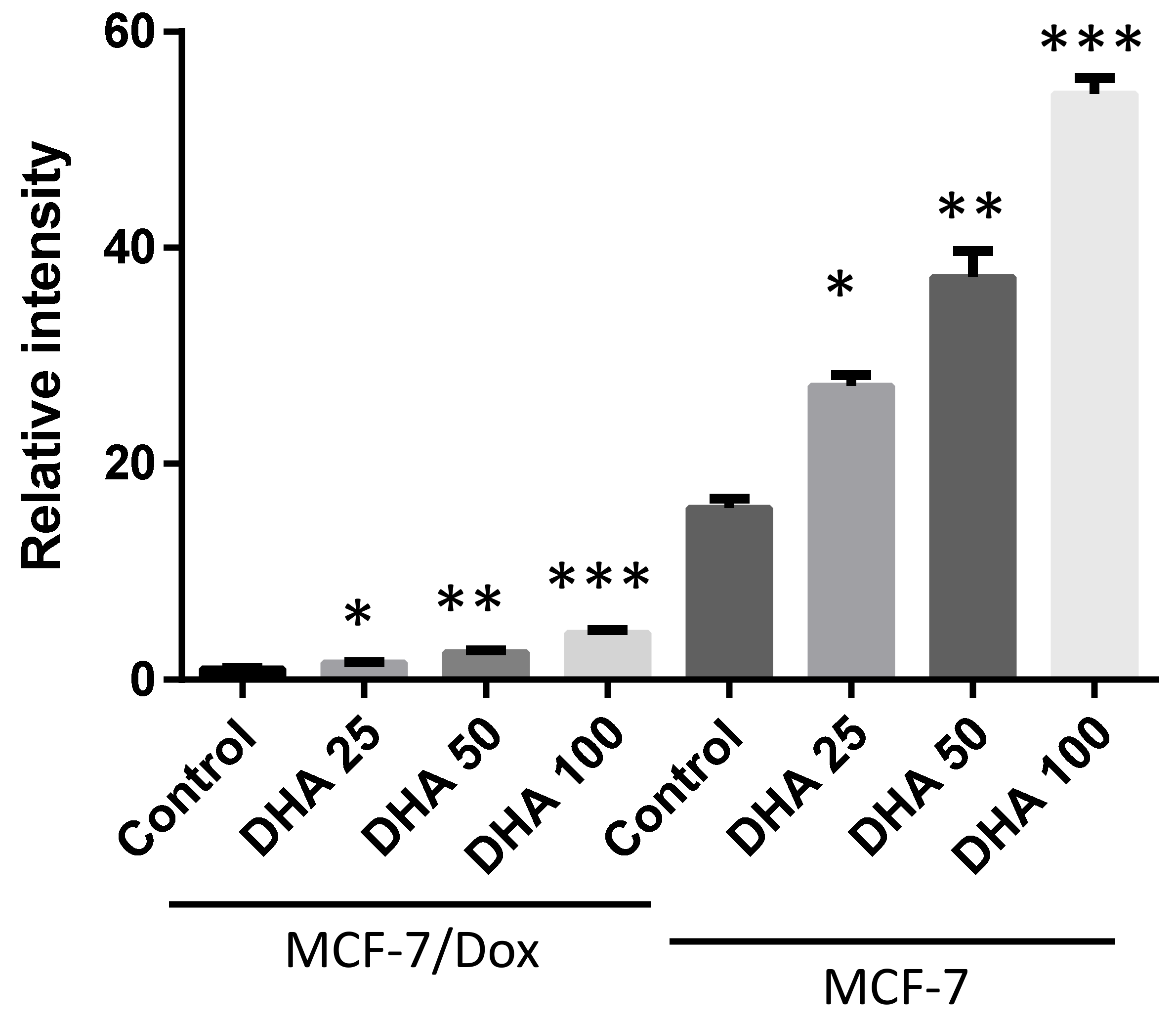
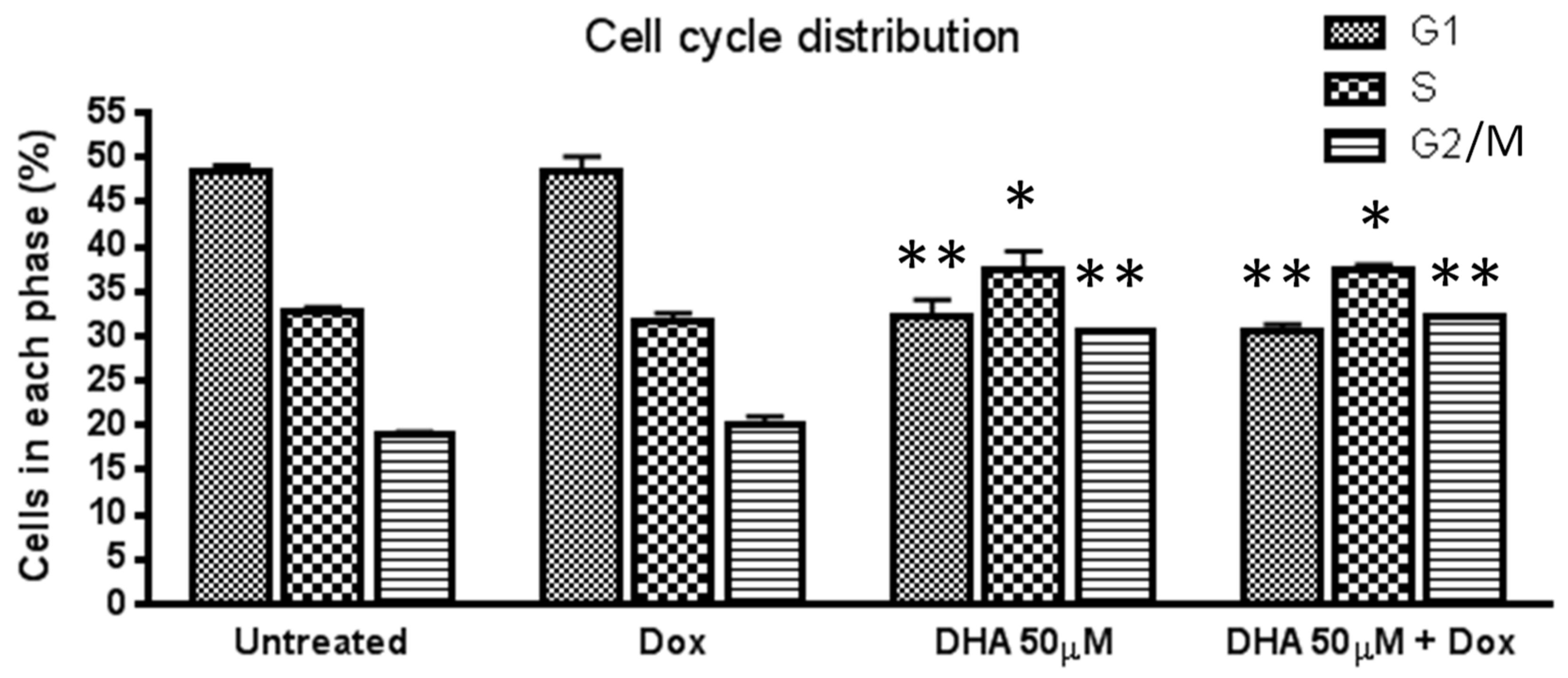
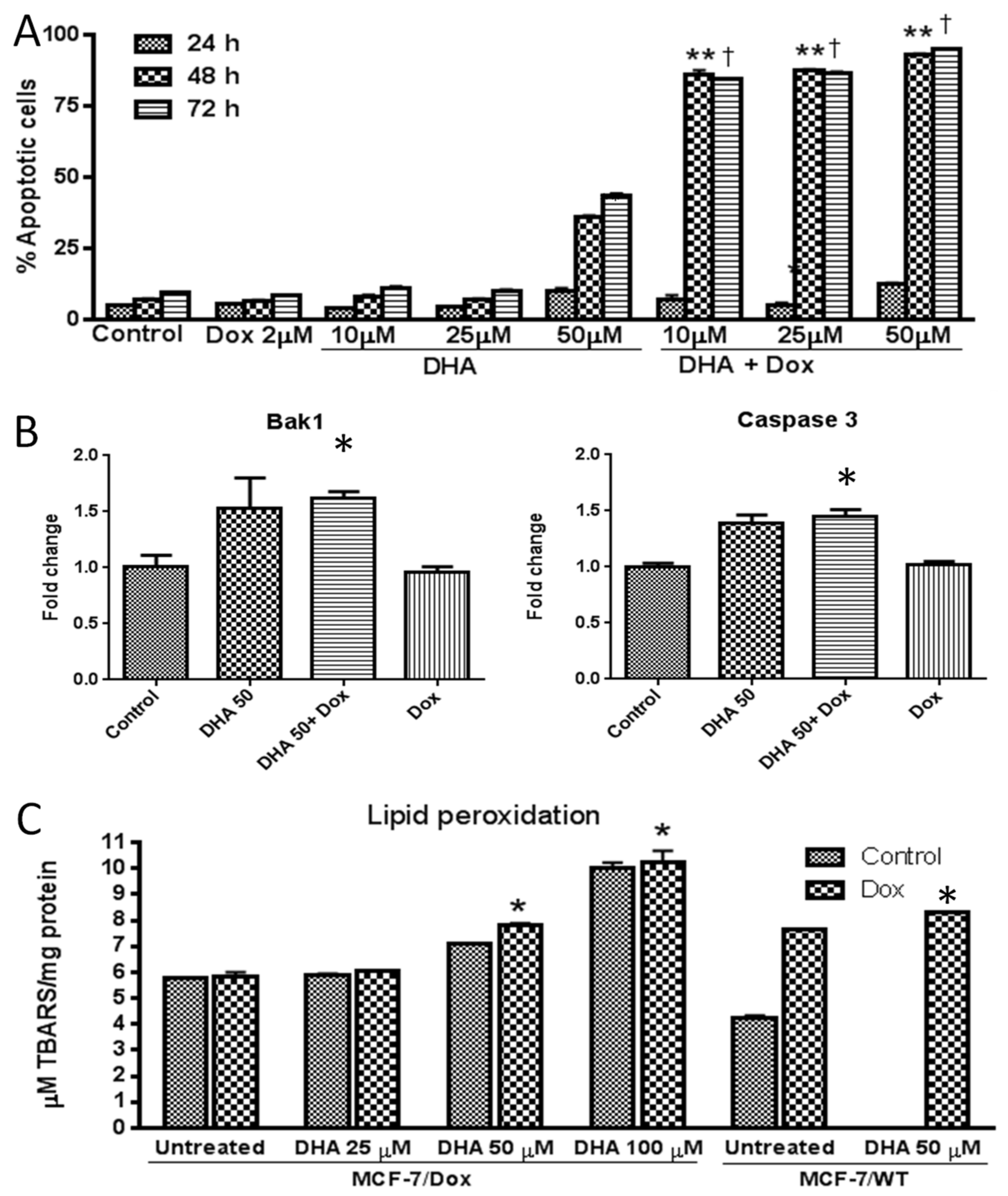
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Satsangi, A.; Roy, S.S.; Satsangi, R.K.; Tolcher, A.W.; Vadlamudi, R.K.; Goins, B.; Ong, J.L. Synthesis of a novel, sequentially active-targeted drug delivery nanoplatform for breast cancer therapy. Biomaterials 2015, 59, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Sharmin, S.; Rahaman, M.M.; Martorell, M.; Sastre-Serra, J.; Sharifi-Rad, J.; Butnariu, M.; Bagiu, I.C.; Bagiu, R.V.; Islam, M.T. Cytotoxicity of synthetic derivatives against breast cancer and multi-drug resistant breast cancer cell lines: A literature-based perspective study. Cancer Cell Int. 2021, 21, 612. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO: Breast Cancer; World Health Organization (WHO) Report 2021; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Liu, H.; Liu, Y.Z.; Zhang, F.; Wang, H.S.; Zhang, G.; Zhou, B.H.; Zuo, Y.L.; Cai, S.H.; Bu, X.Z.; Du, J. Identification of potential pathways involved in the induction of cell cycle arrest and apoptosis by a new 4-arylidene curcumin analogue T63 in lung cancer cells: A comparative proteomic analysis. Mol. Biosyst. 2014, 10, 1320–1331. [Google Scholar] [CrossRef]
- Favaloro, B.; Allocati, N.; Graziano, V.; Di Ilio, C.; De Laurenzi, V. Role of apoptosis in disease. Aging 2012, 4, 330–349. [Google Scholar] [CrossRef]
- Glass, A.G.; Lacey, J.V.; Jr Carreon, J.D.; Hoover, R.N. Breast cancer incidence, 1980–2006: Combined roles of menopausal hormone therapy, screening mammography, and estrogen receptor status. J. Natl. Cancer Inst. 2007, 99, 1152–1161. [Google Scholar] [CrossRef]
- Berry, D.A.; Cronin, K.A.; Plevritis, S.K.; Fryback, D.G.; Clarke, L.; Zelen, M.; Mandelblatt, J.S.; Yakovlev, A.Y.; Habbema, J.D.; Feuer, E.J.; et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N. Engl. J. Med. 2005, 353, 1784–1792. [Google Scholar] [CrossRef]
- Jemal, A.; Ward, E.; Thun, M.J. Recent trends in breast cancer incidence rates by age and tumor characteristics among U.S. women. Breast. Cancer Res. 2007, 9, R28. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zang, C.; Fenner, M.H.; Possinger, K.; Elstner, E. PPARgamma ligands and ATRA inhibit the invasion of human breast cancer cells in vitro. Breast. Cancer Res. Treat. 2003, 79, 63–74. [Google Scholar] [CrossRef]
- Chavez, K.J.; Garimella, S.V.; Lipkowitz, S. Triple negative breast cancer cell lines: One tool in the search for better treatment of triple negative breast cancer. Breast. Dis. 2010, 32, 35–48. [Google Scholar] [CrossRef]
- Wyrebska, A.; Gach, K.; Lewandowska, U.; Szewczyk, K.; Hrabec, E.; Modranka, J.; Jakubowski, R.; Janecki, T.; Szymanski, J.; Janecka, A. Anticancer Activity of New Synthetic alpha-Methylene-delta-Lactones on Two Breast Cancer Cell Lines. Basic. Clin. Pharm. Toxicol. 2013, 113, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.M.; Yeap, S.K.; Abu, N.; Lim, K.L.; Ky, H.; Pauzi, A.Z.M.; Ho, W.Y.; Tan, S.W.; Alan-Ong, H.K.; Zareen, S.; et al. Synthetic curcumin derivative DK1 possessed G2/M arrest and induced apoptosis through accumulation of intracellular ROS in MCF-7 breast cancer cells. Cancer Cell Int. 2017, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Kheirollahi, A.; Pordeli, M.; Safavi, M.; Mashkouri, S.; Naimi-Jamal, M.R.; Ardestani, S.K. Cytotoxic and apoptotic effects of synthetic benzochromene derivatives on human cancer cell lines. Naunyn. Schmiedebergs. Arch Pharm. 2014, 387, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Cameron, I.L.; Munoz, J.; Barnes, C.J.; Hardman, W.E. High dietary level of synthetic vitamin E on lipid peroxidation, membrane fatty acid composition and cytotoxicity in breast cancer xenograft and in mouse host tissue. Cancer Cell Int. 2003, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.D.; Diaz-Cruz, E.S.; Landini, S.; Kim, Y.W.; Brueggemeier, R.W. Evaluation of synthetic isoflavones on cell proliferation, estrogen receptor binding affinity, and apoptosis in human breast cancer cells. J. Steroid. Biochem. Mol. Biol. 2008, 108, 23–31. [Google Scholar] [CrossRef]
- Fabian, C.J.; Kimler, B.F.; Hursting, S.D. Omega-3 fatty acids for breast cancer prevention and survivorship. Breast. Cancer Res. 2015, 17, 62. [Google Scholar] [CrossRef]
- Pizato, N.; Luzete, B.C.; Kiffer, L.; Correa, L.H.; de Oliveira Santos, I.; Assumpcao, J.A.F.; Ito, M.K.; Magalhaes, K.G. Omega-3 docosahexaenoic acid induces pyroptosis cell death in triple-negative breast cancer cells. Sci. Rep. 2018, 8, 1952. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Yang, Y.; Wang, F.; Yang, W.G.; Zhang, J.J.; Zou, Z.Q. Docosahexaenoic acid monoglyceride induces apoptosis and autophagy in breast cancer cells via lipid peroxidation-mediated endoplasmic reticulum stress. J. Food Sci. 2021, 86, 4704–4716. [Google Scholar] [CrossRef]
- Aslan, C.; Maralbashi, S.; Kahroba, H.; Asadi, M.; Soltani-Zangbar, M.S.; Javadian, M.; Shanehbandi, D.; Baradaran, B.; Darabi, M.; Kazemi, T. Docosahexaenoic acid (DHA) inhibits pro-angiogenic effects of breast cancer cells via down-regulating cellular and exosomal expression of angiogenic genes and microRNAs. Life Sci. 2020, 258, 118094. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.M.; Thompson, H.; Vanden-Heuvel, J.P.; Sun, Y.W.; Trushin, N.; Aliaga, C.; Gowda, K.; Amin, S.; Stanley, B.; Manni, A.; et al. Lipoxygenase catalyzed metabolites derived from docosahexaenoic acid are promising antitumor agents against breast cancer. Sci. Rep. 2021, 11, 410. [Google Scholar] [CrossRef] [PubMed]
- Kuban-Jankowska, A.; Gorska-Ponikowska, M.; Sahu, K.K.; Kostrzewa, T.; Wozniak, M.; Tuszynski, J. Docosahexaenoic Acid Inhibits PTP1B Phosphatase and the Viability of MCF-7 Breast Cancer Cells. Nutrients 2019, 11, 2554. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Veigas, J.M.; Williams, P.J.; Fernandes, G. DHA is a more potent inhibitor of breast cancer metastasis to bone and related osteolysis than EPA. Breast. Cancer Res. Treat 2013, 141, 341–352. [Google Scholar] [CrossRef]
- Liscovitch, M.; Lavie, Y. Cancer multidrug resistance: A review of recent drug discovery research. IDrugs 2002, 5, 349–355. [Google Scholar] [PubMed]
- Aller, S.G.; Yu, J.; Ward, A.; Weng, Y.; Chittaboina, S.; Zhuo, R.; Harrell, P.M.; Trinh, Y.T.; Zhang, Q.; Urbatsch, I.L.; et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 2009, 323, 1718–1722. [Google Scholar] [CrossRef] [PubMed]
- Zahreddine, H.; Borden, K.L. Mechanisms and insights into drug resistance in cancer. Front. Pharm. 2013, 4, 28. [Google Scholar] [CrossRef]
- Ling, V. Multidrug resistance: Molecular mechanisms and clinical relevance. Cancer Chemother. Pharm. 1997, 40, S3–S8. [Google Scholar] [CrossRef]
- Ozben, T. Mechanisms and strategies to overcome multiple drug resistance in cancer. FEBS Lett 2006, 580, 2903–2909. [Google Scholar] [CrossRef]
- Higgins, C.F.; Gottesman, M.M. Is the multidrug transporter a flippase? Trends Biochem. Sci. 1992, 17, 18–21. [Google Scholar] [CrossRef]
- Kerbel, R.S.; Kobayashi, H.; Graham, C.H. Intrinsic or acquired drug resistance and metastasis: Are they linked phenotypes? J. Cell Biochem. 1994, 56, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Christowitz, C.; Davis, T.; Isaacs, A.; van Niekerk, G.; Hattingh, S.; Engelbrecht, A.M. Mechanisms of doxorubicin-induced drug resistance and drug resistant tumour growth in a murine breast tumour model. BMC Cancer 2019, 19, 757. [Google Scholar] [CrossRef]
- Conklin, K.A. Chemotherapy-associated oxidative stress: Impact on chemotherapeutic effectiveness. Integr. Cancer Ther. 2004, 3, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.K. Free radicals in anticancer drug pharmacology. Chem. Biol. Interact. 1989, 69, 293–317. [Google Scholar] [CrossRef]
- Gaba, R.C.; Emmadi, R.; Parvinian, A.; Casadaban, L.C. Correlation of Doxorubicin Delivery and Tumor Necrosis after Drug-eluting Bead Transarterial Chemoembolization of Rabbit VX2 Liver Tumors. Radiology 2016, 280, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmcol. 2013, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Surma, M.; Gough, G.; Shi, S.; Lambert-Cheatham, N.; Chang, J.; Shi, J. Dissecting the Mechanisms of Doxorubicin and Oxidative Stress-Induced Cytotoxicity: The Involvement of Actin Cytoskeleton and ROCK1. PLoS ONE 2015, 10, e0131763. [Google Scholar] [CrossRef]
- Chajes, V.; Sattler, W.; Stranzl, A.; Kostner, G.M. Influence of n-3 fatty acids on the growth of human breast cancer cells in vitro: Relationship to peroxides and vitamin-E. Breast Cancer Res. Treat. 1995, 34, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Newell, M.; Patel, D.; Goruk, S.; Field, C.J. Docosahexaenoic Acid Incorporation Is Not Affected by Doxorubicin Chemotherapy in either Whole Cell or Lipid Raft Phospholipids of Breast Cancer Cells in vitro and Tumor Phospholipids in vivo. Lipids 2020, 55, 549–565. [Google Scholar] [CrossRef]
- D’Eliseo, D.; Velotti, F. Omega-3 Fatty Acids and Cancer Cell Cytotoxicity: Implications for Multi-Targeted Cancer Therapy. J. Clin. Med. 2016, 5, 15. [Google Scholar] [CrossRef]
- Ewaschuk, J.B.; Newell, M.; Field, C.J. Docosahexanoic acid improves chemotherapy efficacy by inducing CD95 translocation to lipid rafts in ER(-) breast cancer cells. Lipids 2012, 47, 1019–1030. [Google Scholar] [CrossRef]
- Newell, M.; Brun, M.; Field, C.J. Treatment with DHA Modifies the Response of MDA-MB-231 Breast Cancer Cells and Tumors from nu/nu Mice to Doxorubicin through Apoptosis and Cell Cycle Arrest. J. Nutr. 2019, 149, 46–56. [Google Scholar] [CrossRef]
- Newell, M.; Goruk, S.; Mazurak, V.; Postovit, L.; Field, C.J. Role of docosahexaenoic acid in enhancement of docetaxel action in patient-derived breast cancer xenografts. Breast. Cancer Res. Treat. 2019, 177, 357–367. [Google Scholar] [CrossRef]
- Schley, P.D.; Brindley, D.N.; Field, C.J. (n-3) PUFA alter raft lipid composition and decrease epidermal growth factor receptor levels in lipid rafts of human breast cancer cells. J. Nutr. 2007, 137, 548–553. [Google Scholar] [CrossRef]
- Corsetto, P.A.; Colombo, I.; Kopecka, J.; Rizzo, A.M.; Riganti, C. Omega-3 Long Chain Polyunsaturated Fatty Acids as Sensitizing Agents and Multidrug Resistance Revertants in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2770. [Google Scholar] [CrossRef]
- Germain, E.; Chajes, V.; Cognault, S.; Lhuillery, C.; Bougnoux, P. Enhancement of doxorubicin cytotoxicity by polyunsaturated fatty acids in the human breast tumor cell line MDA-MB-231: Relationship to lipid peroxidation. Int. J. Cancer 1998, 75, 578–583. [Google Scholar] [CrossRef]
- Menendez, J.A.; Lupu, R.; Colomer, R. Exogenous supplementation with omega-3 polyunsaturated fatty acid docosahexaenoic acid (DHA; 22:6n-3) synergistically enhances taxane cytotoxicity and downregulates Her-2/neu (c-erbB-2) oncogene expression in human breast cancer cells. Eur. J. Cancer Prev. 2005, 14, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.S.; Wang, P.; Yamabe, N.; Fukui, M.; Jay, T.; Zhu, B.T. Docosahexaenoic acid induces apoptosis in MCF-7 cells in vitro and in vivo via reactive oxygen species formation and caspase 8 activation. PLoS ONE 2010, 5, e10296. [Google Scholar] [CrossRef]
- Bougnoux, P.; Hajjaji, N.; Ferrasson, M.N.; Giraudeau, B.; Couet, C.; Le Floch, O. Improving outcome of chemotherapy of metastatic breast cancer by docosahexaenoic acid: A phase II trial. Br. J. Cancer 2009, 101, 1978–1985. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Guo, S.; Li, Z. In situ characterizing membrane lipid phenotype of breast cancer cells using mass spectrometry profiling. Sci. Rep. 2015, 5, 11298. [Google Scholar] [CrossRef] [PubMed]
- Stillwell, W.; Shaikh, S.R.; Zerouga, M.; Siddiqui, R.; Wassall, S.R. Docosahexaenoic acid affects cell signaling by altering lipid rafts. Reprod. Nutr. Dev. 2005, 45, 559–579. [Google Scholar] [CrossRef]
- Fodil, M.; Blanckaert, V.; Ulmann, L.; Mimouni, V.; Chenais, B. Contribution of n-3 Long-Chain Polyunsaturated Fatty Acids to the Prevention of Breast Cancer Risk Factors. Int. J. Environ. Res. Public Health 2022, 19, 7936. [Google Scholar] [CrossRef]
- Chen, X.; Li, W.; Ren, J.; Huang, D.; He, W.T.; Song, Y.; Yang, C.; Li, W.; Zheng, X.; Chen, P.; et al. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 2014, 24, 105–121. [Google Scholar] [CrossRef]
- Turk, H.F.; Chapkin, R.S. Membrane lipid raft organization is uniquely modified by n-3 polyunsaturated fatty acids. Prostaglandins. Leukot. Essent. Fat. Acids. 2013, 88, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Biondo, P.D.; Brindley, D.N.; Sawyer, M.B.; Field, C.J. The potential for treatment with dietary long-chain polyunsaturated n-3 fatty acids during chemotherapy. J. Nutr. Biochem. 2008, 19, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K.R.; Kikawa, K.D.; Mouradian, M.; Hernandez, K.; McKinnon, K.M.; Ahwah, S.M.; Pardini, R.S. Docosahexaenoic acid alters epidermal growth factor receptor-related signaling by disrupting its lipid raft association. Carcinogenesis 2010, 31, 1523–1530. [Google Scholar] [CrossRef]
- Chapkin, R.S.; Wang, N.; Fan, Y.Y.; Lupton, J.R.; Prior, I.A. Docosahexaenoic acid alters the size and distribution of cell surface microdomains. Biochim. Biophys. Acta 2008, 1778, 466–471. [Google Scholar] [CrossRef]
- Kim, W.; Fan, Y.Y.; Barhoumi, R.; Smith, R.; McMurray, D.N.; Chapkin, R.S. n-3 polyunsaturated fatty acids suppress the localization and activation of signaling proteins at the immunological synapse in murine CD4+ T cells by affecting lipid raft formation. J. Immunol. 2008, 181, 6236–6243. [Google Scholar] [CrossRef]
- Kauffman, M.K.; Kauffman, M.E.; Zhu, H.; Jia, Z.; Li, Y.R. Fluorescence-Based Assays for Measuring Doxorubicin in Biological Systems. React. Oxyg. Species 2016, 2, 432–439. [Google Scholar] [CrossRef]
- Brown, I.; Lee, J.; Sneddon, A.A.; Cascio, M.G.; Pertwee, R.G.; Wahle, K.W.J.; Rotondo, D.; Heys, S.D. Anticancer effects of n-3 EPA and DHA and their endocannabinoid derivatives on breast cancer cell growth and invasion. Prostaglandins. Leukot. Essent. Fat. Acids 2020, 156, 102024. [Google Scholar] [CrossRef]
- Baumgartner, M.; Sturlan, S.; Roth, E.; Wessner, B.; Bachleitner-Hofmann, T. Enhancement of arsenic trioxide-mediated apoptosis using docosahexaenoic acid in arsenic trioxide-resistant solid tumor cells. Int. J. Cancer 2004, 112, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, L.; Goupille, C.; Blanc, C.; Pinault, M.; Domingo, I.; Guimaraes, C.; Bougnoux, P.; Chevalier, S.; Maheo, K. Long chain n-3 polyunsaturated fatty acids increase the efficacy of docetaxel in mammary cancer cells by downregulating Akt and PKCepsilon/delta-induced ERK pathways. Biochim. Biophys. Acta 2016, 1861, 380–390. [Google Scholar] [CrossRef]
- Vibet, S.; Goupille, C.; Bougnoux, P.; Steghens, J.P.; Gore, J.; Maheo, K. Sensitization by docosahexaenoic acid (DHA) of breast cancer cells to anthracyclines through loss of glutathione peroxidase (GPx1) response. Free Radic. Biol. Med. 2008, 44, 1483–1491. [Google Scholar] [CrossRef]
- Lindskog, M.; Gleissman, H.; Ponthan, F.; Castro, J.; Kogner, P.; Johnsen, J.I. Neuroblastoma cell death in response to docosahexaenoic acid: Sensitization to chemotherapy and arsenic-induced oxidative stress. Int. J. Cancer 2006, 118, 2584–2593. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, C.; Jin, X.; Li, P.; Ye, F.; Zhao, T.; Gong, L.; Li, Q. Genistein enhances the radiosensitivity of breast cancer cells via G(2)/M cell cycle arrest and apoptosis. Molecules 2013, 18, 13200–13217. [Google Scholar] [CrossRef]
- Kimani, S.; Chakraborty, S.; Irene, I.; de la Mare, J.; Edkins, A.; du Toit, A.; Loos, B.; Blanckenberg, A.; Van Niekerk, A.; Costa-Lotufo, L.V.; et al. The palladacycle, BTC2, exhibits anti-breast cancer and breast cancer stem cell activity. Biochem. Pharm. 2021, 190, 114598. [Google Scholar] [CrossRef]
- Gopisetty, M.K.; Adamecz, D.I.; Nagy, F.I.; Baji, A.; Lathira, V.; Szabo, M.R.; Gaspar, R.; Csont, T.; Frank, E.; Kiricsi, M. Androstano-arylpyrimidines: Novel small molecule inhibitors of MDR1 for sensitizing multidrug-resistant breast cancer cells. Eur. J. Pharm. Sci. 2021, 156, 105587. [Google Scholar] [CrossRef]
- Shinde, A.; Kulkoyluoglu Cotul, E.; Chen, H.; Smith, A.; Libring, S.; Solorio, L.; Wendt, M.K. Transglutaminase-2 mediates acquisition of neratinib resistance in metastatic breast cancer. Mol. Biomed. 2022, 3, 19. [Google Scholar] [CrossRef]
- Wu, C.P.; Hung, C.Y.; Murakami, M.; Wu, Y.S.; Lin, C.L.; Huang, Y.H.; Hung, T.H.; Yu, J.S.; Ambudkar, S.V. P-glycoprotein Mediates Resistance to the Anaplastic Lymphoma Kinase Inhiitor Ensartinib in Cancer Cells. Cancers 2022, 14, 2341. [Google Scholar] [CrossRef]
- Herman, J.F.; Mangala, L.S.; Mehta, K. Implications of increased tissue transglutaminase (TG2) expression in drug-resistant breast cancer (MCF-7) cells. Oncogene 2006, 25, 3049–3058. [Google Scholar] [CrossRef]
- Cheng, K.; Wang, X.H.; Hua, Y.T.; Zhang, Y.Z.; Han, Y.; Yang, Z.L. The tissue transglutaminase: A potential target regulating MDR in breast cancer. Eur. Rev. Med. Pharm. Sci. 2020, 24, 6175–6184. [Google Scholar]
- Cabaud, O.; Berger, L.; Crompot, E.; Adelaide, J.; Finetti, P.; Garnier, S.; Guille, A.; Carbuccia, N.; Farina, A.; Agavnian, E.; et al. Overcoming Resistance to Anti-Nectin-4 Antibody-Drug Conjugate. Mol. Cancer Ther. 2022, 21, 1227–1235. [Google Scholar] [CrossRef]
- Robinson, K.; Tiriveedhi, V. Perplexing Role of P-Glycoprotein in Tumor Microenvironment. Front Oncol. 2020, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Jaitak, V. Natural products as multidrug resistance modulators in cancer. Eur. J. Med. Chem. 2019, 176, 268–291. [Google Scholar] [CrossRef]
- Wang, F.; Lv, P.; Gu, Y.; Li, L.; Ge, X.; Guo, G. Galectin-1 knockdown improves drug sensitivity of breast cancer by reducing P-glycoprotein expression through inhibiting the Raf-1/AP-1 signaling pathway. Oncotarget 2017, 8, 25097–25106. [Google Scholar] [CrossRef]
- Kuan, C.Y.; Walker, T.H.; Luo, P.G.; Chen, C.F. Long-chain polyunsaturated fatty acids promote paclitaxel cytotoxicity via inhibition of the MDR1 gene in the human colon cancer Caco-2 cell line. J. Am. Coll. Nutr. 2011, 30, 265–273. [Google Scholar] [CrossRef]
- Maheo, K.; Vibet, S.; Steghens, J.P.; Dartigeas, C.; Lehman, M.; Bougnoux, P.; Gore, J. Differential sensitization of cancer cells to doxorubicin by DHA: A role for lipoperoxidation. Free Radic. Biol. Med. 2005, 39, 742–751. [Google Scholar] [CrossRef]
- Vermonden, P.; Vancoppenolle, M.; Dierge, E.; Mignolet, E.; Cuvelier, G.; Knoops, B.; Page, M.; Debier, C.; Feron, O.; Larondelle, Y. Punicic Acid Triggers Ferroptotic Cell Death in Carcinoma Cells. Nutrients 2021, 13, 2751. [Google Scholar] [CrossRef]
- Dierge, E.; Debock, E.; Guilbaud, C.; Corbet, C.; Mignolet, E.; Mignard, L.; Bastien, E.; Dessy, C.; Larondelle, Y.; Feron, O. Peroxidation of n-3 and n-6 polyunsaturated fatty acids in the acidic tumor environment leads to ferroptosis-mediated anticancer effects. Cell Metab. 2021, 33, 1701–1715.e1705. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Wang, Z.; Zhang, Z.; Cao, Y.; Wei, Z.; Shao, J.; Chen, A.; Zhang, F.; Zheng, S. Dihydroartemisinin alleviates hepatic fibrosis through inducing ferroptosis in hepatic stellate cells. Biofactors 2021, 47, 801–818. [Google Scholar] [CrossRef]
- Shan, K.; Feng, N.; Zhu, D.; Qu, H.; Fu, G.; Li, J.; Cui, J.; Chen, H.; Wang, R.; Qi, Y.; et al. Free docosahexaenoic acid promotes ferroptotic cell death via lipoxygenase dependent and independent pathways in cancer cells. Eur. J. Nutr. 2022, 61, 4059–4075. [Google Scholar] [CrossRef] [PubMed]
- Yurko-Mauro, K.; Van Elswyk, M.; Teo, L. A Scoping Review of Interactions between Omega-3 Long-Chain Polyunsaturated Fatty Acids and Genetic Variation in Relation to Cancer Risk. Nutrients 2020, 12, 1647. [Google Scholar] [CrossRef] [PubMed]
- Newell, M.; Mazurak, V.; Postovit, L.M.; Field, C.J. N-3 Long-Chain Polyunsaturated Fatty Acids, Eicosapentaenoic and Docosahexaenoic Acid, and the Role of Supplementation during Cancer Treatment: A Scoping Review of Current Clinical Evidence. Cancers 2021, 13, 1206. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crovella, S.; Ouhtit, A.; Rahman, S.M.; Rahman, M.M. Docosahexaenoic Acid, a Key Compound for Enhancing Sensitization to Drug in Doxorubicin-Resistant MCF-7 Cell Line. Nutrients 2023, 15, 1658. https://doi.org/10.3390/nu15071658
Crovella S, Ouhtit A, Rahman SM, Rahman MM. Docosahexaenoic Acid, a Key Compound for Enhancing Sensitization to Drug in Doxorubicin-Resistant MCF-7 Cell Line. Nutrients. 2023; 15(7):1658. https://doi.org/10.3390/nu15071658
Chicago/Turabian StyleCrovella, Sergio, Allal Ouhtit, Shaikh Mizanoor Rahman, and Md Mizanur Rahman. 2023. "Docosahexaenoic Acid, a Key Compound for Enhancing Sensitization to Drug in Doxorubicin-Resistant MCF-7 Cell Line" Nutrients 15, no. 7: 1658. https://doi.org/10.3390/nu15071658
APA StyleCrovella, S., Ouhtit, A., Rahman, S. M., & Rahman, M. M. (2023). Docosahexaenoic Acid, a Key Compound for Enhancing Sensitization to Drug in Doxorubicin-Resistant MCF-7 Cell Line. Nutrients, 15(7), 1658. https://doi.org/10.3390/nu15071658









