The Microbiota–Gut–Brain Axis: Psychoneuroimmunological Insights
Abstract
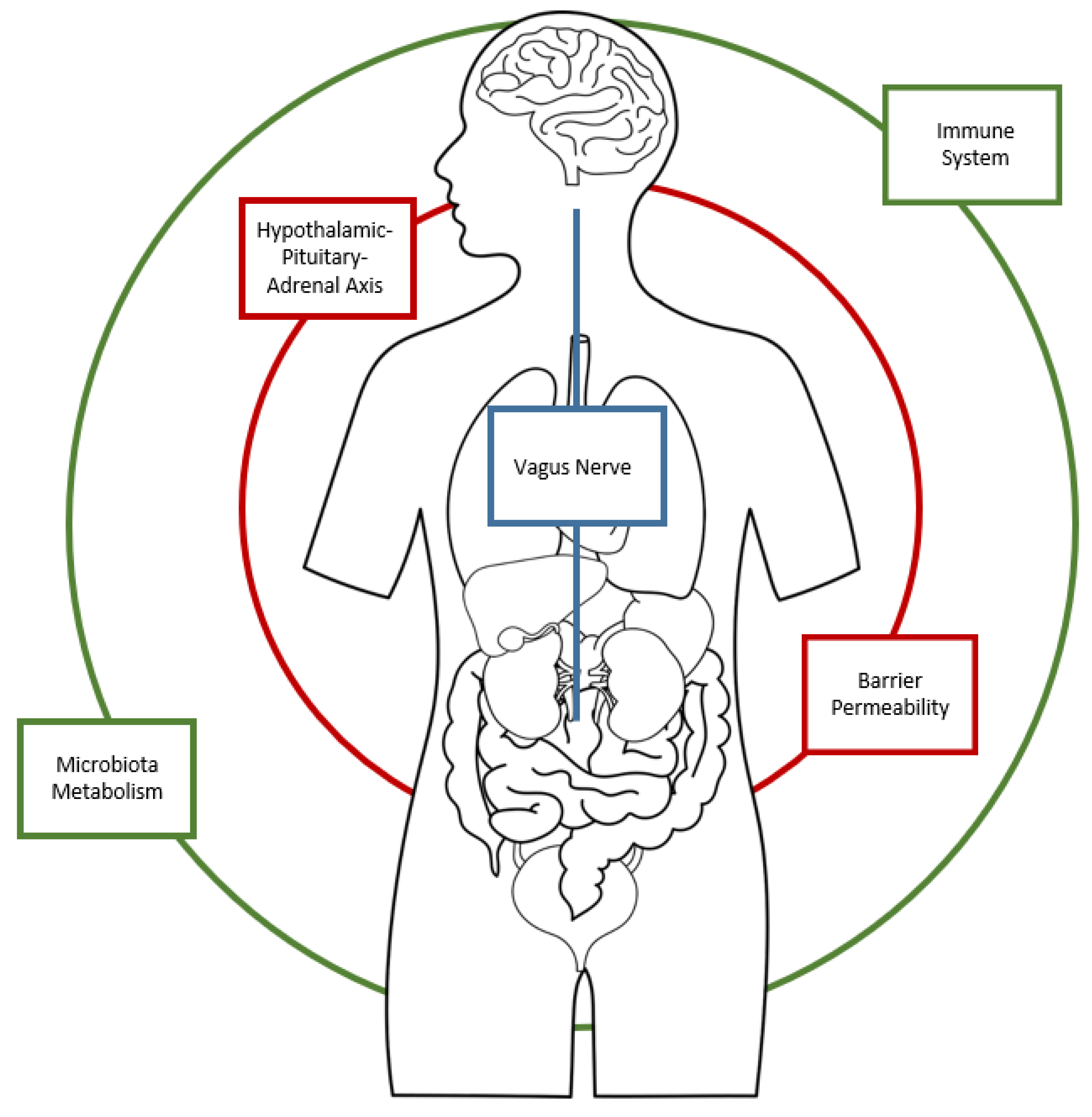
1. Introduction
1.1. The role of the Microbiota in the regulation of the Gut–Brain Axis
1.2. The Role of Autoimmunity
1.3. The Impact of Diet on Microbiota and Mental Health
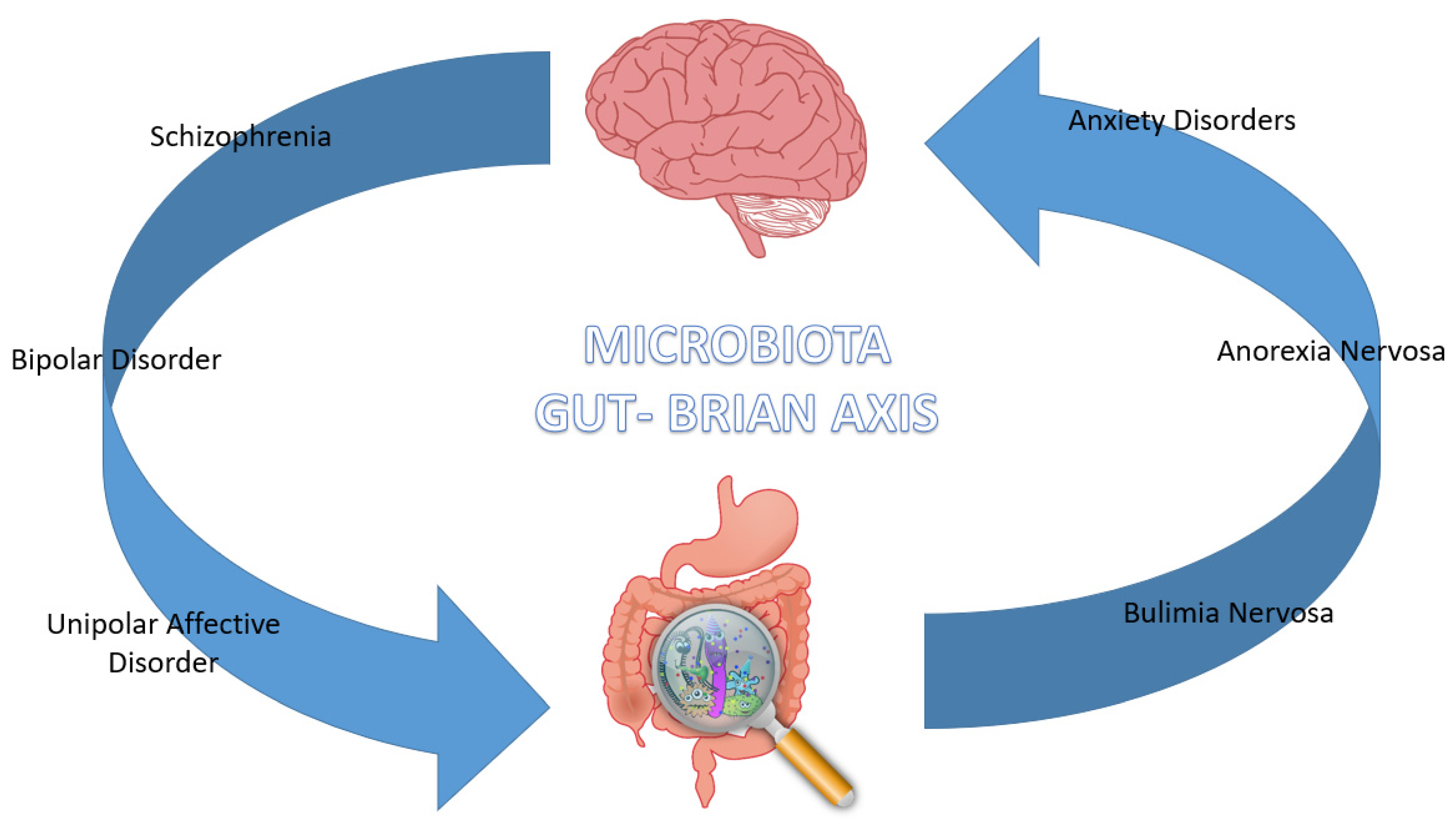
2. The Gut Microbiota in Psychiatric Diseases
2.1. Schizophrenia
2.2. Bipolar Disorder
2.3. Unipolar Affective Disorder
2.4. Anxiety Disorders
2.5. Eating Disorders—Anorexia Nervosa and Bulimia Nervosa
3. Diet and Its Implications
3.1. The Role of Diet in Microbiota Regulation
3.2. The Role of Single Nutrients
3.2.1. Fibers
3.2.2. Vitamins
3.2.3. Carbohydrates
3.2.4. Minerals
3.2.5. Polyphenols
3.3. Different Types of Diets
3.3.1. Western Diet
3.3.2. The Mediterranean Diet
3.3.3. Vegetarian and Vegan Diet
3.4. The Use of Probiotics
3.5. The Fecal Transplant
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- González Olmo, B.M.; Butler, M.J.; Barrientos, R.M. Evolution of the human diet and its impact on gut microbiota, immune responses, and brain health. Nutrients 2021, 13, 196. [Google Scholar] [CrossRef]
- García-Bueno, B.; Caso, J.R.; Leza, J.C. Stress as a neuroinflammatory condition in brain: Damaging and protective mechanisms. Neurosci. Biobehav. Rev. 2008, 32, 1136–1151. [Google Scholar] [CrossRef]
- Tengeler, A.C.; Kozicz, T.; Kiliaan, A.J. Relationship between diet, the gut microbiota, and brain function. Nutr. Rev. 2018, 76, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Gao, X.R.; Peng, L.; Ge, J.F. Crosstalk between the microbiota-gut-brain axis and depression. Heliyon 2020, 6, e04097. [Google Scholar] [CrossRef]
- Sekirov, I. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Neufeld, K.A. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Karlsson, F.H. A closer look at bacteroides: Phylogenetic relationship and genomic implications of a life in the human gut. Microb. Ecol. 2011, 61, 473–485. [Google Scholar] [CrossRef]
- Severance, E.G.; Tveiten, D.; Lindström, L.H.; Yolken, R.H.; Reichelt, K.L. The gut microbiota and the emergence of autoimmunity: Relevance to major psychiatric disorders. Curr. Pharm. Des. 2016, 22, 6076–6086. [Google Scholar] [CrossRef]
- Ismail, A.S.; Hooper, L.V. Epithelial cells and their neighbors. IV. Bacterial contributions to intestinal epithelial barrier integrity. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G779–G784. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Davey, K.J.; Cotter, P.D.; O’Sullivan, O.; Crispie, F.; Dinan, T.G.; Cryan, J.F.; O’Mahony, S.M. Antipsychotics and the gut microbiome: Olanzapine-induced metabolic dysfunction is attenuated by antibiotic administration in the rat. Transl. Psychiatry 2013, 3, e309. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed]
- Fallani, M.; Young, D.; Scott, J.; Norin, E.; Amarri, S.; Adam, R.; Aguilera, M.; Khanna, S.; Gil, A.; Edwards, C.A.; et al. Intestinal microbiota of 6-week-old infants across Europe: Geographic influence beyond delivery mode, breast-feeding, and antibiotics. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Ryan, P.M.; Wiley, N.; Carafa, I.; Sherwin, E.; Moloney, G.; Franciosi, E.; Mandal, R.; Wishart, D.S.; Tuohy, K.; et al. Gamma-aminobutyric acid-producing lactobacilli positively affect metabolism and depressive-like behaviour in a mouse model of metabolic syndrome. Sci. Rep. 2019, 9, 16323. [Google Scholar] [CrossRef]
- Busnelli, M.; Manzini, S.; Chiesa, G. The gut microbiota affects host pathophysiology as an endocrine organ: A focus on cardiovascular disease. Nutrients 2019, 12, 79. [Google Scholar] [CrossRef] [PubMed]
- Nøhr, M.K.; Egerod, K.L.; Christiansen, S.H.; Gille, A.; Offermanns, S.; Schwartz, T.W.; Møller, M. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience 2015, 290, 126–137. [Google Scholar] [CrossRef]
- Waldecker, M.; Kautenburger, T.; Daumann, H.; Busch, C.; Schrenk, D. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J. Nutr. Biochem. 2008, 19, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef]
- Fülling, C.; Dinan, T.G.; Cryan, J.F. Gut microbe to brain signaling: What happens in vagus …. Neuron 2019, 101, 998–1002. [Google Scholar] [CrossRef]
- Wang, Y.; Zhan, G.; Cai, Z.; Jiao, B.; Zhao, Y.; Li, S.; Luo, A. Vagus nerve stimulation in brain diseases: Therapeutic applications and biological mechanisms. Neurosci. Biobehav. Rev. 2021, 127, 37–53. [Google Scholar] [CrossRef]
- Bonaz, B.; Bazin, T.; Pellissier, S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, E.; Gray, K.; Miller, G.; Tyler, R.; Wiers, C.E.; Volkow, N.D.; Wang, G.J. Food addiction: A common neurobiological mechanism with drug abuse. Front. Biosci. 2018, 23, 811–836. [Google Scholar]
- Zhang, P.; Kong, L.; Huang, H.; Pan, Y.; Zhang, D.; Jiang, J.; Shen, Y.; Xi, C.; Lai, J.; Ng, C.H.; et al. Gut microbiota—A potential contributor in the pathogenesis of bipolar disorder. Front. Neurosci. 2022, 16, 830748. [Google Scholar] [CrossRef] [PubMed]
- Scaldaferri, F.; Gerardi, V.; Lopetuso, L.R.; Del Zompo, F.; Mangiola, F.; Boškoski, I.; Bruno, G.; Petito, V.; Laterza, L.; Cammarota, G.; et al. Gut microbial flora, prebiotics, and probiotics in IBD: Their current usage and utility. Biomed. Res. Int. 2013, 2013, 435268. [Google Scholar] [CrossRef] [PubMed]
- Bolon, B. Cellular and molecular mechanisms of autoimmune disease. Toxicol. Pathol. 2012, 40, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. The impact of gut microbiota on brain and behaviour: Implications for psychiatry. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; O’Connell, R.M.; Mazmanian, S.K. Coordination of tolerogenic immune responses by the commensal microbiota. J. Autoimmun. 2010, 34, J220–J225. [Google Scholar] [CrossRef]
- Misiak, B.; Łoniewski, I.; Marlicz, W.; Frydecka, D.; Szulc, A.; Rudzki, L.; Samochowiec, J. The HPA axis dysregulation in severe mental illness: Can we shift the blame to gut microbiota? Prog Neuropsychopharmacol Biol. Psychiatry 2020, 102, 109951. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Xing, C.; Long, W.; Wang, H.Y.; Liu, Q.; Wang, R.F. Impact of microbiota on central nervous system and neurological diseases: The gut-brain axis. J. Neuroinflamm. 2019, 16, 53. [Google Scholar] [CrossRef]
- Doroszkiewicz, J.; Groblewska, M.; Mroczko, B. The Role of Gut Microbiota and Gut-Brain Interplay in Selected Diseases of the Central Nervous System. Int. J. Mol. Sci. 2021, 22, 10028. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Stone, T.W.; Schwarcz, R. The kynurenine pathway: Towards metabolic equilibrium. Neuropharmacology 2017, 112 Pt B, 235–236. [Google Scholar] [CrossRef]
- Schwarcz, R.; Stone, T.W. The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology 2017, 112 Pt B, 237–247. [Google Scholar] [CrossRef]
- Kadriu, B.; Farmer, C.A.; Yuan, P.; Park, L.T.; Deng, Z.D.; Moaddel, R.; Henter, I.D.; Shovestul, B.; Ballard, E.D.; Kraus, C.; et al. The kynurenine pathway and bipolar disorder: Intersection of the monoaminergic and glutamatergic systems and immune response. Mol. Psychiatry 2021, 26, 4085–4095. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.C.; Elmer, G.I.; Bergeron, R.; Albuquerque, E.X.; Guidetti, P.; Wu, H.Q.; Schwarcz, R. Reduction of endogenous kynurenic acid formation enhances extracellular glutamate, hippocampal plasticity, and cognitive behavior. Neuropsychopharmacology 2010, 35, 1734–1742. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- McRorie, J.W., Jr.; McKeown, N.M. Understanding the physics of functional fibers in the gastrointestinal tract: An evidence-based approach to resolving enduring misconceptions about insoluble and soluble fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Addolorato, G.; Capristo, E.; Stefanini, G.F.; Gasbarrini, G. Inflammatory bowel disease: A study of the association between anxiety and depression, physical morbidity, and nutritional status. Scand. J. Gastroenterol. 1997, 32, 1013–1021. [Google Scholar] [CrossRef]
- Addolorato, G.; Capristo, E.; Ghittoni, G.; Valeri, C.; Mascianà, R.; Ancona, C.; Gasbarrini, G. Anxiety but not depression decreases in coeliac patients after one-year gluten-free diet: A longitudinal study. Scand. J. Gastroenterol. 2001, 36, 502–506. [Google Scholar] [CrossRef]
- Meguid, N.A.; Atta, H.M.; Gouda, A.S.; Khalil, R.O. Role of polyunsaturated fatty acids in the management of Egyptian children with autism. Clin. Biochem. 2008, 41, 1044–1048. [Google Scholar] [CrossRef]
- Grosso, G. Nutritional psychiatry: How diet affects brain through gut microbiota. Nutrients 2021, 13, 1282. [Google Scholar] [CrossRef]
- Caso, J.R.; Balanzá-Martínez, V.; Palomo, T.; García-Bueno, B. The microbiota and gut-brain axis: Contributions to the immunopathogenesis of schizophrenia. Curr. Pharm. Des. 2016, 22, 6122–6133. [Google Scholar] [CrossRef]
- Axelsson, R.; Martensson, E.; Alling, C. Impairment of the blood-brain barrier as an aetiological factor in paranoid psychosis. Br. J. Psychiatry 1982, 141, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Sun, Z.; Xie, L.; Liu, S.; Ju, G.; Shi, J.; Shen, Y. Further study of a genetic association between the CLDN5 locus and schizophrenia. Schizophr. Res. 2005, 75, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Schizophrenia Working Group of the Psychiatric Genomics, C. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511, 421–427. [Google Scholar] [CrossRef]
- Leboyer, M.; Oliveira, J.; Tamouza, R.; Groc, L. Is it time for immunopsychiatry in psychotic disorders? Psychopharmacology 2016, 233, 1651–1660. [Google Scholar] [CrossRef]
- Weaver, C.T.; Elson, C.O.; Fouser, L.A.; Kolls, J.K. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu. Rev. Pathol. 2013, 8, 477–512. [Google Scholar] [CrossRef] [PubMed]
- Torrey, E.F.; Bartko, J.J.; Yolken, R.H. Toxoplasma gondii and other risk factors for schizophrenia: An update. Schizophr. Bull. 2012, 38, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Molloy, M.J.; Grainger, J.R.; Bouladoux, N.; Hand, T.W.; Koo, L.Y.; Naik, S.; Quinones, M.; Dzutsev, A.K.; Gao, J.L.; Trinchieri, G.; et al. Intraluminal containment of commensal outgrowth in the gut during infectioninduced dysbiosis. Cell Host Microbe 2013, 14, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Labouesse, M.A.; Langhans, W.; Meyer, U. Long-term pathological consequences of prenatal infection: Beyond brain disorders. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R1–R12. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef]
- Smith, S.E.; Li, J.; Garbett, K.; Mirnics, K.; Patterson, P.H. Maternal immune activation alters fetal brain development through interleukin 6. J. Neurosci. 2007, 27, 10695–10702. [Google Scholar] [CrossRef]
- Severance, E.G.; Yolken, R.H.; Eaton, W.W. Autoimmune diseases, gastrointestinal disorders and the microbiome in schizophrenia: More than a gut feeling. Schizophr. Res. 2016, 176, 23–35. [Google Scholar] [CrossRef]
- Bender, L. Childhood schizophrenia. Psychiatr. Q. 1953, 27, 663–681. [Google Scholar] [CrossRef] [PubMed]
- Graff, H.; Handford, A. Celiac Syndrome in the case histories of five schizophrenics. Psychiatr. Q. 1961, 35, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Dohan, F.C.; Harper, E.H.; Clark, M.H.; Rodrigue, R.B.; Zigas, V. Is schizophrenia rare if grain is rare? Biol. Psychiatry 1984, 19, 385–399. [Google Scholar]
- Purcell, S.M.; Wray, N.R.; Stone, J.L.; Visscher, P.M.; O’Donovan, M.C.; Sullivan, P.F.; Sklar, P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 2009, 460, 748–752. [Google Scholar] [PubMed]
- Corvin, A.; Morris, D.W. Genome-wide association studies: Findings at the major histocompatibility complex locus in psychosis. Biol. Psychiatry 2014, 75, 276–283. [Google Scholar] [CrossRef]
- Van Heel, D.A.; West, J. Recent advances in coeliac disease. Gut 2006, 55, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Addolorato, G.; Stefanini, G.F.; Capristo, E.; Caputo, F.; Gasbarrini, A.; Gasbarrini, G. Anxiety and depression in adult untreated celiac subjects and in patients affected by inflammatory bowel disease: A personality “trait” or a reactive illness? Hepatogastroenterology 1996, 43, 1513–1517. [Google Scholar] [PubMed]
- Addolorato, G.; Mirijello, A.; D’Angelo, C.; Leggio, L.; Ferrulli, A.; Vonghia, L.; Cardone, S.; Leso, V.; Miceli, A.; Gasbarrini, G. Social phobia in coeliac disease. Scand. J. Gastroenterol. 2008, 43, 410–415. [Google Scholar] [CrossRef]
- O’Malley, D.; Quigley, E.M.; Dinan, T.G.; Cryan, J.F. Do interactions between stress and immune responses lead to symptom exacerbations in irritable bowel syndrome? Brain Behav. Immun. 2011, 25, 1333–1341. [Google Scholar] [CrossRef]
- Franceschi, F.; Ojetti, V.; Candelli, M.; Covino, M.; Cardone, S.; Potenza, A.; Simeoni, B.; Gabrielli, M.; Sabia, L.; Gasbarrini, G.; et al. Microbes and Alzheimer’ disease: Lessons from H. pylori and GUT microbiota. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 426–430. [Google Scholar]
- Painold, A.; Mörkl, S.; Kashofer, K.; Halwachs, B.; Dalkner, N.; Bengesser, S.; Birner, A.; Fellendorf, F.; Platzer, M.; Queissner, R.; et al. A step ahead: Exploring the gut microbiota in inpatients with bipolar disorder during a depressive episode. Bipolar Disord. 2019, 21, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Segre, J.A. The human microbiome: Our second genome. Annu. Rev. Genomics Hum. Genet. 2012, 13, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Lucidi, L.; Pettorruso, M.; Vellante, F.; Di Carlo, F.; Ceci, F.; Santovito, M.C.; Di Muzio, I.; Fornaro, M.; Ventriglio, A.; Tomasetti, C.; et al. Gut microbiota and bipolar disorder: An overview on a novel biomarker for diagnosis and treatment. Int. J. Mol. Sci. 2021, 22, 3723. [Google Scholar] [CrossRef]
- Gondalia, S.; Parkinson, L.; Stough, C.; Scholey, A. Gut microbiota and bipolar disorder: A review of mechanisms and potential targets for adjunctive therapy. Psychopharmacology 2019, 236, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- Rieder, R.; Wisniewski, P.J.; Alderman, B.L.; Campbell, S.C. Microbes and mental health: A review. Brain Behav. Immun. 2017, 66, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Fond, G.; Boukouaci, W.; Chevalier, G.; Regnault, A.; Eberl, G.; Hamdani, N.; Dickerson, F.; Macgregor, A.; Boyer, L.; Dargel, A.; et al. The “psychomicrobiotic”: Targeting microbiota in major psychiatric disorders: A systematic review. Pathol. Biol. 2015, 63, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Strakowski, S.M.; Adler, C.M.; Almeida, J.; Altshuler, L.L.; Blumberg, H.P.; Chang, K.D.; Delbello, M.P.; Frangou, S.; McIntosh, A.; Phillips, M.L.; et al. The functional neuroanatomy of bipolar disorder: A consensus model. Bipolar Disord. 2012, 14, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Addolorato, G.; De Lorenzi, G.; Abenavoli, L.; Leggio, L.; Capristo, E.; Gasbarrini, G. Psychological support counselling improves gluten-free diet compliance in coeliac patients with affective disorders. Aliment. Pharmacol. Ther. 2004, 20, 777–782. [Google Scholar] [CrossRef]
- Fond, G.; Loundou, A.; Hamdani, N.; Boukouaci, W.; Dargel, A.; Oliveira, J.; Roger, M.; Tamouza, R.; Leboyer, M.; Boyer, L. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): A systematic review and meta-analysis. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Gao, J. Correlation between anxiety-depression status and cytokines in diarrhea-predominant irritable bowel syndrome. Exp. Ther. Med. 2013, 6, 93–96. [Google Scholar] [CrossRef]
- Bai, Y.M.; Su, T.P.; Tsai, S.J.; Wen-Fei, C.; Li, C.T.; Pei-Chi, T.; Mu-Hong, C. Comparison of inflammatory cytokine levels among type I/type II and manic/ hypomanic/euthymic/depressive states of bipolar disorder. J. Affect. Disord. 2014, 166, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Maes, M. Bipolar disorder: Role of immune-inflammatory cytokines, oxidative and nitrosative stress and tryptophan catabolites. Curr. Psychiatry Rep. 2015, 17, 8. [Google Scholar] [CrossRef]
- Anderson, G.; Jacob, A.; Bellivier, F.; Geoffroy, P.A. Bipolar disorder: The role of the kynurenine and melatonergic pathways. Curr. Pharm. Des. 2016, 22, 987–1012. [Google Scholar] [CrossRef]
- Lugo-Huitrón, R.; Ugalde Muñiz, P.; Pineda, B.; Pedraza-Chaverrí, J.; Ríos, C.; Pérez-de la Cruz, V. Quinolinic acid: An endogenous neurotoxin with multiple targets. Oxid. Med. Cell. Longev. 2013, 2013, 104024. [Google Scholar] [CrossRef]
- Flowers, S.A.; Ward, K.M.; Clark, C.T. The Gut Microbiome in Bipolar Disorder and Pharmacotherapy Management. Neuropsychobiology 2020, 79, 43–49. [Google Scholar] [CrossRef]
- Marano, G.; Traversi, G.; Gaetani, E.; Pola, R.; Claro, A.E.; Mazza, M. Alcohol use disorder and liver injury related to the COVID-19 pandemic. World J. Hepatol. 2022, 14, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Neuman, M.G.; French, S.W.; Zakhari, S.; Malnick, S.; Seitz, H.K.; Cohen, L.B.; Salaspuro, M.; Voinea-Griffin, A.; Barasch, A.; Kirpich, I.A.; et al. Alcohol, microbiome, lifestyle influence alcohol and non-alcoholic organ damage. Exp. Mol. Pathol. 2017, 102, 162–180. [Google Scholar] [CrossRef]
- Legendre, T.; Boudebesse, C.; Henry, C.; Etain, B. Antibiomania: Think of the manic syndrome secondary to antibiotic therapy. Encephale 2017, 43, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015, 9, 392. [Google Scholar] [CrossRef]
- Reininghaus, E.Z.; Wetzlmair, L.C.; Fellendorf, F.T.; Platzer, M.; Queissner, R.; Birner, A.; Pilz, R.; Hamm, C.; Maget, A.; Koidl, C.; et al. The impact of probiotic supplements on cognitive parameters in euthymic individuals with bipolar disorder: A pilot study. Neuropsychobiology 2020, 79, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Institute for Health Metrics and Evaluation (IHME). About IHME. Global Health Data Exchange 2021. Available online: http://www.healthdata.org/about (accessed on 16 January 2023).
- Dash, S.; Clarke, G.; Berk, M.; Jacka, F.N. The gut microbiome and diet in psychiatry: Focus on depression. Curr. Opin. Psychiatry. 2015, 28, 1–6. [Google Scholar] [CrossRef]
- Lach, G.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics 2018, 15, 36–59. [Google Scholar] [CrossRef] [PubMed]
- Winter, G.; Hart, R.A.; Charlesworth, R.P.G.; Sharpley, C.F. Gut microbiome and depression: What we know and what we need to know. Rev. Neurosci. 2018, 29, 629–643. [Google Scholar] [CrossRef] [PubMed]
- Kamada, N.; Seo, S.U.; Chen, G.Y.; Núñez, G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 2013, 13, 321–335. [Google Scholar] [CrossRef]
- Li, N.; Wang, Q.; Wang, Y.; Sun, A.; Lin, Y.; Jin, Y.; Li, X. Fecal microbiota transplantation from chronic unpredictable mild stress mice donors affects anxiety-like and depression-like behavior in recipient mice via the gut microbiota-inflammation-brain axis. Stress 2019, 22, 592–602. [Google Scholar] [CrossRef]
- Simpson, C.A.; Diaz-Arteche, C.; Eliby, D.; Schwartz, O.S.; Simmons, J.G.; Cowan, C.S.M. The gut microbiota in anxiety and depression—A systematic review. Clin. Psychol. Rev. 2021, 83, 101943. [Google Scholar] [CrossRef] [PubMed]
- Barandouzi, Z.A.; Starkweather, A.R.; Henderson, W.A.; Gyamfi, A.; Cong, X.S. Altered Composition of Gut Microbiota in Depression: A Systematic Review. Front. Psychiatry 2020, 11, 541. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Naseribafrouei, A.; Hestad, K.; Avershina, E.; Sekelja, M.; Linløkken, A.; Wilson, R.; Rudi, K. Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil. 2014, 26, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, L.; Wang, X.; Wang, Z.; Zhang, J.; Jiang, R.; Wang, X.; Wang, K.; Liu, Z.; Xia, Z. Similar fecal microbiota signatures in patients with diarrhea-predominant irritable bowel syndrome and patients with depression. Clin. Gastroenterol. Hepatol. 2016, 14, 1602–1611.e5. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Ota, M.; Koga, N.; Hattori, K.; Kunugi, H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016, 202, 254–257. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef]
- Nikolova, V.L.; Smith, M.R.B.; Hall, L.J.; Cleare, A.J.; Stone, J.M.; Young, A.H. Perturbations in Gut Microbiota Composition in Psychiatric Disorders: A Review and Meta-analysis. JAMA Psychiatry 2021, 78, 1343–1354. [Google Scholar] [CrossRef]
- Huang, T.-T.; Lai, J.-B.; Du, Y.-L.; Xu, Y.; Ruan, L.-M.; Hu, S.-H. Current understanding of gut microbiota in mood disorders: An update of human studies. Front. Genet. 2019, 10, 98. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, X.; Li, Z.; Shen, Y.; Shi, X.; Wang, L.; Li, G.; Yuan, Y.; Wang, J.; Zhang, Y. Possible association of Firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 3329. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, W.; Zheng, H.-M.; Li, P.; McDonald, D.; Sheng, H.-F.; Chen, M.-X.; Chen, Z.-H.; Ji, G.-Y.; Mujagond, P. Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat. Med. 2018, 24, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Bonder, M.J.; Kurilshikov, A.; Tigchelaar, E.F.; Mujagic, Z.; Imhann, F.; Vila, A.V.; Deelen, P.; Vatanen, T.; Schirmer, M.; Smeekens, S.P. The effect of host genetics on the gut microbiome. Nat. Genet. 2016, 48, 1407–1412. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Jeffery, I.B. Gut microbiota and aging. Science 2015, 350, 1214–1215. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Borodovitsyna, O.; Flamini, M.; Chandler, D. Noradrenergic Modulation of Cognition in Health and Disease. Neural Plast. 2017, 2017, 6031478. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, signaling, and association with neurological diseases. Cell Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Kleinridders, A.; Pothos, E.N. Impact of brain insulin signaling on dopamine function, food intake, reward, and emotional behavior. Curr. Nutr. Rep. 2019, 8, 83–91. [Google Scholar] [CrossRef]
- Zhao, L.; Zheng, S.; Su, G.; Lu, X.; Yang, J.; Xiong, Z.; Wu, C. In vivo study on the neurotransmitters and their metabolites change in depressive disorder rat plasma by ultra high performance liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. B 2015, 988, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Yadid, G.; Friedman, A. Dynamics of the dopaminergic system as a key component to the understanding of depression. Prog. Brain Res. 2008, 172, 265–286. [Google Scholar] [PubMed]
- Willner, P.; Hale, A.S.; Argyropoulos, S. Dopaminergic mechanism of antidepressant action in depressed patients. J. Affect. Disord. 2005, 86, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.M. Essential Psychopharmacology: Neuroscientific Basis and Practical Applications; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Fidalgo, T.M.; Morales-Quezada, J.L.; Muzy, G.S.; Chiavetta, N.M.; Mendonca, M.E.; Santana, M.V.; Goncalves, O.F.; Brunoni, A.R.; Fregni, F. Biological Markers in Noninvasive Brain Stimulation Trials in Major Depressive Disorder. J. ECT 2013, 30, 47–61. [Google Scholar] [CrossRef]
- González-Arancibia, C.; Urrutia-Piñones, J.; Illanes-González, J.; Martinez-Pinto, J.; Sotomayor-Zárate, R.; Julio-Pieper, M.; Bravo, J.A. Do your gut microbes affect your brain dopamine? Psychopharmacology 2019, 236, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Rogers, G.; Keating, D.; Young, R.; Wong, M.; Licinio, J.; Wesselingh, S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry 2016, 21, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Tellez, L.A.; Perkins, M.H.; Perez, I.O.; Qu, T.; Ferreira, J.; Ferreira, T.L.; Quinn, D.; Liu, Z.-W.; Gao, X.-B. A neural circuit for gut-induced reward. Cell 2018, 175, 665.e623–678.e623. [Google Scholar] [CrossRef]
- Freestone, P.P.; Williams, P.H.; Haigh, R.D.; Maggs, A.F.; Neal, C.P.; Lyte, M. Growth stimulation of intestinal commensal Escherichia coli by catecholamines: A possible contributory factor in trauma-induced sepsis. Shock 2002, 18, 465–470. [Google Scholar] [CrossRef]
- Bansal, T.; Englert, D.; Lee, J.; Hegde, M.; Wood, T.K.; Jayaraman, A. Differential effects of epinephrine, norepinephrine, and indole on Escherichia coli O157:H7 chemotaxis, colonization, and gene expression. Infect. Immun. 2007, 75, 4597–4607. [Google Scholar] [CrossRef]
- O’Donnell, P.M.; Aviles, H.; Lyte, M.; Sonnenfeld, G. Enhancement of in vitro growth of pathogenic bacteria by norepinephrine: Importance of inoculum density and role of transferrin. Appl. Environ. Microbiol. 2006, 72, 5097–5099. [Google Scholar] [CrossRef] [PubMed]
- Averina, O.V.; Danilenko, V.N. Human intestinal microbiota: Role in development and functioning of the nervous system. Microbiology 2017, 86, 1–18. [Google Scholar] [CrossRef]
- Shishov, V.; Kirovskaya, T.; Kudrin, V.; Oleskin, A. Amine neuromediators, their precursors, and oxidation products in the culture of Escherichia coli K-12. Appl. Biochem. Microbiol. 2009, 45, 494–497. [Google Scholar] [CrossRef]
- Hoban, A.E.; Moloney, R.D.; Golubeva, A.V.; Neufeld, K.M.; O’Sullivan, O.; Patterson, E.; Stanton, C.; Dinan, T.G.; Clarke, G.; Cryan, J.F. Behavioural and neurochemical consequences of chronic gut microbiota depletion during adulthood in the rat. Neuroscience 2016, 339, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.F.; Hsu, C.C.; Chou, G.T.; Hsu, J.S.; Liong, M.T.; Tsai, Y.C. Lactobacillus paracasei PS23 reduced early-life stress abnormalities in maternal separation mouse model. Benef Microbes 2019, 10, 425–436. [Google Scholar] [CrossRef]
- Wei, C.L.; Wang, S.; Yen, J.T.; Cheng, Y.F.; Liao, C.L.; Hsu, C.C.; Wu, C.C.; Tsai, Y.C. Antidepressant-like activities of live and heat-killed Lactobacillus paracasei PS23 in chronic corticosterone-treated mice and possible mechanisms. Brain Res. 2019, 1711, 202–213. [Google Scholar] [CrossRef]
- Huang, F.; Wu, X. Brain Neurotransmitter Modulation by Gut Microbiota in Anxiety and Depression. Front. Cell. Dev. Biol. 2021, 9, 649103. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Jašarević, E.; Hill, E.M.; Kane, P.J.; Rutt, L.; Gyles, T.; Folts, L.; Rock, K.D.; Howard, C.D.; Morrison, K.E.; Ravel, J.; et al. The composition of human vaginal microbiota transferred at birth affects offspring health in a mouse model. Nat. Commun. 2021, 12, 6289. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stilling, R.M.; Stanton, C.; Cryan, J.F. Collective unconscious: How gut microbes shape human behavior. J. Psychiatr. Res. 2015, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Özoğul, F. Production of biogenic amines by Morganella morganii, Klebsiella pneumoniae and Hafnia alvei using a rapid HPLC method. Eur. Food Res. Technol. 2004, 219, 465–469. [Google Scholar] [CrossRef]
- Özoğul, F.; Kuley, E.; Özoğul, Y.; Özoğul, İ. The function of lactic acid bacteria on biogenic amines production by food-borne pathogens in arginine decarboxylase broth. Food Sci. Technol. Res. 2012, 18, 795–804. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef]
- Wang, S.; Egan, M.; Ryan, C.A.; Boyaval, P.; Dempsey, E.M.; Ross, R.P.; Stanton, C. A good start in life is important-perinatal factors dictate early microbiota development and longer term maturation. FEMS Microbiol. Rev. 2020, 44, 763–781. [Google Scholar] [CrossRef]
- Weiss, S.J.; Leung, C. Maternal depressive symptoms, poverty, and young motherhood increase the odds of early depressive and anxiety disorders for children born prematurely. Infant. Ment. Health J. 2021, 42, 586–602. [Google Scholar] [CrossRef] [PubMed]
- Bear, T.; Dalziel, J.; Coad, J.; Roy, N.; Butts, C.; Gopal, P. The Microbiome-Gut-Brain Axis and Resilience to Developing Anxiety or Depression under Stress. Microorganisms 2021, 9, 723. [Google Scholar] [CrossRef] [PubMed]
- Sanders, A.; Rackers, H.; Kimmel, M. A role for the microbiome in mother-infant interaction and perinatal depression. Int. Rev. Psychiatry 2019, 31, 280–294. [Google Scholar] [CrossRef]
- Van den Bergh, B.R.H.; van den Heuvel, M.I.; Lahti, M.; Braeken, M.; de Rooij, S.R.; Entringer, S.; Hoyer, D.; Roseboom, T.; Räikkönen, K.; King, S.; et al. Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neurosci. Biobehav. Rev. 2020, 117, 26–64. [Google Scholar] [CrossRef]
- Jašarević, E.; Bale, T.L. Prenatal and postnatal contributions of the maternal microbiome on offspring programming. Front. Neuroendocrinol. 2019, 55, 100797. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, K.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2011, 23, 255-e119. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Bruch, H. Perceptual and conceptual disturbances in anorexia nervosa. Psychosom. Med. 1962, 24, 187–194. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). Eating Disorders: Recognition and Treatment Full Guideline. 2017. Available online: https://www.nice.org.uk/guidance/ng69/evidence/full-guideline-pdf-161214767896 (accessed on 16 January 2023).
- Hilbert, A.; Hoek, H.W.; Schmidt, R. Evidence-based clinical guidelines for eating disorders: International comparison. Curr. Opin. Psychiatry 2017, 30, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Galmiche, M.; Déchelotte, P.; Lambert, G.; Tavolacci, M.P. Prevalence of eating disorders over the 2000–2018 period: A systematic literature review. Am. J. Clin. Nutr. 2019, 109, 1402–1413. [Google Scholar] [CrossRef] [PubMed]
- Hay, P. Current approach to eating disorders: A clinical update. Intern Med. J. 2020, 50, 24–29. [Google Scholar] [CrossRef]
- Fetissov, S.O. Role of the gut microbiota in host appetite control: Bacterial growth to animal feeding behaviour. Nat. Rev. Endocrinol. 2017, 13, 11–25. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.; Sandhu, K.V.; Bastiaanssen, T.F.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Roubalová, R.; Procházková, P.; Papežová, H.; Smitka, K.; Bilej, M.; Tlaskalová-Hogenová, H. Anorexia nervosa: Gut microbiota-immune-brain interactions. Clin. Nutr. 2020, 39, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Fetissov, S.O.; Déchelotte, P. The new link between gut-brain axis and neuropsychiatric disorders. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Breton, J.; Jacquemot, J.; Yaker, L.; Leclerc, C.; Connil, N.; Feuilloley, M.; Déchelotte, P.; Fetissov, S.O. Host Starvation and Female Sex Influence Enterobacterial ClpB Production: A Possible Link to the Etiology of Eating Disorders. Microorganisms 2020, 8, 530. [Google Scholar] [CrossRef]
- Smitka, K.; Prochazkova, P.; Roubalova, R.; Dvorak, J.; Papezova, H.; Hill, M.; Pokorny, J.; Kittnar, O.; Bilej, M.; Tlaskalova-Hogenova, H. Current Aspects of the Role of Autoantibodies Directed Against Appetite-Regulating Hormones and the Gut Microbiome in Eating Disorders. Front. Endocrinol. 2021, 12, 613983. [Google Scholar] [CrossRef] [PubMed]
- Fetissov, S.O.; Sinno, M.H.; Coëffier, M.; Bole-Feysot, C.; Ducrotté, P.; Hökfelt, T.; Déchelotte, P. Autoantibodies against appetite-regulating peptide hormones and neuropeptides: Putative modulation by gut microflora. Nutrition 2008, 24, 348–359. [Google Scholar] [CrossRef]
- Lucas, N.; Legrand, R.; Bôle-Feysot, C.; Breton, J.; Coëffier, M.; Akkermann, K.; Järv, A.; Harro, J.; Déchelotte, P.; Fetissov, S.O. Immunoglobulin G modulation of the melanocortin 4 receptor signaling in obesity and eating disorders. Transl. Psychiatry 2019, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Fetissov, S.O.; Hökfelt, T. On the origin of eating disorders: Altered signaling between gut microbiota, adaptive immunity and the brain melanocortin system regulating feeding behavior. Curr. Opin. Pharmacol. 2019, 48, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Wheatland, R. Chronic ACTH autoantibodies are a significant pathological factor in the disruption of the hypothalamic-pituitary-adrenal axis in chronic fatigue syndrome, anorexia nervosa and major depression. Med. Hypotheses 2005, 65, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, J.M.; Fetissov, S.O.; Legrand, R.; Claeyssens, S.; Hoekstra, P.J.; Verhulst, F.C.; Van Oort, F.V. Corticotropin (ACTH)-reactive immunoglobulins in adolescents in relation to antisocial behavior and stress-induced cortisol response. The TRAILS study. Psychoneuroendocrinology 2013, 38, 3039–3047. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.K.; Brownley, K.A.; Bardone-Cone, A.M.; Bulik, C.M.; Baker, J.H. Associations of Stress and Appetite Hormones with Binge Eating in Females with Anorexia Nervosa after Weight Restoration: A Longitudinal Study. J. Pers. Med. 2021, 11, 1020. [Google Scholar] [CrossRef] [PubMed]
- Holden, R.J.; Pakula, I.S. Tumor necrosis factor-alpha: Is there a continuum of liability between stress, anxiety states and anorexia nervosa? Med. Hypotheses 1999, 52, 155–162. [Google Scholar] [CrossRef]
- Patsalos, O.; Dalton, B.; Himmerich, H. Effects of IL-6 Signaling Pathway Inhibition on Weight and BMI: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 6290. [Google Scholar] [CrossRef]
- Patsalos, O.; Dalton, B.; Leppanen, J.; Ibrahim, M.A.A.; Himmerich, H. Impact of TNF-α Inhibitors on Body Weight and BMI: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2020, 11, 481. [Google Scholar] [CrossRef]
- Solmi, M.; Santonastaso, P.; Caccaro, R.; Favaro, A. A case of anorexia nervosa with comorbid Crohn’s disease: Beneficial effects of anti-TNF-α therapy? Int. J. Eat. Disord. 2013, 46, 639–641. [Google Scholar] [CrossRef] [PubMed]
- Barber, J.; Sheeran, T.; Mulherin, D. Anti-tumour necrosis factor treatment in a patient with anorexia nervosa and juvenile idiopathic arthritis. Ann. Rheum. Dis. 2003, 62, 490–491. [Google Scholar] [CrossRef]
- Caspani, G.; Swann, J. Small talk: Microbial metabolites involved in the signaling from microbiota to brain. Curr. Opin. Pharmacol. 2019, 48, 99–106. [Google Scholar] [CrossRef]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 2020, 11, 135–157. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. All disease begins in the (leaky) gut: Role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Research 2020, 9, F1000 Faculty Rev–69. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Morrison, D.J.; Frost, G. Control of appetite and energy intake by SCFA: What are the potential underlying mechanisms? Proc. Nutr. Soc. 2015, 74, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, B.; O’Toole, J.; Lask, B. PANDAS and anorexia nervosa—A spotters’ guide: Suggestions for medical assessment. Eur. Eat. Disord. Rev. 2010, 8, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef] [PubMed]
- Stark, P.L.; Lee, A. The microbial ecology of the large bowel of breast-fed and formula-fed infants during the first year of life. J. Med. Microbiol. 1982, 15, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Evrensel, A.; Ceylan, M.E. The gut-brain axis: The missing link in depression. Clin. Psychopharmacol. Neurosci. 2015, 13, 239–244. [Google Scholar] [CrossRef]
- Tanaka, K.; Farooqui, A.A.; Siddiqi, N.J.; Alhomida, A.S.; Ong, W.Y. Effects of docosahexaenoic acid on neurotransmission. Biomol. Ther. 2012, 20, 152–157. [Google Scholar] [CrossRef]
- Do Rosario, V.A.; Fernandes, R.; Trindade, E.B. Vegetarian diets and gut microbiota: Important shifts in markers of metabolism and cardiovascular disease. Nutr. Rev. 2016, 74, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Ferrocino, I.; Di Cagno, R.; De Angelis, M.; Turroni, S.; Vannini, L.; Bancalari, E.; Rantsiou, K.; Cardinali, G.; Neviani, E.; Cocolin, L. Fecal microbiota in healthy subjects following omnivore, vegetarian and vegan diets: Culturable populations and rRNA DGGE profiling. PLoS ONE 2015, 10, e0128669. [Google Scholar] [CrossRef] [PubMed]
- Pomponi, M.; Janiri, L.; La Torre, G.; Di Stasio, E.; Di Nicola, M.; Mazza, M.; Martinotti, G.; Bria, P.; Lippa, S.; Natili, R.; et al. Plasma levels of n-3 fatty acids in bipolar patients: Deficit restricted to DHA. J. Psychiatr. Res. 2013, 47, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The impact of western diet and nutrients on the microbiota and immune response at mucosal interfaces. Front. Immunol. 2017, 8, 838. [Google Scholar] [CrossRef]
- Moreira, A.P.B.; Texeira, T.F.S.; Ferreira, A.B.; Do Carmo Gouveia Peluzio, M.; De Cássia Gonçalves Alfenas, R. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br. J. Nutr. 2012, 108, 801–809. [Google Scholar] [CrossRef]
- Cazettes, F.; Cohen, J.I.; Yau, P.L.; Talbot, H.; Convit, A. Obesity-mediated inflammation may damage the brain circuit that regulates food intake. Brain Res. 2011, 1373, 101–109. [Google Scholar] [CrossRef]
- Chen, L.L.; Abbaspour, A.; Mkoma, G.F.; Bulik, C.M.; Rück, C.; Djurfeldt, D. Gut microbiota in psychiatric disorders: A systematic review. Psychosom. Med. 2021, 83, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Liu, J.; Cetinbas, M.; Sadreyev, R.; Koh, M.; Huang, H.; Adeseye, A.; He, P.; Zhu, J.; Russell, H.; et al. New and preliminary evidence on altered oral and gut microbiota in individuals with autism spectrum disorder (ASD): Implications for ASD diagnosis and subtyping based on microbial biomarkers. Nutrients 2019, 11, 2128. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Raza, G.S.; Putaala, H.; Hibberd, A.A.; Alhoniemi, E.; Tiihonen, K.; Mäkelä, K.A.; Herzig, K.H. Polydextrose changes the gut microbiome and attenuates fasting triglyceride and cholesterol levels in Western diet fed mice. Sci. Rep. 2017, 7, 5294. [Google Scholar] [CrossRef]
- Allen, A.P.; Hutch, W.; Borre, Y.E.; Kennedy, P.J.; Temko, A.; Boylan, G.; Murphy, E.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Bifidobacterium longum 1714 as a translational psychobiotic: Modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl. Psychiatry 2016, 6, e939. [Google Scholar] [CrossRef]
- Messaoudi, M.; Rozan, P.; Nejdi, A.; Hidalgo, S.; Desor, D. Behavioural and cognitive effects of oligofructose-enriched inulin in rats. Br. J. Nutr. 2005, 93 (Suppl. 1), S27–S30. [Google Scholar] [CrossRef] [PubMed]
- Vissers, L.E.; Dalmeijer, G.W.; Boer, J.M.; Monique Verschuren, W.M.; van der Schouw, Y.T.; Beulens, J.W. Intake of dietary phylloquinone and menaquinones and risk of stroke. J. Am. Heart Assoc. 2013, 2, e000455. [Google Scholar] [CrossRef] [PubMed]
- Karl, J.P.; Meydani, M.; Barnett, J.B.; Vanegas, S.M.; Barger, K.; Fu, X.; Goldin, B.; Kane, A.; Rasmussen, H.; Vangay, P.; et al. Fecal concentrations of bacterially derived vitamin K forms are associated with gut microbiota composition but not plasma or fecal cytokine concentrations in healthy adults. Am. J. Clin. Nutr. 2017, 106, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Gancheva, S.M.; Zhelyazkova-Savova, M.D. Vitamin K2 improves anxiety and depression but not cognition in rats with metabolic syndrome: A role of blood glucose? Folia Med. 2016, 58, 264–272. [Google Scholar] [CrossRef]
- Lee, H.; Ko, G. Antiviral effect of vitamin A on norovirus infection via modulation of the gut microbiome. Sci. Rep. 2016, 6, 25835. [Google Scholar] [CrossRef] [PubMed]
- Lupp, C.; Robertson, M.L.; Wickham, M.E.; Sekirov, I.; Champion, O.L.; Gaynor, E.C.; Finlay, B.B. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of enterobacteriaceae. Cell Host Microbe 2007, 2, 119–129. [Google Scholar] [CrossRef]
- Reed, S.; Neuman, H.; Moscovich, S.; Glahn, R.P.; Koren, O.; Tako, E. Chronic zinc deficiency alters chick gut microbiota composition and function. Nutrients 2015, 7, 9768–9784. [Google Scholar] [CrossRef] [PubMed]
- Koudoufio, M.; Desjardins, Y.; Feldman, F.; Spahis, S.; Delvin, E.; Levy, E. Insight into polyphenol and gut microbiota crosstalk: Are their metabolites the key to understand protective effects against metabolic disorders? Antioxidants 2020, 9, 982. [Google Scholar] [CrossRef]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota–Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef] [PubMed]
- Man, A.W.C.; Zhou, Y.; Xia, N.; Li, H. Involvement of Gut Microbiota, Microbial Metabolites and Interaction with Polyphenol in Host Immunometabolism. Nutrients 2020, 12, 3054. [Google Scholar] [CrossRef] [PubMed]
- Colica, C.; Di Renzo, L.; Trombetta, D.; Smeriglio, A.; Bernardini, S.; Cioccoloni, G.; Costa De Miranda, R.; Gualtieri, P.; Sinibaldi Salimei, P.; De Lorenzo, A. Antioxidant Effects of a Hydroxytyrosol-Based Pharmaceutical Formulation on Body Composition, Metabolic State, and Gene Expression: A Randomized Double-Blinded, Placebo-Controlled Crossover Trial. Oxid. Med. Cell. Longev. 2017, 2017, 2473495. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Martín-Peláez, S.; Mosele, J.I.; Pizarro, N.; Farràs, M.; de la Torre, R.; Subirana, I.; Pérez-Cano, F.J.; Castañer, O.; Solà, R.; Fernandez-Castillejo, S.; et al. Effect of virgin olive oil and thyme phenolic compounds on blood lipid profile: Implications of human gut microbiota. Eur. J. Nutr. 2017, 56, 119–131. [Google Scholar] [CrossRef]
- Qiao, Y.; Sun, J.; Xia, S.; Tang, X.; Shi, Y.; Le, G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014, 5, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Piao, M.; Song, Y. Dietary Quercetin Increases Colonic Microbial Diversity and Attenuates Colitis Severity in Citrobacter rodentium-Infected Mice. Front. Microbiol. 2019, 10, 1092. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gomez, B.; Lezama, A.; Amigo-Benavent, M.; Ullate, M.; Herrero, M.; Martín, M.Á.; Mesa, M.D.; del Castillo, M.D. Insights on the Health Benefits of the Bioactive Compounds of Coffee Silverskin Extract. J. Funct. Foods 2016, 25, 197–207. [Google Scholar] [CrossRef]
- Barros Silva, R.; Santos, N.A.G.; Martins, N.M.; Ferreira, D.A.S.; Barbosa, F.; Oliveira Souza, V.C.; Kinoshita, Â.; Baffa, O.; Del-Bel, E.; Santos, A.C. Caffeic Acid Phenethyl Ester Protects against the Dopaminergic Neuronal Loss Induced by 6-Hydroxydopamine in Rats. Neuroscience 2013, 233, 86–94. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Uranga, J.A.; del Castillo, M.D.; Abalo, R. Effects of Coffee and Its Components on the Gastrointestinal Tract and the Brain–Gut Axis. Nutrients 2021, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Gainey, S.J.; Kwakwa, K.A.; Bray, J.K.; Pillote, M.M.; Tir, V.L.; Towers, A.E.; Freund, G.G. Short-term high-fat diet (HFD) induced anxiety-like behaviors and cognitive impairment are improved with treatment by glyburide. Front. Behav. Neurosci. 2016, 10, 156. [Google Scholar] [CrossRef]
- Richardson, A.J. Omega-3 fatty acids in ADHD and related neurodevelopmental disorders. Int. Rev. Psychiatry 2006, 18, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.; Pomponi, M.; Janiri, L.; Bria, P.; Mazza, S. Omega-3 fatty acids and antioxidants in neurological and psychiatric diseases: An overview. Prog Neuropsychopharmacol Biol. Psychiatry 2007, 31, 12–26. [Google Scholar] [CrossRef]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef]
- Losasso, C.; Eckert, E.M.; Mastrorilli, E.; Villiger, J.; Mancin, M.; Patuzzi, I.; Di Cesare, A.; Cibin, V.; Barrucci, F.; Pernthaler, J.; et al. Assessing the Influence of Vegan, Vegetarian and Omnivore Oriented Westernized Dietary Styles on Human gut microbiota: A cross sectional study. Front. Microbiol. 2018, 9, 317. [Google Scholar] [CrossRef] [PubMed]
- Klimenko, N.S.; Tyakht, A.V.; Popenko, A.S.; Vasiliev, A.S.; Altukhov, I.A.; Ischenko, D.S.; Shashkova, T.I.; Efimova, D.A.; Nikogosov, D.A.; Osipenko, D.A.; et al. Microbiome responses to an uncontrolled short-term diet intervention in the frame of the citizen science project. Nutrients 2018, 10, 576. [Google Scholar] [CrossRef]
- Verdam, F.J.; Fuentes, S.; de Jonge, C.; Zoetendal, E.G.; Erbil, R.; Greve, J.W.; Buurman, W.A.; de Vos, W.M.; Rensen, S.S. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obes. Silver Spring Md. 2013, 21, E607–E615. [Google Scholar] [CrossRef] [PubMed]
- Glick-Bauer, M.; Yeh, M.-C. The health advantage of a vegan diet: Exploring the gut microbiota connection. Nutrients 2014, 6, 4822–4838. [Google Scholar] [CrossRef]
- Figueroa-Gonzalez, I.; Quijano, G.; Ramirez, G.; Cruz-Guerrero, A. Probiotics and prebiotics-perspectives and challenges. J. Sci. Food Agric. 2011, 91, 1341–1348. [Google Scholar] [CrossRef]
- Anandharaj, M.; Sivasankari, B.; Rani, R.P. Effects of probiotics, prebiotics, and synbiotics on hypercholesterolemia: A review. Chin. J. Biol. 2014, 2014, 572754. [Google Scholar] [CrossRef]
- Singh, K.; Kallali, B.; Kumar, A.; Thaker, V. Probiotics: A review. Asian Pac. J. Trop. Biomed. 2011, 1, S287–S290. [Google Scholar] [CrossRef]
- Maurya, P.; Mogra, R.; Bajpai, P. Probiotics: An approach towards health and disease. Trends Biosci. 2014, 7, 3107–3113. [Google Scholar]
- Bowman, C.C.; Rasley, A.; Tranguch, S.L.; Marriott, I. Cultured astrocytes express toll-like receptors for bacterial products. Glia 2003, 43, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Bsibsi, M.; Ravid, R.; Gveric, D.; van Noort, J.M. Broad expression of Toll-like receptors in the human central nervous system. J. Neuropathol. Exp. Neurol. 2002, 61, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, F.B.; Stallings, C.; Origoni, A.; Katsafanas, E.; Savage, C.L.; Schweinfurth, L.A.; Goga, J.; Khushalani, S.; Yolken, R.H. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: A randomized, placebo-controlled trial. Prim. Care Companion CNS Disord. 2014, 16, 26294. [Google Scholar] [CrossRef]
- Gasbarrini, G.; Bonvicini, F.; Gramenzi, A. Probiotics History. J. Clin. Gastroenterol. 2016, 50, S116–S119. [Google Scholar] [CrossRef]
- Mangiola, F.; Ianiro, G.; Franceschi, F.; Fagiuoli, S.; Gasbarrini, G.; Gasbarrini, A. Gut microbiota in autism and mood disorders. World J. Gastroenterol. 2016, 22, 361–368. [Google Scholar] [CrossRef] [PubMed]
| Type of Diet | Impact on Microbiota | References |
|---|---|---|
| Western diet | Decreased levels of intestinal microbiotic diversity (reduced number of members of the phylum Bacteroidetes has been found, along with higher levels of Proteobacteria and Firmicutes). | Tengeler et al., 2018 [3] |
| Increase in fatty acids levels leads to pro-inflammatory state, through the already-mentioned effect of altered microbiota on intestinal wall permeability (increased susceptibility to psychiatric and neurological diseases). | Evrensel et al., 2015 [174] | |
| Mediterranean diet | Increased diversity of microbiome composition, with a decreased concentration of Firmicutes, Proteobacteria and Clostridia, and an increase in Bifidobacteria and Lactobacillus. | Singh et al., 2017 [39] |
| Reduced inflammation levels, decreased cytokines release and a greater modulation on intestinal permeability. | Tanaka et al., [175] | |
| Vegetarian and vegan diet | Decreased representation of Enterobacteriaceae and increase of Bacteroides and Prevotella | do Rosario et al., 2016 [176] |
| Reduction in Bacteroides Fragilis and Clostridium with subsequent decrease of risk factors for chronic inflammation | Ferrocino et al., 2015 [177] |
| Psychiatric Disorder | Impact on Microbiota | References |
|---|---|---|
| Schizophrenia | Increased immune activation against dietary proteins and pathogens and continuous exposure to antigens predisposing the digestive system to chronic inflammation; alteration of permeability of the intestinal blood barrier and an increased risk of passage into the systemic circulation of bacterial and food peptides; increased inflammatory state with production of pro-inflammatory cytokines. | Caso et al., 2016 [44] |
| Stimulation of Th17 cells in response to external stimuli, with consequent gastrointestinal inflammation causing intestinal dysbiosis. Maternal infections during pregnancy inducing a pro-inflammatory activation state responsible of metabolic consequences in the long period (reduced glycemic regulation, insulin resistance, increased body weight). Association of schizophrenia with irritable bowel syndrome influenced by gut microbiota, through bacterial translocation. | Torrey et al., 2012 [50] Labouesse et al., 2015 [52] Severance et al., 2016 [55] | |
| Bipolar Disorder | Lower proportion of Faecalibacterium corresponding to a worsening of the pathology. | Painold et al., 2017 [66] |
| Greater representation of the phylum Actinobacteria, with higher concentration of Prevotella and Enterobacter species, and gram-positive bacteria Atopobium Cluster, Clostridium, Flavinofractor. Prevotella more represented in bipolar type 1 patients, while Collinsella more abundant in bipolar type 2 patients. Production of neuroactive kynurenine, capable of inhibit the synthesis of 5-HT and interfere with the secretion of dopamine and GABA. Synaptic pruning could be modified by a direct effect of gut microbiota on microglial cells, especially in the ventral prefrontal and limbic cortex. | Gondalia et al., 2019 [69] Lucidi et al. 2021 [68] Schwarcz et al., 2017 [33] Strakowski et al., 2012 [72] | |
| Unipolar Affective Disorder | Intestinal bacteria can change function of the hypotalamic–pituitary–andrenal axis (HPA) leading to an increased concentration of cortisol and pro-inflammatory molecules: the proinflammatory state increases the intestinal barrier permeability, facilitating the access to the bloodstream for gram-negative bacteria and inducing chronic inflammation in the central nervous system. The possible microbial profile in depressed patients can be defined by a reduction in the concentration of Firmicutes, Bacteroides and Proteobacteria, or by increased levels of Actinobacteria and Fusobacteria, Prevotellaceae and Lachnospiraceae. | Li et al., 2019 [91] Barandouzi et al., 2020 [93] |
| Decreased concentrations of Bifidobacterium, Firmicutes, Lactobacillus, Faecalibacterium and Ruminococcus and increased concentrations of Proteobacteria, Bacteroides and Prevotella in the gut microbiota of depressed patients. | Liu et al., 2016 [97] | |
| Anxiety Disorders | Stressful events during intrauterine life and early childhood are associated with intestinal dysbiosis in the unborn child and in the mother. Alterations in the microbiota associated with increased concentrations of circulating corticosterone in response to stressful events, with transmission of stressed-altered maternal microbiota have long-term effects on gene expression at level of the hypothalamic paraventricular nucleus. The microbiota could regulate the serotonin system in the brain, influencing the hypotalamic–pituitary–andrenal axis and modifying gene expression at hippocampal and hypothalamic levels. | Jašarević et al., 2021 [131] Neufeld et al., 2011 [142] |
| Anorexia Nervosa | Increased concentration of bacteria of the species Clostridia, Enterobacteriaceae and M. Smithii and reduction in the species Roseburia. The intestinal microbiota transforms the proceeds of the diet into a large variety of products including vitamins, amino acid derivatives and short-chain fatty acids, able to modulate the permeability of the blood–brain barrier. | Roubalová et al., 2020 [151] Parker et al., 2020 [167] |
| Bulimia Nervosa | Increased levels of caseinolytic proteinase B, produced by E. Coli, which in turn stimulate autoimmunity response. The activity of the centers of appetite is complexly regulated by an elaborate neuroimmunoendocrine communication system, with the microbiota regulating the activity of adipose tissue and general homeostasis. | Smitka et al., 2021 [154] Lucas et al., 2019 [156] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marano, G.; Mazza, M.; Lisci, F.M.; Ciliberto, M.; Traversi, G.; Kotzalidis, G.D.; De Berardis, D.; Laterza, L.; Sani, G.; Gasbarrini, A.; et al. The Microbiota–Gut–Brain Axis: Psychoneuroimmunological Insights. Nutrients 2023, 15, 1496. https://doi.org/10.3390/nu15061496
Marano G, Mazza M, Lisci FM, Ciliberto M, Traversi G, Kotzalidis GD, De Berardis D, Laterza L, Sani G, Gasbarrini A, et al. The Microbiota–Gut–Brain Axis: Psychoneuroimmunological Insights. Nutrients. 2023; 15(6):1496. https://doi.org/10.3390/nu15061496
Chicago/Turabian StyleMarano, Giuseppe, Marianna Mazza, Francesco Maria Lisci, Michele Ciliberto, Gianandrea Traversi, Georgios Demetrios Kotzalidis, Domenico De Berardis, Lucrezia Laterza, Gabriele Sani, Antonio Gasbarrini, and et al. 2023. "The Microbiota–Gut–Brain Axis: Psychoneuroimmunological Insights" Nutrients 15, no. 6: 1496. https://doi.org/10.3390/nu15061496
APA StyleMarano, G., Mazza, M., Lisci, F. M., Ciliberto, M., Traversi, G., Kotzalidis, G. D., De Berardis, D., Laterza, L., Sani, G., Gasbarrini, A., & Gaetani, E. (2023). The Microbiota–Gut–Brain Axis: Psychoneuroimmunological Insights. Nutrients, 15(6), 1496. https://doi.org/10.3390/nu15061496














