A Review of Low-Density Lipoprotein-Lowering Diets in the Age of Anti-Sense Technology
Abstract
1. Introduction
2. Source of Evidence
3. LDL and Atherosclerotic Cardiovascular Disease
4. Evaluation of Diet on LDLc
4.1. Dietary Fat and Fatty Acids
4.2. Dietary Cholesterol
4.3. Other Dietary Approaches
4.4. Total Diet
4.5. Implications for HDL and Triglyceride Concentrations
4.6. Summarizing the Potential Reduction in LDLc by Dietary Means
5. Recent Pharmacological Advances for Lowering LDLc
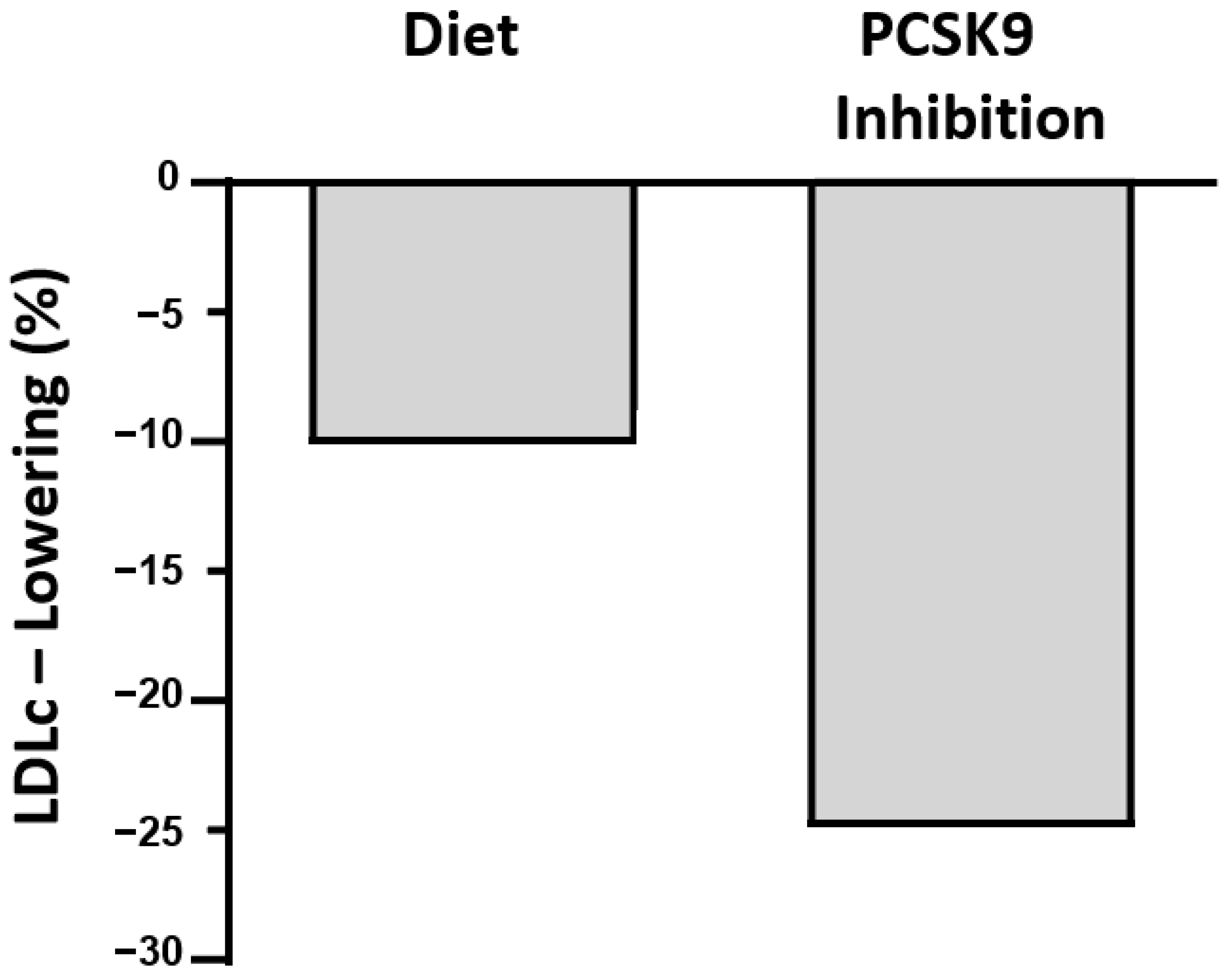
6. Conclusions (Figure 1)
- LDLc is lowered by a variety of dietary interventions that achieve as much as a 20% reduction. Adequate substitutions of saturated fatty acids by polyunsaturated fatty acids have shown, on average, about a 10% lowering. Other interventions reduce LDLc by about 5% each and include reductions in cholesterol consumption and supplementing with plant sterols and other nutrients.
- Further research should focus on combinations of foods, specific nutrients, and supplements aiming for total LDLc reductions of 20%, which would be competitive with simple pharmacological therapies such as statins or ezetimibe. For the large population with mild-to-moderate hypercholesterolemia, successful interventions through diet are more desirable but more difficult to achieve currently than pharmacologically. Enthusiastic support from health professionals is essential. Appropriate manufacturing and pricing of foods fortified to lower LDLc is required.
- The advent of drugs more potent than statins, such as the siRNA inhibitor of PCSK9 that lowers LDLc by >25% and requires only twice-yearly subcutaneous injections, will be competitive with diet once it becomes affordable to most patients.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pirillo, A.; Casula, M.; Olmastroni, E.; Norata, G.D.; Catapano, A.L. Global epidemiology of dyslipidaemias. Nat. Rev. Cardiol. 2021, 18, 689–700. [Google Scholar] [CrossRef]
- World Health Organization. Noncommunicable Diseases: Risk Factors. The Global Health Observatory. 2021. Available online: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/ncd-risk-factors (accessed on 21 February 2023).
- Global Health Data Exchange. GBD Results Tool. Institute for Health Metrics and Evaluation. 2021. Available online: http://ghdx.healthdata.org/gbd-results-tool (accessed on 21 February 2023).
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed]
- Cholesterol Treatment Trialists, C.; Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Catapano, A.L.; Corsini, A.; Manzato, E.; Werba, J.P.; Catena, G.; Cetin, I.; Cicero, A.F.G.; Cignarella, A.; Colivicchi, F.; et al. LDL-cholesterol control in the primary prevention of cardiovascular diseases: An expert opinion for clinicians and health professionals. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Keys, A.; Anderson, J.T.; Grande, F. Prediction of serum-cholesterol responses of man to changes in fats in the diet. Lancet 1957, 273, 959–966. [Google Scholar] [CrossRef]
- Keys, A.; Anderson, J.T.; Grande, F. Serum cholesterol response to changes in the diet: IV. Particular saturated fatty acids in the diet. Metabolism 1965, 14, 776–787. [Google Scholar] [CrossRef]
- Keys, A.; Parlin, R.W. Serum cholesterol response to changes in dietary lipids. Am. J. Clin. Nutr. 1966, 19, 175–181. [Google Scholar] [CrossRef]
- Hegsted, D.M.; McGandy, R.B.; Myers, M.L.; Stare, F.J. Quantitative effects of dietary fat on serum cholesterol in man. Am. J. Clin. Nutr. 1965, 17, 281–295. [Google Scholar] [CrossRef]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): A prospective cohort study. Lancet 2017, 390, 2050–2062. [Google Scholar] [CrossRef]
- Grundy, S.M. Comparison of monounsaturated fatty acids and carbohydrates for lowering plasma cholesterol. N. Engl. J. Med. 1986, 314, 745–748. [Google Scholar] [CrossRef]
- Nestel, P.; Clifton, P.; Noakes, M. Effects of increasing dietary palmitoleic acid compared with palmitic and oleic acids on plasma lipids of hypercholesterolemic men. J. Lipid Res. 1994, 35, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Stonehouse, W.; Sergi, D.; Benassi-Evans, B.; James-Martin, G.; Johnson, N.; Thompson, C.H.; Abeywardena, M. Eucaloric diets enriched in palm olein, cocoa butter, and soybean oil did not differentially affect liver fat concentration in healthy participants: A 16-week randomized controlled trial. Am. J. Clin. Nutr. 2021, 113, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Sellem, L.; Flourakis, M.; Jackson, K.G.; Joris, P.J.; Lumley, J.; Lohner, S.; Mensink, R.P.; Soedamah-Muthu, S.S.; Lovegrove, J.A. Impact of Replacement of Individual Dietary SFAs on Circulating Lipids and Other Biomarkers of Cardiometabolic Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials in Humans. Adv. Nutr. 2022, 13, 1200–1225. [Google Scholar] [CrossRef]
- Noakes, M.; Nestel, P.J.; Clifton, P.M. Modifying the fatty acid profile of dairy products through feedlot technology lowers plasma cholesterol of humans consuming the products. Am. J. Clin. Nutr. 1996, 63, 42–46. [Google Scholar] [CrossRef]
- Nestel, P.J.; Noakes, M.; Belling, G.B.; McArthur, R.; Clifton, R.M.; Abbey, M. Plasma cholesterol-lowering potential of edible-oil blends suitable for commercial use. Am. J. Clin. Nutr. 1992, 55, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Bonanome, A.; Grundy, S.M. Effect of dietary stearic acid on plasma cholesterol and lipoprotein levels. N. Engl. J. Med. 1988, 318, 1244–1248. [Google Scholar] [CrossRef] [PubMed]
- Panth, N.; Abbott, K.A.; Dias, C.B.; Wynne, K.; Garg, M.L. Differential effects of medium- and long-chain saturated fatty acids on blood lipid profile: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018, 108, 675–687. [Google Scholar] [CrossRef]
- Zock, P.L.; de Vries, J.H.; Katan, M.B. Impact of myristic acid versus palmitic acid on serum lipid and lipoprotein levels in healthy women and men. Arterioscler. Thromb. 1994, 14, 567–575. [Google Scholar] [CrossRef]
- Denke, M.A.; Grundy, S.M. Comparison of effects of lauric acid and palmitic acid on plasma lipids and lipoproteins. Am. J. Clin. Nutr. 1992, 56, 895–898. [Google Scholar] [CrossRef]
- Tholstrup, T.; Hjerpsted, J.; Raff, M. Palm olein increases plasma cholesterol moderately compared with olive oil in healthy individuals. Am. J. Clin. Nutr. 2011, 94, 1426–1432. [Google Scholar] [CrossRef]
- Brassard, D.; Tessier-Grenier, M.; Allaire, J.; Rajendiran, E.; She, Y.; Ramprasath, V.; Gigleux, I.; Talbot, D.; Levy, E.; Tremblay, A.; et al. Comparison of the impact of SFAs from cheese and butter on cardiometabolic risk factors: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Tholstrup, T.; Hoy, C.E.; Andersen, L.N.; Christensen, R.D.; Sandstrom, B. Does fat in milk, butter and cheese affect blood lipids and cholesterol differently? J. Am. Coll. Nutr. 2004, 23, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Hjerpsted, J.; Leedo, E.; Tholstrup, T. Cheese intake in large amounts lowers LDL-cholesterol concentrations compared with butter intake of equal fat content. Am. J. Clin. Nutr. 2011, 94, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Nestel, P.J.; Mori, T.A. Dairy Foods: Is Its Cardiovascular Risk Profile Changing? Curr. Atheroscler. Rep. 2022, 24, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Eyres, L.; Eyres, M.F.; Chisholm, A.; Brown, R.C. Coconut oil consumption and cardiovascular risk factors in humans. Nutr. Rev. 2016, 74, 267–280. [Google Scholar] [CrossRef]
- Sun, Y.; Neelakantan, N.; Wu, Y.; Lote-Oke, R.; Pan, A.; van Dam, R.M. Palm Oil Consumption Increases LDL Cholesterol Compared with Vegetable Oils Low in Saturated Fat in a Meta-Analysis of Clinical Trials. J. Nutr. 2015, 145, 1549–1558. [Google Scholar] [CrossRef]
- Cox, C.; Mann, J.; Sutherland, W.; Chisholm, A.; Skeaff, M. Effects of coconut oil, butter, and safflower oil on lipids and lipoproteins in persons with moderately elevated cholesterol levels. J. Lipid Res. 1995, 36, 1787–1795. [Google Scholar] [CrossRef]
- Mensink, R.P.; Katan, M.B. Effect of dietary trans fatty acids on high-density and low-density lipoprotein cholesterol levels in healthy subjects. N. Engl. J. Med. 1990, 323, 439–445. [Google Scholar] [CrossRef]
- Chardigny, J.M.; Destaillats, F.; Malpuech-Brugere, C.; Moulin, J.; Bauman, D.E.; Lock, A.L.; Barbano, D.M.; Mensink, R.P.; Bezelgues, J.B.; Chaumont, P.; et al. Do trans fatty acids from industrially produced sources and from natural sources have the same effect on cardiovascular disease risk factors in healthy subjects? Results of the trans Fatty Acids Collaboration (TRANSFACT) study. Am. J. Clin. Nutr. 2008, 87, 558–566. [Google Scholar] [CrossRef]
- Motard-Belanger, A.; Charest, A.; Grenier, G.; Paquin, P.; Chouinard, Y.; Lemieux, S.; Couture, P.; Lamarche, B. Study of the effect of trans fatty acids from ruminants on blood lipids and other risk factors for cardiovascular disease. Am. J. Clin. Nutr. 2008, 87, 593–599. [Google Scholar] [CrossRef]
- Willett, W.; Mozaffarian, D. Ruminant or industrial sources of trans fatty acids: Public health issue or food label skirmish? Am. J. Clin. Nutr. 2008, 87, 515–516. [Google Scholar] [CrossRef] [PubMed]
- Dreon, D.M.; Fernstrom, H.A.; Campos, H.; Blanche, P.; Williams, P.T.; Krauss, R.M. Change in dietary saturated fat intake is correlated with change in mass of large low-density-lipoprotein particles in men. Am. J. Clin. Nutr. 1998, 67, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Froyen, E. The effects of fat consumption on low-density lipoprotein particle size in healthy individuals: A narrative review. Lipids Health Dis. 2021, 20, 86. [Google Scholar] [CrossRef] [PubMed]
- Rajendiran, E.; Lamarche, B.; She, Y.; Ramprasath, V.; Eck, P.; Brassard, D.; Gigleux, I.; Levy, E.; Tremblay, A.; Couture, P.; et al. A combination of single nucleotide polymorphisms is associated with the interindividual variability in the blood lipid response to dietary fatty acid consumption in a randomized clinical trial. Am. J. Clin. Nutr. 2021, 114, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Griffin, B.A.; Mensink, R.P.; Lovegrove, J.A. Does variation in serum LDL-cholesterol response to dietary fatty acids help explain the controversy over fat quality and cardiovascular disease risk? Atherosclerosis 2021, 328, 108–113. [Google Scholar] [CrossRef]
- Ludwig, D.S.; Willett, W.C.; Volek, J.S.; Neuhouser, M.L. Dietary fat: From foe to friend? Science 2018, 362, 764–770. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, E596–E646. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Carson, J.A.S.; Lichtenstein, A.H.; Anderson, C.A.M.; Appel, L.J.; Kris-Etherton, P.M.; Meyer, K.A.; Petersen, K.; Polonsky, T.; Van Horn, L.; American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; et al. Dietary Cholesterol and Cardiovascular Risk: A Science Advisory From the American Heart Association. Circulation 2020, 141, e39–e53. [Google Scholar] [CrossRef]
- Katan, M.B.; Beynen, A.C. Characteristics of human hypo- and hyperresponders to dietary cholesterol. Am. J. Epidemiol. 1987, 125, 387–399. [Google Scholar] [CrossRef]
- Vincent, M.J.; Allen, B.; Palacios, O.M.; Haber, L.T.; Maki, K.C. Meta-regression analysis of the effects of dietary cholesterol intake on LDL and HDL cholesterol. Am. J. Clin. Nutr. 2019, 109, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Zhong, V.W.; Van Horn, L.; Cornelis, M.C.; Wilkins, J.T.; Ning, H.; Carnethon, M.R.; Greenland, P.; Mentz, R.J.; Tucker, K.L.; Zhao, L.; et al. Associations of Dietary Cholesterol or Egg Consumption with Incident Cardiovascular Disease and Mortality. JAMA 2019, 321, 1081–1095. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.; Raman, G.; Vishwanathan, R.; Jacques, P.F.; Johnson, E.J. Dietary cholesterol and cardiovascular disease: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2015, 102, 276–294. [Google Scholar] [CrossRef] [PubMed]
- Stellaard, F. From Dietary Cholesterol to Blood Cholesterol, Physiological Lipid Fluxes, and Cholesterol Homeostasis. Nutrients 2022, 14, 1643. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Kendall, C.W.; Faulkner, D.A.; Nguyen, T.; Kemp, T.; Marchie, A.; Wong, J.M.; de Souza, R.; Emam, A.; Vidgen, E.; et al. Assessment of the longer-term effects of a dietary portfolio of cholesterol-lowering foods in hypercholesterolemia. Am. J. Clin. Nutr. 2006, 83, 582–591. [Google Scholar] [CrossRef]
- Laffin, L.J.; Bruemmer, D.; Garcia, M.; Brennan, D.M.; McErlean, E.; Jacoby, D.S.; Michos, E.D.; Ridker, P.M.; Wang, T.Y.; Watson, K.E.; et al. Comparative Effects of Low-Dose Rosuvastatin, Placebo, and Dietary Supplements on Lipids and Inflammatory Biomarkers. J. Am. Coll. Cardiol. 2023, 81, 1–12. [Google Scholar] [CrossRef]
- Clifton, P.M.; Noakes, M.; Sullivan, D.; Erichsen, N.; Ross, D.; Annison, G.; Fassoulakis, A.; Cehun, M.; Nestel, P. Cholesterol-lowering effects of plant sterol esters differ in milk, yoghurt, bread and cereal. Eur. J. Clin. Nutr. 2004, 58, 503–509. [Google Scholar] [CrossRef]
- Blanco Mejia, S.; Messina, M.; Li, S.S.; Viguiliouk, E.; Chiavaroli, L.; Khan, T.A.; Srichaikul, K.; Mirrahimi, A.; Sievenpiper, J.L.; Kris-Etherton, P.; et al. A Meta-Analysis of 46 Studies Identified by the FDA Demonstrates that Soy Protein Decreases Circulating LDL and Total Cholesterol Concentrations in Adults. J. Nutr. 2019, 149, 968–981. [Google Scholar] [CrossRef]
- Del Gobbo, L.C.; Falk, M.C.; Feldman, R.; Lewis, K.; Mozaffarian, D. Effects of tree nuts on blood lipids, apolipoproteins, and blood pressure: Systematic review, meta-analysis, and dose-response of 61 controlled intervention trials. Am. J. Clin. Nutr. 2015, 102, 1347–1356. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wolever, T.M.; Rao, A.V.; Hegele, R.A.; Mitchell, S.J.; Ransom, T.P.; Boctor, D.L.; Spadafora, P.J.; Jenkins, A.L.; Mehling, C.; et al. Effect on blood lipids of very high intakes of fiber in diets low in saturated fat and cholesterol. N. Engl. J. Med. 1993, 329, 21–26. [Google Scholar] [CrossRef]
- Swain, J.F.; Rouse, I.L.; Curley, C.B.; Sacks, F.M. Comparison of the effects of oat bran and low-fiber wheat on serum lipoprotein levels and blood pressure. N. Engl. J. Med. 1990, 322, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Nestel, P.J.; Beilin, L.J.; Clifton, P.M.; Watts, G.F.; Mori, T.A. Practical Guidance for Food Consumption to Prevent Cardiovascular Disease. Heart Lung Circ. 2021, 30, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Nestel, P.J.; Mori, T.A. Dietary patterns, dietary nutrients and cardiovascular disease. Rev. Cardiovasc. Med. 2022, 23, 17. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, E.J.; Lamon-Fava, S.; Ausman, L.M.; Ordovas, J.M.; Clevidence, B.A.; Judd, J.T.; Goldin, B.R.; Woods, M.; Gorbach, S.; Lichtenstein, A.H. Individual variability in lipoprotein cholesterol response to National Cholesterol Education Program Step 2 diets. Am. J. Clin. Nutr. 1997, 65, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Fujita, M.; Ikewaki, K. HDL Functions-Current Status and Future Perspectives. Biomolecules 2023, 13, 105. [Google Scholar] [CrossRef]
- Abbey, M.; Nestel, P.J. Plasma cholesteryl ester transfer protein activity is increased when trans-elaidic acid is substituted for cis-oleic acid in the diet. Atherosclerosis 1994, 106, 99–107. [Google Scholar] [CrossRef]
- Holmes, M.V.; Asselbergs, F.W.; Palmer, T.M.; Drenos, F.; Lanktree, M.B.; Nelson, C.P.; Dale, C.E.; Padmanabhan, S.; Finan, C.; Swerdlow, D.I.; et al. Mendelian randomization of blood lipids for coronary heart disease. Eur. Heart J. 2015, 36, 539–550. [Google Scholar] [CrossRef]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef]
- Miller, M.; Stone, N.J.; Ballantyne, C.; Bittner, V.; Criqui, M.H.; Ginsberg, H.N.; Goldberg, A.C.; Howard, W.J.; Jacobson, M.S.; Kris-Etherton, P.M.; et al. Triglycerides and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2011, 123, 2292–2333. [Google Scholar] [CrossRef]
- Virani, S.S.; Morris, P.B.; Agarwala, A.; Ballantyne, C.M.; Birtcher, K.K.; Kris-Etherton, P.M.; Ladden-Stirling, A.B.; Miller, M.; Orringer, C.E.; Stone, N.J. 2021 ACC Expert Consensus Decision Pathway on the Management of ASCVD Risk Reduction in Patients With Persistent Hypertriglyceridemia: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2021, 78, 960–993. [Google Scholar] [CrossRef]
- Foster, G.D.; Wyatt, H.R.; Hill, J.O.; Makris, A.P.; Rosenbaum, D.L.; Brill, C.; Stein, R.I.; Mohammed, B.S.; Miller, B.; Rader, D.J.; et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: A randomized trial. Ann. Intern. Med. 2010, 153, 147–157. [Google Scholar] [CrossRef]
- Clifton, P.M.; Noakes, M.; Keogh, J.B. Very low-fat (12%) and high monounsaturated fat (35%) diets do not differentially affect abdominal fat loss in overweight, nondiabetic women. J. Nutr. 2004, 134, 1741–1745. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, A.; Petersen, K.S.; Jafari, F.; Kris-Etherton, P.M. Dietary management of dyslipidemia and the impact of dietary patterns on lipid disorders. Prog. Cardiovasc. Dis. 2022, 75, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Theroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef]
- Seidah, N.G.; Awan, Z.; Chretien, M.; Mbikay, M. PCSK9: A key modulator of cardiovascular health. Circ. Res. 2014, 114, 1022–1036. [Google Scholar] [CrossRef]
- Landmesser, U.; Chapman, M.J.; Farnier, M.; Gencer, B.; Gielen, S.; Hovingh, G.K.; Luscher, T.F.; Sinning, D.; Tokgozoglu, L.; Wiklund, O.; et al. European Society of Cardiology/European Atherosclerosis Society Task Force consensus statement on proprotein convertase subtilisin/kexin type 9 inhibitors: Practical guidance for use in patients at very high cardiovascular risk. Eur. Heart J. 2017, 38, 2245–2255. [Google Scholar] [CrossRef]
- Myasoedova, V.A.; Rimbert, A.; Camera, M.; Le May, C.; Capoulade, R.; Cariou, B.; Poggio, P. LDL lowering effect of PCSK9 inhibition is reduced in women. Eur. Heart J. Cardiovasc. Pharmacother. 2023; in press. [Google Scholar] [CrossRef]
- Sosnowska, B.; Adach, W.; Surma, S.; Rosenson, R.S.; Banach, M. Evinacumab, an ANGPTL3 Inhibitor, in the Treatment of Dyslipidemia. J. Clin. Med. 2023, 12, 168. [Google Scholar] [CrossRef]
- Wright, R.S.; Ray, K.K.; Raal, F.J.; Kallend, D.G.; Jaros, M.; Koenig, W.; Leiter, L.A.; Landmesser, U.; Schwartz, G.G.; Friedman, A.; et al. Pooled Patient-Level Analysis of Inclisiran Trials in Patients With Familial Hypercholesterolemia or Atherosclerosis. J. Am. Coll. Cardiol. 2021, 77, 1182–1193. [Google Scholar] [CrossRef]
- Li, Z.F.; Wu, N.Q. The Progression of Treatment for Refractory Hypercholesterolemia: Focus on the Prospect of Gene Therapy. Front. Genet. 2022, 13, 911429. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.J.; Lee, R.G.; Brandt, T.A.; Tai, L.J.; Fu, W.; Peralta, R.; Yu, R.; Hurh, E.; Paz, E.; McEvoy, B.W.; et al. Cardiovascular and Metabolic Effects of ANGPTL3 Antisense Oligonucleotides. N. Engl. J. Med. 2017, 377, 222–232. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nestel, P.J.; Mori, T.A. A Review of Low-Density Lipoprotein-Lowering Diets in the Age of Anti-Sense Technology. Nutrients 2023, 15, 1249. https://doi.org/10.3390/nu15051249
Nestel PJ, Mori TA. A Review of Low-Density Lipoprotein-Lowering Diets in the Age of Anti-Sense Technology. Nutrients. 2023; 15(5):1249. https://doi.org/10.3390/nu15051249
Chicago/Turabian StyleNestel, Paul J., and Trevor A. Mori. 2023. "A Review of Low-Density Lipoprotein-Lowering Diets in the Age of Anti-Sense Technology" Nutrients 15, no. 5: 1249. https://doi.org/10.3390/nu15051249
APA StyleNestel, P. J., & Mori, T. A. (2023). A Review of Low-Density Lipoprotein-Lowering Diets in the Age of Anti-Sense Technology. Nutrients, 15(5), 1249. https://doi.org/10.3390/nu15051249






