Alleviation Syndrome of High-Cholesterol-Diet-Induced Hypercholesterolemia in Mice by Intervention with Lactiplantibacillus plantarum WLPL21 via Regulation of Cholesterol Metabolism and Transportation as Well as Gut Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Cultures
2.2. Animals and Experimental Design
2.3. Serum and Hepatic Lipids
2.4. Fecal Total Bile Acid
2.5. Histological Analysis
2.6. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.7. SCFAs Analysis
2.8. Analysis of Gut Microbiota by 16S rRNA Sequencing
2.9. Statistical Analysis
3. Results
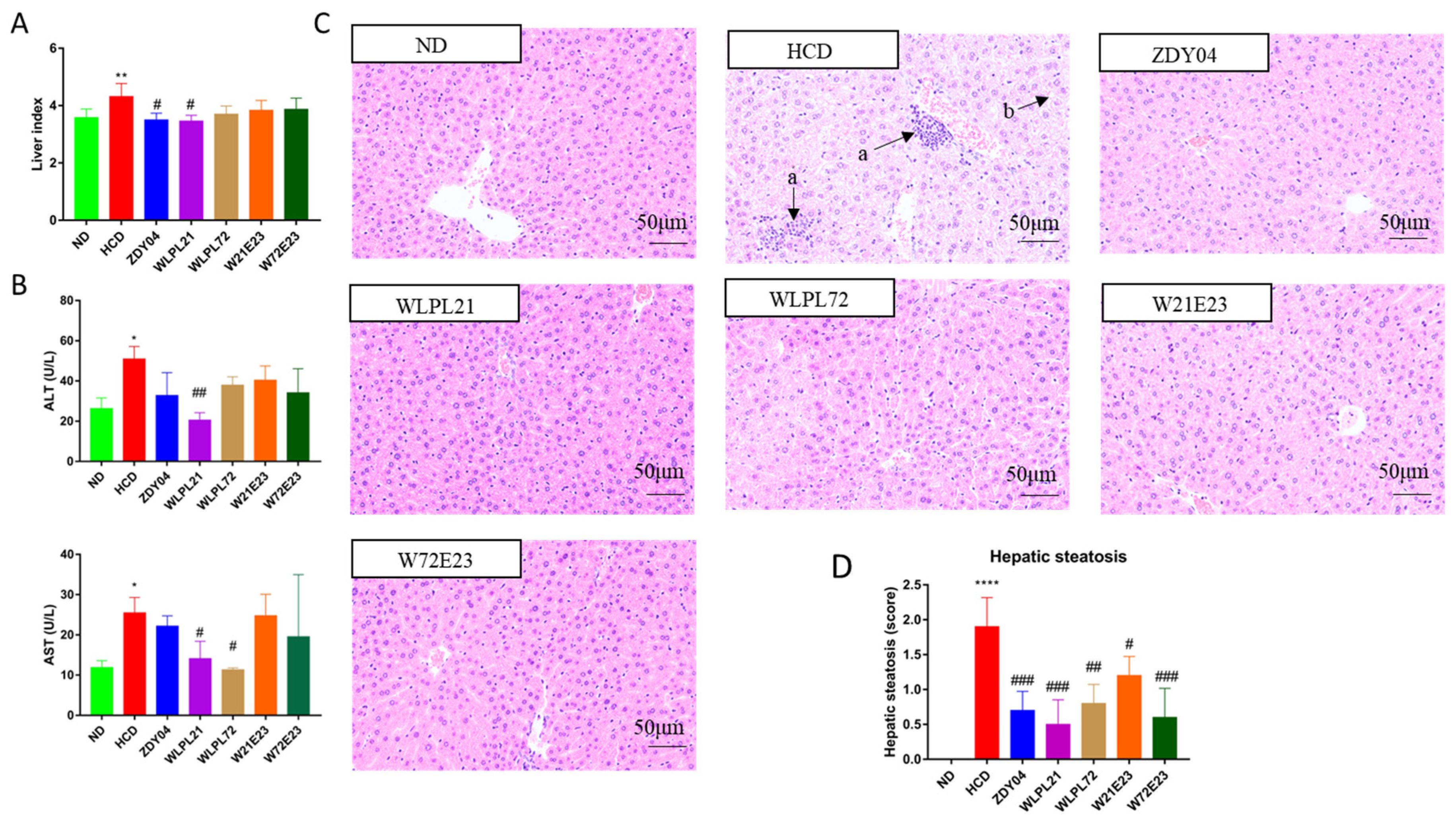
3.1. Probiotics Reduced Cholesterol Levels in Serum and Liver
3.2. Probiotics Alleviated Hepatic Injury Caused by HCD
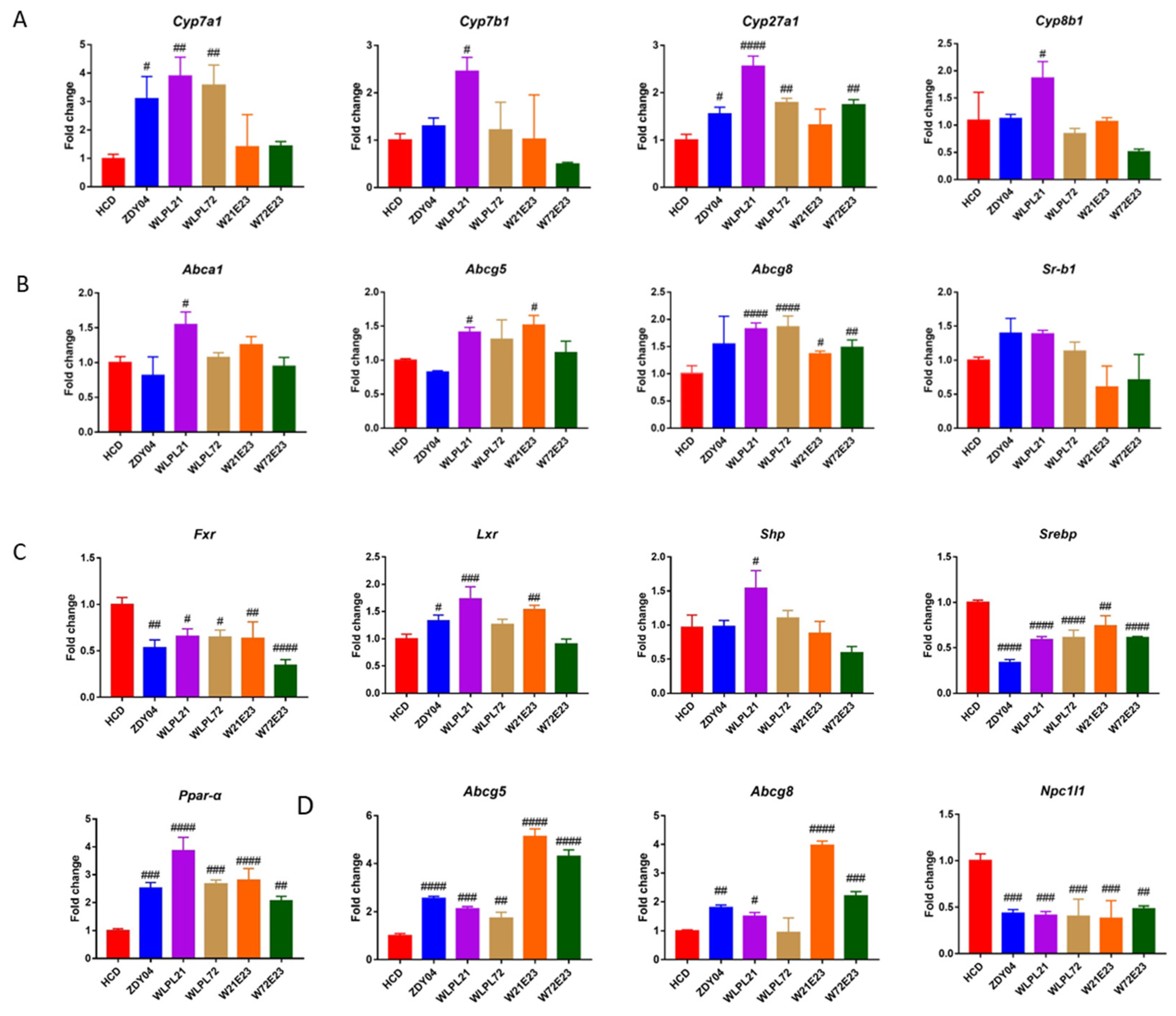
3.3. Probiotics Regulated Cholesterol Metabolism in the Liver and Cholesterol Transportation in the Liver and Ileum
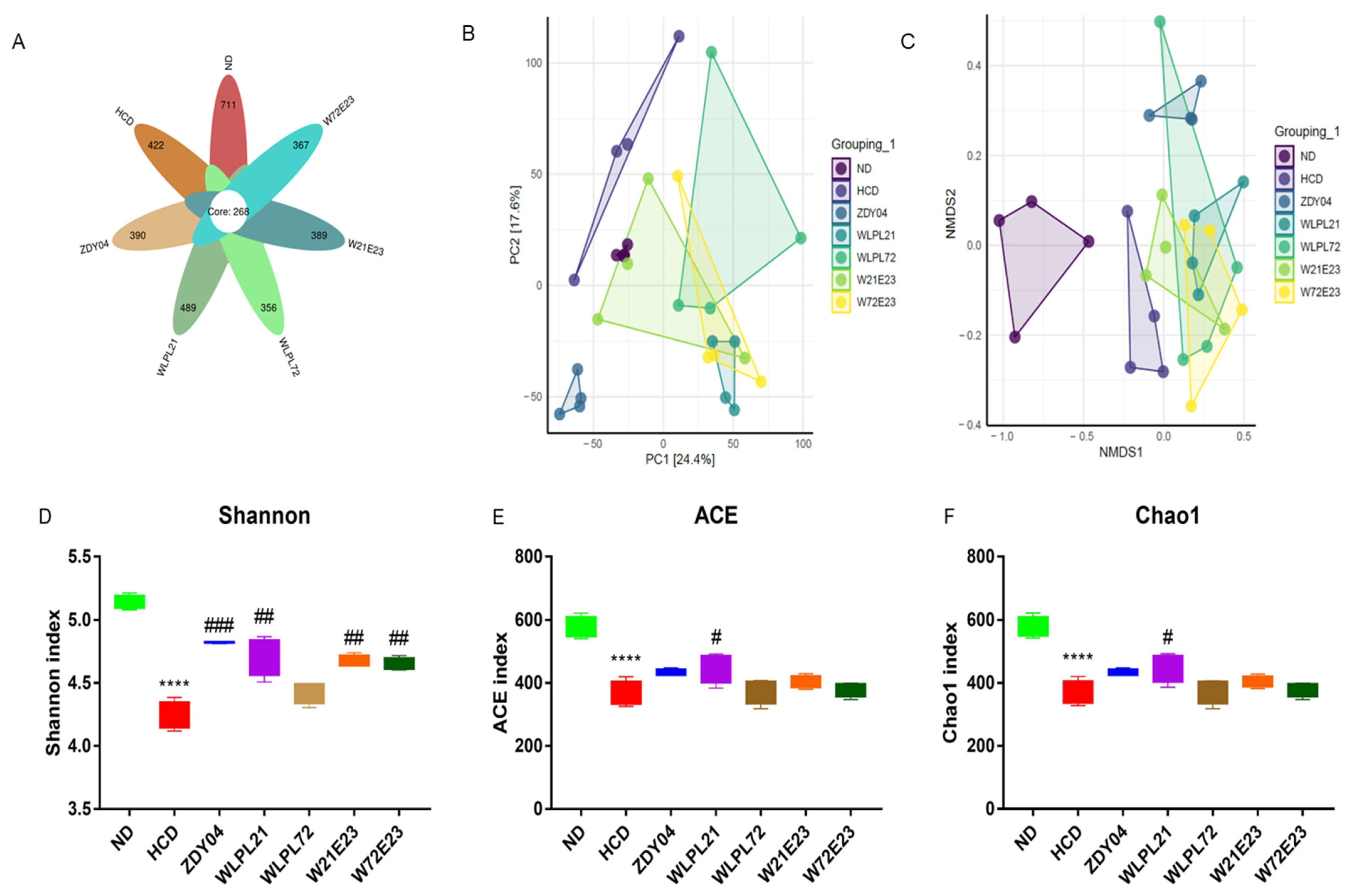
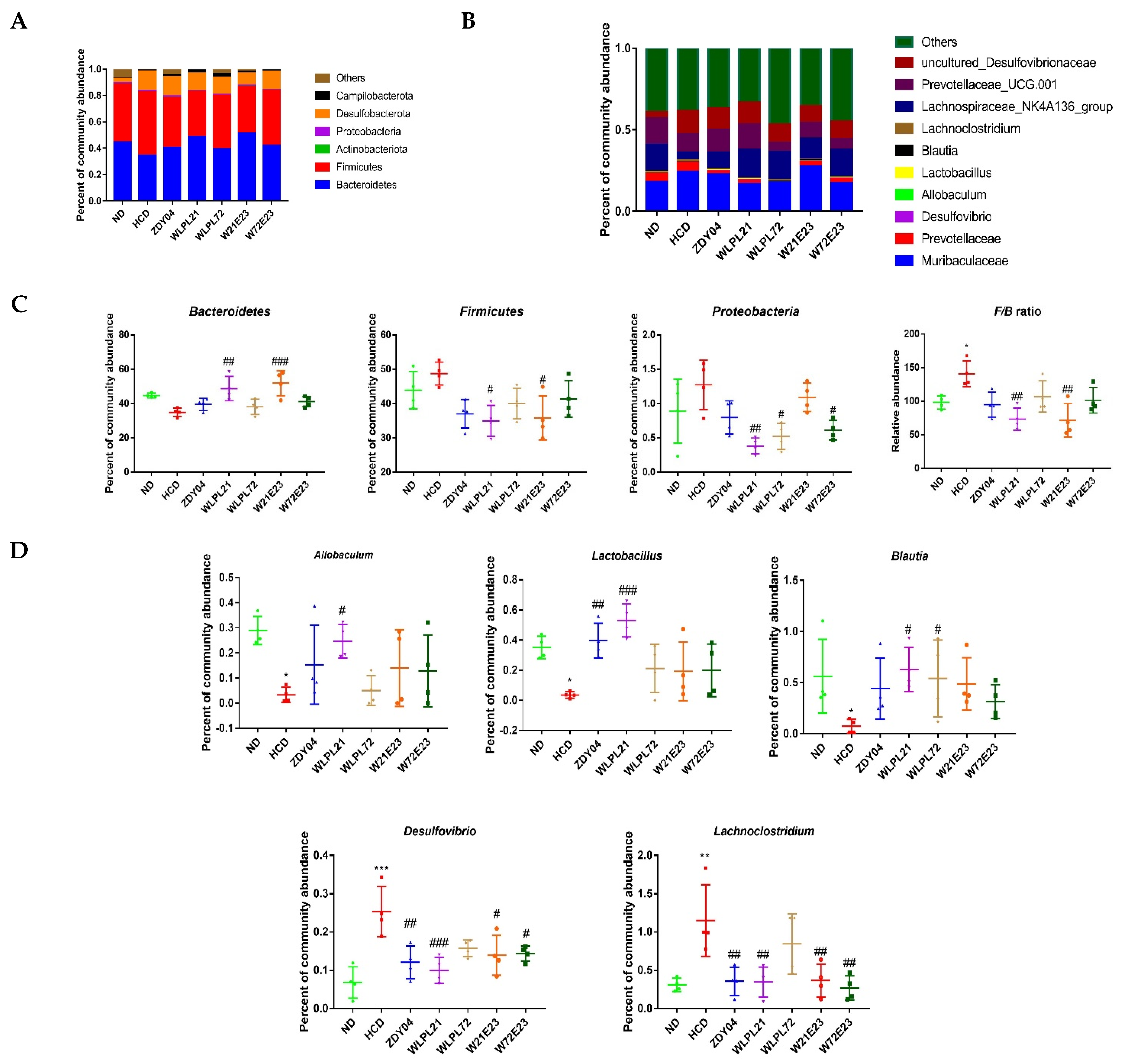
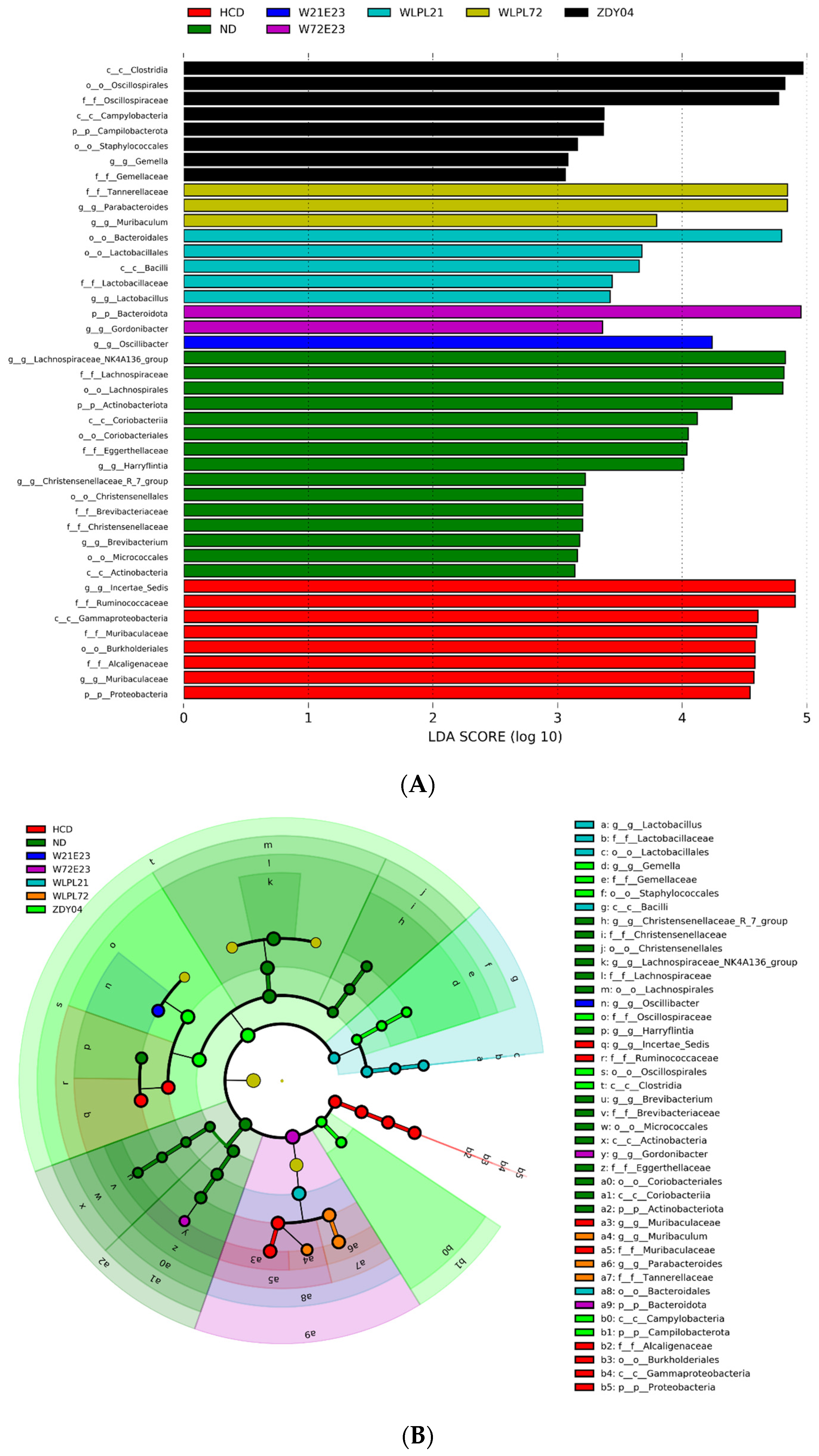
3.4. Probiotics Modulated the Composition of Gut Microbiota and Cecum SCFA Level
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sugiyama, D.; Turin, T.C.; Yeasmin, F.; Rumana, N.; Watanabe, M.; Higashiyama, A.; Takegami, M.; Kokubo, Y.; Okamura, T.; Miyamoto, Y. Hypercholesterolemia and Lifetime Risk of Coronary Heart Disease in the General Japanese Population: Results from the Suita Cohort Study. J. Atheroscler. Thromb. 2020, 27, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Mu, F.; Rich-Edwards, J.; Rimm, E.B.; Spiegelman, D.; Forman, J.P.; Missmer, S.A. Association between Endometriosis and Hypercholesterolemia or Hypertension. Hypertension 2017, 70, 59–65. [Google Scholar] [CrossRef]
- Paquette, M.; Bernard, S.; Ruel, I.; Blank, D.W.; Genest, J.; Baass, A. Diabetes is associated with an increased risk of cardiovascular disease in patients with familial hypercholesterolemia. J. Clin. Lipidol. 2019, 13, 123–128. [Google Scholar] [CrossRef]
- Emanuelsson, F.; Nordestgaard, B.G.; Benn, M. Familial Hypercholesterolemia and Risk of Peripheral Arterial Disease and Chronic Kidney Disease. J. Clin. Endocrinol. Metab. 2018, 103, 4491–4500. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Huang, W.; Xia, Y.; Xiong, Z.; Ai, L. Cholesterol-lowering potentials of Lactobacillus strain overexpression of bile salt hydrolase on high cholesterol diet-induced hypercholesterolemic mice. Food Funct. 2019, 10, 1684–1695. [Google Scholar] [CrossRef]
- WHO. Cardiovascular Diseases (CVDs). 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 11 June 2021).
- Grundy, S.M. Does Dietary Cholesterol Matter? Curr. Atheroscler. Rep. 2016, 18, 68. [Google Scholar] [CrossRef] [PubMed]
- Denke, M.A.; Sempos, C.T.; Grundy, S.M. Excess Body Weight. Arch. Intern. Med. 1993, 153, 1093–1103. [Google Scholar] [CrossRef]
- Nasi, M.; Patrizi, G.; Pizzi, C.; Landolfo, M.; Boriani, G.; Cas, A.D.; Cicero, A.F.; Fogacci, F.; Rapezzi, C.; Sisca, G.; et al. The role of physical activity in individuals with cardiovascular risk factors. J. Cardiovasc. Med. 2019, 20, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Anagnostis, P.; Stevenson, J.C.; Crook, D.; Johnston, D.G.; Godsland, I.F. Effects of menopause, gender and age on lipids and high-density lipoprotein cholesterol subfractions. Maturitas 2015, 81, 62–68. [Google Scholar] [CrossRef]
- Zhang, M.; Deng, Q.; Wang, L.; Huang, Z.; Zhou, M.; Li, Y.; Zhao, Z.; Zhang, Y.; Wang, L. Prevalence of dyslipidemia and achievement of low-density lipoprotein cholesterol targets in Chinese adults: A nationally representative survey of 163,641 adults. Int. J. Cardiol. 2018, 260, 196–203. [Google Scholar] [CrossRef]
- Ruscica, M.; Ferri, N.; Banach, M.; Sirtori, C.R.; Corsini, A. Side effects of statins: From pathophysiology and epidemiology to diagnostic and therapeutic implications. Cardiovasc. Res. 2022, 118, 3288–3304. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Q.; Li, S.B.; Xie, B. Simvastatin is Efficacious in Treating Cirrhosis. J. Clin. Gastroenterol. 2022, 56, e303–e312. [Google Scholar] [CrossRef]
- Yu, X.-H.; Zhang, D.; Zheng, X.-L.; Tang, C.-K. Cholesterol transport system: An integrated cholesterol transport model involved in atherosclerosis. Prog. Lipid Res. 2018, 73, 65–91. [Google Scholar] [CrossRef]
- Houten, S.M.; Watanabe, M.; Auwerx, J. Endocrine functions of bile acids. EMBO J. 2006, 25, 1419–1425. [Google Scholar] [CrossRef]
- Yu, L.; Lu, H.; Yang, X.; Li, R.; Shi, J.; Yu, Y.; Ma, C.; Sun, F.; Zhang, S.; Zhang, F. Diosgenin alleviates hypercholesterolemia via SRB1/CES-1/CYP7A1/FXR pathway in high-fat diet-fed rats. Toxicol. Appl. Pharmacol. 2020, 412, 115388. [Google Scholar] [CrossRef]
- Liang, X.; Lv, Y.; Zhang, Z.; Yi, H.; Liu, T.; Li, R.; Yu, Z.; Zhang, L. Study on intestinal survival and cholesterol metabolism of probiotics. LWT 2020, 124, 109132. [Google Scholar] [CrossRef]
- Yang, L.; Xie, X.; Li, Y.; Wu, L.; Fan, C.; Liang, T.; Xi, Y.; Yang, S.; Li, H.; Zhang, J.; et al. Evaluation of the Cholesterol-Lowering Mechanism of Enterococcus faecium Strain 132 and Lactobacillus paracasei Strain 201 in Hypercholesterolemia Rats. Nutrients 2021, 13, 1982. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zou, K.; Zhan, Y.; Cai, Y.; Zhang, Z.; Tao, X.; Qiu, L.; Wei, H. Enterococcus faecium GEFA01 alleviates hypercholesterolemia by promoting reverse cholesterol transportation via modulating the gut microbiota-SCFA axis. Front. Nutr. 2022, 9, 1020734. [Google Scholar] [CrossRef]
- Hassan, A.; Din, A.U.; Zhu, Y.; Zhang, K.; Li, T.; Wang, Y.; Xu, S.; Lei, H.; Yu, X.; Wang, G. Anti-atherosclerotic effects of Lactobacillus plantarum ATCC 14917 in ApoE−/− mice through modulation of proinflammatory cytokines and oxidative stress. Appl. Microbiol. Biotechnol. 2020, 104, 6337–6350. [Google Scholar] [CrossRef]
- Li, X.; Xiao, Y.; Song, L.; Huang, Y.; Chu, Q.; Zhu, S.; Lu, S.; Hou, L.; Li, Z.; Li, J.; et al. Effect of Lactobacillus plantarum HT121 on serum lipid profile, gut microbiota, and liver transcriptome and metabolomics in a high-cholesterol diet–induced hypercholesterolemia rat model. Nutrition 2020, 79–80, 110966. [Google Scholar] [CrossRef]
- Qiu, L.; Tao, X.; Xiong, H.; Yu, J.; Wei, H. Lactobacillus plantarum ZDY04 exhibits a strain-specific property of lowering TMAO via the modulation of gut microbiota in mice. Food Funct. 2018, 9, 4299–4309. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, F.; Xu, D.; Zhang, Z.; Xu, F.; Tao, X.; Qiu, L.; Wei, H. Enterococcus faecium WEFA23 from infants lessens high-fat-diet-induced hyperlipidemia via cholesterol 7-alpha-hydroxylase gene by altering the composition of gut microbiota in rats. J. Dairy Sci. 2018, 101, 7757–7767. [Google Scholar] [CrossRef]
- Zhang, F.; Qiu, L.; Xu, X.; Liu, Z.; Zhan, H.; Tao, X.; Shah, N.P.; Wei, H. Beneficial effects of probiotic cholesterol-lowering strain of Enterococcus faecium WEFA23 from infants on diet-induced metabolic syndrome in rats. J. Dairy Sci. 2017, 100, 1618–1628. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, Q.; Mi, X.; Qiu, L.; Tao, X.; Zhang, Z.; Xia, J.; Wu, Q.; Wei, H. Ripened Puerh Tea Extract Promotes Gut Microbiota Resilience against Dextran Sulfate Sodium Induced Colitis. J. Agric. Food Chem. 2021, 69, 2190–2203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chen, H.; Jia, J.; Ye, X.; Ding, H.; Zhan, Y. The CYP17A1 gene polymorphisms are associated with hypercholesterolemia in Han Chinese. J. Gene Med. 2019, 21, e3102. [Google Scholar] [CrossRef]
- Chen, Z.-C.; Shin, S.-J.; Kuo, K.-K.; Lin, K.-D.; Yu, M.-L.; Hsiao, P.-J. Significant association of ABCG8:D19H gene polymorphism with hypercholesterolemia and insulin resistance. J. Hum. Genet. 2008, 53, 757–763. [Google Scholar] [CrossRef]
- Heo, W.; Lee, E.S.; Cho, H.T.; Kim, J.H.; Lee, J.H.; Yoon, S.M.; Kwon, H.T.; Yang, S.; Kim, Y.-J. Lactobacillus plantarum LRCC 5273 isolated from Kimchi ameliorates diet-induced hypercholesterolemia in C57BL/6 mice. Biosci. Biotechnol. Biochem. 2018, 82, 1964–1972. [Google Scholar] [CrossRef]
- Qu, T.; Yang, L.; Wang, Y.; Jiang, B.; Shen, M.; Ren, D. Reduction of serum cholesterol and its mechanism by Lactobacillus plantarum H6 screened from local fermented food products. Food Funct. 2020, 11, 1397–1409. [Google Scholar] [CrossRef]
- Zheng, F.; Wang, Z.; Stanton, C.; Ross, R.P.; Zhao, J.; Zhang, H.; Yang, B.; Chen, W. Lactobacillus rhamnosus FJSYC4-1 and Lactobacillus reuteri FGSZY33L6 alleviate metabolic syndrome via gut microbiota regulation. Food Funct. 2021, 12, 3919–3930. [Google Scholar] [CrossRef]
- Yan, J.; Li, J.; Xue, Q.; Xie, S.; Jiang, J.; Li, P.; Du, B. Bacillus sp. DU-106 ameliorates type 2 diabetes by modulating gut microbiota in high-fat-fed and streptozotocin-induced mice. J. Appl. Microbiol. 2022, 133, 3126–3138. [Google Scholar] [CrossRef]
- Yang, D.; Lyu, W.; Hu, Z.; Gao, J.; Zheng, Z.; Wang, W.; Firrman, J.; Ren, D. Probiotic Effects of Lactobacillus fermentum ZJUIDS06 and Lactobacillus plantarum ZY08 on Hypercholesteremic Golden Hamsters. Front. Nutr. 2021, 8, 705763. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, M.; Ma, Y.; Yang, Y.; Cheng, Y.; Ma, H.; Ren, D.; Chen, P. Regulation of viable/inactivated/lysed probiotic Lactobacillus plantarum H6 on intestinal microbiota and metabolites in hypercholesterolemic mice. NPJ Sci. Food 2022, 6, 50. [Google Scholar] [CrossRef]
- Yamasaki, M.; Minesaki, M.; Iwakiri, A.; Miyamoto, Y.; Ogawa, K.; Nishiyama, K.; Tsend-Ayush, C.; Oyunsuren, T.; Li, Y.; Nakano, T.; et al. Lactobacillus plantarum 06CC2 reduces hepatic cholesterol levels and modulates bile acid deconjugation in Balb/c mice fed a high-cholesterol diet. Food Sci. Nutr. 2020, 8, 6164–6173. [Google Scholar] [CrossRef]
- Park, S.; Kang, J.; Choi, S.; Park, H.; Hwang, E.; Kang, Y.; Kim, A.; Holzapfel, W.; Ji, Y. Cholesterol-lowering effect of Lactobacillus rhamnosus BFE5264 and its influence on the gut microbiome and propionate level in a murine model. PLoS ONE 2018, 13, e0203150. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, S.; Cui, J.; Guo, T.; Zhang, J. Effect of bile salt hydrolase-active Lactobacillus plantarum Y15 on high cholesterol diet induced hypercholesterolemic mice. CyTA-J. Food 2021, 19, 408–417. [Google Scholar] [CrossRef]




| Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| Gapdh | AGGTCGGTGTGAACGGATTTG | GGGGTCGTTGATGGCAACA |
| Cyp27a1 | GCCTTGGAAGCCATCACCTA | AGATCTGATGAAGGCGGCAG |
| Cyp7b1 | CCCTGCGTGACGAAATTGAC | AGAATAGTGCTTTCCAGGCAGA |
| Cyp7a1 | AGCAACTAAACAACCTGCCAGTA | GTCCGGATATTCAAGGATGCA |
| Cyp8b1 | TTGCAAATGCTGCCTCAACC | AGTGGGAAATTAACAGTCGCA |
| Abca1 | GTTGGTCTCCAGAAGGTATT | TTCAGGATGTCCATGTTGT |
| Abcg5 | TCAATGAGTTTTACGGCCTGAA | GCACATCGGGTGATTTAGCA |
| Abcg8 | TGCCCACCTTCCACATGTC | ATGAAGCCGGCAGTAAGGTAGA |
| Sr-b1 | TGTACTGCCTAACATCTTGGTCC | ACTGTGCGGTTCATAAAAGCA |
| Fxr | TGAGAACCCACAGCATTTCG | GCGTGGTGATGGTTGAATGTC |
| Lxr | CTCAATGCCTGATGTTTCTCCT | TCCAACCCTATCCCTAAAGCAA |
| Shp | CGATCCTCTTCAACCCAGATG | AGGGCTCCAAGACTTCACACA |
| Srebp1c | GGAGCCATGGATTGCACATT | GGCCCGGGAAGTCACTGT |
| Ppar-α | AACATCGAGTGTCGAATATGTGG | CCGAATAGTTCGCCGAAAGAA |
| Npc1l1 | TTTCTAGGGGCCCTGACCTC | TTGAAAAGCAGCACACGACG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.; Qiu, L.; He, Y.; Tao, X.; Zhang, Z.; Wei, H. Alleviation Syndrome of High-Cholesterol-Diet-Induced Hypercholesterolemia in Mice by Intervention with Lactiplantibacillus plantarum WLPL21 via Regulation of Cholesterol Metabolism and Transportation as Well as Gut Microbiota. Nutrients 2023, 15, 2600. https://doi.org/10.3390/nu15112600
Zhao K, Qiu L, He Y, Tao X, Zhang Z, Wei H. Alleviation Syndrome of High-Cholesterol-Diet-Induced Hypercholesterolemia in Mice by Intervention with Lactiplantibacillus plantarum WLPL21 via Regulation of Cholesterol Metabolism and Transportation as Well as Gut Microbiota. Nutrients. 2023; 15(11):2600. https://doi.org/10.3390/nu15112600
Chicago/Turabian StyleZhao, Kui, Liang Qiu, Yao He, Xueying Tao, Zhihong Zhang, and Hua Wei. 2023. "Alleviation Syndrome of High-Cholesterol-Diet-Induced Hypercholesterolemia in Mice by Intervention with Lactiplantibacillus plantarum WLPL21 via Regulation of Cholesterol Metabolism and Transportation as Well as Gut Microbiota" Nutrients 15, no. 11: 2600. https://doi.org/10.3390/nu15112600
APA StyleZhao, K., Qiu, L., He, Y., Tao, X., Zhang, Z., & Wei, H. (2023). Alleviation Syndrome of High-Cholesterol-Diet-Induced Hypercholesterolemia in Mice by Intervention with Lactiplantibacillus plantarum WLPL21 via Regulation of Cholesterol Metabolism and Transportation as Well as Gut Microbiota. Nutrients, 15(11), 2600. https://doi.org/10.3390/nu15112600




