The Effects of Probiotics/Synbiotics on Glucose and Lipid Metabolism in Women with Gestational Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Method
2.1. Study Protocol
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Data Extraction and Quality Assessment
2.5. Statistical Analysis
3. Results
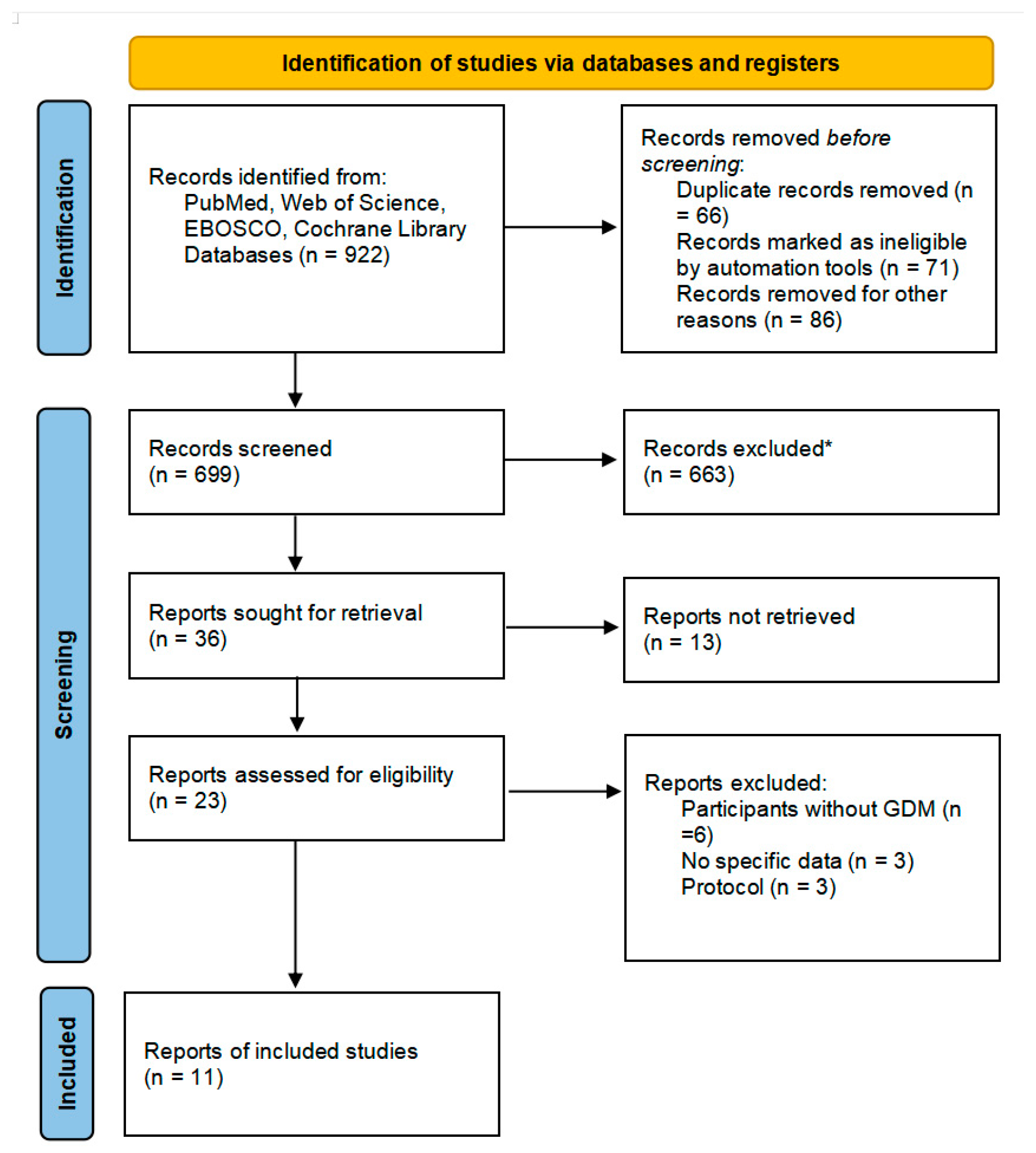
3.1. Study Selection
3.2. Description of Included Studies
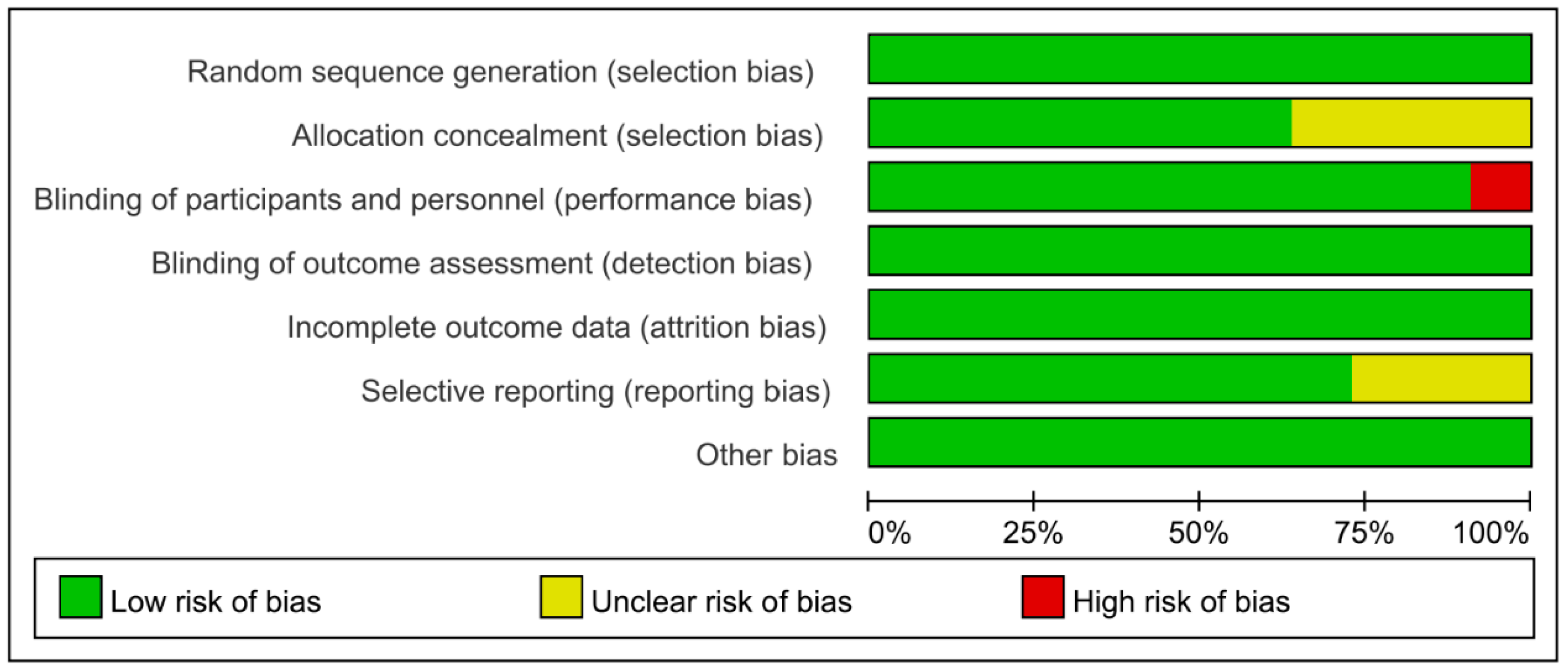
3.3. Risk of Bias of Included Studies and Quality of Evidence
3.4. Glucose Control
3.4.1. Fasting Plasma Glucose
3.4.2. Insulin
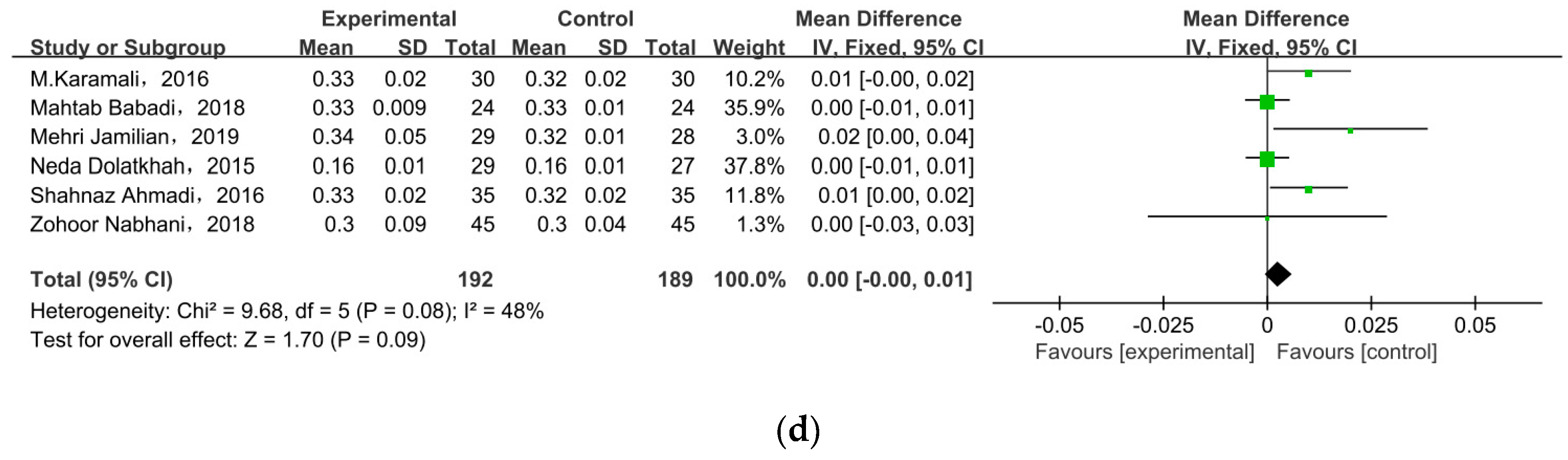
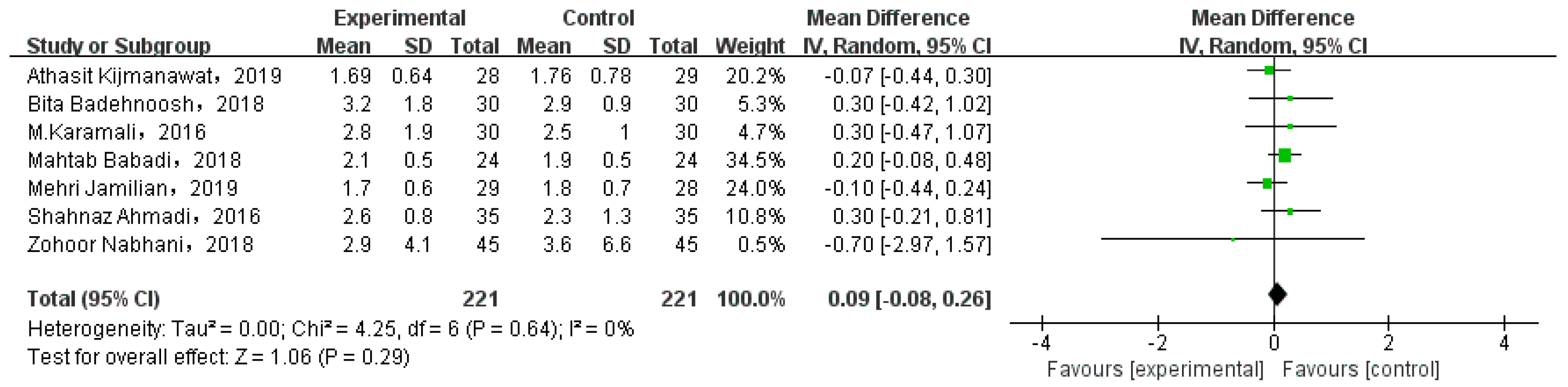
3.5. Lipid Profiles
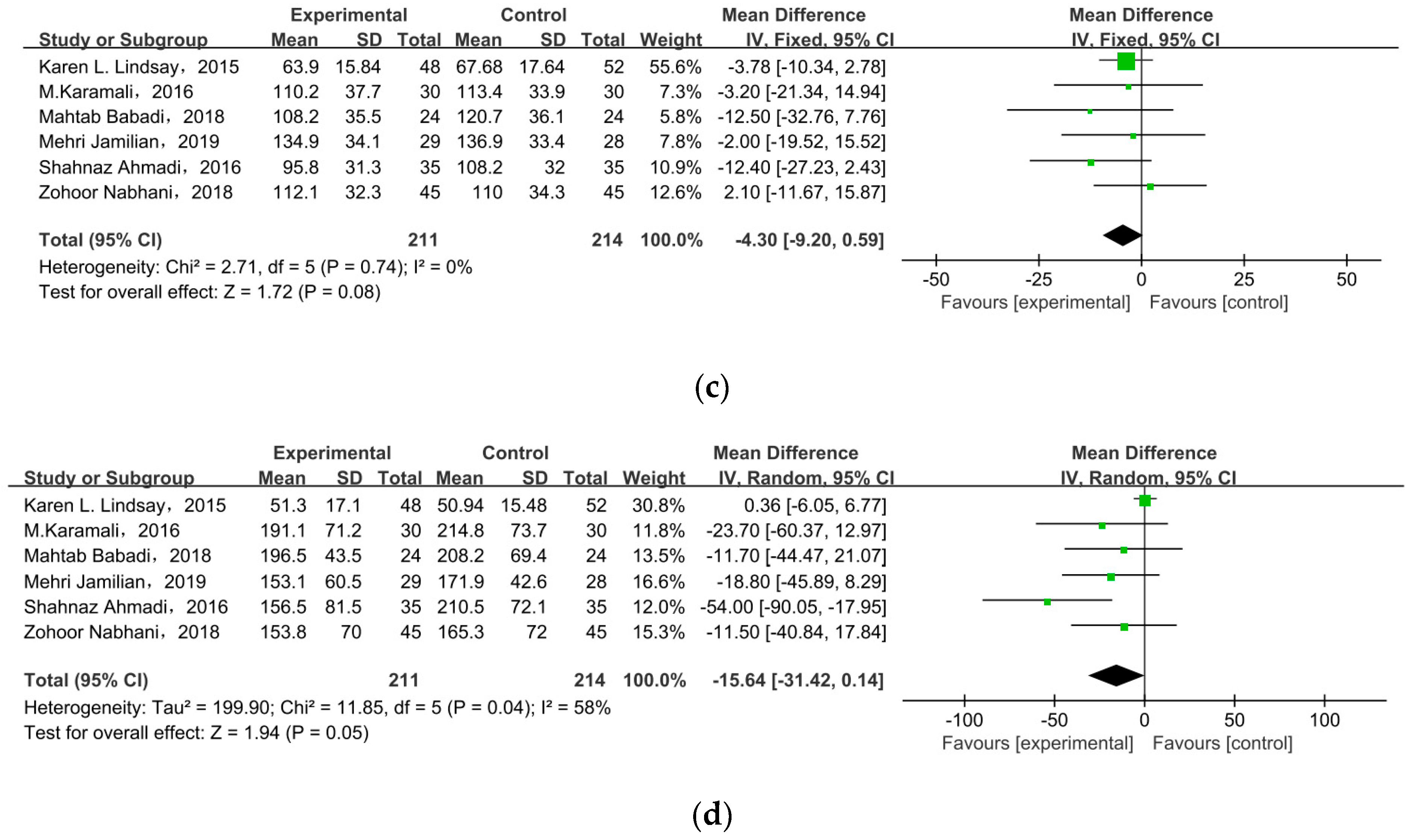
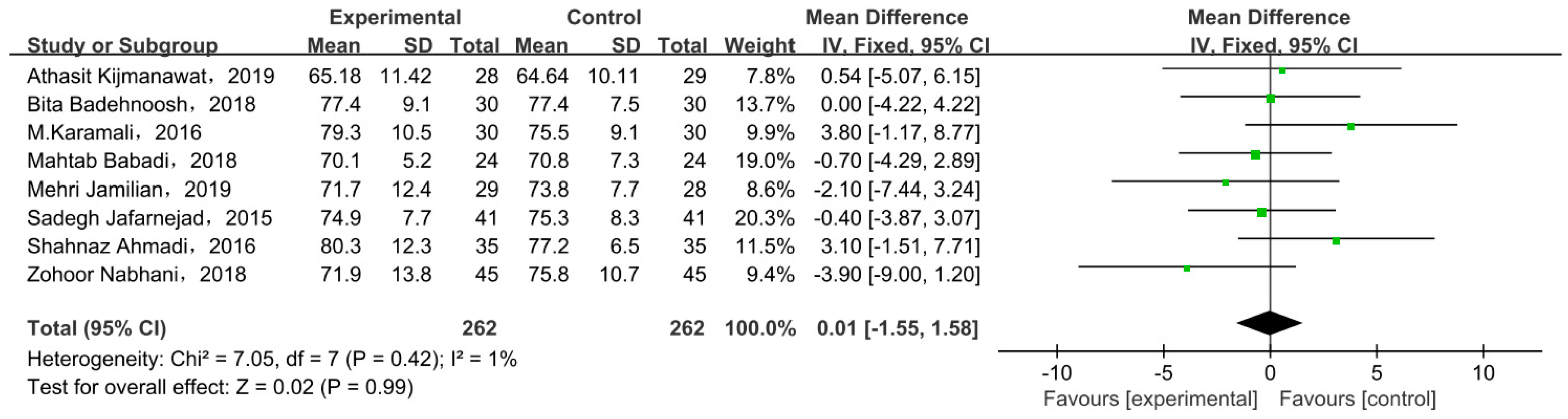
3.6. Body Weight
3.6.1. Weight at End of Trial
3.6.2. Gestational Weight Gain
3.7. Subgroup Analysis
4. Discussion
5. Strength and Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wan, J.; Ma, J. Efficacy of dietary supplements targeting gut microbiota in the prevention and treatment of gestational diabetes mellitus. Front. Microbiol. 2022, 13, 927883. [Google Scholar] [CrossRef]
- Homayouni, A.; Bagheri, N.; Mohammad-Alizadeh-Charandabi, S.; Kashani, N.; Mobaraki-Asl, N.; Mirghafurvand, M.; Asgharian, H.; Ansari, F.; Pourjafar, H. Prevention of Gestational Diabetes Mellitus (GDM) and Probiotics: Mechanism of Action: A Review. Curr. Diabetes Rev. 2020, 16, 538–545. [Google Scholar]
- Quaresima, P.; Saccone, G.; Pellegrino, R.; Vaccarisi, S.; Taranto, L.; Mazzulla, R.; Bernardo, S.; Venturella, R.; Di Carlo, C.; Morelli, M. Incidental diagnosis of a pancreatic adenocarcinoma in a woman affected by gestational diabetes mellitus: Case report and literature review. Am. J. Obstet. Gynecol. MFM 2021, 3, 100471. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, M.; Chiefari, E.; Tocci, V.; Greco, E.; Foti, D.; Brunetti, A. Gestational diabetes: Implications for fetal growth, intervention timing, and treatment options. Curr. Opin. Pharmacol. 2021, 60, 1–10. [Google Scholar] [CrossRef]
- Ornoy, A.; Becker, M.; Weinstein-Fudim, L.; Ergaz, Z. Diabetes during Pregnancy: A Maternal Disease Complicating the Course of Pregnancy with Long-Term Deleterious Effects on the Offspring. A Clinical Review. Int. J. Mol. Sci. 2021, 22, 2965. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Melhorn, S.; Olerich, K.L.W.; Angelo, B.; Chow, T.; Xiang, A.; Schur, A.E.; Page, A.K. Exposure to Gestational Diabetes Mellitus Prior to 26 Weeks Is Related to the Presence of Mediobasal Hypothalamic Gliosis in Children. Diabetes 2022, 71, 2552–2556. [Google Scholar] [CrossRef] [PubMed]
- Desoye, G.; Nolan, C.J. The fetal glucose steal: An underappreciated phenomenon in diabetic pregnancy. Diabetologia 2016, 59, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Lowe, W.J.; Scholtens, D.M.; Kuang, A.; Linder, B.; Lawrence, M.J.; Lebenthal, Y.; McCance, D.; Hamilton, J.; Nodzenski, M.; Talbot, O. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): Maternal Gestational Diabetes Mellitus and Childhood Glucose Metabolism. Diabetes Care 2019, 42, 372–380. [Google Scholar] [CrossRef]
- Huang, L.; Thonusin, C.; Chattipakorn, N.; Chattipakorn, C.S. Impacts of gut microbiota on gestational diabetes mellitus: A comprehensive review. Eur. J. Nutr. 2021, 60, 2343–2360. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Hu, X.; Hu, X.; Zhang, J.; Zhang, H.; Wang, J.; Su, S.; Wang, Y.; Lyu, Z. The effect of gestational diabetes mellitus on fetal right heart growth in late-term pregnancy: A prospective study. Echocardiography 2022, 39, 1101–1112. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Li, Y.; Huang, S.; Zhang, L.; Cao, C.; Baker, N.P.; Tong, C.; Zheng, P.; Qi, H. Altered gut bacterial and metabolic signatures and their interaction in gestational diabetes mellitus. Gut Microbes 2020, 12, 1–13. [Google Scholar] [CrossRef]
- Davidson, S.J.; Barrett, H.L.; Price, S.A.; Callaway, K.L.; Nitert, D.M. Probiotics for preventing gestational diabetes. Cochrane Database Syst. Rev. 2021, 4, D9951. [Google Scholar]
- Liu, H.; Pan, L.L.; Lv, S.; Yang, Q.; Zhang, H.; Chen, W.; Lv, Z.; Sun, J. Alterations of Gut Microbiota and Blood Lipidome in Gestational Diabetes Mellitus With Hyperlipidemia. Front. Physiol. 2019, 10, 1015. [Google Scholar] [CrossRef] [PubMed]
- Mahdizade, A.M.; Teymouri, S.; Fazlalian, T.; Asadollahi, P.; Afifirad, R.; Sabaghan, M.; Valizadeh, F.; Ghanavati, R.; Darbandi, A. The effect of probiotics on gestational diabetes and its complications in pregnant mother and newborn: A systematic review and meta-analysis during 2010–2020. J. Clin. Lab. Anal. 2022, 36, e24326. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, X.; Zhang, W. The Effect of Metformin Therapy for Preventing Gestational Diabetes Mellitus in Women with Polycystic Ovary Syndrome: A Meta-Analysis. Exp. Clin. Endocrinol. Diabetes 2020, 128, 199–205. [Google Scholar] [CrossRef]
- Huifen, Z.; Yaping, X.; Meijing, Z.; Huibin, H.; Chunhong, L.; Fengfeng, H.; Yaping, Z. Effects of moderate-intensity resistance exercise on blood glucose and pregnancy outcome in patients with gestational diabetes mellitus: A randomized controlled trial. J. Diabetes Complications 2022, 36, 108186. [Google Scholar] [CrossRef]
- Rasmussen, L.; Poulsen, C.W.; Kampmann, U.; Smedegaard, B.S.; Ovesen, G.P.; Fuglsang, J. Diet and Healthy Lifestyle in the Management of Gestational Diabetes Mellitus. Nutrients 2020, 12, 3050. [Google Scholar] [CrossRef]
- Rad, A.H.; Abbasalizadeh, S.; Vazifekhah, S.; Abbasalizadeh, F.; Hassanalilou, T.; Bastani, P.; Ejtahed, S.H.; Soroush, R.V.; Javadi, M.; Mortazavian, M.A. The Future of Diabetes Management by Healthy Probiotic Microorganisms. Curr. Diabetes Rev. 2017, 13, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Masulli, M.; Vitacolonna, E.; Fraticelli, F.; Pepa, D.G.; Mannucci, E.; Monami, M. Effects of probiotic supplementation during pregnancy on metabolic outcomes: A systematic review and meta-analysis of randomized controlled trials. Diabetes Res. Clin. Pract. 2020, 162, 108111. [Google Scholar] [CrossRef] [PubMed]
- Kunasegaran, T.; Balasubramaniam, V.; Arasoo, V.; Palanisamy, U.; Ramadas, A. The Modulation of Gut Microbiota Composition in the Pathophysiology of Gestational Diabetes Mellitus: A Systematic Review. Biology 2021, 10, 1027. [Google Scholar] [CrossRef]
- Kijmanawat, A.; Panburana, P.; Reutrakul, S.; Tangshewinsirikul, C. Effects of probiotic supplements on insulin resistance in gestational diabetes mellitus: A double-blind randomized controlled trial. J. Diabetes Investig. 2019, 10, 163–170. [Google Scholar] [CrossRef]
- Lindsay, K.L.; Brennan, L.; Kennelly, M.A.; Maguire, C.O.; Smith, T.; Curran, S.; Coffey, M.; Foley, E.M.; Hatunic, M.; Shanahan, F. Impact of probiotics in women with gestational diabetes mellitus on metabolic health: A randomized controlled trial. Am. J. Obstet. Gynecol. 2015, 212, 491–496. [Google Scholar]
- Rethlefsen, M.L.; Page, M.J. PRISMA 2020 and PRISMA-S: Common questions on tracking records and the flow diagram. J. Med. Libr. Assoc. 2022, 110, 253–257. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37 (Suppl. S1), S81–S90. [Google Scholar] [CrossRef]
- Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, A.T.; Catalano, C.A.; Damm, P.; Dyer, R.A.; Leiva, A.; Hod, M.; Kitzmiler, L.J. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef]
- Carpenter, M.W.; Coustan, D.R. Criteria for screening tests for gestational diabetes. Am. J. Obstet. Gynecol. 1982, 144, 768–773. [Google Scholar] [CrossRef]
- Badehnoosh, B.; Karamali, M.; Zarrati, M.; Jamilian, M.; Bahmani, F.; Tajabadi-Ebrahimi, M.; Jafari, P.; Rahmani, E.; Asemi, Z. The effects of probiotic supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. J. Matern.-Fetal Neonatal Med. 2018, 31, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Sahhaf Ebrahimi, F.; Homayouni Rad, A.; Mosen, M.; Abbasalizadeh, F.; Tabrizi, A.; Khalili, L. Effect of L. Effect of L. acidophilus and B. lactis on blood glucose in women with gestational diabetes mellitus: A randomized placebo-controlled trial. Diabetol. Metab. Syndr. 2019, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Karamali, M.; Dadkhah, F.; Sadrkhanlou, M.; Jamilian, M.; Ahmadi, S.; Tajabadi-Ebrahimi, M.; Asemi, Z. Effects of probiotic supplementation on glycaemic control and lipid profiles in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Diabetes Metab. 2016, 42, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Babadi, M.; Khorshidi, A.; Aghadavood, E.; Samimi, M.; Kavossian, E.; Bahmani, F.; Mafi, A.; Shafabakhsh, R.; Satari, M.; Asemi, Z. The Effects of Probiotic Supplementation on Genetic and Metabolic Profiles in Patients with Gestational Diabetes Mellitus: A Randomized, Double-Blind, Placebo-Controlled Trial. Probiotics Antimicrob. Proteins 2019, 11, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Amirani, E.; Asemi, Z. The effects of vitamin D and probiotic co-supplementation on glucose homeostasis, inflammation, oxidative stress and pregnancy outcomes in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 2098–2105. [Google Scholar] [CrossRef] [PubMed]
- Dolatkhah, N.; Hajifaraji, M.; Abbasalizadeh, F.; Aghamohammadzadeh, N.; Mehrabi, Y.; Mesgari Abbasi, M. Is there a value for probiotic supplements in gestational diabetes mellitus? A randomized clinical trial. J. Health Popul. Nutr. 2015, 33, 25. [Google Scholar] [CrossRef] [PubMed]
- Jafarnejad, S.; Saremi, S.; Jafarnejad, F.; Arab, A. Effects of a Multispecies Probiotic Mixture on Glycemic Control and Inflammatory Status in Women with Gestational Diabetes: A Randomized Controlled Clinical Trial. J. Nutr. Metab. 2016, 2016, 5190846. [Google Scholar] [CrossRef]
- Ahmadi, S.; Jamilian, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Asemi, Z. The effects of synbiotic supplementation on markers of insulin metabolism and lipid profiles in gestational diabetes: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2016, 116, 1394–1401. [Google Scholar] [CrossRef]
- Nabhani, Z.; Hezaveh, S.J.G.; Razmpoosh, E.; Asghari-Jafarabadi, M.; Gargari, B.P. The effects of synbiotic supplementation on insulin resistance/sensitivity, lipid profile and total antioxidant capacity in women with gestational diabetes mellitus: A randomized double-blind placebo controlled clinical trial. Diabetes Res. Clin. Pract. 2018, 138, 149–157. [Google Scholar] [CrossRef]
- Feng, C.; Adebero, T.; DePaul, G.V.; Vafaei, A.; Norman, E.K.; Auais, M. A Systematic Review and Meta-Analysis of Exercise Interventions and Use of Exercise Principles to Reduce Fear of Falling in Community-Dwelling Older Adults. Phys Ther. 2022, 102. [Google Scholar] [CrossRef]
- Catalano, P.M.; Tyzbir, E.D.; Roman, N.M.; Amini, S.B.; Sims, E.A. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am. J. Obstet. Gynecol. 1991, 165, 1667–1672. [Google Scholar] [CrossRef]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [PubMed]
- Kautzky-Willer, A.; Krssak, M.; Winzer, C.; Pacini, G.; Tura, A.; Farhan, S.; Wagner, O.; Brabant, G.; Horn, R.; Stingl, H. Increased intramyocellular lipid concentration identifies impaired glucose metabolism in women with previous gestational diabetes. Diabetes 2003, 52, 244–251. [Google Scholar] [CrossRef]
- Stefanovski, D.; Punjabi, N.M.; Boston, R.C.; Watanabe, R.M. Insulin Action, Glucose Homeostasis and Free Fatty Acid Metabolism: Insights From a Novel Model. Front. Endocrinol. 2021, 12, 625701. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, Z.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb. Cell Factories 2020, 19, 23. [Google Scholar] [CrossRef]
- Taylor, B.L.; Woodfall, G.E.; Sheedy, K.E.; O Riley, M.L.; Rainbow, K.A.; Bramwell, E.L.; Kellow, N.J. Effect of Probiotics on Metabolic Outcomes in Pregnant Women with Gestational Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2017, 9, 461. [Google Scholar] [CrossRef]
- Christiansen, C.B.; Gabe, M.B.N.; Svendsen, B.; Dragsted, L.O.; Rosenkilde, M.M.; Holst, J.J. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G53–G65. [Google Scholar] [CrossRef]
- Jayashree, B.; Bibin, Y.S.; Prabhu, D.; Shanthirani, C.S.; Gokulakrishnan, K.; Lakshmi, B.S.; Mohan, V.; Balasubramanyam, M. Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Mol. Cell. Biochem. 2014, 388, 203–210. [Google Scholar] [CrossRef]
- Fuller, M.; Priyadarshini, M.; Gibbons, S.M.; Angueira, A.R.; Brodsky, M.; Hayes, G.M.; Kovatcheva-Datchary, P.; Bäckhed, F.; Gilbert, J.A. The short-chain fatty acid receptor, FFA2, contributes to gestational glucose homeostasis. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E840–E851. [Google Scholar] [CrossRef]
- Li, H.; Zhou, D.; Gan, R.; Huang, S.; Zhao, C.; Shang, A.; Xu, X.; Li, H. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, P.; Farhangi, M.A.; Tavakoli, F.; Aliasgarzadeh, A.; Akbari, A.M. Impact of prebiotic supplementation on T-cell subsets and their related cytokines, anthropometric features and blood pressure in patients with type 2 diabetes mellitus: A randomized placebo-controlled Trial. Complement. Ther. Med. 2016, 24, 6–102. [Google Scholar] [CrossRef] [PubMed]
- Tuohy, K.M.; Fava, F.; Viola, R. ‘The way to a man’s heart is through his gut microbiota’--dietary pro- and prebiotics for the management of cardiovascular risk. Proc. Nutr. Soc. 2014, 73, 172–185. [Google Scholar] [CrossRef]
- Salles, B.; Cioffi, D.; Ferreira, S. Probiotics supplementation and insulin resistance: A systematic review. Diabetol. Metab. Syndr. 2020, 12, 98. [Google Scholar] [CrossRef] [PubMed]
- Swartwout, B.; Luo, X.M. Implications of Probiotics on the Maternal-Neonatal Interface: Gut Microbiota, Immunomodulation, and Autoimmunity. Front. Immunol. 2018, 9, 2840. [Google Scholar] [CrossRef] [PubMed]


| Number | Author, Year | Country | Diagnostic Method | Diagnostic Criteria | Duration (Weeks) | Frequency of Treatment | Sample Size (Probiotic/Placebo) | Mean Age (Years) (Probiotic/Placebo) | Intervention | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Athasit Kijmanawat 2019 [21] | Thailand | 2 h 75 g oral glucose tolerance test (OGTT) | Based on International Association of Diabetes and Pregnancy Study Groups | 4 | 1 capsule/day | 28/29 | 32.50 ± 5.02/30.72 ± 5.05 | 1 × 109 CFU Lactobacillus acidophilus, Bifidobacterium bifidum | ①, ②, ③, ⑨, ⑩ |
| 2 | Bita Badehnoosh 2018 [27] | Iran | 2 h 75 g oral glucose tolerance test (OGTT) | Based on the American Diabetes Association guidelines | 6 | 1 capsule/day | 30/30 | 28.8 ± 5.4/27.8 ± 3.7 | 2 × 109 CFU/g each; Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum | ①, ⑨, ⑩ |
| 3 | Farnaz Sahhaf Ebrahimi 2019 [28] | Iran | 2 h 75 g oral glucose tolerance test (OGTT) | Unknown | 8 | 300 mg/day | 42/42 | 31.64 ± 5.97/31.61 ± 5.49 | 1 × 106 CFU/g; Lactobacillus acidophilus, Bifdobacterium lactis | ① |
| 4 | Karen L. Lindsay 2015 [22] | Ireland | 3 h 100 g oral glucose tolerance test | Based on O’Sullivan’s diagnostic criteria | 4–6 | 1 capsule/day | 57/58 | 33.5 ± 5.0/32.6 ± 4.5 | 1 × 109 CFU/g; Lactobacillus salivarius UCC118 | ①, ②, ③, ⑤, ⑥, ⑦, ⑧ |
| 5 | Karamali 2016 [29] | Iran | 2 h 75 g oral glucose tolerance test (OGTT) | Based on American Diabetes Association guidelines | 6 | 1 capsule/day | 30/30 | 31.8 ± 6.0/29.7 ± 4.0 | 2 × 109 CFU/g; L. acidophilus, L. casei, B. bifidum | ①, ②, ③, ④, ⑤, ⑥, ⑦, ⑧, ⑨, ⑩ |
| 6 | Mahtab Babadi 2018 [30] | Iran | 2 h 75 g oral glucose tolerance test (OGTT) | Based on the American Diabetes Association guidelines | 6 | 1 capsule/day | 24/24 | 28.8 ± 4.3/29.0 ± 4.2 | 2 × 109 CFU/g; Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, Lactobacillus fermentum | ①, ②, ③, ④, ⑤, ⑥, ⑦, ⑧, ⑨, ⑩ |
| 7 | Mehri Jamilian 2019 [31] | Iran | 2 h 75 g oral glucose tolerance test (OGTT) | Based on the American Diabetes Association guidelines | 6 | 1 capsule/day | 29/28 | 31.2 ± 5.9/29.9 ± 3.7 | 2 × 109 CFU/g each; Lactobacillus acidophilus, Bifidobacterium bifidum, L. reuteri, Lactobacillus fermentum | ①, ②, ③, ④, ⑤, ⑥, ⑦, ⑧, ⑨, ⑩ |
| 8 | Neda Dolatkhah 2015 [32] | Turkey | By either a gynecologist or an internal medicine specialist | Unknown | 8 | 1 capsule/day | 29/27 | 28.14 ± 6.24/26.48 ± 5.23 | >1 × 109 CFU/g each; Lactobacillus acidophilus LA-5, Bifidobacterium BB-12, Streptococcus thermophilus STY-31, Lactobacillus delbrueckii bulgaricus LBY-27 | ①, ②, ③, ④,⑩ |
| 9 | Sadegh Jafarnejad 2016 [33] | Iran | 2 h 75 g oral glucose tolerance test (OGTT) | Based on the American Diabetes Association guidelines | 8 | 2 capsule/day | 41/41 | 32.4 ± 3.1/31.9 ± 4.0 | 112.5 × 109 CFU/capsule Streptococcus thermophilus, Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, and Lactobacillus delbrueckii subsp. Bulgaricus and microcrystalline cellulose, stearic acid, magnesium stearate, vegetable material (hydroxypropyl methylcellulose), silicon dioxide | ①, ②, ③, ⑨ |
| 10 | Shahnaz Ahmadi 2016 [34] | Iran | 2 h 75 g oral glucose tolerance test (OGTT) | Based on the American Diabetes Association guidelines | 6 | 1 capsule/day | 35/35 | 28.5 ± 5.8/28.7 ± 3.4 | 2 × 109 CFU/g each Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum plus 0.8 g inulin | ①, ②, ③, ④, ⑤, ⑥, ⑦, ⑧, ⑨, ⑩ |
| 11 | Zohoor Nabhani 2018 [35] | Iran | 2 h 75 g oral glucose tolerance test (OGTT) | Based on the American Diabetes Association guidelines | 6 | 500 mg/day | 45/45 | 29.4 ± 5.8/30.3 ± 5.6 | L. acidophilus (5 × 1010 CFU/g), L. plantarum (1.5 × 1010 CFU/g), L. fermentum (7 × 109 CFU/g), L. gasseri (2 × 1010 CFU/g), and 38.5 mg of FOS | ①, ②, ③, ④, ⑤, ⑥, ⑦, ⑧, ⑨, ⑩ |
| Outcomes | Significant Improvement in Outcomes | p |
|---|---|---|
| FPG (mg/dL) | Y | 0.02 |
| FSI (mU/L) | Y | 0.0003 |
| HOMA-IR | Y | 0.02 |
| QUICKI | N | 0.09 |
| TC (mg/dL) | Y | 0.02 |
| HDL cholesterol (mg/dL) | N | 0.33 |
| LDL cholesterol (mg/dL) | N | 0.08 |
| TG (mg/dL) | N | 0.05 |
| Weight at end of trial (kg) | N | 0.99 |
| GWG (kg) | N | 0.29 |
| Subgroup | Studies | Participants | Mean Difference (95% CI) | Heterogeneity (I2%) | p |
|---|---|---|---|---|---|
| FPG | |||||
| Duration < 8weeks | 8 | 557 | −2.14 (−4.23, −0.05) | 53 | 0.05 |
| Duration ≥ 8weeks | 3 | 222 | −2.73 (−7.08, 1.63) | 91 | 0.22 |
| Probiotic | 8 | 537 | −3.77 (−5.37, −2.17) | 39 | <0.00001 |
| Synbiotic | 3 | 242 | 0.70 (−0.79, 2.19) | 0 | 0.35 |
| Dose < 2 × 109 CFU/g | 4 | 312 | −3.40 (−6.10, −0.70) | 68 | 0.01 |
| Dose ≥ 2 × 109 CFU/g | 7 | 467 | −1.63 (−3.97, 0.70) | 63 | 0.17 |
| FSI | |||||
| Duration < 8weeks | 7 | 497 | −2.20 (−3.61, −0.79) | 43 | 0.002 |
| Duration ≥ 8weeks | 2 | 138 | −3.18 (−7.81, 1.44) | 94 | 0.18 |
| Probiotic | 6 | 393 | −1.37 (−2.29, −0.45) | 36 | 0.003 |
| Synbiotic | 3 | 242 | −4.65 (−7.33, −1.97) | 51 | 0.0007 |
| Dose < 2 × 109 CFU/g | 3 | 228 | −0/96(-1.20, −0.72) | 0 | <0.00001 |
| Dose ≥ 2 × 109 CFU/g | 6 | 407 | −3.70 (−5.51, −1.89) | 57 | <0.0001 |
| HOMA-IR | |||||
| Duration < 8weeks | 7 | 497 | −0.34 (−0.58, −0.10) | 75 | 0.006 |
| Duration ≥ 8weeks | 2 | 138 | −0.30 (−0.35, −0.24) | 89 | <0.00001 |
| Probiotic | 6 | 393 | −0.30 (−0.35, −0.25) | 43 | <0.00001 |
| Synbiotic | 3 | 242 | −0.36 (−0.78, 0.05) | 92 | 0.08 |
| Dose < 2 × 109 CFU/g | 3 | 228 | −0.29 (−0.34, −0.24) | 0 | <0.00001 |
| Dose ≥ 2 × 109 CFU/g | 6 | 407 | −0.57 (−0.84, −0.30) | 83 | <0.0001 |
| HDL cholesterol | |||||
| Probiotic | 4 | 280 | −0.96 (−4.44, 2.52) | 60 | 0.59 |
| Synbiotic | 2 | 160 | −2.94 (−12.94, 7.05) | 89 | 0.56 |
| TG | |||||
| Probiotic | 4 | 280 | −5.15 (−15.79, 5.49) | 19 | 0.34 |
| Synbiotic | 2 | 160 | −31.41 (−72.97, 10.16) | 69 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, J.; Guo, X.; Zhou, Y.; Cao, G. The Effects of Probiotics/Synbiotics on Glucose and Lipid Metabolism in Women with Gestational Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2023, 15, 1375. https://doi.org/10.3390/nu15061375
Mu J, Guo X, Zhou Y, Cao G. The Effects of Probiotics/Synbiotics on Glucose and Lipid Metabolism in Women with Gestational Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Nutrients. 2023; 15(6):1375. https://doi.org/10.3390/nu15061375
Chicago/Turabian StyleMu, Jinhao, Xian Guo, Yanbing Zhou, and Guoxia Cao. 2023. "The Effects of Probiotics/Synbiotics on Glucose and Lipid Metabolism in Women with Gestational Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials" Nutrients 15, no. 6: 1375. https://doi.org/10.3390/nu15061375
APA StyleMu, J., Guo, X., Zhou, Y., & Cao, G. (2023). The Effects of Probiotics/Synbiotics on Glucose and Lipid Metabolism in Women with Gestational Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Nutrients, 15(6), 1375. https://doi.org/10.3390/nu15061375







