Effects of Wild Blueberries on Fat Oxidation Rates in Aerobically Trained Males
Abstract
1. Introduction
2. Materials and Methods
3. Results
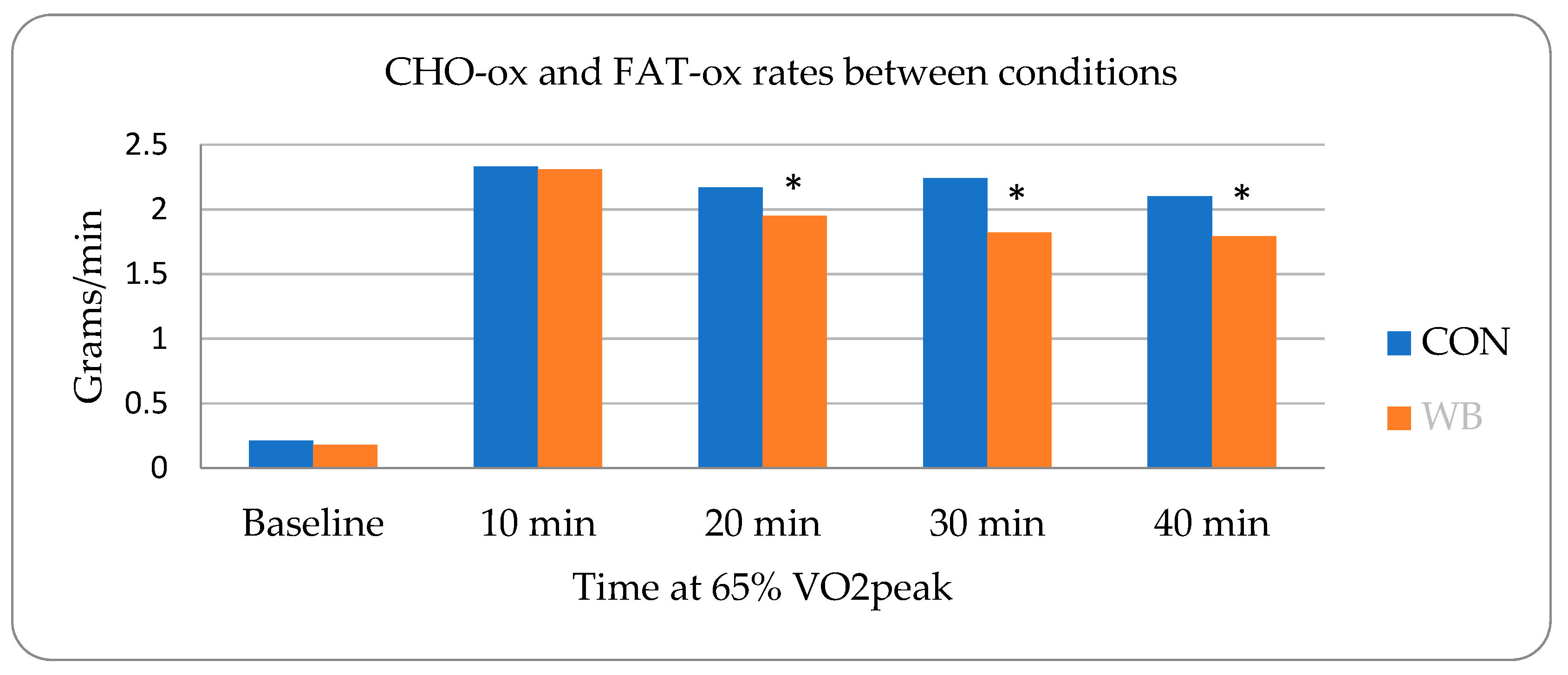
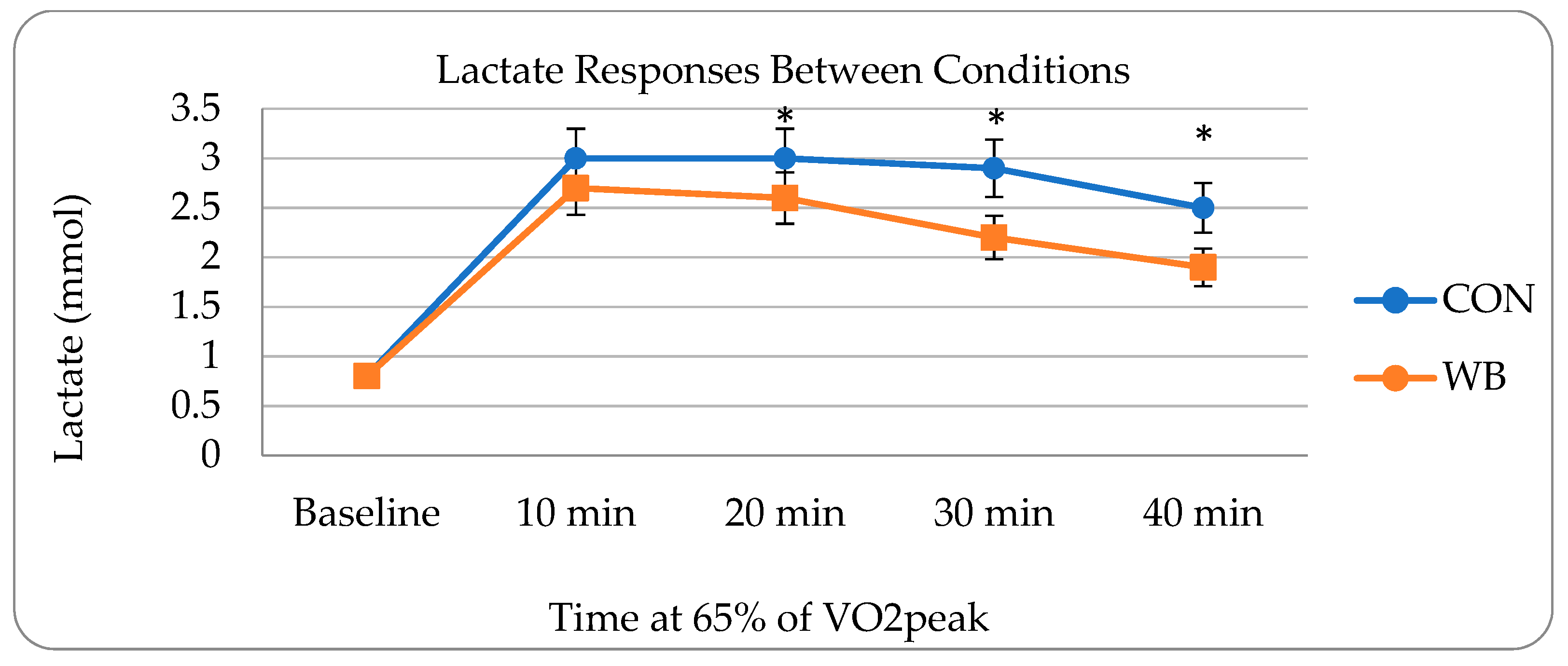
3.1. Metabolic Assessments
3.2. Dietary Intake
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horowitz, J.F. Fatty acid mobilization from adipose tissue during exercise. Trends Endocrinol. Metab. 2003, 14, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.F.; Klein, S. Lipid metabolism during endurance exercise. Am. J. Clin. Nutr. 2000, 72 (Suppl. S2), 558S–563S. [Google Scholar] [CrossRef]
- Brooks, G.A. Mammalian fuel utilization during sustained exercise. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1998, 120, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E. Regulation of fat metabolism in skeletal muscle. Ann. N. Y. Acad. Sci. 2006, 967, 217–235. [Google Scholar] [CrossRef] [PubMed]
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. Endocrinol. 1993, 265, E380–E391. [Google Scholar] [CrossRef]
- Spriet, L.L. New insights into the interaction of carbohydrate and fat metabolism during exercise. Sport. Med. 2014, 44, 87–96. [Google Scholar] [CrossRef]
- Loon, L.J.; Greenhaff, P.L.; Constantin-Teodosiu, D.; Saris, W.H.; Wagenmakers, A.J. The effects of increasing exercise intensity on muscle fuel utilization in humans. J. Physiol. 2001, 536, 295–304. [Google Scholar] [CrossRef]
- Burke, L.M.; Ross, M.L.; Garvican-Lewis, L.A.; Welvaert, M.; Heikura, I.A.; Forbes, S.G.; Mirtschin, J.G.; Cato, L.E.; Strobel, N.; Sharma, A.P.; et al. Low carbohydrate, high fat diet impairs exercise economy and negates the performance benefit from intensified training in elite race walkers. J. Physiol. 2017, 595, 2785–2807. [Google Scholar] [CrossRef]
- Hargreaves, M.; Hawley, J.A.; Jeukendrup, A.E. Pre-exercise carbohydrate and fat ingestion; effects on metabolism and performance. J. Sport. Sci. 2004, 22, 31–38. [Google Scholar] [CrossRef]
- Hosseinian, F.S.; Beta, T. Saskatoon and wild blueberries have higher anthocyanin contents than other Manitoba berries. J. Agric. Food Chem. 2007, 55, 10832–10838. [Google Scholar] [CrossRef]
- Clifford, T.; Howatson, G.; West, D.; Stevenson, E. The potential benefits of red beetroot supplementation in health and disease. Nutrients 2015, 7, 2801–2822. [Google Scholar] [CrossRef]
- Domínguez, R.; Cuenca, E.; Maté-Muñoz, J.; García-Fernández, P.; Serra-Paya, N.; Estevan, M.; Garnacho-Castaño, M. Effects of beetroot juice supplementation on cardiorespiratory endurance in athletes. A systematic review. Nutrients 2017, 9, 43. [Google Scholar] [CrossRef]
- Kim, J.; Park, Y. Combined effects of phytochemicals and exercise on fatty acid oxidation. J. Exerc. Nutrition. Biochem. 2016, 20, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Horio, F.; Uchida, K.; Aoki, H.; Osawa, T. Dietary cyanidin 3-O-beta-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 2003, 133, 2125–2130. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wilkes, S.E.; Rogers, T.R.; Khanal, R.C.; Wu, X.; Howard, L.R. Purified blueberry anthocyanins and blueberry juice alter development of obesity in mice fed an obesogenic high-fat diet. J. Agric. Food Chem. 2010, 58, 3970–3976. [Google Scholar] [CrossRef]
- Solverson, P.M.; Rumpler, W.V.; Leger, J.L.; Redan, B.W.; Ferruzzi, M.G.; Baer, D.J.; Castonguay, T.W.; Novotny, J.A. Blackberry Feeding Increases Fat Oxidation and Improves Insulin Sensitivity in Overweight and Obese Males. Nutrients 2018, 10, 1048. [Google Scholar] [CrossRef]
- Wu, T.; Tang, Q.; Gao, Z.; Yu, Z.; Song, J.; Zheng, X.; Chen, W. Blueberry and mulberry juice prevent obesity development in C57BL/6 mice. PLoS ONE 2013, 8, e77585. [Google Scholar] [CrossRef]
- Rupasinghe, H.V.; Sekhon-Loodu, S.; Mantso, T.; Panayiotidis, M.I. Phytochemicals in regulating fatty acid β-oxidation: Potential underlying mechanisms and their involvement in obesity and weight loss. Pharmacol. Ther. 2016, 165, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, M.J.; Inoue, S.; Horio, F.; Tsuda, T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J. Nutr. 2010, 140, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Towler, M.C.; Hardie, D.G. AMP-Activated Protein Kinase in Metabolic Control and Insulin Signalling. Circ. Res. 2007, 100, 328–341. [Google Scholar] [CrossRef]
- Matsumoto, H.; Takenami, E.; Iwasaki-Kurashige, K.; Osada, T.; Katsumura, T.; Hamaoka, T. Effects of blackcurrant anthocyanin intake on peripheral muscle circulation during typing work in humans. Eur. J. Appl. Physiol. 2004, 94, 36–45. [Google Scholar] [CrossRef]
- Venables, M.C.; Hulston, C.J.; Cox, H.R.; Jeukendrup, A.E. Green tea extract ingestion, fat oxidation, and glucose tolerance in healthy humans. Am. J. Clin. Nutr. 2008, 87, 778–784. [Google Scholar] [CrossRef]
- Cook, M.D.; Myers, S.D.; Blacker, S.D.; Willems, M.E. New Zealand blackcurrant extract improves cycling performance and fat oxidation in cyclists. Eur. J. Appl. Physiol. 2015, 115, 2357–2365. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.D.; Myers, S.D.; Gault, M.L.; Edwards, V.C.; Willems, M.E. Dose effects of New Zealand blackcurrant on substrate oxidation and physiological responses during prolonged cycling. Eur. J. Appl. Physiol. 2017, 117, 1207–1216. [Google Scholar] [CrossRef]
- Strauss, J.A.; Willems, M.E.; Shepherd, S.O. New Zealand blackcurrant extract enhances fat oxidation during prolonged cycling in endurance-trained females. Eur. J. Appl. Physiol. 2018, 118, 1265–1272. [Google Scholar] [CrossRef]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The case for anthocyanin consumption to promote human health: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef]
- Riso, P.; Klimis-Zacas, D.; Bo’, C.D.; Martini, D.; Campolo, J.; Vendrame, S.; Moller, P.; Loft, S.; De Maria, R.; Porrini, M. Effect of a wild blueberry (Vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. Eur. J. Nutr. 2012, 52, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, S.; Guglielmetti, S.; Riso, P.; Arioli, S.; Klimis-Zacas, D.; Porrini, M. Six-Week Consumption of a Wild Blueberry Powder Drink Increases Bifidobacteria in the Human Gut. J. Agric. Food Chem. 2011, 59, 12815–12820. [Google Scholar] [CrossRef]
- Riebe, D.; Ehrman, J.K.; Liguori, G.; Magal, M. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2018; pp. 73–77. [Google Scholar]
- Dulloo, A.G.; Duret, C.; Rohrer, D.; Girardier, L.; Mensi, N.; Fathi, M.; Chantre, P.; Vandermander, J. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-hour energy expenditure and fat oxidation in humans. Am. J. Clin. Nutr. 1999, 70, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Figiel, A.; Oszmiański, J. Effect of Drying Methods with the Application of Vacuum Microwaves on the Bioactive Compounds, Color, and Antioxidant Activity of Strawberry Fruits. J. Agric. Food Chem. 2009, 57, 1337–1343. [Google Scholar] [CrossRef]
- Burnley, M.; Doust, J.H.; Vanhatalo, A. A 3-min All-Out Test to Determine Peak Oxygen Uptake and the Maximal Steady State. Med. Sci. Sports Exerc. 2006, 38, 1995–2003. [Google Scholar] [CrossRef]
- Achten, J.G.M.; Jeukendrup, A.E. Determination of the exercise intensity that elicits maximal fat oxidation. Med. Sci. Sports Exerc. 2002, 34, 92–97. [Google Scholar] [CrossRef]
- Crouter, S.E.; Antczak, A.; Hudak, J.R.; Dellavalle, D.M.; Haas, J.D. Accuracy and reliability of the Parvo Medics TrueOne 2400 and MedGraphics VO2000 metabolic systems. Eur. J. Appl. Physiol. 2006, 98, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E.; Wallis, G.A. Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int. J. Sports Med. 2005, 26, S28–S37. [Google Scholar] [CrossRef] [PubMed]
- Desai, T.; Bottoms, L.; Roberts, M. The effects of Montmorency tart cherry juice supplementation and FATMAX exercise on fat oxidation rates and cardio-metabolic markers in healthy humans. Eur. J. Appl. Physiol. 2018, 118, 2523–2539. [Google Scholar] [CrossRef]
- Vieira, A.; Costa, R.; Macedo, R.; Coconcelli, L.; Kruel, L. Effects of aerobic exercise performed in fasted v. fed state on fat and carbohydrate metabolism in adults: A systematic review and meta-analysis. Br. J. Nutr. 2016, 116, 1153–1164. [Google Scholar] [CrossRef]
- Bloedon, T.K.; Braithwaite, R.E.; Carson, I.A.; Klimis-Zacas, D.; Lehnhard, R.A. Impact of anthocyanin-rich whole fruit consumption on exercise-induced oxidative stress and inflammation: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 630–645. [Google Scholar] [CrossRef]
- Willems, M.E.; Cousins, L.; Williams, D.; Blacker, S.D. Beneficial effects of New Zealand blackcurrant extract on maximal sprint speed during the Loughborough intermittent shuttle test. Sports 2016, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.; Desgranges, C.; Chèze, C.; Vercauteren, J.; Freslon, J. Vasorelaxant effects of grape polyphenols in rat isolated aorta. Possible involvement of a purinergic pathway. Fundam. Clin. Pharmacol. 2003, 17, 673–681. [Google Scholar] [CrossRef]
- Ziberna, L.; Lunder, M.; Tramer, F.; Drevenšek, G.; Passamonti, S. The endothelial plasma membrane transporter bilitranslocase mediates rat aortic vasodilation induced by anthocyanins. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 68–74. [Google Scholar] [CrossRef]
- Paton, C.D.; Hopkins, W.G. Variation in performance of elite cyclists from race to race. Eur. J. Sport Sci. 2006, 6, 23–31. [Google Scholar] [CrossRef]
| Variable | First Session | Third Session | p-Value |
|---|---|---|---|
| Age | 26.55 ± 7.95 | ||
| Height (cm) | 180.16 ± 3.42 | ||
| Weight (kg) | 74.74 ± 8.22 | 74.91 ± 8.53 | 0.563 |
| Body fat (%) | 10.15 ± 3.37 | 10.15 ± 3.27 | 0.983 |
| Waist circumference (cm) | 79.41 ± 6.36 | 78.68 ± 5.81 | 0.195 |
| VO2peak (mL/kg/min) | 54.43 ± 7.99 |
| Variable | Condition | Baseline | 10 min | 20 min | 30 min | 40 min | p-Value |
|---|---|---|---|---|---|---|---|
| HR | C | 69 ± 10 | 155 ± 17 | 144 ± 44 | 162 ± 17 | 162 ± 17 | |
| WB | 67 ± 7 | 155 ± 13 | 157 ± 15 | 160 ± 14 | 160 ± 14 | 0.434 | |
| Power | C | 191 ± 24 | 185 ± 25 | 179 ± 25 | 177 ± 24 | ||
| WB | 190 ± 26 | 183 ± 27 | 181 ± 26 | 177 ± 26 | 0.263 | ||
| Cadence | C | 82 ± 6 | 83 ± 6 | 83 ± 7 | 85 ± 9 | ||
| WB | 77 ± 25 | 83 ± 10 | 83 ± 10 | 83 ± 9 | 0.489 | ||
| RPE | C | 5 ± 1 | 6 ± 2 | 7 ± 2 | 7 ± 3 | ||
| WB | 5 ± 1 | 6 ± 2 | 7 ± 2 | 7 ± 2 | 0.230 | ||
| RER | C | 0.86 ± 0.06 | 0.92 ± 0.03 | 0.90 ± 0.03 | 0.90 ± 0.03 | 0.90 ± 0.04 | |
| WB | 0.83 ± 0.04 | 0.91 ± 0.03 | 0.88 ± 0.03 | 0.87 ± 0.03 | 0.86 ± 0.03 | 0.426 | |
| Ve | C | 10.9 ± 1.9 | 67.7 ± 7.5 | 68.5 ± 7.2 | 69.7 ± 9.1 | 69.1 ± 9.5 | |
| WB | 10.0 ± 2.1 | 68.7 ± 8.9 | 66.9 ± 9.6 | 66.7 ± 9.6 | 66.5 ± 8.0 | 0.302 |
| CON | DRI Met (%) | WB | DRI Met% | ANOVA | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | p Value | |
| Total Kcal | 2450 | 952 | 75 | 30 | 2458 | 939 | 84 | 33 | 0.948 |
| Kcal/kg | 33.5 | 14.3 | 74 | 29 | 33.6 | 14.4 | 85 | 33 | 0.939 |
| CHO (g) | 270.3 | 23.4 | 64 | 23 | 324.4 | 69.5 | 82 | 23 | 0.138 |
| Total Fat (g) | 103.3 | 52.4 | 109 | 59 | 109.5 | 66.7 | 119 | 61 | 0.812 |
| MUFAs (g) | 29.7 | 23.3 | 106 | 80 | 30.9 | 23.6 | 99 | 68 | 0.906 |
| PUFAs (g) | 15.7 | 12.5 | 51 | 42 | 17.1 | 14 | 51 | 36 | 0.806 |
| SFAs (g) | 30.2 | 14.6 | 97 | 53 | 33.3 | 19.1 | 110 | 58 | 0.665 |
| Protein (g) | 106.1 | 43.4 | 184 | 68 | 105.9 | 31.9 | 169 | 68 | 0.991 |
| CHO (%) | 45.6 | 7.92 | 87 | 19 | 56.4 | 12.8 | 102 | 21 | 0.00024 * |
| Fat (%) | 36.9 | 6.9 | 144 | 57 | 37.3 | 10.8 | 138 | 49 | 0.840 |
| Protein (%) | 17.2 | 4.3 | 106 | 34 | 18.2 | 3.5 | 103 | 22 | 0.561 |
| CHO (g/kg) | 3.67 | 1.4 | 160 | 47 | 4.4 | 1.2 | 83 | 23 | 0.0043 * |
| Total fat (g/kg) | 1.5 | 0.8 | 112 | 60 | 1.5 | 0.9 | 117 | 60 | 0.605 |
| Protein (g/kg) | 1.4 | 0.6 | 177 | 73 | 1.4 | 0.5 | 183 | 55 | 0.917 |
| Dietary fiber (g) | 32.2 | 21.8 | 75 | 52 | 44.2 | 14.9 | 92 | 31 | 0.145 |
| Added sugars (g) | 6.1 | 8.6 | 8 | 12 | 6.5 | 9.4 | 6 | 9 | 0.914 |
| Vitamin A (IU) | 8837 | 7009 | 294 | 233 | 11,356 | 15,493 | 362 | 528 | 0.595 |
| Vitamin E (mg) | 12.1 | 13.6 | 80 | 91 | 13.4 | 13.1 | 72 | 43 | 0.429 |
| Vitamin C (mg) | 86.4 | 61 | 96 | 68 | 84.5 | 88.6 | 71 | 67 | 0.943 |
| Vitamin D (IU) | 113.7 | 43.4 | 39 | 69 | 153.9 | 104.1 | 27 | 16 | 0.263 |
| Vitamin K (mcg) | 185.4 | 235 | 154 | 196 | 326.8 | 763 | 118 | 179 | 0.551 |
| Vitamin B1 (mg) | 1.5 | 0.8 | 128 | 71 | 1.1 | 0.6 | 91 | 40 | 0.207 |
| Vitamin B2 (mg) | 2 | 0.9 | 170 | 96 | 1.1 | 0.6 | 155 | 70 | 0.0130 * |
| Vitamin B3 (mg) | 25.2 | 16.4 | 135 | 101 | 24.9 | 17.9 | 150 | 112 | 0.982 |
| Vitamin B6 (mg) | 2.6 | 1.9 | 223 | 186 | 2.7 | 2.2 | 195 | 172 | 0.904 |
| Folate (mcg) | 433.9 | 1.9 | 108 | 85 | 409.9 | 323.2 | 89 | 51 | 0.867 |
| Vitamin B12 (mcg) | 4.3 | 2.4 | 181 | 99 | 5.3 | 5.6 | 229 | 227 | 0.602 |
| Selenium (mcg) | 87.4 | 49.6 | 158 | 90 | 93.6 | 25.7 | 174 | 48 | 0.715 |
| Zinc (mg) | 9.7 | 5.1 | 88 | 47 | 9.5 | 2.9 | 87 | 32 | 0.905 |
| Iron (mg) | 16.7 | 7.6 | 206 | 93 | 16.2 | 7.1 | 205 | 87 | 0.866 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilolla, K.D.; Armendariz, J.; Burrus, B.M.; Baston, D.S.; McCarthy, K.A.; Bloedon, T.K. Effects of Wild Blueberries on Fat Oxidation Rates in Aerobically Trained Males. Nutrients 2023, 15, 1339. https://doi.org/10.3390/nu15061339
Pilolla KD, Armendariz J, Burrus BM, Baston DS, McCarthy KA, Bloedon TK. Effects of Wild Blueberries on Fat Oxidation Rates in Aerobically Trained Males. Nutrients. 2023; 15(6):1339. https://doi.org/10.3390/nu15061339
Chicago/Turabian StylePilolla, Kari D., Jessie Armendariz, Boe M. Burrus, David S. Baston, Karli A. McCarthy, and Taylor K. Bloedon. 2023. "Effects of Wild Blueberries on Fat Oxidation Rates in Aerobically Trained Males" Nutrients 15, no. 6: 1339. https://doi.org/10.3390/nu15061339
APA StylePilolla, K. D., Armendariz, J., Burrus, B. M., Baston, D. S., McCarthy, K. A., & Bloedon, T. K. (2023). Effects of Wild Blueberries on Fat Oxidation Rates in Aerobically Trained Males. Nutrients, 15(6), 1339. https://doi.org/10.3390/nu15061339






