Elevated C-Reactive Protein Levels Modify the Effect of Magnesium on Depressive Symptoms: A Population-Based Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Assessment of Serum Magnesium
2.3. Assessment of C-Reactive Protein
2.4. Other Covariates
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. The Global Burden of Disease: 2004 Update; World Health Organization: Geneva, Switzerland, 2008.
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Ménard, C.; Hodes, G.E.; Russo, S.J. Pathogenesis of depression: Insights from human and rodent studies. Neuroscience 2016, 321, 138–162. [Google Scholar] [CrossRef]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and Longer-Term Outcomes in Depressed Outpatients Requiring One or Several Treatment Steps: A STAR*D Report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef]
- Derom, M.-L. Magnesium and depression: A systematic review. Nutr. Neurosci. 2013, 16, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, W.; Xin, X.; Song, X.; Zhang, D. Association of total zinc, iron, copper and selenium intakes with depression in the US adults. J. Affect. Disord. 2018, 228, 68–74. [Google Scholar] [CrossRef]
- Tarleton, E.K. The Association between Serum Magnesium Levels and Depression in an Adult Primary Care Population. Nutrients 2019, 11, 1475. [Google Scholar] [CrossRef]
- de Baaij, J.H.; Hoenderop, J.G.; Bindels, R.J. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Workinger, J.L.; Doyle, R.P.; Bortz, J. Challenges in the Diagnosis of Magnesium Status. Nutrients 2018, 10, 1202. [Google Scholar] [CrossRef] [PubMed]
- Rosanoff, A. Perspective: US Adult Magnesium Requirements Need Updating: Impacts of Rising Body Weights and Data-Derived Variance. Adv. Nutr. 2021, 12, 298–304. [Google Scholar] [CrossRef]
- Wang, J.L.; Weng, Y.L.; Pan, W.H.; Kao, M.D. Trends and nutritional status for magnesium in Taiwan from NAHSIT 1993 to 2008. Asia Pac. J. Clin. Nutr. 2011, 20, 266–274. [Google Scholar]
- Pickering, G.; Mazur, A.; Trousselard, M.; Bienkowski, P.; Yaltsewa, N.; Amessou, M.; Noah, L.; Pouteau, E. Magnesium status and stress: The vicious circle concept revisited. Nutrients 2020, 12, 3672. [Google Scholar] [CrossRef] [PubMed]
- Sartori, S.B.; Whittle, N.; Hetzenauer, A.; Singewald, N. Magnesium deficiency induces anxiety and HPA axis dysregulation: Modulation by therapeutic drug treatment. Neuropharmacology 2012, 62, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Minner, B.; Suy, E.; Vandervorst, C.; Raus, J. Coexisting dysregulations of both the sympathoadrenal system and hypothalamic-pituitary-adrenal-axis in melancholia. J. Neural Transm. 1991, 85, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Eby III, G.A.; Eby, K.L. Magnesium for treatment-resistant depression: A review and hypothesis. Med. Hypotheses 2010, 74, 649–660. [Google Scholar] [CrossRef]
- Newport, D.J.; Carpenter, L.L.; McDonald, W.M.; Potash, J.B.; Tohen, M.; Nemeroff, C.B.; The APA Council of Research Task Force on Novel Biomarkers and Treatments. Ketamine and other NMDA antagonists: Early clinical trials and possible mechanisms in depression. Am. J. Psychiatry 2015, 172, 950–966. [Google Scholar] [CrossRef]
- Lang, U.E.; Beglinger, C.; Schweinfurth, N.; Walter, M.; Borgwardt, S. Nutritional aspects of depression. Cell. Physiol. Biochem. 2015, 37, 1029–1043. [Google Scholar] [CrossRef]
- Tarleton, E.K.; Littenberg, B. Magnesium intake and depression in adults. J. Am. Board Fam. Med. 2015, 28, 249–256. [Google Scholar] [CrossRef]
- Sun, C.; Wang, R.; Li, Z.; Zhang, D. Dietary magnesium intake and risk of depression. J. Affect. Disord. 2019, 246, 627–632. [Google Scholar] [CrossRef]
- Fang, X.; Wang, K.; Han, D.; He, X.; Wei, J.; Zhao, L.; Imam, M.U.; Ping, Z.; Li, Y.; Xu, Y.; et al. Dietary magnesium intake and the risk of cardiovascular disease, type 2 diabetes, and all-cause mortality: A dose–response meta-analysis of prospective cohort studies. BMC Med. 2016, 14, 210. [Google Scholar] [CrossRef]
- Han, H.; Fang, X.; Wei, X.; Liu, Y.; Jin, Z.; Chen, Q.; Fan, Z.; Aaseth, J.; Hiyoshi, A.; He, J.; et al. Dose-response relationship between dietary magnesium intake, serum magnesium concentration and risk of hypertension: A systematic review and meta-analysis of prospective cohort studies. Nutr. J. 2017, 16, 26. [Google Scholar] [CrossRef]
- Asbaghi, O.; Moradi, S.; Kashkooli, S.; Zobeiri, M.; Nezamoleslami, S.; Lazaridi, A.-V.; Miraghajani, M. The effects of oral magnesium supplementation on glycemic control in patients with type 2 diabetes: A systematic review and dose-response meta-analysis of controlled clinical trials. Br. J. Nutr. 2022, 128, 2363–2372. [Google Scholar] [CrossRef]
- Li, B.; Lv, J.; Wang, W.; Zhang, D. Dietary magnesium and calcium intake and risk of depression in the general population: A meta-analysis. Aust. N. Z. J. Psychiatry 2017, 51, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Szkup, M.; Jurczak, A.; Brodowska, A.; Brodowska, A.; Noceń, I.; Chlubek, D.; Laszczyńska, M.; Karakiewicz, B.; Grochans, E. Analysis of relations between the level of Mg, Zn, Ca, Cu, and Fe and depressiveness in postmenopausal women. Biol. Trace Elem. Res. 2017, 176, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Islam, M.R.; Shalahuddin Qusar, M.; Islam, M.S.; Kabir, M.H.; Mustafizur Rahman, G.; Islam, M.S.; Hasnat, A. Alterations of serum macro-minerals and trace elements are associated with major depressive disorder: A case-control study. BMC Psychiatry 2018, 18, 94. [Google Scholar] [CrossRef]
- Botturi, A.; Ciappolino, V.; Delvecchio, G.; Boscutti, A.; Viscardi, B.; Brambilla, P. The role and the effect of magnesium in mental disorders: A systematic review. Nutrients 2020, 12, 1661. [Google Scholar] [CrossRef]
- Chou, M.H.; Yang, Y.K.; Wang, J.D.; Lin, C.Y.; Lin, S.H. The Association of Serum and Dietary Magnesium with Depressive Symptoms. Nutrients 2023, 15, 774. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Belvedere, M.; Dominguez, L.J. Magnesium homeostasis and aging. Magnes. Res. 2009, 22, 235–246. [Google Scholar] [CrossRef]
- Barbagallo, M.; Veronese, N.; Dominguez, L.J. Magnesium in Type 2 Diabetes Mellitus, Obesity, and Metabolic Syndrome. Nutrients 2022, 14, 714. [Google Scholar] [CrossRef]
- Hunt, C.; Macedo, E.C.T.; Suchting, R.; de Dios, C.; Cuellar Leal, V.A.; Soares, J.C.; Dantzer, R.; Teixeira, A.L.; Selvaraj, S. Effect of immune activation on the kynurenine pathway and depression symptoms—A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2020, 118, 514–523. [Google Scholar] [CrossRef]
- Maier, J.A.; Castiglioni, S.; Locatelli, L.; Zocchi, M.; Mazur, A. Magnesium and inflammation: Advances and perspectives. Semin. Cell Dev. Biol. 2021, 115, 37–44. [Google Scholar] [CrossRef]
- McNally, L.; Bhagwagar, Z.; Hannestad, J. Inflammation, glutamate, and glia in depression: A literature review. CNS Spectr. 2008, 13, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Wium-Andersen, M.K.; Ørsted, D.D.; Nielsen, S.F.; Nordestgaard, B.G. Elevated C-reactive protein levels, psychological distress, and depression in 73, 131 individuals. JAMA Psychiatry 2013, 70, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Felger, J.C.; Haroon, E.; Patel, T.A.; Goldsmith, D.R.; Wommack, E.C.; Woolwine, B.J.; Le, N.A.; Feinberg, R.; Tansey, M.G.; Miller, A.H. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol. Psychiatry 2020, 25, 1301–1311. [Google Scholar] [CrossRef]
- Ridker, P.M. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 2003, 107, 363–369. [Google Scholar] [CrossRef]
- Louis, E.; Vermeire, S.; Rutgeerts, P.; De Vos, M.; Van Gossum, A.; Pescatore, P.; Fiasse, R.; Pelckmans, P.; Reynaert, H.; D’Haens, G.; et al. A positive response to infliximab in Crohn disease: Association with a higher systemic inflammation before treatment but not with -308 TNF gene polymorphism. Scand. J. Gastroenterol. 2002, 37, 818–824. [Google Scholar] [CrossRef]
- Arends, S.; Brouwer, E.; van der Veer, E.; Groen, H.; Leijsma, M.K.; Houtman, P.M.; Th, A.J.T.L.; Kallenberg, C.G.; Spoorenberg, A. Baseline predictors of response and discontinuation of tumor necrosis factor-alpha blocking therapy in ankylosing spondylitis: A prospective longitudinal observational cohort study. Arthritis Res. Ther. 2011, 13, R94. [Google Scholar] [CrossRef]
- Raison, C.L.; Rutherford, R.E.; Woolwine, B.J.; Shuo, C.; Schettler, P.; Drake, D.F.; Haroon, E.; Miller, A.H. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: The role of baseline inflammatory biomarkers. JAMA Psychiatry 2013, 70, 31–41. [Google Scholar] [CrossRef]
- Lee, M.B.; Liao, S.C.; Lee, Y.J.; Wu, C.H.; Tseng, M.C.; Gau, S.F.; Rau, C.L. Development and verification of validity and reliability of a short screening instrument to identify psychiatric morbidity. J. Formos. Med. Assoc. 2003, 102, 687–694. [Google Scholar]
- Li, R.; Hertzmark, E.; Louie, M.; Chen, L.; Spiegelman, D. The Sas Lgtphcurv9 Macro; Channing Laboratory: Boston, MA, USA, 2011. [Google Scholar]
- Yary, T. Dietary magnesium intake and the incidence of depression: A 20-year follow-up study. J. Affect. Disord. 2016, 193, 94–98. [Google Scholar] [CrossRef]
- Rosanoff, A. The high heart health value of drinking-water magnesium. Med. Hypotheses 2013, 81, 1063–1065. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, R.; Della Porta, M.; Manoni, M.; Iotti, S.; Pinotti, L.; Maier, J.A. Going to the roots of reduced magnesium dietary intake: A tradeoff between climate changes and sources. Heliyon 2020, 6, e05390. [Google Scholar] [CrossRef] [PubMed]
- Seeman, M.V. Psychopathology in women and men: Focus on female hormones. Am. J. Psychiatry 1997, 154, 1641–1647. [Google Scholar] [CrossRef]
- Dullo, P.; Vedi, N. Changes in serum calcium, magnesium and inorganic phosphorus levels during different phases of the menstrual cycle. J. Hum. Reprod. Sci. 2008, 1, 77–80. [Google Scholar] [CrossRef]
- Krishnadas, R.; Cavanagh, J. Depression: An inflammatory illness? J. Neurol. Neurosurg. Psychiatry 2012, 83, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Toth, P.P.; Banach, M. C-reactive Protein Is Associated with Prevalence of the Metabolic Syndrome, Hypertension, and Diabetes Mellitus in US Adults. Angiology 2018, 69, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, C.; Costa, G.M.; Santarella, L.; Flacco, M.E.; Capasso, L.; Bert, F.; Manzoli, L. Depression symptoms and the progression of carotid intima-media thickness: A 5-year follow-up study. Atherosclerosis 2014, 233, 530–536. [Google Scholar] [CrossRef]



| Variable | Total Sample (n = 2196) | Depression (Continuous Variable) 1 | Depression (Binary Variable) 2 | |||
|---|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Depression < 2, Mean ± SD | Depression ≥ 2, Mean ± SD | p Value 3 | ||
| Serum Mg (mg/dL) | 2.14 ± 0.19 | 2.14 (0.27) | 2.14 ± 0.19 | 2.10 ± 0.21 | 0.018 * | |
| Age | 53.39 ± 17.36 | 54.00 (27.00) | 53.75 ± 17.41 | 48.30 ± 15.85 | <0.001 * | |
| n (%) | Mean ± SD | p Value 3 | Depression < 2, n (%) | Depression ≥ 2, n (%) | ||
| CRP | ||||||
| <5 mg/L | 1976 (89.98) | 0.34 ± 0.67 | 0.092 | 1855 (90.44) | 121 (83.45) | 0.007 * |
| ≥5 mg/L | 220 (10.02) | 0.44 ± 0.86 | 196 (9.56) | 24 (16.55) | ||
| Sex | ||||||
| Women | 1127 (51.32) | 0.38 ± 0.70 | 0.023 * | 1050 (51.19) | 77 (53.10) | 0.647 |
| Men | 1069 (48.68) | 0.32 ± 0.69 | 1001 (48.81) | 68 (46.90) | ||
| BMI | 0.003 * | 0.043 * | ||||
| <18 | 118 (5.37) | 0.42 ± 0.66 | 111 (5.42) | 7 (4.83) | ||
| 18–24 | 933 (42.49) | 0.40 ± 0.76 | 857 (41.78) | 76 (52.41) | ||
| >24 | 1145 (52.14) | 0.30 ± 0.63 | 1083 (52.80) | 62 (42.76) | ||
| Education level | ||||||
| None | 209 (9.52) | 0.37 ± 0.72 | 0.457 | 194 (9.46) | 15 (10.34) | 0.248 |
| Below SHS | 936 (42.62) | 0.33 ± 0.67 | 885 (43.15) | 51 (35.17) | ||
| SHS | 585 (26.64) | 0.38 ± 0.72 | 538 (26.23) | 47 (32.41) | ||
| Above SHS | 466 (21.22) | 0.35 ± 0.71 | 434 (21.16) | 32 (22.07) | ||
| Income level | ||||||
| No income | 533 (24.27) | 0.40 ± 0.75 | 0.055 | 489 (23.84) | 44 (30.34) | 0.011 * |
| TWD 20,000 and below | 842 (38.34) | 0.31 ± 0.64 | 803 (39.15) | 39 (26.90) | ||
| TWD 20,001–50,000 | 569 (25.91) | 0.38 ± 0.71 | 525 (25.60) | 44 (30.34) | ||
| TWD 50,001–70,000 | 153 (6.97) | 0.25 ± 0.65 | 146 (7.12) | 7 (4.83) | ||
| TWD 70,001 and above | 99 (4.51) | 0.38 ± 0.74 | 88 (4.29) | 11 (7.59) | ||
| Physical activity status | ||||||
| No | 978 (44.55) | 0.40 ± 0.76 | 0.010 * | 898 (43.78) | 80 (55.17) | 0.028 * |
| Yes–walking | 615 (27.95) | 0.30 ± 0.61 | 583 (28.43) | 32 (22.07) | ||
| Yes–more than walking 4 | 603 (27.50) | 0.32 ± 0.66 | 570 (27.79) | 33 (22.76) | ||
| Alcohol use | ||||||
| Non-drinker | 1186 (54.01) | 0.34 ± 0.67 | 0.198 | 1111 (54.17) | 75 (51.72) | 0.073 |
| Former drinker | 178 (8.11) | 0.44 ± 0.85 | 159 (7.75) | 19 (13.10) | ||
| Current drinker | 832 (37.89) | 0.35 ± 0.69 | 781 (38.08) | 51 (35.17) | ||
| Smoking Status | ||||||
| Non-smoker | 1493 (67.99) | 0.34 ± 0.65 | 0.223 | 1407 (68.60) | 86 (59.31) | 0.034 * |
| Former smoker | 272 (12.39) | 0.33 ± 0.70 | 253 (12.34) | 19 (13.10) | ||
| Current smoker | 431 (19.63) | 0.40 ± 0.83 | 391 (19.06) | 40 (27.59) | ||
| Diabetes mellitus | ||||||
| No | 2007 (91.39) | 0.35 ± 0.69 | 0.818 | 1874 (91.37) | 133 (91.72) | 0.883 |
| Yes | 189 (8.61) | 0.34 ± 0.69 | 177 (8.63) | 12 (8.28) | ||
| Hypertension | ||||||
| No | 1665 (75.82) | 0.36 ± 0.72 | 0.088 | 1550 (75.57) | 115 (79.31) | 0.310 |
| Yes | 531 (24.18) | 0.31 ± 0.60 | 501 (24.43) | 30 (20.69) | ||
| Kidney disease | ||||||
| No | 2156 (98.18) | 0.35 ± 0.69 | 0.279 | 2017 (98.34) | 139 (95.86) | 0.031 * |
| Yes | 40 (1.82) | 0.50 ± 0.88 | 34 (1.66) | 6 (4.14) | ||
| Variable | Depression (Continuous Variable) 1 | Depression (Binary Variable) 2 | ||||
|---|---|---|---|---|---|---|
| β | p Value | 95% CI | OR | p Value | 95% CI | |
| Crude | ||||||
| Serum Mg (mg/dL) | −0.26 | 0.001 * | (−0.41, −0.11) | 0.32 | 0.008 * | (0.14, 0.74) |
| CRP ≥ 5 mg/L 3 | 0.10 | 0.040 | (0.01, 0.20) | 1.88 | 0.008 * | (1.18, 2.98) |
| Model 1 | ||||||
| Serum Mg (mg/dL) | −0.23 | 0.004 * | (−0.39, −0.08) | 0.36 | 0.018 * | (0.15, 0.84) |
| CRP ≥ 5 mg/L | 0.14 | 0.005 * | (0.04, 0.24) | 2.23 | 0.001 * | (1.37, 3.61) |
| Model 2 | ||||||
| Serum Mg (mg/dL) | −0.23 | 0.004 * | (−0.38, −0.07) | 0.37 | 0.022 * | (0.16, 0.87) |
| CRP ≥ 5 mg/L | 0.14 | 0.005 * | (0.04, 0.24) | 2.19 | 0.002 * | (1.35, 3.55) |
| Variable | Depression (Continuous Variable) 1 | Depression (Binary Variable) 2 | ||||
|---|---|---|---|---|---|---|
| β | p Value | 95% CI | OR | p Value | 95% CI | |
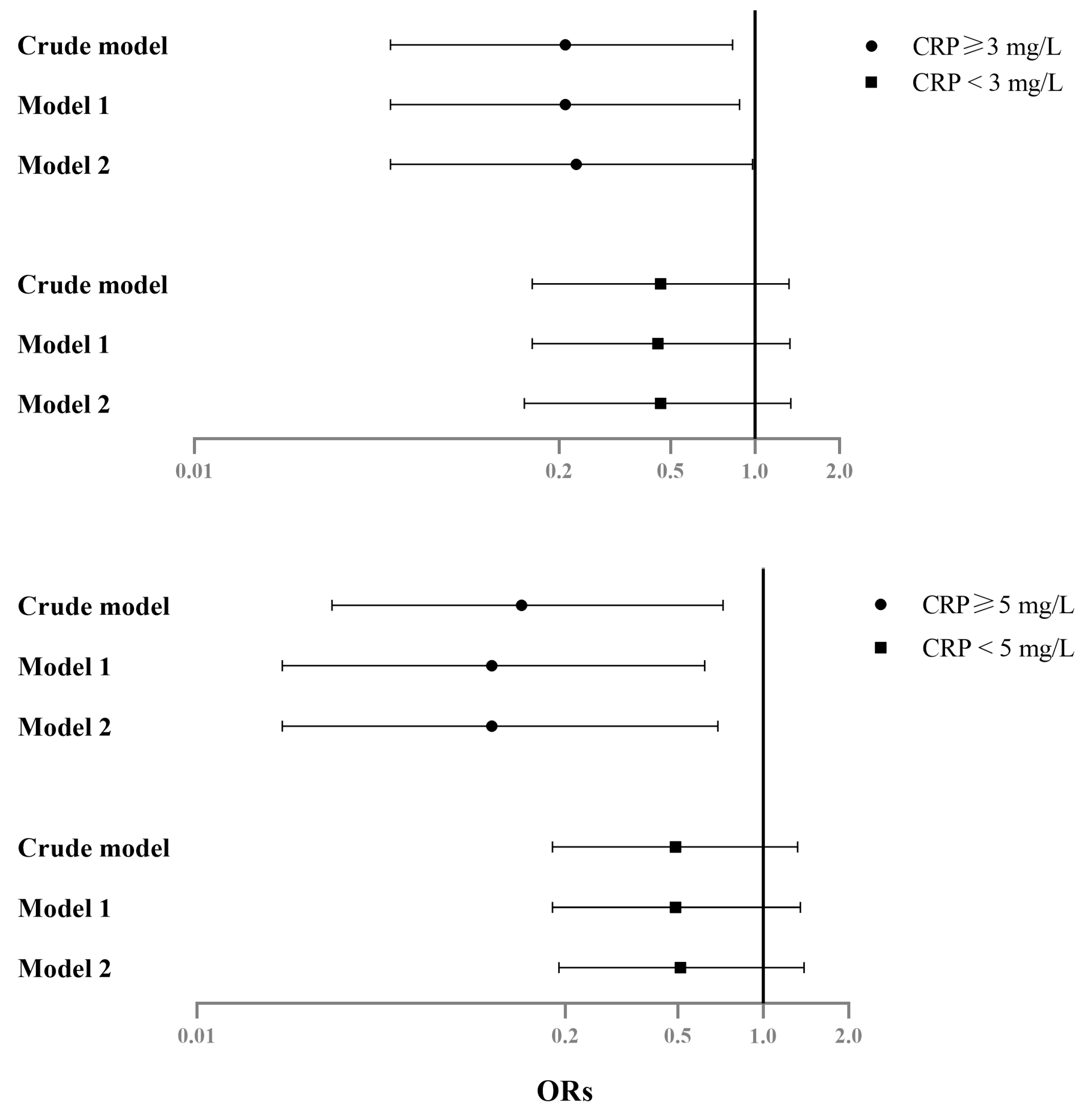
| Subgroup analysis (among CRP ≥ 5 mg/L) | ||||||
| Crude | ||||||
| Serum Mg (mg/dL) | −0.56 | 0.019 * | (−1.02, −0.09) | 0.14 | 0.018 * | (0.03, 0.72) |
| Model 1 | ||||||
| Serum Mg (mg/dL) | −0.54 | 0.025 * | (−1.01, −0.07) | 0.11 | 0.013 * | (0.02, 0.62) |
| Model 2 | ||||||
| Serum Mg (mg/dL) | −0.50 | 0.039 * | (−0.97, −0.03) | 0.11 | 0.018 * | (0.02, 0.69) |
| Subgroup analysis (among CRP < 5 mg/L) | ||||||
| Crude | ||||||
| Serum Mg (mg/dL) | −0.19 | 0.025 * | (−0.35, −0.02) | 0.49 | 0.159 | (0.18, 1.32) |
| Model 1 | ||||||
| Serum Mg (mg/dL) | −0.17 | 0.042 * | (−0.34, −0.01) | 0.49 | 0.170 | (0.18, 1.35) |
| Model 2 | ||||||
| Serum Mg (mg/dL) | −0.17 | 0.045 * | (−0.33, −0.00) | 0.51 | 0.186 | (0.19, 1.39) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, M.-H.; Yang, Y.K.; Wang, J.-D.; Lin, C.-Y.; Lin, S.-H. Elevated C-Reactive Protein Levels Modify the Effect of Magnesium on Depressive Symptoms: A Population-Based Study. Nutrients 2023, 15, 1560. https://doi.org/10.3390/nu15071560
Chou M-H, Yang YK, Wang J-D, Lin C-Y, Lin S-H. Elevated C-Reactive Protein Levels Modify the Effect of Magnesium on Depressive Symptoms: A Population-Based Study. Nutrients. 2023; 15(7):1560. https://doi.org/10.3390/nu15071560
Chicago/Turabian StyleChou, Ming-Hui, Yen Kuang Yang, Jung-Der Wang, Chung-Ying Lin, and Sheng-Hsiang Lin. 2023. "Elevated C-Reactive Protein Levels Modify the Effect of Magnesium on Depressive Symptoms: A Population-Based Study" Nutrients 15, no. 7: 1560. https://doi.org/10.3390/nu15071560
APA StyleChou, M.-H., Yang, Y. K., Wang, J.-D., Lin, C.-Y., & Lin, S.-H. (2023). Elevated C-Reactive Protein Levels Modify the Effect of Magnesium on Depressive Symptoms: A Population-Based Study. Nutrients, 15(7), 1560. https://doi.org/10.3390/nu15071560







