Radiological Benefits of Vitamin D Status and Supplementation in Patients with MS—A Two-Year Prospective Observational Cohort Study
Abstract
1. Introduction
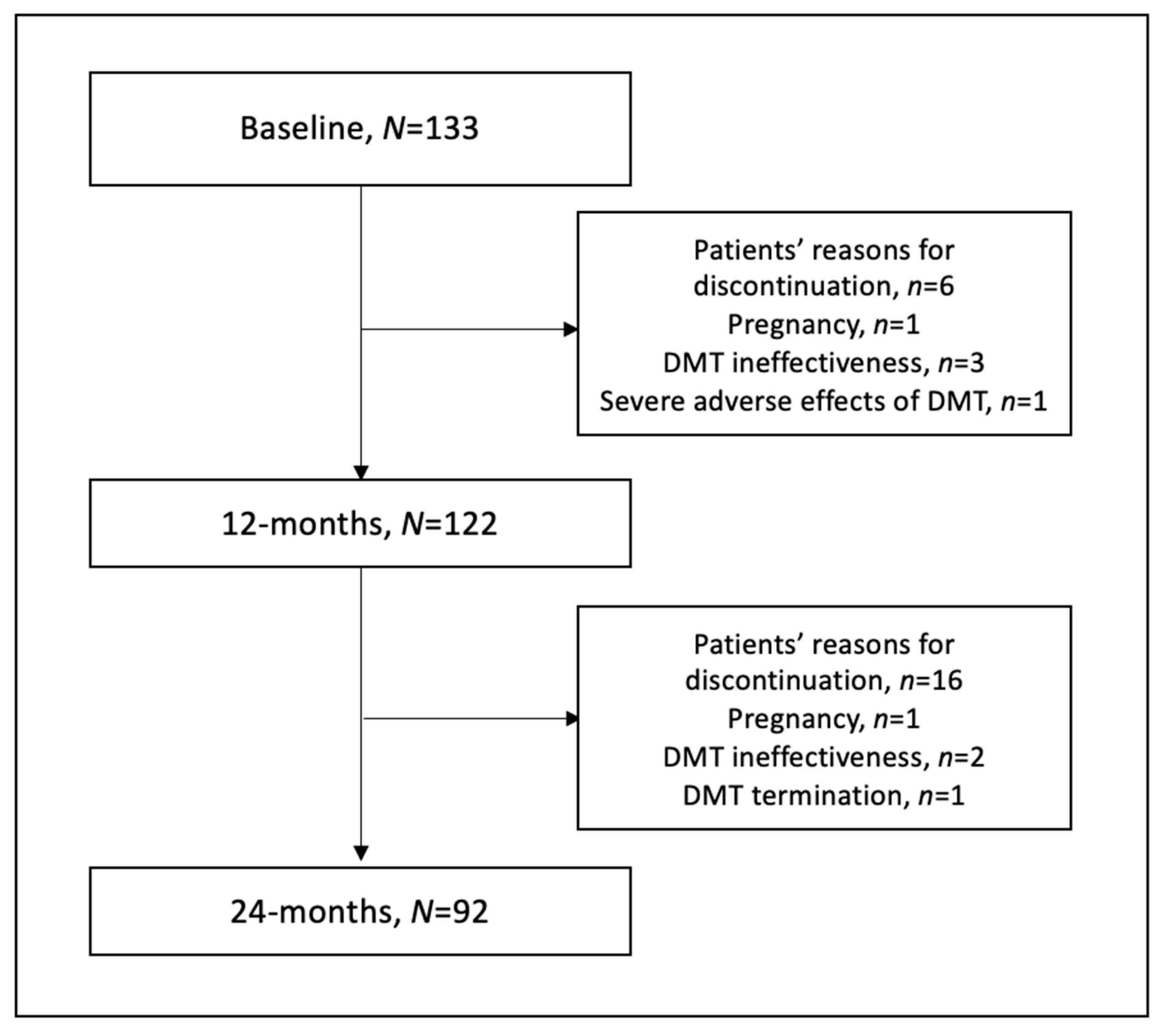
2. Materials and Methods
- Diagnosed with MS (RRMS, SPMS and PPMS) according to the 2010 or 2017 revised McDonald criteria;
- Aged over 18 years;
- EDSS ≤ 6.5;
- Stable treatment with DMT in a clinical trial or drug prescription program for at least one year at the baseline (interferon beta, glatiramer acetate, dimethyl fumarate, teriflunomide, natalizumab, fingolimod, ocrelizumab, alemtuzumab and cladribine).
- Relapse in the last 4 weeks;
- EDSS > 6.5;
- Pregnancy or breast-feeding;
- Acute or chronic renal failure;
- Hypercalcemia in medical history;
- DMT notable ineffectiveness is defined as at least two new T2-w lesions and one GEL in MRI and two relapses in a year; DMT discontinuation/termination; or DMT severe adverse effect.
- EDSS change;
- Number of new relapses;
- Time to relapse;
- Number of new or enlarged T2-w lesions in MRI;
- Number of GELs in MRI.
3. Results
3.1. Vitamin D Status and Supplementation
3.2. Vitamin D and Clinical Outcomes
3.3. Vitamin D and Radiological Outcomes
3.4. Vitamin D and Disease-Modifying Therapy
4. Discussion
4.1. Vitamin D Status and Supplementation
4.2. Vitamin D and Clinical Outcomes
4.3. Vitamin D and Radiological Outcomes
4.4. Vitamin D and Disease-Modifying Therapy
4.5. Study Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gil, Á.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and Novel Actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Bivona, G.; Agnello, L.; Ciaccio, M. The immunological implication of the new vitamin D metabolism. Cent. Eur. J. Immunol. 2018, 43, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef]
- Provvedini, D.M.; Tsoukas, C.D.; Deftos, L.J.; Manolagas, S.C. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science 1983, 221, 1181–1183. [Google Scholar] [CrossRef] [PubMed]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and immune function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef]
- Bellan, M.; Andreoli, L.; Mele, C.; Sainaghi, P.P.; Rigamonti, C.; Piantoni, S.; De Benedittis, C.; Aimaretti, G.; Pirisi, M.; Marzullo, P. Pathophysiological Role and Therapeutic Implications of Vitamin D in Autoimmunity: Focus on Chronic Autoimmune Diseases. Nutrients 2020, 12, 789. [Google Scholar] [CrossRef]
- Ascherio, A.; Munger, K. Epidemiology of Multiple Sclerosis: From Risk Factors to Prevention—An Update. Semin. Neurol. 2016, 36, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Rhead, B.; Bäärnhielm, M.; Gianfrancesco, M.; Mok, A.; Shao, X.; Quach, H.; Shen, L.; Schaefer, C.; Link, J.; Gyllenberg, A.; et al. Mendelian randomization shows a causal effect of low vitamin D on multiple sclerosis risk. Neurol. Genet. 2016, 2, e97. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Mendelian randomization study updates the effect of 25-hydroxyvitamin D levels on the risk of multiple sclerosis. J. Transl. Med. 2022, 20, 3. [Google Scholar] [CrossRef]
- Munger, K.L.; Levin, L.I.; Hollis, B.W.; Howard, N.S.; Ascherio, A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006, 296, 2832–2838. [Google Scholar] [CrossRef]
- Munger, K.L.; Zhang, S.M.; O’Reilly, E.; Hernán, M.A.; Olek, M.J.; Willett, W.C.; Ascherio, A. Vitamin D intake and incidence of multiple sclerosis. Neurology 2004, 62, 60–65. [Google Scholar] [CrossRef]
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef] [PubMed]
- Browne, P.; Chandraratna, D.; Angood, C.; Tremlett, H.; Baker, C.; Taylor, B.V.; Thompson, A.J. Atlas of multiple sclerosis 2013: A growing global problem with widespread inequity. Neurology 2014, 83, 1022–1024. [Google Scholar] [CrossRef]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. J. 2020, 26, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Lublin, F.D.; Coetzee, T.; Cohen, J.A.; Marrie, R.A.; Thompson, A.J.; International Advisory Committee on Clinical Trials in MS. The 2013 clinical course descriptors for multiple sclerosis. A clarification. Neurology 2020, 94, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Hemond, C.C.; Bakshi, R. Magnetic Resonance Imaging in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a028969. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.L.; Cree, B.A.C. Treatment of Multiple Sclerosis: A Review. Am. J. Med. 2020, 133, 1380–1390.e2. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.B.; Nashold, F.E.; Spach, K.M.; Hayes, C.E. 1,25-hydroxyvitamin D3 reverses experimental autoimmune encephalomyelitis by inhibiting chemokine synthesis and monocyte trafficking. J. Neurosci. Res. 2007, 85, 2480–2490. [Google Scholar] [CrossRef] [PubMed]
- Sotirchos, E.S.; Bhargava, P.; Eckstein, C.; Van Haren, K.; Baynes, M.; Ntranos, A.; Gocke, A.; Steinman, L.; Mowry, E.M.; Calabresi, P.A. Safety and Immunologic Effects of High- vs Low-Dose Cholecalciferol in Multiple Sclerosis. Neurology 2016, 86, 382–390. [Google Scholar] [CrossRef]
- Mosayebi, G.; Ghazavi, A.; Ghasami, K.; Jand, Y.; Kokhaei, P. Therapeutic effect of vitamin D3 in multiple sclerosis patients. Immunol. Investig. 2011, 40, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Walawska-Hrycek, A.; Galus, W.; Hrycek, E.; Kaczmarczyk, A.; Krzystanek, E. The Impact of Vitamin D Low Doses on Its Serum Level and Cytokine Profile in Multiple Sclerosis Patients. J. Clin. Med. 2021, 10, 2781. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, A.G.; Errea, O.; van Wijngaarden, P.; Gonzalez, G.A.; Kerninon, C.; Jarjour, A.A.; Lewis, H.J.; Jones, C.A.; Nait-Oumesmar, B.; Zhao, C.; et al. Vitamin D receptor-retinoid X receptor heterodimer signaling regulates oligodendrocyte progenitor cell differentiation. J. Cell Biol. 2015, 211, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pinedo, U.; Cuevas, J.A.; Benito-Martín, M.S.; Moreno-Jiménez, L.; Esteban-Garcia, N.; Torre-Fuentes, L.; Matías-Guiu, J.A.; Pytel, V.; Montero, P.; Matías-Guiu, J. Vitamin D increases remyelination by promoting oligodendrocyte lineage differentiation. Brain Behav. 2020, 10, e01498. [Google Scholar] [CrossRef]
- Martínez-Lapiscina, E.H.; Mahatanan, R.; Lee, C.-H.; Charoenpong, P.; Hong, J.-P. Associations of serum 25(OH) vitamin D levels with clinical and radiological outcomes in multiple sclerosis, a systematic review and meta-analysis. J. Neurol. Sci. 2020, 411, 116668. [Google Scholar] [CrossRef] [PubMed]
- Moosazadeh, M.; Nabinezhad-Male, F.; Afshari, M.; Nasehi, M.M.; Shabani, M.; Kheradmand, M.; Aghaei, I. Vitamin D status and disability among patients with multiple sclerosis: A systematic review and meta-analysis. AIMS Neurosci. 2021, 8, 239–253. [Google Scholar] [CrossRef]
- Jagannath, V.A.; Filippini, G.; Di Pietrantonj, C.; Asokan, G.V.; Robak, E.W.; Whamond, L.; Robinson, S.A. Vitamin D for the management of multiple sclerosis. Cochrane Database Syst. Rev. 2018, 9, CD008422. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, L.; Clarke, L.; Khalilidehkordi, E.; Butzkueven, H.; Taylor, B.; Broadley, S.A. Vitamin D for the treatment of multiple sclerosis: A meta-analysis. J. Neurol. 2018, 265, 2893–2905. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Guo, L.; Jiang, C.; Yang, X.; Huang, J. The Effect of Different Administration Time and Dosage of Vitamin D Supplementation in Patients with Multiple Sclerosis: A Meta-Analysis of Randomized Controlled Trials. Neuroimmunomodulation 2021, 28, 118–128. [Google Scholar] [CrossRef]
- Hanaei, S.; Ali Sahraian, M.; Mohammadifar, M.; Ramagopalan, S.V.; Ghajarzadeh, M. Effect of Vitamin D Supplements on Relapse Rate and Expanded Disability Status Scale (EDSS) in Multiple Sclerosis (MS): A Systematic Review and Meta-Analysis. Int. J. Prev. Med. 2021, 12, 42. [Google Scholar]
- Doosti-Irani, A.; Tamtaji, O.R.; Mansournia, M.A.; Mobarhan, M.G.; Ferns, G.; Kakhaki, R.D.; Shahmirzadi, A.R.; Asemi, Z. The effects of vitamin D supplementation on expanded disability status scale in people with multiple sclerosis: A critical, systematic review and meta-analysis of randomized controlled trials. Clin. Neurol. Neurosurg. 2019, 187, 105564. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; He, L.; Liu, L.; Zhu, J.; Jin, T. The efficacy of vitamin D in multiple sclerosis: A meta-analysis. Mult. Scler. Relat. Disord. 2018, 23, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Fatima, M.; Lamis, A.; Siddiqui, S.W.; Ashok, T.; Patni, N.; Fadiora, O.E. Therapeutic Role of Vitamin D in Multiple Sclerosis: An Essentially Contested Concept. Cureus 2022, 14, e26186. [Google Scholar] [CrossRef]
- Gandhi, F.; Jhaveri, S.; Avanthika, C.; Singh, A.; Jain, N.; Gulraiz, A.; Shah, P.; Nasir, F. Impact of Vitamin D Supplementation in Multiple Sclerosis. Cureus 2021, 13, e18487. [Google Scholar] [CrossRef]
- Sintzel, M.B.; Rametta, M.; Reder, A.T. Vitamin D and Multiple Sclerosis: A Comprehensive Review. Neurol. Ther. 2018, 7, 59–85. [Google Scholar] [CrossRef]
- Pierrot-Deseilligny, C.; Souberbielle, J.-C. Vitamin D and multiple sclerosis: An update. Mult. Scler. Relat. Disord. 2017, 14, 35–45. [Google Scholar] [CrossRef]
- Rusińska, A.; Płudowski, P.; Walczak, M.; Borszewska-Kornacka, M.K.; Bossowski, A.; Chlebna-Sokół, D.; Czech-Kowalska, J.; Dobrzańska, A.; Franek, E.; Helwich, E.; et al. Vitamin D Supplementation Guidelines for General Population and Groups at Risk of Vitamin D Deficiency in Poland—Recommendations of the Polish Society of Pediatric Endocrinology and Diabetes and the Expert Panel with Participation of National Specialist Consultant and Representatives of Scientific Societies—2018 Update. Front. Endocrinol. Lausanne 2018, 9, 246. [Google Scholar] [PubMed]
- Płudowski, P.; Karczmarewicz, E.; Bayer, M.; Carter, G.; Chlebna-Sokół, D.; Czech-Kowalska, J.; Dębski, R.; Decsi, T.; Do-brzańska, A.; Franek, E.; et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe—Recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynol. Pol. 2013, 64, 319–327. [Google Scholar] [CrossRef]
- Holmøy, T.; Torkildsen, Ø.; Myhr, K.-M.; Løken-Amsrud, K.I. Vitamin D supplementation and monitoring in multiple sclerosis: Who, when and wherefore. Acta Neurol. Scand. Suppl. 2012, 126, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Boltjes, R.; Knippenberg, S.; Gerlach, O.; Hupperts, R.; Damoiseaux, J. Vitamin D supplementation in multiple sclerosis: An expert opinion based on the review of current evidence. Expert Rev. Neurother. 2021, 21, 715–725. [Google Scholar] [CrossRef]
- Galus, W.; Walawska-Hrycek, A.; Rzepka, M.; Krzystanek, E. Vitamin D Supplementation Practices among Multiple Sclerosis Patients and Professionals. J. Clin. Med. 2022, 11, 7278. [Google Scholar] [CrossRef] [PubMed]
- Pape, K.; Steffen, F.; Zipp, F.; Bittner, S. Supplementary medication in multiple sclerosis: Real-world experience and potential interference with neurofilament light chain measurement. Mult. Scler. J. Exp. Transl. Clin. 2020, 6, 2055217320936318. [Google Scholar] [CrossRef]
- Masulo, L.; Papas, M.A.; Cotugna, N.; Baker, S.; Mahoney, L.; Trabulsi, J. Complemtary and alternative medicine use and nutrient intake among individuals with multiple sclerosis in the United States. J. Community Health 2015, 40, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, Y.D.; Adoni, T.; Alves-Leon, S.V.; Apostolos-Pereira, S.L.; Arruda, W.O.; Brooks, J.B.; Cal, H.S.; Damasceno, C.A.; Gama, P.D.; Goncalves, M.V.; et al. No correlation was observed between vitamin D levels and disability of patients with multiple sclerosis between latitudes 18° and 30° South. Arq. Neuropsiquiatr. 2017, 75, 3–8. [Google Scholar] [CrossRef]
- Rito, Y.; Flores, J.; Fernández-Aguilar, A.; Escalante-Membrillo, C.; Barboza, M.A.; Amezcua, L.; Corona, T. Vitamin D and disability in relapsing—Remitting multiple sclerosis in patients with a Mexican background. Acta Neurol. Belg. 2018, 118, 47–52. [Google Scholar] [CrossRef]
- Brola, W.; Sobolewski, P.; Szczuchniak, W.; Góral, A.; Fudala, M.; Przybylski, W.; Opara, J. Association of seasonal serum 25-hydroxyvitamin D levels with disability and relapses in relapsing-remitting multiple sclerosis. Eur. J. Clin. Nutr. 2016, 70, 995–999. [Google Scholar] [CrossRef]
- Thouvenot, E.; Orsini, M.; Daures, J.-P.; Camu, W. Vitamin D is associated with degree of disability in patients with fully ambulatory relapsing-remitting multiple sclerosis. Eur. J. Neurol. 2015, 22, 564–569. [Google Scholar] [CrossRef]
- Harandi, A.A.; Shahbeigi, S.; Pakdaman, H.; Fereshtehnejad, S.-M.; Nikravesh, E.; Jalilzadeh, R. Association of serum 25(OH) vitamin D3 concentration with severity of multiple sclerosis. Iran. J. Neurol. 2012, 11, 54–58. [Google Scholar] [PubMed]
- van der Mei, I.A.F.; Ponsonby, A.-L.; Dwyer, T.; Blizzard, L.; Taylor, B.V.; Kilpatrick, T.; Butzkueven, H.; McMichael, A.J. Vitamin D levels in people with multiple sclerosis and community controls in Tasmania, Australia. J. Neurol. 2007, 254, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.F.; Hackett, C.T.; Dworek, D.C.; Schramke, C.J. Low vitamin D level is associated with higher relapse rate in natalizumab treated MS patients. J. Neurol. Sci. 2013, 330, 27–31. [Google Scholar] [CrossRef]
- Wang, C.; Zeng, Z.; Wang, B.; Guo, S. Lower 25-hydroxyvitamin D is associated with higher relapse risk in patients with relapsing-remitting multiple sclerosis. J. Nutr. Health Aging 2018, 22, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Runia, T.F.; Hop, W.C.J.; de Rijke, Y.B.; Buljevac, D.; Hintzen, R.Q. Lower serum vitamin D levels are associated with a higher relapse risk in multiple sclerosis. Neurology 2012, 79, 261–266. [Google Scholar] [CrossRef]
- Simpson, S.; Taylor, B.; Blizzard, L.; Ponsonby, A.L.; Pittas, F.; Tremlett, H.; Dwyer, T.; Gies, P.; van der Mei, I. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann. Neurol. 2010, 68, 193–203. [Google Scholar]
- Burton, J.M.; Kimball, S.; Vieth, R.; Bar-Or, A.; Dosch, H.M.; Cheung, R.; Gagne, D.; D’Souza, C.; Ursell, M.; O’Connor, P. A phase I/II dose-escalation trial of vitamin D3 and calcium in multiple sclerosis. Neurology 2010, 74, 1852–1859. [Google Scholar] [CrossRef]
- Shaygannejad, V.; Janghorbani, M.; Ashtari, F.; Dehghan, H. Effects of adjunct low-dose vitamin d on relapsing-remitting multiple sclerosis progression: Preliminary findings of a randomized placebo-controlled trial. Mult. Scler. Int. 2012, 2012, 452541. [Google Scholar] [CrossRef] [PubMed]
- Kampman, M.T.; Steffensen, L.H.; Mellgren, S.I.; Jørgensen, L. Effect of vitamin D3 supplementation on relapses, disease progression, and measures of function in persons with multiple sclerosis: Exploratory outcomes from a double-blind randomised controlled trial. Mult. Scler. 2012, 18, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Golan, D.; Halhal, B.; Glass-Marmor, L.; Staun-Ram, E.; Rozenberg, O.; Lavi, I.; Dishon, S.; Barak, M.; Ish-Shalom, S.; Miller, A. Vitamin D supplementation for patients with multiple sclerosis treated with interferon-beta: A randomized controlled trial assessing the effect on flu-like symptoms and immunomodulatory properties. BMC Neurol. 2013, 13, 60. [Google Scholar] [CrossRef]
- Ascherio, A.; Munger, K.L.; White, R.; Köchert, K.; Simon, K.C.; Polman, C.H.; Freedman, M.S.; Hartung, H.P.; Miller, D.H.; Montalbán, X.; et al. Vitamin D as an early predictor of multiple sclerosis activity and progression. JAMA Neurol. 2014, 71, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.J.; van der Mei, I.; Lucas, R.M.; Ponsonby, A.-L.; Broadley, S.; Blizzard, L.; Taylor, B.; Ausimmune/AusLong Investigators Group; Dear, K.; Dwyer, T.; et al. Sun exposure across the life course significantly modulates early multiple sclerosis clinical course. Front. Neurol. 2018, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Rotstein, D.L.; Healy, B.C.; Malik, M.T.; Carruthers, R.L.; Musallam, A.J.; Kivisakk, P.; Weiner, H.L.; Glanz, B.; Chitnis, T. Effect of vitamin D on MS activity by disease-modifying therapy class. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e167. [Google Scholar] [CrossRef]
- Mowry, E.M.; Waubant, E.; McCulloch, C.E.; Okuda, D.T.; Evangelista, A.A.; Lincoln, R.R.; Gourraud, P.A.; Brenneman, D.; Owen, M.C.; Qualley, P.; et al. Vitamin D status predicts new brain magnetic resonance imaging activity in multiple sclerosis. Ann. Neurol. 2012, 72, 234–240. [Google Scholar] [CrossRef]
- Camu, W.; Lehert, P.; Pierrot-Deseilligny, C.; Hautecoeur, P.; Besserve, A.; Deleglise, A.-S.J.; Payet, M.; Thouvenot, E.; Souberbielle, J.C. Cholecalciferol in relapsing-remitting MS: A randomized clinical trial (CHOLINE). Neurol. Neuroimmunol. Neuroinflamm. 2019, 6, e597. [Google Scholar] [CrossRef]
- Hupperts, R.; Smolders, J.; Vieth, R.; Holmøy, T.; Marhardt, K.; Schluep, M.; Killestein, J.; Barkhof, F.; Beelke, M.; Grimaldi, L.M.E.; et al. Randomized trial of daily high-dose vitamin D3 in patients with RRMS receiving subcutaneous interferon β-1a. Neurology 2019, 93, e1906–e1916. [Google Scholar] [CrossRef]
- Soilu-Hänninen, M.; Aivo, J.; Lindström, B.M.; Elovaara, I.; Sumelahti, M.L.; Färkkilä, M.; Tienari, P.; Atula, S.; Sarasoja, T.; Herrala, L.; et al. A randomised, double blind, placebo controlled trial with vitamin D3 as an add on treatment to interferon-1b in patients with multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2012, 83, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Derakhshandi, H.; Etemadifar, M.; Feizi, A.; Abtahi, S.-H.; Minagar, A.; Abtahi, M.-A.; Abtahi, Z.-A.; Dehghani, A.; Sajjadi, S.; Tabrizi, N. Preventive effect of vitamin D3 supplementation on conversion of optic neuritis to clinically definite multiple sclerosis: A double blind, randomized, placebo-controlled pilot clinical trial. Acta Neurol. Belg. 2013, 113, 257–263. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, K.; Sulaimani, J.; Basdeo, S.A.; Kinsella, K.; Jordan, S.; Kenny, O.; Kelly, S.B.; Murphy, D.; Heffernan, E.; Killeen, R.P.; et al. Effects of vitamin D in clinically isolated syndrome and healthy control participants: A double-blind randomised controlled trial. Mult. Scler. J. Exp. Transl. Clin. 2017, 3, 2055217317727296. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.S.; Liu, Y.; Gray, O.M.; Baker, J.E.; Kolbe, S.C.; Ditchfield, M.R.; Egan, G.F.; Mitchell, P.J.; Harrison, L.C.; Butzkueven, H.; et al. A randomized trial of high-dose vitamin D2 in relapsing-remitting multiple sclerosis. Neurology 2011, 77, 1611–1618. [Google Scholar] [CrossRef]
| Author, Year [Ref.] | Number of Eligible RCTs | Study Group/Control Group | Clinical and Radiological Outcomes | |||
|---|---|---|---|---|---|---|
| Relapses/ ARRs | EDSS | GELs | New or Enlarged T2 Lesions | |||
| Jagannath, 2018 [27] | 12 | 464/469 | NS | NS | NS | - |
| McLaughlin, 2018 [28] | 12 | 479/468 | NS | NS | NS | NS |
| Yuan, 2021 [29] | 9 | 356/362 | NS | NS | - | - |
| Hanaei, 2021 [30] | 9 | 245/247 | NS | NS | - | - |
| Doosti-Irani, 2019 [31] | 6 | 166/165 | - | NS | - | - |
| Zheng, 2018 [32] | 6 | 169/168 | NS | NS | - | - |
| Characteristics | Baseline (N = 133) | 12 Months (N = 120) | 24 Months (N = 90) |
|---|---|---|---|
| Men/Women (n, %) | 37 (27.8%)/96 (72.2%) | 35 (29.2%)/85 (70.8%) | 29 (32.2%)/61 (77.8%) |
| EDSS (median, min–max) | 2.5 (0–6.5) | 2.5 (0–6.5) | 2.5 (1–6.5) |
| Vitamin D serum level (mean ± SD) | 35.77 ± 25.23 ng/mL | 37.96 ± 24.20 ng/mL | 37.42 ± 21.293 ng/mL |
| Patients with supplementation (mean) | 42.03 ng/mL | 43.32 ng/mL | 42.31 ng/mL |
| Patients without supplementation (mean) | 20.12 ng/mL | 25.71 ng/mL | 27.91 ng/mL |
| DMT (n, %) | |||
| Interferon-beta | 22 (16.5%) | 21 (15.8%) | 11 (8.3%) |
| Glatiramer acetate | 10 (7.5%) | 7 (5.3%) | 7 (5.3%) |
| Dimethyl fumarate | 65 (48.9%) | 58 (43.6%) | 44 (33.1%) |
| Teriflunomide | 15 (11.3%) | 14 (10.5%) | 14 (10.5%) |
| Fingolimod | 11 (8.3%) | 9 (6.8%) | 8 (6.0%) |
| Natalizumab | 8 (6.0%) | 9 (6.8%) | 8 (6.0%) |
| Ocrelizumab | 1 (0.8%) | 1 (0.8%) | 1 (0.8%) |
| Alemtuzumab | 1 (0.8%) | 1 (0.8%) | 0 (0.0%) |
| Patients with Supplementation | Patients without Supplementation | |||||
|---|---|---|---|---|---|---|
| Baseline (n = 95) | 12 Months (n = 86) | 24 Months (n = 62) | Baseline (n = 38) | 12 Months (n = 36) | 24 Months (n = 30) | |
| Vitamin D serum level (mean ± SD) (ng/mL) | 42.58 ± 26.89 | 43.81 ± 25.58 | 42.31 ± 21.80 | 20.58 ± 11.88 | 25.45 ± 15.04 | 27.40 ± 16.03 |
| Severe deficiency (n, %) | 1 (1.05%) | 0 (0%) | 1 (1.61%) | 8 (21.1%) | 5 (13.9%) | 3 (10.0%) |
| Deficiency (n, %) | 10 (10.53%) | 7 (7.37%) | 7 (11.29%) | 14 (36.8%) | 12 (33.3%) | 8 (26.7%) |
| Suboptimal (n, %) | 20 (21.05%) | 19 (20.0%) | 9 (14.52%) | 8 (21.1%) | 9 (25%) | 10 (33.3%) |
| Optimal (n, %) | 41 (43.16%) | 38 (40.0%) | 28 (45.16%) | 7 (18.4%) | 6 (11.1%) | 6 (10.0%) |
| High (n, %) | 19 (20%) | 20 (21.05%) | 16 (25.81) | 1 (2.6%) | 4 (11.1) | 3 (10.0%) |
| Toxic (n, %) | 4 (4.21%) | 2 (2.11%) | 1 (1.6%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Patients without Supplementation | Patients with Supplementation | ||||
|---|---|---|---|---|---|
| 24 Months of Observation | Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | p |
| EDSS | 2.9 (±1.6) | 2.5 (1.5–4.5) | 2.7 (±1.4) | 2.5 (1.5–3.5) | 0.61548 |
| New relapses | 0.2 (±0.5) | 0.0 (0.0–0.0) | 0.1(±0.4) | 0.0 (0.0–0.0) | 0.69798 |
| Number of new T2 | 0.8 (+0.4) | 0.0 (0.0–1.0) | 0.4 (±1.1) | 0.0 (0.0–0.0) | 0.03443 |
| Number of GELs | 0.8 (+0.0) | 0.0 (0.0–1.0) | 0.0 (±11) | 0.0 (0.0–1.0) | 0.09407 |
| Number of 12-Month Periods with Optimal or Higher Vitamin D Serum Level (>30 ng/mL) | |||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||
| Radiological Outcomes | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | p |
| Number of new T2 | |||||
| Baseline | 0.3 (±0.6) | 0.3 (±0.4) | 0.4 (±1.2) | 0.2 (±0.4) | 0.20610 |
| 12 months | 0.3 (±0.7) | 0.3 (±0.6) | 0.2 (±0.5) | 0.1 (±0.2) | 0.28050 |
| 24 months | 0.4 (±1.1) | 0.4 (±0.6) | 0.6 (±1.2) | 0.1 (±0.2) | 0.04440 |
| Cumulative | 1.1 (±1.2) | 1.0 (±0.6) | 1.2 (±1.9) | 0.4 (±0.4) | 0.04570 |
| Number of GELs | |||||
| Baseline | 0.2 (±0.6) | 0.5 (±1.0) | 0.3 (±1.1) | 0.1 (±0.3) | 0.00620 |
| 12 months | 0.3 (±0.8) | 0.2 (±0.8) | 0.2 (±0.8) | 0.1 (±0.3) | 0.54180 |
| 24 months | 0.1 (±0.6) | 0.3 (±0.6) | 0.0 (±0.0) | 0.2 (±0.5) | 0.45940 |
| Cumulative | 0.5 (±1.3) | 0.8 (±1.5) | 0.6 (±1.4) | 0.3 (±0.9) | 0.12770 |
| Patients without Supplementation | Patients with Supplementation | |||||
|---|---|---|---|---|---|---|
| 24 Months of Observation | Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | p | |
| Interferon beta glatiramer acetate, dimethyl fumarate, teriflunomide. | New relapses | 0.7 (±1.3) | 0.0 (0.0–1.0) | 0.6(±1.3) | 0.0 (0.0–2.0) | 0.58962 |
| Number of new T2 | 0.9 (±1.6) | 0.0 (0.0–1.0) | 0.4 (±1.0) | 0.0 (0.0–2.0) | 0.04041 | |
| Number of GELs | 0.8 (±1.7) | 0.0 (0.0–1.0) | 0.4 (±11) | 0.0 (0.0–1.0) | 0.07645 | |
| Fingolimod, natalizumab, ocrelizumab, alemtuzumab. | New relapses | 0.8 (±1.2) | 0.0 (0.0–0.0) | 1.2 (±1.2) | 0.0 (0.0–0.0) | 0.78988 |
| Number of new T2 | 0.5 (±0.8) | 0.0 (0.0–1.0) | 0.5 (±1.3) | 0.0 (0.0–0.0) | 0.73330 | |
| Number of GELs | 0.3 (±0.5) | 0.0 (0.0–1.0) | 0.7 (±1.6) | 0.0 (0.0–1.0) | 0.20511 | |
| Author, Year [Ref.] | Study Group/Control Group | Vitamin D Doses | Vitamin D Form | Time of Supplementation [Months] | Radiological Outcomes in Patients with MS | |
|---|---|---|---|---|---|---|
| Study Group | Control Group | |||||
| Camu, 2019 [62] | 45/45 | 100,000 IU/5 days | Placebo | D3 | 24 | Reduction in new T1 lesions (p = 0.025); reduction in T1 lesion volume (p = 0.031) |
| Derakhshand, 2013 [65] | 15/15 | 50,000 IU/week | Placebo | D3 | 12 | NS |
| Golan, 2013 [57] | 24/21 | 4370 IU/day | 800 IU/day | D3 | 12 | NS |
| Hupperts, 2019 [63] | 113/116 | 14,007 IU/day | Placebo | D3 | 12 | Reduction of GELs (p = 0.0045); reduction in increase of total T2 lesion volume (p = 0.035) |
| Mosayebi, 2011 [21] | 26/33 | 300,000 IU/month (i.m.) | Placebo | D3 | 6 | NS |
| O’Connell *, 2017 [66] | 23/9 | 5000 IU/day or 10,000 IU/day | Placebo | D3 | 6 | NS |
| Soilu-Hanninen, 2012 [64] | 34/32 | 20,000 IU/week | Placebo | D3 | 12 | Reduction of GELs (p = 0.004) |
| Stein, 2011 [67] | 11/12 | 6000–12,000 IU/day + 1000 IU/day | Placebo | D2 | 6 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galus, W.; Chmiela, T.; Walawska-Hrycek, A.; Krzystanek, E. Radiological Benefits of Vitamin D Status and Supplementation in Patients with MS—A Two-Year Prospective Observational Cohort Study. Nutrients 2023, 15, 1465. https://doi.org/10.3390/nu15061465
Galus W, Chmiela T, Walawska-Hrycek A, Krzystanek E. Radiological Benefits of Vitamin D Status and Supplementation in Patients with MS—A Two-Year Prospective Observational Cohort Study. Nutrients. 2023; 15(6):1465. https://doi.org/10.3390/nu15061465
Chicago/Turabian StyleGalus, Weronika, Tomasz Chmiela, Anna Walawska-Hrycek, and Ewa Krzystanek. 2023. "Radiological Benefits of Vitamin D Status and Supplementation in Patients with MS—A Two-Year Prospective Observational Cohort Study" Nutrients 15, no. 6: 1465. https://doi.org/10.3390/nu15061465
APA StyleGalus, W., Chmiela, T., Walawska-Hrycek, A., & Krzystanek, E. (2023). Radiological Benefits of Vitamin D Status and Supplementation in Patients with MS—A Two-Year Prospective Observational Cohort Study. Nutrients, 15(6), 1465. https://doi.org/10.3390/nu15061465








