Pre-, pro-, syn-, and Postbiotics in Infant Formulas: What Are the Immune Benefits for Infants?
Abstract
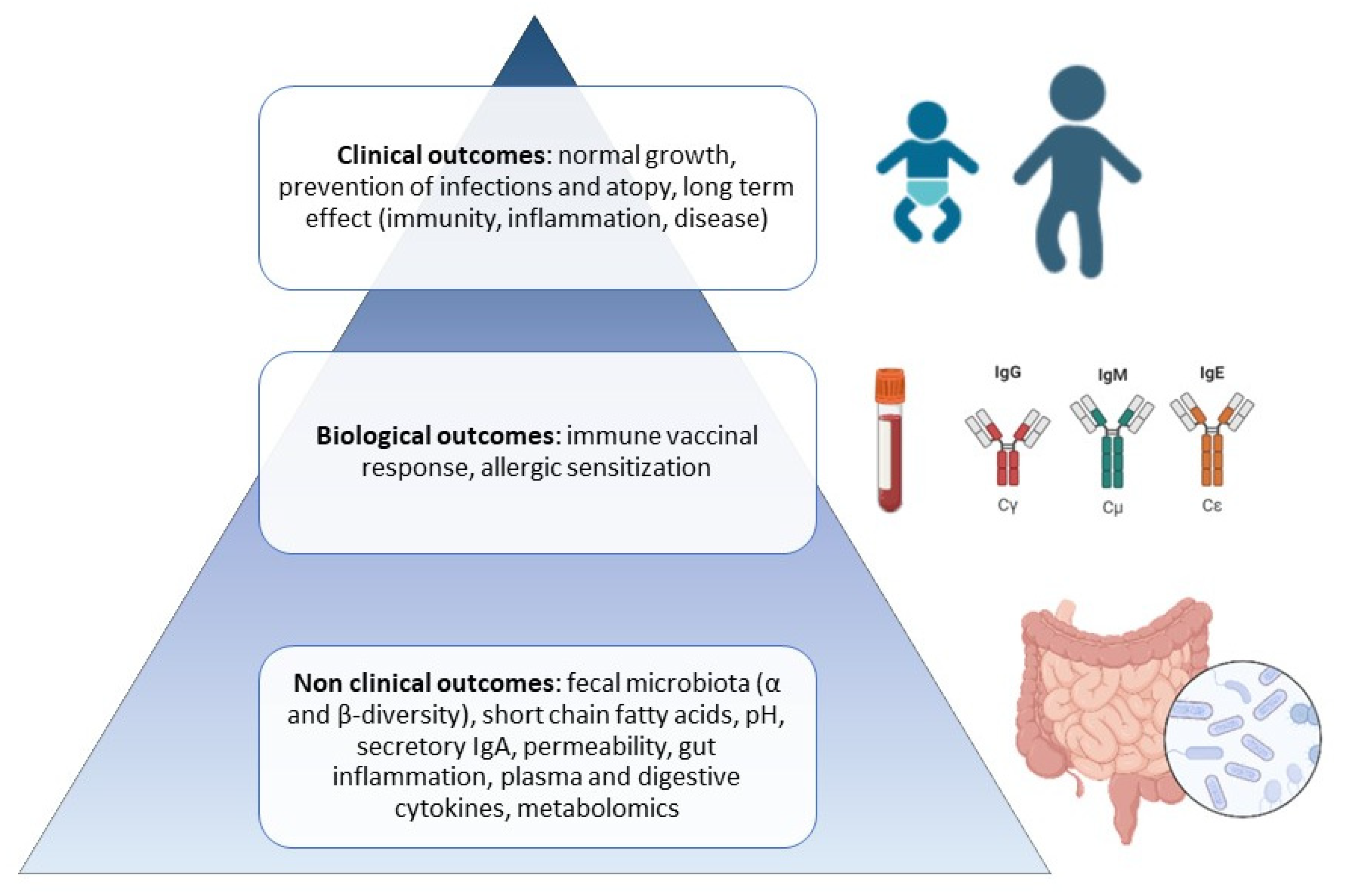
:1. Introduction
2. Methods
3. Results
3.1. Probiotics
3.1.1. Definition
3.1.2. Bifidobacterium animalis sp. lactis Bb-12 and B. lactis CNCM I-3446
3.1.3. Lactobacillus casei CRL431 and B. lactis Bb-12
3.1.4. Lactobacillus paracasei sp. paracasei, strain F19 (F19)
3.1.5. Lactobacillus reuteri DSM 17938
3.1.6. Lactobacillus rhamnosus GG (LGG)
3.1.7. Lactobacillus fermentum CECT5716
3.1.8. Bifidobacterium breve CECT7263
3.1.9. Bifidobacterium longum sp. infantis CECT7210 (B. infantis IM1)
3.1.10. Bifidobacterium animalis sp. lactis HN019
3.1.11. Lacticaseibacillus rhamnosus HN001
3.1.12. Other Bifidobacteria
3.2. Prebiotics
3.2.1. Definition
3.2.2. HMOs
3.2.3. GOSs
3.2.4. FOSs
3.2.5. GOSs/FOSs at a Ratio of 9:1
3.2.6. GOSs and/or FOSs
3.3. Synbiotics
3.3.1. Definition
3.3.2. Bifidobacterium animalis sp. lactis Bb-12 and Bovine-Milk-Derived Oligosaccharides (BMOs)
3.3.3. B. lactis animalis sp. lactis Bb-12 and GOSs/FOSs
3.3.4. Bifidobacterium breve (Bb) M-16V and GOSs/FOSs
3.3.5. Lactobacillus fermentum CECT5716 and GOSs
3.3.6. Lactobacillus reuteri DSM 17,938 and 2′FL
3.3.7. Bifidobacterium longum ATCC BAA-999 (Bl999) and Lactobacillus rhamnosus CGMCC 1.3724 (LPR) ± BMOs
3.3.8. Lactobacillus paracasei sp. paracasei strain F19 and GOSs/FOSs
3.3.9. Lactobacillus rhamnosus LCS- 742, Bifidobacterium longum sp. infantis M63, and GOSs/FOSs
3.3.10. B. infantis IM1, L. rhamnosus LCS-742, FOSs, and Inulin
3.4. Postbiotics
3.4.1. Definition
3.4.2. Postbiotics Produced by Lactobacillus paracasei (CBA L74)
3.4.3. Postbiotics Produced by Bifidobacterium breve C50 and Streptococcus thermophilus ST065
3.4.4. Postbiotics produced by Bifidobacterium animalis sp. lactis CECT 8145 BPL1TM
4. Discussion and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macpherson, A.J.; Harris, N.L. Interactions between Commensal Intestinal Bacteria and the Immune System. Nat. Rev. Immunol. 2004, 4, 477–485. [Google Scholar]
- Corthésy, B. Multi-Faceted Functions of Secretory IgA at Mucosal Surfaces. Front. Immunol. 2013, 4, 185. [Google Scholar]
- Arrieta, M.C.; Stiemsma, L.T.; Amenyogbe, N.; Brown, E.; Finlay, B. The Intestinal Microbiome in Early Life: Health and Disease. Front. Immunol. 2014, 5, 427. [Google Scholar]
- Korpela, K. Impact of Delivery Mode on Infant Gut Microbiota. Ann. Nutr. Metab 2021, 77, 11–19. [Google Scholar]
- Ríos-Covian, D.; Langella, P.; Martín, R. From Short-to Long-Term Effects of c-Section Delivery on Microbiome Establishment and Host Health. Microorganisms 2021, 9, 2122. [Google Scholar]
- Stewart, C.J.; Ajami, N.J.; O’brien, J.L.; Hutchinson, D.S.; Smith, D.P.; Wong, M.C.; Ross, M.C.; Lloyd, R.E.; Doddapaneni, H.; Metcalf, G.A.; et al. Temporal Development of the Gut Microbiome in Early Childhood from the TEDDY Study. Nature 2018, 563, 583–588. [Google Scholar]
- Bakker-Zierikzee, A.M.; Van Tol, E.A.F.; Kroes, H.; Alles, M.S.; Kok, F.J.; Bindels, J.G. Faecal SIgA Secretion in Infants Fed on Pre- or Probiotic Infant Formula. Pediatr. Allergy Immunol. 2006, 17, 134–140. [Google Scholar]
- Xiao, L.; Gong, C.; Ding, Y.; Ding, G.; Xu, X.; Deng, C.; Ze, X.; Malard, P.; Ben, X. Probiotics Maintain Intestinal Secretory Immunoglobulin A Levels in Healthy Formula-Fed Infants: A Randomised, Double-Blind, Placebo-Controlled Study. Benef. Microbes 2019, 10, 729–739. [Google Scholar]
- Berdi, M.; de Lauzon-Guillain, B.; Forhan, A.; Castelli, F.A.; Fenaille, F.; Charles, M.A.; Heude, B.; Junot, C.; Adel-Patient, K. Immune Components of Early Breastmilk: Association with Maternal Factors and with Reported Food Allergy in Childhood. Pediatr. Allergy Immunol. 2019, 30, 107–116. [Google Scholar]
- Adjibade, M.; Davisse-Paturet, C.; Divaret-Chauveau, A.; Adel-Patient, K.; Raherison, C.; Dufourg, M.-N.; Lioret, S.; Charles, M.-A.; De Lauzon-Guillain, B. Enrichment of Formula in Probiotics or Prebiotics and Risk of Infection and Allergic Diseases up to Age 5.5 Years in the Nationwide ELFE Cohort. J. Nutr. 2022, 152, 1138–1148. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar]
- Martín, R.; Langella, P. Emerging Health Concepts in the Probiotics Field: Streamlining the Definitions. Front. Microbiol. 2019, 10, 1047. [Google Scholar]
- Skórka, A.; Pieścik-Lech, M.; Kołodziej, M.; Szajewska, H. To Add or Not to Add Probiotics to Infant Formulae? An Updated Systematic Review. Benef. Microbes 2017, 8, 717–725. [Google Scholar]
- Holscher, H.D.; Czerkies, L.A.; Cekola, P.; Litov, R.; Benbow, M.; Santema, S.; Alexander, D.D.; Perez, V.; Sun, S.; Saavedra, J.M.; et al. Bifidobacterium Lactis Bb12 Enhances Intestinal Antibody Response in Formula-Fed Infants: A Randomized, Double-Blind, Controlled Trial. J. Parenter Enter. Nutr. 2012, 36, 106S–117S. [Google Scholar]
- Chouraqui, J.P.; Van Egroo, L.D.; Fichot, M.C. Acidified Milk Formula Supplemented With Bifidobacterium Lactis: Impact on Infant Diarrhea in Residential Care Settings. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 288–292. [Google Scholar]
- Fukushima, Y.; Kawata, Y.; Hara, H.; Terada, A.; Mitsuoka, T. Effect of a Probiotic Formula on Intestinal Immunoglobulin A Production in Healthy Children. Int. J. Food Microbiol. 1998, 42, 39–44. [Google Scholar]
- Baglatzi, L.; Gavrili, S.; Stamouli, K.; Zachaki, S.; Favre, L.; Pecquet, S.; Benyacoub, J.; Costalos, C. Effect of Infant Formula Containing a Low Dose of the Probiotic Bifidobacterium Lactis CNCM I-3446 on Immune and Gut Functions in C-Section Delivered Babies: A Pilot Study. Clin. Med. Insights Pediatr. 2016, 10, 11–19. [Google Scholar]
- Dupont, C.; Hol, J.; Nieuwenhuis, E.E.S. An Extensively Hydrolysed Casein-Based Formula for Infants with Cows’ Milk Protein Allergy: Tolerance/Hypo-Allergenicity and Growth Catch-Up. Br. J. Nutr. 2015, 113, 1102–1112. [Google Scholar]
- Li, X.; Peng, Y.; Li, Z.; Christensen, B.; Heckmann, A.B.; Lagerqvist, C.; Stenlund, H.; Lonnerdal, B.; Hernell, O.; West, C.E. Serum Cytokine Patterns Are Modulated in Infants Fed Formula with Probiotics or Milk Fat Globule Membranes: A Randomized Controlled Trial. PLoS ONE 2021, 16, e0251293. [Google Scholar]
- Zeevenhooven, J.; Browne, P.D.; LHoir, M.P.; Weerth, C.; Benninga, M.A. Infant Colic: Mechanisms and Management. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 479–496. [Google Scholar]
- Garcia Rodenas, C.L.; Lepage, M.; Ngom-Bru, C.; Fotiou, A.; Papagaroufalis, K.; Berger, B. Effect of Formula Containing Lactobacillus Reuteri DSM 17938 on Fecal Microbiota of Infants Born by Cesarean-Section. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 681–687. [Google Scholar]
- Sánchez-Valverde, F.; Etayo, V.; Gil, F.; Aznal, E.; Martínez, D.; Amézqueta, A.; Mendizábal, M.; Galbete, A.; Pastor, N.; Vanderhoof, J. Factors Associated with the Development of Immune Tolerance in Children with Cow’s Milk Allergy. Int. Arch. Allergy Immunol. 2019, 179, 290–296. [Google Scholar]
- Berni Canani, R.; Nocerino, R.; Terrin, G.; Frediani, T.; Lucarelli, S.; Cosenza, L.; Passariello, A.; Leone, L.; Granata, V.; Di Costanzo, M.; et al. Formula Selection for Management of Children with Cow’s Milk Allergy Influences the Rate of Acquisition of Tolerance: A Prospective Multicenter Study. J. Pediatr. 2013, 163, 771–777.e1. [Google Scholar]
- Berni Canani, R.; Di Costanzo, M.; Bedogni, G.; Amoroso, A.; Cosenza, L.; Di Scala, C.; Granata, V.; Nocerino, R. Extensively Hydrolyzed Casein Formula Containing Lactobacillus Rhamnosus GG Reduces the Occurrence of Other Allergic Manifestations in Children with Cow’s Milk Allergy: 3-Year Randomized Controlled Trial. J. Allergy Clin Immunol. 2017, 139, 1906–1913. [Google Scholar]
- Nocerino, R.; Bedogni, G.; Carucci, L.; Cosenza, L.; Cozzolino, T.; Paparo, L.; Palazzo, S.; Riva, L.; Verduci, E.; Berni Canani, R. The Impact of Formula Choice for the Management of Pediatric Cow’s Milk Allergy on the Occurrence of Other Allergic Manifestations: The Atopic March Cohort Study. J. Pediatr. 2021, 232, 183–191.e3. [Google Scholar]
- Berni Canani, R.; Nocerino, R.; Terrin, G.; Coruzzo, A.; Cosenza, L.; Leone, L.; Troncone, R. Effect of Lactobacillus GG on Tolerance Acquisition in Infants with Cow’s Milk Allergy: A Randomized Trial. J. Allergy Clin Immunol. 2012, 129, 580–582. [Google Scholar]
- Berni Canani, R.; De Filippis, F.; Nocerino, R.; Paparo, L.; Scala, C.D.; Cosenza, L.; Della Gatta, G.; Calignano, A.; De Caro, C.; Laiola, M.; et al. Gut Microbiota Composition and Butyrate Production in Children Affected by Non-IgE-Mediated Cow’s Milk Allergy. Sci. Rep. 2018, 8, 12500. [Google Scholar]
- Berni Canani, R.; Sangwan, N.; Stefka, A.T.; Nocerino, R.; Paparo, L.; Aitoro, R.; Calignano, A.; Khan, A.A.; Gilbert, J.A.; Nagler, C.R. Lactobacillus Rhamnosus GG-Supplemented Formula Expands Butyrate-Producing Bacterial Strains in Food Allergic Infants. ISME J. 2015, 10, 742–750. [Google Scholar]
- Paparo, L.; Nocerino, R.; Cosenza, L.; Aitoro, R.; D’Argenio, V.; Del Monaco, V.; Di Scala, C.; Amoroso, A.; Di Costanzo, M.; Salvatore, F.; et al. Epigenetic Features of FoxP3 in Children with Cow’s Milk Allergy. Clin. Epigenetics 2016, 8, 4–9. [Google Scholar]
- Paparo, L.; Nocerino, R.; Bruno, C.; Di Scala, C.; Cosenza, L.; Bedogni, G.; Di Costanzo, M.; Mennini, M.; D’Argenio, V.; Salvatore, F.; et al. Randomized Controlled Trial on the Influence of Dietary Intervention on Epigenetic Mechanisms in Children with Cow’s Milk Allergy: The EPICMA Study. Sci. Rep. 2019, 9, 2828. [Google Scholar]
- Scalabrin, D.; Harris, C.; Johnston, W.; Berseth, C. Long-Term Safety Assessment in Children Who Received Hydrolyzed Protein Formulas with Lactobacillus Rhamnosus GG: A 5-Year Follow-Up. Eur. J. Pediatr. 2017, 176, 217–224. [Google Scholar]
- Shulman, R.J.; Chichlowski, M.; Orozco, F.G.; Harris, C.L.; Wampler, J.L.; Bokulich, N.A.; Berseth, C.L. Infant Behavioral State and Stool Microbiome in Infants Receiving Lactocaseibacillus Rhamnosus GG in Formula: Randomized Controlled Trial. BMC Pediatr. 2022, 22, 580. [Google Scholar]
- Martín, R.; Olivares, M.; Marín, M.L.; Fernández, L.; Xaus, J.; Rodríguez, J.M. Probiotic Potential of 3 Lactobacilli Strains Isolated from Breast Milk. J. Hum. Lact. 2005, 21, 8–17. [Google Scholar]
- Maldonado, J.; Gil-Campos, M.; Maldonado-Lobón, J.A.; Benavides, M.R.; Flores-Rojas, K.; Jaldo, R.; Jiménez Del Barco, I.; Bolívar, V.; Valero, A.D.; Prados, E.; et al. Evaluation of the Safety, Tolerance and Efficacy of 1-Year Consumption of Infant Formula Supplemented with Lactobacillus Fermentum CECT5716 Lc40 or Bifidobacterium Breve CECT7263: A Randomized Controlled Trial. BMC Pediatr. 2019, 19, 361. [Google Scholar]
- Escribano, J.; Ferré, N.; Gispert-Llaurado, M.; Luque, V.; Rubio-Torrents, C.; Zaragoza-Jordana, M.; Polanco, I.; Codoñer, F.M.; Chenoll, E.; Morera, M.; et al. Bifidobacterium Longum Subsp Infantis CECT7210-Supplemented Formula Reduces Diarrhea in Healthy Infants: A Randomized Controlled Trial. Pediatr. Res. 2018, 83, 1120–1128. [Google Scholar]
- Dekker, J.; Quilter, M.; Qian, H. Comparison of Two Probiotics in Follow-on Formula: Bifidobacterium Animalis Subsp. Lactis HN019 Reduced Upper Respiratory Tract Infections in Chinese Infants. Benef. Microbes 2022, 13, 341–353. [Google Scholar]
- Bazanella, M.; Maier, T.V.; Clavel, T.; Lagkouvardos, I.; Lucio, M.; Maldonado-Gòmez, M.X.; Autran, C.; Walter, J.; Bode, L.; Schmitt-Kopplin, P.; et al. Randomized Controlled Trial on the Impact of Early-Life Intervention with Bifidobacteria on the Healthy Infant Fecal Microbiota and Metabolome. Am. J. Clin. Nutr. 2017, 106, 1274–1286. [Google Scholar]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar]
- Bedu-Ferrari, C.; Biscarrat, P.; Langella, P.; Cherbuy, C. Prebiotics and the Human Gut Microbiota: From Breakdown Mechanisms to the Impact on Metabolic Health. Nutrients 2022, 14, 2096. [Google Scholar]
- Sencio, V.; Barthelemy, A.; Tavares, L.P.; Ferreira, P.; Teixeira, M.M.; Ois, F.; Correspondence, T.; Machado, M.G.; Soulard, D.; Cuinat, C.; et al. Gut Dysbiosis during Influenza Contributes to Pulmonary Pneumococcal Superinfection through Altered Short-Chain Fatty Acid Production. Cell Rep. 2020, 30, 2934–2947.e6. [Google Scholar]
- Sencio, V.; Gallerand, A.; Machado, G.; Deruyter, L. Influenza Virus Infection Impairs the Gut’ s Barrier Properties and Favors Secondary Enteric Bacterial Infection through Reduced Production of Short-Chain Fatty Acids. Infect Immun. 2021, 89, e00734-20. [Google Scholar]
- Venter, C.; Meyer, R.W.; Greenhawt, M.; Pali-Schöll, I.; Nwaru, B.; Roduit, C.; Untersmayr, E.; Adel-Patient, K.; Agache, I.; Agostoni, C.; et al. Role of Dietary Fiber in Promoting Immune Health—An EAACI Position Paper. Allergy 2022, 77, 3185–3198. [Google Scholar]
- Vandenplas, Y.; De Greef, E.; Veereman, G. Prebiotics in Infant Formula. Gut Microbes 2014, 5, 681–687. [Google Scholar]
- Bode, L. Human Milk Oligosaccharides: Structure and Functions. In Milk, Mucosal Immunity and the Microbiome: Impact on the Neonate; Ogra, P.L., Walker, W.A., Lönnerdal, B., Eds.; Nestlé Nutrition Institute Workshop Series; Karger: Basel, Switzerland, 2020; Volume 94, pp. 115–123. [Google Scholar] [CrossRef]
- Sekerel, B.E.; Bingol, G.; Cullu Cokugras, F.; Cokugras, H.; Kansu, A.; Ozen, H.; Tamay, Z. An Expert Panel Statement on the Beneficial Effects of Human Milk Oligosaccharides (HMOs) in Early Life and Potential Utility of HMO-Supplemented Infant Formula in Cow’s Milk Protein Allergy. J. Asthma Allergy 2021, 14, 1147–1164. [Google Scholar]
- Alliet, P.; Vandenplas, Y.; Roggero, P.; Jespers, S.N.J.; Peeters, S.; Stalens, J.P.; Kortman, G.A.M.; Amico, M.; Berger, B.; Sprenger, N.; et al. Safety and Efficacy of a Probiotic-Containing Infant Formula Supplemented with 2’-Fucosyllactose: A Double-Blind Randomized Controlled Trial. Nutr. J. 2022, 21, 11. [Google Scholar]
- Kong, C.; Faas, M.M.; De Vos, P.; Akkerman, R. Impact of Dietary Fibers in Infant Formulas on Gut Microbiota and the Intestinal Immune Barrier. Food Funct. 2020, 11, 9445–9467. [Google Scholar]
- Goehring, K.C.; Marriage, B.J.; Oliver, J.S.; Wilder, J.A.; Barrett, E.G.; Buck, R.H. Similar to Those Who Are Breastfed, Infants Fed a Formula Containing 2’-Fucosyllactose Have Lower Inflammatory Cytokines in a Randomized Controlled Trial. J. Nutr. 2016, 146, 2559–2566. [Google Scholar]
- Puccio, G.; Alliet, P.; Cajozzo, C.; Janssens, E.; Corsello, G.; Sprenger, N.; Wernimont, S.; Egli, D.; Gosoniu, L.; Steenhout, P. Effects of Infant Formula with Human Milk Oligosaccharides on Growth and Morbidity: A Randomized Multicenter Trial. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 624–631. [Google Scholar]
- Berger, B.; Porta, N.; Foata, F.; Grathwohl, D.; Delley, M.; Moine, D.; Charpagne, A.; Siegwald, L.; Descombes, P.; Alliet, P.; et al. Linking Human Milk Oligosaccharides, Infant Fecal Community Types, and Later Risk to Require Antibiotics. MBio 2020, 11, e03196-19. [Google Scholar]
- Parschat, K.; Melsaether, C.; Jäpelt, K.R.; Jennewein, S. Clinical Evaluation of 16-Week Supplementation with Tolerability, Safety, and Effect on Growth. Nutrients 2021, 13, 2871. [Google Scholar]
- Lasekan, J.; Choe, Y.; Dvoretskiy, S.; Devitt, A.; Zhang, S.; Mackey, A.; Wulf, K.; Buck, R.; Steele, C.; Johnson, M.; et al. Growth and Gastrointestinal Tolerance in Healthy Term Infants Fed Milk-Based Infant Formula Supplemented with Five Human Milk Oligosaccharides (HMOs): A Randomized Multicenter Trial. Nutrients 2022, 14, 2625. [Google Scholar]
- Leung, T.F.; Ulfman, L.H.; Chong, M.K.C.; Hon, K.L.; Khouw, I.M.S.L.; Chan, P.K.S.; Delsing, D.J.; Kortman, G.A.M.; Bovee-Oudenhoven, I.M.J. A Randomized Controlled Trial of Different Young Child Formulas on Upper Respiratory and Gastrointestinal Tract Infections in Chinese Toddlers. Pediatr. Allergy Immunol. 2020, 31, 745–754. [Google Scholar]
- Vandenplas, Y.; Zołnowska, M.; Berni Canani, R.; Ludam, S.; Tengelyi, Z.; Moreno-Alvarez, A.; Goh, A.E.N.; Gosoniu, M.L.; Kirwan, B.; Tadi, M.; et al. Effects of an Extensively Hydrolyzed Formula Supplemented with Two Human Milk Oligosaccharides on Growth, Tolerability, Safety and Infection Risk in Infants with Cow’s Milk Protein Allergy: A Randomized, Multi-Center Trial. Nutrients 2022, 14, 530. [Google Scholar]
- Sierra, C.; Bernal, M.J.; Blasco, J.; Martínez, R.; Dalmau, J.; Ortuño, I.; Espín, B.; Vasallo, M.I.; Gil, D.; Vidal, M.L.; et al. Prebiotic Effect during the First Year of Life in Healthy Infants Fed Formula Containing GOS as the Only Prebiotic: A Multicentre, Randomised, Double-Blind and Placebo-Controlled Trial. Eur. J. Nutr. 2015, 54, 89–99. [Google Scholar]
- Giovannini, M.; Verduci, E.; Gregori, D.; Ballali, S.; Soldi, S.; Ghisleni, D.; Riva, E. Prebiotic Effect of an Infant Formula Supplemented with Galacto-Oligosaccharides: Randomized Multicenter Trial. J. Am. Coll. Nutr. 2014, 33, 385–393. [Google Scholar]
- Boženský, J.; Hill, M.; Zelenka, R.; Skýba, T. Prebiotics Do Not Influence the Severity of Atopic Dermatitis in Infants: A Randomised Controlled Trial. PLoS ONE 2015, 10, e0142897. [Google Scholar]
- Scalabrin, D.M.F.; Mitmesser, S.H.; Welling, G.W.; Harris, C.L.; Marunycz, J.D.; Walker, D.C.; Bos, N.A.; Tölkkö, S.; Salminen, S.; Vanderhoof, J.A. New Prebiotic Blend of Polydextrose and Galacto-Oligosaccharides Has a Bifidogenic Effect in Young Infants. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 343–352. [Google Scholar]
- Salminen, S.; Endo, A.; Isolauri, E.; Scalabrin, D. Early Gut Colonization with Lactobacilli and Staphylococcus in Infants: The Hygiene Hypothesis Extended. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 80–86. [Google Scholar]
- Ranucci, G.; Buccigrossi, V.; Borgia, E.; Piacentini, D.; Visentin, F.; Cantarutti, L.; Baiardi, P.; Felisi, M.; Spagnuolo, M.I.; Zanconato, S.; et al. Galacto-Oligosaccharide/Polidextrose Enriched Formula Protects against Respiratory Infections in Infants at High Risk of Atopy: A Randomized Clinical Trial. Nutrients 2018, 10, 286. [Google Scholar]
- Wernimont, S.; Northington, R.; Kullen, M.J.; Yao, M.; Bettler, J. Effect of an α-Lactalbumin-Enriched Infant Formula Supplemented with Oligofructose on Fecal Microbiota, Stool Characteristics, and Hydration Status: A Randomized, Double-Blind, Controlled Trial. Clin Pediatr. 2015, 54, 359–370. [Google Scholar]
- Closa-Monasterolo, R.; Gispert-Llaurado, M.; Luque, V.; Ferre, N.; Rubio-Torrents, C.; Zaragoza-Jordana, M.; Escribano, J. Safety and Efficacy of Inulin and Oligofructose Supplementation in Infant Formula: Results from a Randomized Clinical Trial. Clin. Nutr. 2013, 32, 918–927. [Google Scholar]
- Neumer, F.; Urraca, O.; Alonso, J.; Palencia, J.; Varea, V.; Theis, S.; Rodriguez-Palmero, M.; Moreno-Muñoz, J.A.; Guarner, F.; Veereman, G.; et al. Long-term Safety and Efficacy of Prebiotic Enriched Infant Formula—A Randomized Controlled Trial. Nutrients 2021, 13, 1276. [Google Scholar]
- Xia, Q.; Williams, T.; Hustead, D.; Price, P.; Morrison, M.; Yu, Z. Quantitative Analysis of Intestinal Bacterial Populations from Term Infants Fed Formula Supplemented with Fructo-Oligosaccharides. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 314–320. [Google Scholar]
- Bruzzese, E.; Volpicelli, M.; Squeglia, V.; Bruzzese, D.; Salvini, F.; Bisceglia, M.; Lionetti, P.; Cinquetti, M.; Iacono, G.; Amarri, S.; et al. A Formula Containing Galacto- and Fructo-Oligosaccharides Prevents Intestinal and Extra-Intestinal Infections: An Observational Study. Clin. Nutr. 2009, 28, 156–161. [Google Scholar]
- Shahramian, I.; Kalvandi, G.; Javaherizadeh, H.; Khalili, M.; Noori, N.M.; Delaramnasab, M.; Bazi, A. The Effects of Prebiotic Supplementation on Weight Gain, Diarrhoea, Constipation, Fever and Respiratory Tract Infections in the First Year of Life. J. Paediatr. Child Health 2018, 54, 875–880. [Google Scholar]
- Grüber, C.; Van Stuivenberg, M.; Mosca, F.; Moro, G.; Chirico, G.; Braegger, C.P.; Riedler, J.; Yavuz, Y.; Boehm, G.; Wahn, U. Immunoactive Prebiotics Transiently Prevent Occurrence of Early Atopic Dermatitis among Low-Atopy-Risk Infants. J. Allergy Clin. Immunol. 2015, 136, 1696–1698.e1. [Google Scholar]
- Holscher, H.D.; Faust, K.L.; Czerkies, L.A.; Litov, R.; Ziegler, E.E.; Lessin, H.; Hatch, T.; Sun, S.; Tappenden, K.A. Effects of Prebiotic-Containing Infant Formula on Gastrointestinal Tolerance and Fecal Microbiota in a Randomized Controlled Trial. J. Parenter. Enter. Nutr. 2012, 36, 95–105. [Google Scholar]
- Wopereis, H.; Sim, K.; Shaw, A.; Warner, J.O.; Knol, J.; Kroll, J.S. Intestinal Microbiota in Infants at High Risk for Allergy: Effects of Prebiotics and Role in Eczema Development. J. Allergy Clin. Immunol. 2018, 141, 1334–1342.e5. [Google Scholar]
- Boyle, R.J.; Tang, L.-K.; Chiang, W.C.; Chua, M.C.; Ismail, I.; Nauta, A.; Hourihane, B.; Smith, P.; Gold, M.; Ziegler, J.; et al. Prebiotic-Supplemented Partially Hydrolysed Cow’s Milk Formula for the Prevention of Eczema in High-Risk Infants: A Randomized Controlled Trial On Behalf of the PATCH Study Investigators. Allergy 2016, 71, 701–710. [Google Scholar]
- Moro, G.; Arslanoglu, S.; Stahl, B.; Jelinek, J.; Wahn, U.; Boehm, G. A Mixture of Prebiotic Oligosaccharides Reduces the Incidence of Atopic Dermatitis during the First Six Months of Age. Arch Dis. Child. 2006, 91, 814–819. [Google Scholar]
- Van Hoffen, E.; Ruiter, B.; Faber, J.; M’Rabet, L.; Knol, E.F.; Stahl, B.; Arslanoglu, S.; Moro, G.; Boehm, G.; Garssen, J. A Specific Mixture of Short-Chain Galacto-Oligosaccharides and Long-Chain Fructo-Oligosaccharides Induces a Beneficial Immunoglobulin Profile in Infants at High Risk for Allergy. Allergy 2009, 64, 484–487. [Google Scholar]
- Arslanoglu, S.; Moro, G.E.; Schmitt, J.; Tandoi, L.; Rizzardi, S.; Boehm, G. Early Dietary Intervention with a Mixture of Prebiotic Oligosaccharides Reduces the Incidence of Allergic Manifestations and Infections during the First Two Years of Life. J. Nutr. 2008, 138, 1091–1095. [Google Scholar]
- Arslanoglu, S. Early Neutral Prebiotic Oligosaccharide Supplementaion Reduces the Incidence of Some Allergic Manifestations in the First 5 Years of Life. J. Biol. Regul. Homeost. Agents 2012, 25, 49–59. [Google Scholar]
- Zhu, B.; Zheng, S.; Lin, K.; Xu, X.; Lv, L.; Zhao, Z.; Shao, J. Effects of Infant Formula Supplemented With Prebiotics and OPO on Infancy Fecal Microbiota: A Pilot Randomized Clinical Trial. Front. Cell. Infect. Microbiol. 2021, 11, 650407. [Google Scholar]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar]
- EFSA Panel on Dietetic Products Nutrition and Allergies Scientific Opinion on the Substantiation of Health Claims Related to Live Yoghurt Cultures and Improved Lactose Digestion (ID 1143, 2976) Pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 4254.
- Simeoni, U.; Berger, B.; Junick, J.; Blaut, M.; Pecquet, S.; Rezzonico, E.; Grathwohl, D.; Sprenger, N.; Brüssow, H.; Szajewska, H.; et al. Gut Microbiota Analysis Reveals a Marked Shift to Bifidobacteria by a Starter Infant Formula Containing a Synbiotic of Bovine Milk-Derived Oligosaccharides and Bifidobacterium Animalis Subsp. Lactis CNCM I-3446. Environ. Microbiol. 2016, 18, 2185–2195. [Google Scholar]
- Radke, M.; Picaud, J.C.; Loui, A.; Cambonie, G.; Faas, D.; Lafeber, H.N.; De Groot, N.; Pecquet, S.S.; Steenhout, P.G.; Hascoet, J.M. Starter Formula Enriched in Prebiotics and Probiotics Ensures Normal Growth of Infants and Promotes Gut Health: A Randomized Clinical Trial. Pediatr. Res. 2017, 81, 622–631. [Google Scholar]
- Castanet, M.; Costalos, C.; Haiden, N.; Hascoet, J.M.; Berger, B.; Sprenger, N.; Grathwohl, D.; Brüssow, H.; De Groot, N.; Steenhout, P.; et al. Early Effect of Supplemented Infant Formulae on Intestinal Biomarkers and Microbiota: A Randomized Clinical Trial. Nutrients 2020, 12, 1481. [Google Scholar]
- Bocquet, A.; Lachambre, E.; Kempf, C.; Beck, L. Effect of Infant and Follow-on Formulas Containing B. Lactis and Galacto-and Fructo-Oligosaccharides on Infection in Healthy Term Infants. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 180–187. [Google Scholar]
- Wong, C.B.; Iwabuchi, N.; Xiao, J.-Z. Exploring the Science behind Bifidobacterium Breve M-16V in Infant Health. Nutrients 2019, 11, 1724. [Google Scholar]
- Chua, M.C.; Ben-Amor, K.; Lay, C.; Neo, A.G.E.; Chiang, W.C.; Rao, R.; Chew, C.; Chaithongwongwatthana, S.; Khemapech, N.; Knol, J.; et al. Effect of Synbiotic on the Gut Microbiota of Cesarean Delivered Infants: A Randomized, Double-Blind, Multicenter Study. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 102–106. [Google Scholar]
- Lay, C.; Chu, C.W.; Purbojati, R.W.; Acerbi, E.; Drautz-Moses, D.I.; de Sessions, P.F.; Jie, S.; Ho, E.; Kok, Y.J.; Bi, X.; et al. A Synbiotic Intervention Modulates Meta-Omics Signatures of Gut Redox Potential and Acidity in Elective Caesarean Born Infants. BMC Microbiol. 2021, 21, 191. [Google Scholar]
- Kosuwon, P.; Lao-Araya, M.; Uthaisangsook, S.; Lay, C.; Bindels, J.; Knol, J.; Chatchatee, P. A Synbiotic Mixture of ScGOS/LcFOS and Bifidobacterium Breve M-16V Increases Faecal Bifidobacterium in Healthy Young Children. Benef. Microbes 2018, 9, 541–552. [Google Scholar]
- Van Der Aa, L.B.; Heymans, H.S.; Van Aalderen, W.M.; Sillevis Smitt, J.H.; Knol, J.; Ben Amor, K.; Goossens, D.A.; Sprikkelman, A.B. Effect of a New Synbiotic Mixture on Atopic Dermatitis in Infants: A Randomized-Controlled Trial. Clin. Exp. Allergy 2010, 40, 795–804. [Google Scholar]
- Van Der Aa, L.B.; Van Aalderen, W.M.C.; Heymans, H.S.A.; Henk Sillevis Smitt, J.; Nauta, A.J.; Knippels, L.M.J.; Ben Amor, K.; Sprikkelman, A.B. Synbiotics Prevent Asthma-like Symptoms in Infants with Atopic Dermatitis. Allergy 2011, 66, 170–177. [Google Scholar]
- De Kivit, S.; Saeland, E.; Kraneveld, A.D.; Van De Kant, H.J.G.; Schouten, B.; Van Esch, B.C.A.M.; Knol, J.; Sprikkelman, A.B.; Van Der Aa, L.B.; Knippels, L.M.J.; et al. Galectin-9 Induced by Dietary Synbiotics Is Involved in Suppression of Allergic Symptoms in Mice and Humans. Allergy 2012, 67, 343–352. [Google Scholar]
- van der Aa, L.B.; Lutter, R.; Heymans, H.S.A.; Smids, B.S.; Dekker, T.; van Aalderen, W.M.C.; Sillevis Smitt, J.H.; Knippels, L.M.J.; Garssen, J.; Nauta, A.J.; et al. No Detectable Beneficial Systemic Immunomodulatory Effects of a Specific Synbiotic Mixture in Infants with Atopic Dermatitis. Clin. Exp. Allergy 2012, 42, 531–539. [Google Scholar]
- Sorensen, K.; Cawood, A.L.; Gibson, G.R.; Cooke, L.H.; Stratton, R.J. Amino Acid Formula Containing Synbiotics in Infants with Cow’s Milk Protein Allergy: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 935. [Google Scholar]
- Fox, A.T.; Wopereis, H.; Van Ampting, M.T.J.; Oude Nijhuis, M.M.; Butt, A.M.; Peroni, D.G.; Vandenplas, Y.; Candy, D.C.A.; Shah, N.; West, C.E.; et al. A Specific Synbiotic-Containing Amino Acid-Based Formula in Dietary Management of Cow’s Milk Allergy: A Randomized Controlled Trial. Clin. Transl. Allergy 2019, 9, 5. [Google Scholar]
- Candy, D.C.A.; Van Ampting, M.T.J.; Oude Nijhuis, M.M.; Butt, A.M.; Peroni, D.G.; Vandenplas, Y.; Fox, A.T.; Shah, N.; West, C.E.; Garssen, J.; et al. A Synbiotic-Containing Amino-Acid-Based Formula Improves Gut Microbiota in Non-IgE-Mediated Allergic Infants. Pediatr Res 2018, 83, 677–686. [Google Scholar]
- Wopereis, H.; J van Ampting, M.T.; Cetinyurek-Yavuz, A.; Slump, R.; A Candy, D.C.; Butt, A.M.; Peroni, D.G.; Vandenplas, Y.; Fox, A.T.; Shah, N.; et al. A Specific Synbiotic-Containing Amino Acid-Based Formula Restores Gut Microbiota in Non-IgE Mediated Cow’s Milk Allergic Infants: A Randomized Controlled Trial. Clin Transl Allergy 2019, 9, 1–13. [Google Scholar]
- Chatchatee, P.; Nowak-Wegrzyn, A.; Lange, L.; Benjaponpitak, S.; Chong, K.W.; Sangsupawanich, P.; van Ampting, M.T.J.; Oude Nijhuis, M.M.; Harthoorn, L.F.; Langford, J.E.; et al. Tolerance Development in Cow’s Milk–Allergic Infants Receiving Amino Acid–Based Formula: A Randomized Controlled Trial. J. Allergy Clin. Immunol. 2022, 149, 650–658.e5. [Google Scholar]
- Gil-Campos, M.; López, M.Á.; Rodriguez-Benítez, M.V.; Romero, J.; Roncero, I.; Linares, M.D.; Maldonado, J.; López-Huertas, E.; Berwind, R.; Ritzenthaler, K.L.; et al. Lactobacillus Fermentum CECT 5716 Is Safe and Well Tolerated in Infants of 1-6 Months of Age: A Randomized Controlled Trial. Pharmacol. Res. 2012, 65, 231–238. [Google Scholar]
- Maldonado-Lobón, J.A.; Gil-Campos, M.; Maldonado, J.; López-Huertas, E.; Flores-Rojas, K.; Valero, A.D.; Rodríguez-Benítez, M.V.; Bañuelos, O.; Lara-Villoslada, F.; Fonollá, J.; et al. Long-Term Safety of Early Consumption of Lactobacillus Fermentum CECT5716: A 3-Year Follow-up of a Randomized Controlled Trial. Pharmacol. Res. 2015, 95–96, 12–19. [Google Scholar]
- Maldonado, J.; Cañabate, F.; Sempere, L.; Vela, F.; Sánchez, A.R.; Narbona, E.; López-Huertas, E.; Geerlings, A.; Valero, A.D.; Olivares, M.; et al. Human Milk Probiotic Lactobacillus Fermentum CECT5716 Reduces the Incidence of Gastrointestinal and Upper Respiratory Tract Infections in Infants. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 55–61. [Google Scholar]
- Meli, F.; Puccio, G.; Cajozzo, C.; Ricottone, G.L.; Pecquet, S.; Sprenger, N.; Steenhout, P. Growth and Safety Evaluation of Infant Formulae Containing Oligosaccharides Derived from Bovine Milk: A Randomized, Double-Blind, Noninferiority Trial. BMC Pediatr. 2014, 14, 306. [Google Scholar]
- Szajewska, H.; Ruszczyński, M.; Szymański, H.; Sadowska-Krawczenko, I.; Piwowarczyk, A.; Rasmussen, P.B.; Kristensen, M.B.; West, C.E.; Hernell, O. Effects of Infant Formula Supplemented with Prebiotics Compared with Synbiotics on Growth up to the Age of 12 Mo: A Randomized Controlled Trial. Pediatr. Res. 2017, 81, 752–758. [Google Scholar]
- Rozé, J.C.; Barbarot, S.; Butel, M.J.; Kapel, N.; Waligora-Dupriet, A.J.; De Montgolfier, I.; Leblanc, M.; Godon, N.; Soulaines, P.; Darmaun, D.; et al. An α-Lactalbumin-Enriched and Symbiotic-Supplemented v. a Standard Infant Formula: A Multicentre, Double-Blind, Randomised Trial. Br. J. Nutr. 2012, 107, 1616–1622. [Google Scholar]
- Cerdó, T.; Ruíz, A.; Acuña, I.; Nieto-Ruiz, A.; Diéguez, E.; Sepúlveda-Valbuena, N.; Escudero-Marín, M.; García-Santos, J.A.; García-Ricobaraza, M.; Herrmann, F.; et al. A Synbiotics, Long Chain Polyunsaturated Fatty Acids, and Milk Fat Globule Membranes Supplemented Formula Modulates Microbiota Maturation and Neurodevelopment. Clin. Nutr. 2022, 41, 1697–1711. [Google Scholar]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar]
- Roggero, P.; Liotto, N.; Pozzi, C.; Braga, D.; Troisi, J.; Menis, C.; Giannì, M.L.; Berni Canani, R.; Paparo, L.; Nocerino, R.; et al. Analysis of Immune, Microbiota and Metabolome Maturation in Infants in a Clinical Trial of Lactobacillus Paracasei CBA L74-Fermented Formula. Nat. Commun. 2020, 11, 2703. [Google Scholar]
- Zagato, E.; Mileti, E.; Massimiliano, L.; Fasano, F.; Budelli, A.; Penna, G.; Rescigno, M. Lactobacillus Paracasei CBA L74 Metabolic Products and Fermented Milk for Infant Formula Have Anti-Inflammatory Activity on Dendritic Cells in Vitro and Protective Effects against Colitis and an Enteric Pathogen in Vivo. PLoS ONE 2014, 9, e87615. [Google Scholar]
- Corsello, G.; Carta, M.; Marinello, R.; Picca, M.; De Marco, G.; Micillo, M.; Ferrara, D.; Vigneri, P.; Cecere, G.; Ferri, P.; et al. Preventive Effect of Cow’s Milk Fermented with Lactobacillus Paracasei CBA L74 on Common Infectious Diseases in Children: A Multicenter Randomized Controlled Trial. Nutrients 2017, 9, 669. [Google Scholar]
- Nocerino, R.; Paparo, L.; Terrin, G.; Pezzella, V.; Amoroso, A.; Cosenza, L.; Cecere, G.; De Marco, G.; Micillo, M.; Albano, F.; et al. Cow’s Milk and Rice Fermented with Lactobacillus Paracasei CBA L74 Prevent Infectious Diseases in Children: A Randomized Controlled Trial. Clin. Nutr. 2017, 36, 118–125. [Google Scholar]
- Ménard, S.; Candalh, C.; Bambou, J.C.; Terpend, K.; Cerf-Bensussan, N.; Heyman, M. Lactic Acid Bacteria Secrete Metabolites Retaining Anti-Inflammatory Properties after Intestinal Transport. Gut 2004, 53, 821–828. [Google Scholar]
- Thibault, H.; Aubert-Jacquin, C.; Goulet, O. Effects of Long-Term Consumption of a Fermented Infant Formula (with Bifidobacterium Breve C50 and Streptococcus Thermophilus 065) on Acute Diarrhea in Healthy Infants. J. Pediatr. Gastroenterol. Nutr. 2004, 39, 147–152. [Google Scholar]
- Morisset, M.; Aubert-Jacquin, C.; Soulaines, P.; Moneret-Vautrin, D.A.; Dupont, C. A Non-Hydrolyzed, Fermented Milk Formula Reduces Digestive and Respiratory Events in Infants at High Risk of Allergy. Eur. J. Clin. Nutr. 2011, 65, 175–183. [Google Scholar]
- Indrio, F.; Ladisa, G.; Mautone, A.; Montagna, O. Effect of a Fermented Formula on Thymus Size and Stool PH in Healthy Term Infants. Pediatr. Res 2007, 62, 98–100. [Google Scholar]
- Béghin, L.; Tims, S.; Roelofs, M.; Rougé, C.; Oozeer, R.; Rakza, T.; Chirico, G.; Roeselers, G.; Knol, J.; Rozé, J.C.; et al. Fermented Infant Formula (with Bifidobacterium Breve C50 and Streptococcus Thermophilus O65) with Prebiotic Oligosaccharides Is Safe and Modulates the Gut Microbiota towards a Microbiota Closer to That of Breastfed Infants. Clin. Nutr. 2021, 40, 778–787. [Google Scholar]
- Rodriguez-Herrera, A.; Tims, S.; Polman, J.; Rubio, R.P.; Hoyos, A.M.; Agosti, M.; Lista, G.; Corvaglia, L.T.; Knol, J.; Roeselers, G.; et al. Early-Life Fecal Microbiome and Metabolome Dynamics in Response to an Intervention with Infant Formula Containing Specific Prebiotics and Postbiotics. Am. J. Physiol. Gastrointest Liver Physiol. 2022, 322, G571–G582. [Google Scholar]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Morales, J.; de la Torre, A.I.C.; García-García, A.; de Prado, C.N.; Coronel-Rodríguez, C.; Crespo, C.; Ortega, E.; Martín-Pérez, E.; et al. Effects of a Novel Infant Formula on Weight Gain, Body Composition, Safety and Tolerability to Infants: The INNOVA 2020 Study. Nutrients 2023, 15, 147. [Google Scholar]
- Ribeiro, T.C.M.; Costa-Ribeiro, H.; Almeida, P.S.; Pontes, M.V.; Leite, M.E.Q.; Filadelfo, L.R.; Khoury, J.C.; Bean, J.A.; Mitmesser, S.H.; Vanderhoof, J.A.; et al. Stool Pattern Changes in Toddlers Consuming a Follow-on Formula Supplemented with Polydextrose and Galactooligosaccharides. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 288–290. [Google Scholar]
- Osborn, D.; Sinn, J. Prebiotics in Infants for Prevention of Allergy. Cochrane Database Syst. Rev. 2013, CD006474. [Google Scholar] [CrossRef]
- Skórka, A.; Piescik-Lech, M.; Kolodziej, M.; Szajewska, H. Infant Formulae Supplemented with Prebiotics: Are They Better than Unsupplemented Formula? An Updated Systematic Review. Br. J. Nutr. 2018, 119, 810–825. [Google Scholar]
- Braegger, C.; Chmielewska, A.; Decsi, T.; Kolacek, S.; Mihatsch, W.; Moreno, L.; Pieścik, M.; Puntis, J.; Shamir, R.; Szajewska, H.; et al. Supplementation of Infant Formula with Probiotics and/or Prebiotics: A Systematic Review and Comment by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 238–250. [Google Scholar]

| Probiotics | Dose and Duration | Clinical Effects | References |
|---|---|---|---|
| Bb-12 | 106 CFU/g in fermented and acidified formula (S. thermophilus and L. helveticus) T0: before 8 months of life Period: at least 4 months | Lower incidence of acute gastroenteritis | [15] |
| 104 to 107 CFU/g Period: 0–12 months of age | Similar prevalence of acute gastroenteritis before 6 months of age | [17] | |
| Bb-12 with L. casei CRL431 | 107 CFU/g each (in extensively hydrolyzed casein formula) T0: before 6 months Duration: 6 months | Similar duration of cow’s milk allergy | [18] |
| L. reuteri DSM 17938 | retrospective observational cohort | No prevention of respiratory diseases up to 5 years of age | [10] |
| LGG | 1.4 × 107 CFU/100 mL (in extensively hydrolyzed casein formula) Start: 1–12 months of age Duration: until acquisition of tolerance to cow’s milk |
| [22,23,24,25,26] |
| L. fermentum CECT5716 | 107 CFU/g Period: 1–12 months of age | Lower incidence and shorter duration of diarrhea | [34] |
| retrospective observational cohort | Higher risk of upper respiratory tract infection if consumption discontinued between 2 and 10 months of age, whereas no additional risk for daily consumption | [10] | |
| B. breve CECT7263 | 107 CFU/g Period: 1–12 months of age | Similar incidence and duration of respiratory and gastrointestinal infections during the first year of life | [34] |
| B. infantis IM1 | 107 CFU/g Start: before 3 months of life Duration: 12 weeks | No significant effect on diarrhea | [35] |
| B. animalis sp. lactis HN019 | 106 CFU/g Start: 6–12 months of age Duration: 12 weeks | Fewer physician-confirmed infections and fewer parentally reported infections | [36] |
| L. rhamnosus HN001 | 106 CFU/g Start: 6–12 months of age Duration: 12 weeks | No significant effect on infections | [36] |
| Mix of Bifidobacteria (B. bifidum, B. breve, B. longum, and B. longum sp. infantis) | 107 CFU/g Period: 0–12 months of age | No significant effect on episodes of fever, diarrhea, or antibiotics recourse | [37] |
| Prebiotics | Dose and Duration | Clinical Effects | References |
|---|---|---|---|
| HMOs | 2′FL (1 g/L) and LNnT (0.5 g/L) Period: 0–6 months of age | Fewer respiratory infections, less use of antibiotics and antipyretics before the age of 1 year | [49] |
| 5-HMO mix (2′FL at 2.99 g/L, LNnT at 1.5 g/L, 3FL at 0.75 g/L, 6′SL at 0.28 g/L, and 3′SL at 0.23 g/L) Period: 0–4 months of age | No significant effect on infections and infestations | [51] | |
| 2′FL at 3 g/L, LNnT at 1.5 g/L, 3FL at 0.8 g/L, 6′SL at 0.3 g/L, and 3′SL at 0.2 g/L Period: 0–4 months of age | Less recourse to healthcare professionals for illness before 3 months of age | [52] | |
| Combination of GOSs (4 g/L), TGF-β (9.9 or 15 µg/L), lactoferrin (0 to 1.7 g/L), immunoglobulins (0 to 1 g/L), milk fat (0.5 to 17 g/L), and 2′FL (0 or 3 g/L) (4 groups) Period: 1–2.5 years of age |
| [53] | |
| 2′FL at 1 g/L and LNnT at 0.5 g/L (in extensive whey protein hydrolyzate) Start: 0–6 months of age End: 12 months of age |
| [54] | |
| GOSs | 5 g/L (in partially hydrolyzed formula) Period: 1–6 months of age | No specific effect of GOSs on atopic dermatitis | [57] |
| GOSs at 2 g/L with PDX at 2 g/L Period: 0–11 months of age |
| [60] | |
| retrospective observational cohort | Lower risk of upper respiratory tract infections up to 5.5 years of age with early consumption of GOSs compared to infants never supplemented | [10] | |
| GOS/FOS Ratio of 9:1 | 4 g/L Start: 0–4 months of age End: 12 months of age |
| [65] |
| ? g/L Period: 0–12 months |
| [66] | |
| 6.8 g/L Start: before 2 months of age End: 12 months of age | Decreased rate of atopic dermatitis in the first year of life; no sustained effect after stopping supplementation | [67] | |
| 6.8 g/L with acidic oligosaccharide at 1.2 g/L (in partial whey protein hydrolyzate) Period: 0–6 months of age | No prevention of atopic dermatitis at 12 months | [70] | |
| 8 g/L Period: 0–6 months of age |
| [73,74] | |
| GOSs/FOSs | retrospective observational cohort | No association between consumption of GOSs/FOSs at 2 months and occurrence of respiratory disease up to 5.5 years of age | [10] |
| Synbiotics | Dose and Duration | Clinical Effects | References |
|---|---|---|---|
| Bb-12 with oligosaccharides | 107 CFU/g with BMOs at 8 g/L Period: 0–6 months of age | Similar rates of diarrhea and febrile infections | [79] |
| 107 CFU/g with GOSs/FOSs at 4g/L (ratio of 9:1) Period: 0–12 months of age | Similar rates of respiratory tract and gastrointestinal infections and antibiotic use | [81] | |
| Bb M-16V with oligosaccharides | 7.5 × 108 CFU/100 mL with GOSs/FOSs (8 g/L) Period: 0–3 months of age | Less atopic dermatitis | [83] |
| 1.3 × 109 CFU/100 mL with GOSs/FOSs (8 g/L, 9:1) Start: before 7 months of age Duration: 12 weeks |
| [86,87] | |
| 1.47 × 109 CFU/100 mL with FOSs and long-chain inulin (6.3 g/L, 9:1) (in amino-acid-based formula) Period: 0–12 months of age Duration: from 8 weeks to 12 months |
| [90,91,92,94] | |
| L. fermentum CECT5716 with GOSs | 107 CFU/g with 3 g/L Period: 1–6 months of age |
| [96] |
| 2 × 108 CFU/day with 4 g/L Period: 6–12 months of age |
| [97] | |
| L. paracasei F19 with GOSs/FOSs | 109 CFU/L with GOSs at 5.4 g/L and FOSs at 0.61 g/L Period: 1–4 months of age | Fewer respiratory tract infections during the 0–12-month period | [99] |
| L. rhamnosus LCS-742 with B. longum sp. infantis M63 and GOSs/FOSs | LCS-742 at 1.4 × 108 CFU/100 mL, M63 at 1.4 × 108 CFU/100 mL, GOSs at 4 g/L, and FOSs at 0.2 g/L Period: 0–6 months | Less occurrence of atopic dermatitis | [100] |
| Postbiotics | Duration | Clinical Effects | Reference |
|---|---|---|---|
| B. breve C50 with S. thermophilus ST065 | Start: 4–6 months of age Duration: 5 months | Lower severity (hospitalization, dehydration, medical consultations, prescription for oral rehydration solutions) but similar incidence of acute gastroenteritis | [108] |
| Period: 0–12 months of age | Less cow’s milk sensitization and fewer digestive or respiratory allergic symptoms | [109] | |
| B. animalis sp. lactis CECT 8145 BPL1TM | Period: 0–12 months of age | Less atopic dermatitis and fewer bronchitis and bronchiolitis episodes | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemoine, A.; Tounian, P.; Adel-Patient, K.; Thomas, M. Pre-, pro-, syn-, and Postbiotics in Infant Formulas: What Are the Immune Benefits for Infants? Nutrients 2023, 15, 1231. https://doi.org/10.3390/nu15051231
Lemoine A, Tounian P, Adel-Patient K, Thomas M. Pre-, pro-, syn-, and Postbiotics in Infant Formulas: What Are the Immune Benefits for Infants? Nutrients. 2023; 15(5):1231. https://doi.org/10.3390/nu15051231
Chicago/Turabian StyleLemoine, Anaïs, Patrick Tounian, Karine Adel-Patient, and Muriel Thomas. 2023. "Pre-, pro-, syn-, and Postbiotics in Infant Formulas: What Are the Immune Benefits for Infants?" Nutrients 15, no. 5: 1231. https://doi.org/10.3390/nu15051231
APA StyleLemoine, A., Tounian, P., Adel-Patient, K., & Thomas, M. (2023). Pre-, pro-, syn-, and Postbiotics in Infant Formulas: What Are the Immune Benefits for Infants? Nutrients, 15(5), 1231. https://doi.org/10.3390/nu15051231






