Body Mass Index Measured Repeatedly over 42 Years as a Risk Factor for Ischemic Stroke: The HUNT Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Exposure Assessment
2.3. Outcome Assessment
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Body Mass Index and Risk for Ischemic Stroke
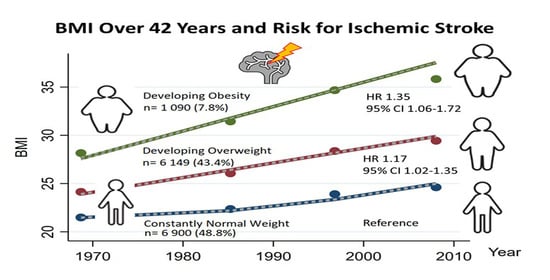
3.2. Change in Body Mass Index and Risk for Ischemic Stroke
4. Discussion
Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vangen-Lonne, A.M.; Wilsgaard, T.; Johnsen, S.H.; Lochen, M.L.; Njolstad, I.; Mathiesen, E.B. Declining Incidence of Ischemic Stroke: What Is the Impact of Changing Risk Factors? The Tromso Study 1995 to 2012. Stroke 2017, 48, 544–550. [Google Scholar] [CrossRef]
- Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration; Lu, Y.; Hajifathalian, K.; Ezzati, M.; Woodward, M.; Rimm, E.B.; Danaei, G. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: A pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet 2014, 383, 970–983. [Google Scholar] [CrossRef]
- Stevens, J.; Erber, E.; Truesdale, K.P.; Wang, C.H.; Cai, J. Long- and short-term weight change and incident coronary heart disease and ischemic stroke: The Atherosclerosis Risk in Communities Study. Am. J. Epidemiol. 2013, 178, 239–248. [Google Scholar] [CrossRef]
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- DALYs, G.B.D.; Collaborators, H. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1603–1658. [Google Scholar] [CrossRef]
- Van Vliet-Ostaptchouk, J.V.; Nuotio, M.L.; Slagter, S.N.; Doiron, D.; Fischer, K.; Foco, L.; Gaye, A.; Gogele, M.; Heier, M.; Hiekkalinna, T.; et al. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: A collaborative analysis of ten large cohort studies. BMC Endocr. Disord. 2014, 14, 9. [Google Scholar] [CrossRef]
- World Health Organization. Regional Office for Europe. WHO European Regional Obesity Report 2022; World Health Organization. Regional Office for Europe: Copenhagen, Denmark, 2022. [Google Scholar]
- Liu, X.; Zhang, D.; Liu, Y.; Sun, X.; Hou, Y.; Wang, B.; Ren, Y.; Zhao, Y.; Han, C.; Cheng, C.; et al. A J-shaped relation of BMI and stroke: Systematic review and dose-response meta-analysis of 4.43 million participants. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 1092–1099. [Google Scholar] [CrossRef]
- Strazzullo, P.; D'Elia, L.; Cairella, G.; Garbagnati, F.; Cappuccio, F.P.; Scalfi, L. Excess body weight and incidence of stroke: Meta-analysis of prospective studies with 2 million participants. Stroke 2010, 41, e418–e426. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Song, M.; Manson, J.E.; Giovannucci, E.L.; Hu, F.B. Group-Based Trajectory of Body Shape From Ages 5 to 55 Years and Cardiometabolic Disease Risk in 2 US Cohorts. Am. J. Epidemiol. 2017, 186, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Rexrode, K.M.; Hennekens, C.H.; Willett, W.C.; Colditz, G.A.; Stampfer, M.J.; Rich-Edwards, J.W.; Speizer, F.E.; Manson, J.E. A prospective study of body mass index, weight change, and risk of stroke in women. JAMA 1997, 277, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Dahl, A.K.; Reynolds, C.A. Accuracy of recalled body weight--a study with 20-years of follow-up. Obesity (Silver Spring) 2013, 21, 1293–1298. [Google Scholar] [CrossRef]
- Engeland, A.; Bjorge, T.; Tverdal, A.; Sogaard, A.J. Obesity in adolescence and adulthood and the risk of adult mortality. Epidemiology 2004, 15, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Holmen, J.; Midthjell, K.; Krüger, Ø.; Langhammer, A.; Holmen, T.L.; Bratberg, G.H.; Vatten, L.; Lund-Larsen, P.G. The Nord-Trøndelag Health Study 1995–97 (HUNT 2): Objectives, contents, methods and participation. Norsk Epidemiol. 2003, 13, 19–32. [Google Scholar]
- Krokstad, S.; Langhammer, A.; Hveem, K.; Holmen, T.; Midthjell, K.; Stene, T.; Bratberg, G.; Heggland, J.; Holmen, J. Cohort Profile: The HUNT Study, Norway. Int. J. Epidemiol. 2012, 42, 968–977. [Google Scholar] [CrossRef]
- Langhammer, A.; Krokstad, S.; Romundstad, P.; Heggland, J.; Holmen, J. The HUNT study: Participation is associated with survival and depends on socioeconomic status, diseases and symptoms. BMC Med. Res. Methodol. 2012, 12, 143. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ 2000, 320, 1240–1243. [Google Scholar] [CrossRef]
- Cole, T.J.; Flegal, K.M.; Nicholls, D.; Jackson, A.A. Body mass index cut offs to define thinness in children and adolescents: International survey. BMJ 2007, 335, 194. [Google Scholar] [CrossRef]
- Jones, B.L.; Nagin, D.S. A Note on a Stata Plugin for Estimating Group-based Trajectory Models. Sociol. Methods Res. 2013, 42, 608–613. [Google Scholar] [CrossRef]
- Horn, J.W.; Romundstad, S.; Ellekjaer, H.; Janszky, I.; Horn, J. Low grade albuminuria as a risk factor for subtypes of stroke—the HUNT Study in Norway. BMC Neurol. 2020, 20, 170. [Google Scholar] [CrossRef]
- Ellekjaer, H.; Holmen, J.; Kruger, O.; Terent, A. Identification of incident stroke in Norway: Hospital discharge data compared with a population-based stroke register. Stroke 1999, 30, 56–60. [Google Scholar] [CrossRef]
- Statistics Norway. Classification of Occupations. Available online: https://www.ssb.no/en/klass/klassifikasjoner/7 (accessed on 27 March 2020).
- Melgarejo, J.D.; Thijs, L.; Wei, D.M.; Bursztyn, M.; Yang, W.Y.; Li, Y.; Asayama, K.; Hansen, T.W.; Kikuya, M.; Ohkubo, T.; et al. Relative and Absolute Risk to Guide the Management of Pulse Pressure, an Age-Related Cardiovascular Risk Factor. Am. J. Hypertens. 2021, 34, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.F.; Streja, E.; Zahmatkesh, G.; Streja, D.; Kashyap, M.; Moradi, H.; Molnar, M.Z.; Reddy, U.; Amin, A.N.; Kovesdy, C.P.; et al. Reverse Epidemiology of Traditional Cardiovascular Risk Factors in the Geriatric Population. J. Am. Med. Dir. Assoc. 2015, 16, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Walsh, C.E.; Johnson, M.P.; Belsky, D.W.; Reason, M.; Curran, P.; Aiello, A.E.; Chanti-Ketterl, M.; Harris, K.M. Life-course trajectories of body mass index from adolescence to old age: Racial and educational disparities. Proc. Natl. Acad. Sci. USA 2021, 118, e2020167118. [Google Scholar] [CrossRef] [PubMed]
- Saito, I.; Iso, H.; Kokubo, Y.; Inoue, M.; Tsugane, S. Body mass index, weight change and risk of stroke and stroke subtypes: The Japan Public Health Center-based prospective (JPHC) study. Int. J. Obes. (Lond.) 2011, 35, 283–291. [Google Scholar] [CrossRef]
- Cho, J.H.; Rhee, E.J.; Park, S.E.; Kwon, H.; Jung, J.H.; Han, K.D.; Park, Y.G.; Yoo, S.J.; Kim, Y.H.; Lee, W.Y. Maintenance of body weight is an important determinant for the risk of ischemic stroke: A nationwide population-based cohort study. PLoS ONE 2019, 14, e0210153. [Google Scholar] [CrossRef]
- Bangalore, S.; Fayyad, R.; Laskey, R.; DeMicco, D.A.; Messerli, F.H.; Waters, D.D. Body-Weight Fluctuations and Outcomes in Coronary Disease. N. Engl. J. Med. 2017, 376, 1332–1340. [Google Scholar] [CrossRef]
- Arboix, A. Cardiovascular risk factors for acute stroke: Risk profiles in the different subtypes of ischemic stroke. World J. Clin. Cases 2015, 3, 418–429. [Google Scholar] [CrossRef]
- Matsuo, T.; Sairenchi, T.; Suzuki, K.; Tanaka, K.; Muto, T. Long-term stable obesity increases risk of hypertension. Int. J. Obes. (Lond.) 2011, 35, 1056–1062. [Google Scholar] [CrossRef]
- Wilson, P.W.; D’Agostino, R.B.; Sullivan, L.; Parise, H.; Kannel, W.B. Overweight and obesity as determinants of cardiovascular risk: The Framingham experience. Arch. Intern. Med. 2002, 162, 1867–1872. [Google Scholar] [CrossRef]
- Esmaili, S.; Hemmati, M.; Karamian, M. Physiological role of adiponectin in different tissues: A review. Arch. Physiol. Biochem. 2020, 126, 67–73. [Google Scholar] [CrossRef]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. J. Cardiothorac. Surg. 2016, 50, e1–e88. [Google Scholar] [CrossRef] [PubMed]
- Nalliah, C.J.; Sanders, P.; Kottkamp, H.; Kalman, J.M. The role of obesity in atrial fibrillation. Eur. Heart J. 2015, 37, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Barone, D.A.; Krieger, A.C. Stroke and obstructive sleep apnea: A review. Curr. Atheroscler. Rep. 2013, 15, 334. [Google Scholar] [CrossRef] [PubMed]
- Varmdal, T.; Bakken, I.J.; Janszky, I.; Wethal, T.; Ellekjaer, H.; Rohweder, G.; Fjaertoft, H.; Ebbing, M.; Bonaa, K.H. Comparison of the validity of stroke diagnoses in a medical quality register and an administrative health register. Scand. J. Public Health 2016, 44, 143–149. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation, Geneva, Switzerland, 8–11 December 2008; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Huxley, R.; Mendis, S.; Zheleznyakov, E.; Reddy, S.; Chan, J. Body mass index, waist circumference and waist:hip ratio as predictors of cardiovascular risk—A review of the literature. Eur. J. Clin. Nutr. 2010, 64, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Nagin, D.S. Handbook of Quantitative Criminology; Springer: New York, NY, USA, 2010. [Google Scholar]
- Nagin, D.S. Group-Based Modeling of Development; Harvard University Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Nagin, D.S.; Odgers, C.L. Group-Based Trajectory Modeling in Clinical Research. Annu. Rev. Clin. Psychol. 2010, 6, 109–138. [Google Scholar] [CrossRef]
- Twisk, J.; Hoekstra, T. Classifying developmental trajectories over time should be done with great caution: A comparison between methods. J. Clin. Epidemiol. 2012, 65, 1078–1087. [Google Scholar] [CrossRef]
- Schwarz, G. Estimating the Dimension of a Model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]


| Age at HUNT3 (years) | 65.2 (9.3) |
| Female, n (%) | 7838 (55.4) |
| BMI67 (kg/m2) | 23.2 (3.1) |
| BMI85 (kg/m2) | 24.7 (3.3) |
| BMI96 (kg/m2) | 26.7 (3.8) |
| BMI07 (kg/m2) | 27.6 (4.2) |
| SBP (mmHg) missing n = 40, (0.3%) | 137.5 (19.4) |
| DBP (mmHg) missing n = 40, (0.3%) | 75.3 (11.4) |
| Hypertension, n (%) | 10,550 (75.2) |
| Diabetes mellitus, n (%) | 1018 (7.2) |
| Total cholesterol (mmol/L) missing n = 318 (2.2%) | 5.76 (1.12) |
| HDL cholesterol (mmol/L) missing n = 318 (2.2%) | 1.38 (0.37) |
| Triglycerides (mmol/L) missing n= 141 (1%) | 1.71 (0.95) |
| Smoking status: | |
| Never, n (%) | 5528 (39.1) |
| Former, n (%) | 5681 (40.2) |
| Occasionally, n (%) | 748 (5.3) |
| Current, n (%) | 2182 (15.4) |
| Alcohol consumption: | |
| Abstainers, n (%) | 3526 (24.9) |
| Light drinkers, n (%) | 7810 (55.2) |
| Moderate drinkers, n (%) | 2589 (18.3) |
| Heavy drinkers, n (%) | 214 (1.5) |
| Education: | |
| Lower secondary, n (%) | 6242 (44.2) |
| Upper secondary, n (%) | 5637 (40.0) |
| Tertiary, n (%) | 2260 (16.0) |
| Physical inactivity | |
| Inactive, n (%) | 2840 (20.1) |
| Active, n (%) | 11,299 (79.9) |
| Marital Status: | |
| Single, n (%) | 782 (5.5) |
| Married, Cohabitant, n (%) | 9962 (70.5) |
| Widow, Divorced, Separated, n (%) | 3395 (24.0) |
| Participants with number of chronic diseases: | |
| No, n (%) | 7010 (49.6) |
| One, n (%) | 2810 (19.9) |
| Two, n (%) | 2159 (15.3) |
| Three, n (%) | 1214 (8.6) |
| Four, n (%) | 572 (4.0) |
| Fife or more, n (%) | 374 (2.6) |
| Mean BMI (kg/m2) | N 14,139 | Events 856 | Person Years 152,843 | HR § | 95% CI | HR ‖ | 95% CI | HR # | 95% CI |
|---|---|---|---|---|---|---|---|---|---|
| Average BMI from BMI67 to the end of follow up with repeated updated exposure | |||||||||
| <18.5 | 36 | 1 | 405 | 0.60 | 0.08–4.24 | 0.55 | 0.08–3.90 | 0.57 | 0.08–4.09 |
| 18.5–<25 | 7182 | 363 | 78,735 | 1 | (Ref) | 1 | (Ref) | 1 | (Ref) |
| 25–29.9 | 5895 | 420 | 62,756 | 1.23 | 1.07–1.42 | 1.23 | 1.06–1.42 | 1.19 | 0.99–1.43 |
| ≥30 | 1026 | 72 | 10,947 | 1.27 | 0.98–1.63 | 1.22 | 0.94–1.58 | 1.14 | 0.79–1.64 |
| Average BMI from BMI67 to baseline HUNT-3 | |||||||||
| <18.5 | 65 | 1 | 742 | 0.46 | 0.06–3.25 | 0.41 | 0.06–2.95 | 0.41 | 0.06–2.96 |
| 18.5–<25 | 8477 | 401 | 93,931 | 1 | (Ref) | 1 | (Ref) | 1 | (Ref) |
| 25–29.9 | 4865 | 393 | 50,683 | 1.30 | 1.13–1.50 | 1.29 | 1.11–1.48 | 1.29 | 1.08–1.52 |
| ≥30 | 732 | 61 | 7487 | 1.33 | 1.01–1.75 | 1.27 | 0.96–1.67 | 1.27 | 0.90–1.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horn, J.W.; Feng, T.; Mørkedal, B.; Aune, D.; Strand, L.B.; Horn, J.; Mukamal, K.J.; Janszky, I. Body Mass Index Measured Repeatedly over 42 Years as a Risk Factor for Ischemic Stroke: The HUNT Study. Nutrients 2023, 15, 1232. https://doi.org/10.3390/nu15051232
Horn JW, Feng T, Mørkedal B, Aune D, Strand LB, Horn J, Mukamal KJ, Janszky I. Body Mass Index Measured Repeatedly over 42 Years as a Risk Factor for Ischemic Stroke: The HUNT Study. Nutrients. 2023; 15(5):1232. https://doi.org/10.3390/nu15051232
Chicago/Turabian StyleHorn, Jens W., Tingting Feng, Bjørn Mørkedal, Dagfinn Aune, Linn Beate Strand, Julie Horn, Kenneth J. Mukamal, and Imre Janszky. 2023. "Body Mass Index Measured Repeatedly over 42 Years as a Risk Factor for Ischemic Stroke: The HUNT Study" Nutrients 15, no. 5: 1232. https://doi.org/10.3390/nu15051232
APA StyleHorn, J. W., Feng, T., Mørkedal, B., Aune, D., Strand, L. B., Horn, J., Mukamal, K. J., & Janszky, I. (2023). Body Mass Index Measured Repeatedly over 42 Years as a Risk Factor for Ischemic Stroke: The HUNT Study. Nutrients, 15(5), 1232. https://doi.org/10.3390/nu15051232