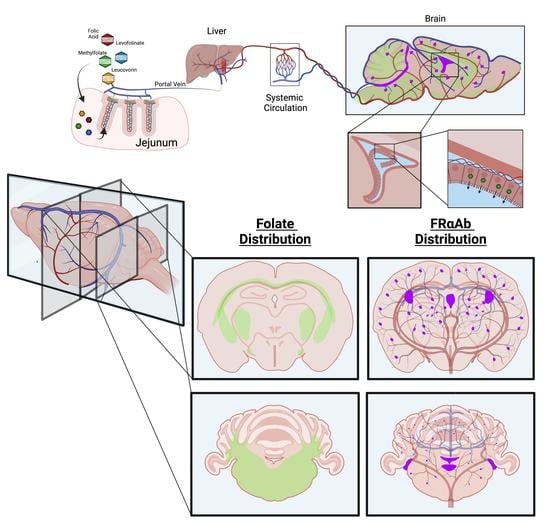
Brain Uptake of Folate Forms in the Presence of Folate Receptor Alpha Antibodies in Young Rats: Folate and Antibody Distribution
Abstract
1. Introduction
2. Materials and Methods
2.1. Folate Compounds Used in the Study
2.2. FRαAb and 3H-PGA Distribution in PND 13 Pups
2.3. Brain Uptake of B-PGA in PND 23 Pups
2.4. Brain Localization of FRαAb in PND 23 Pups
2.5. Absorption and Tissue Distribution of 3H-PGA in PND23 Pups
2.6. Dosing of PND23 Rats with Folate Compounds
2.7. Statistical Analysis
3. Results
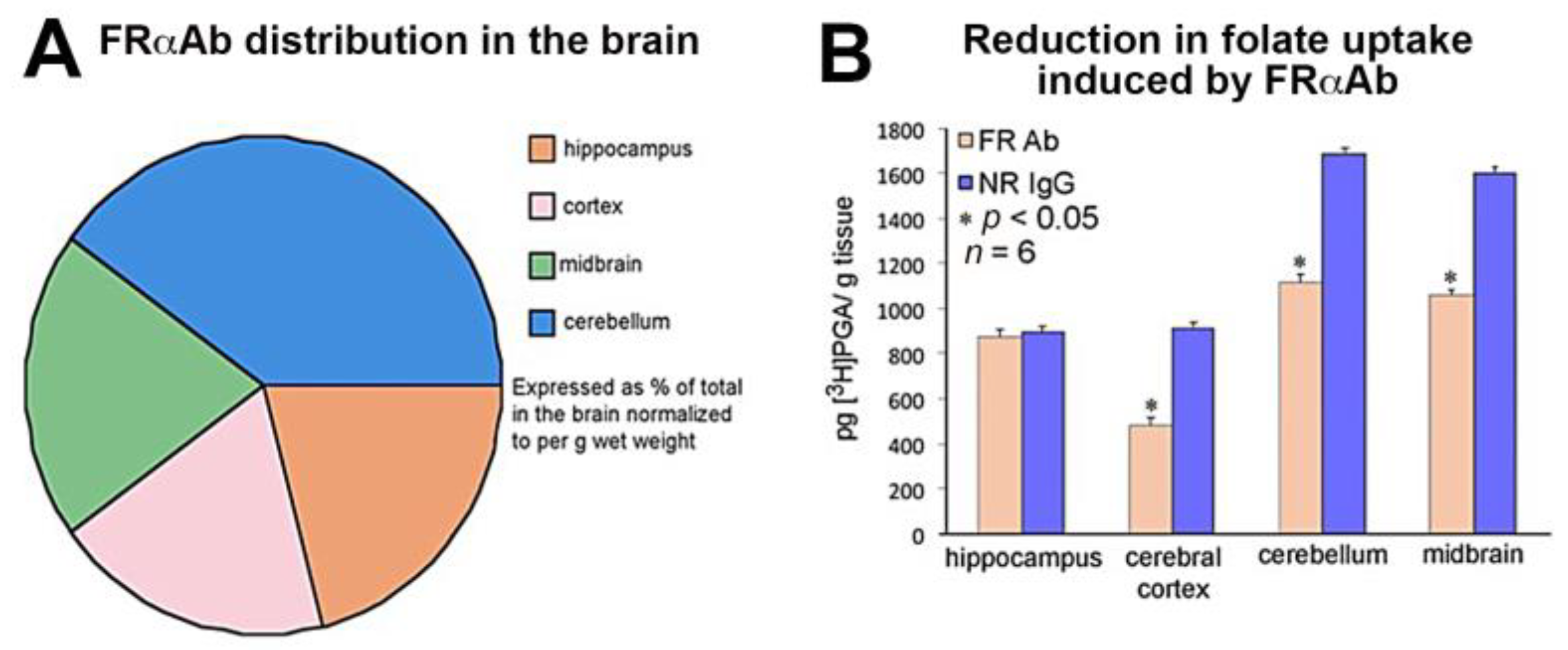
3.1. Distribution of FRαAb and Blocking of Folate Uptake in PND 13 Rat Brain
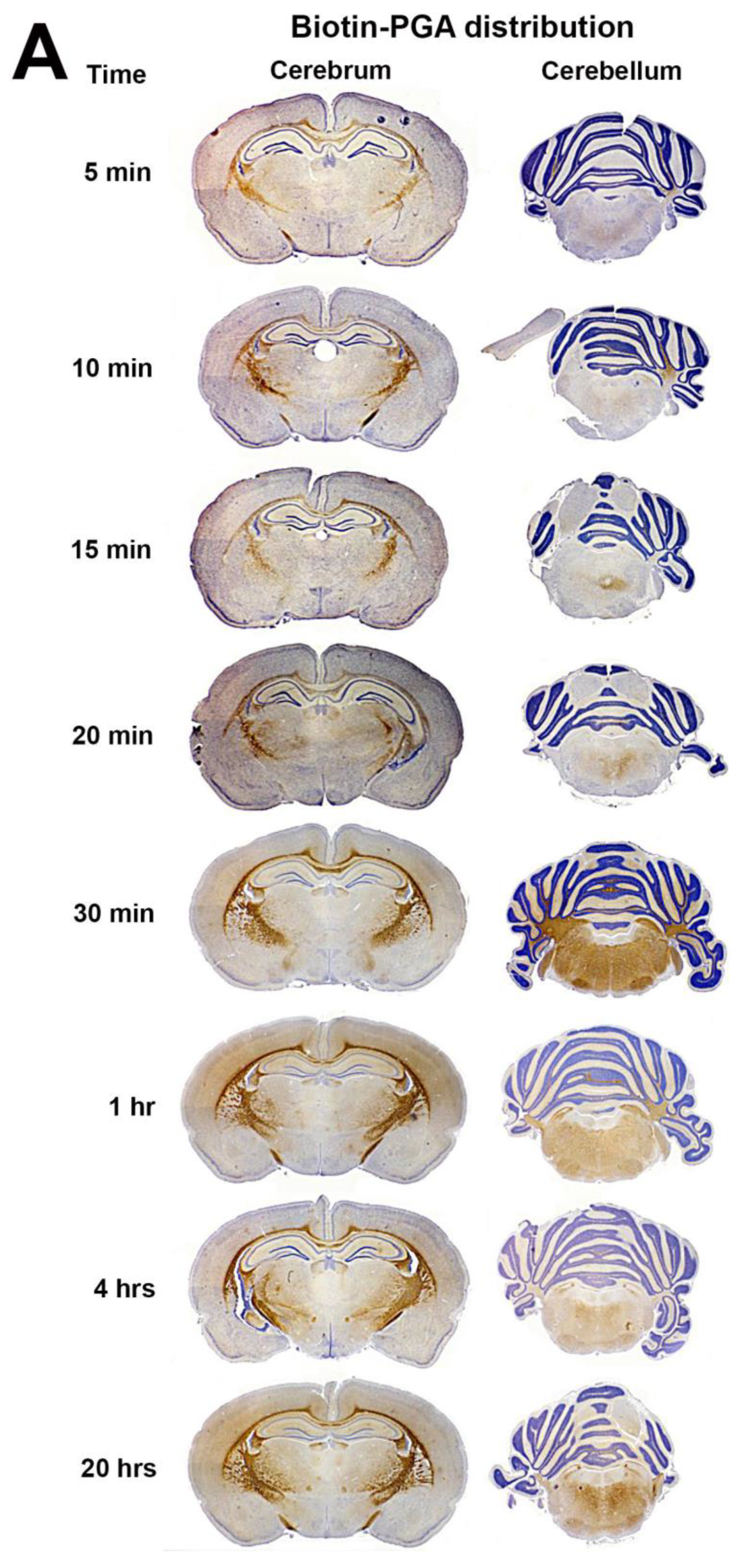
3.2. Biotin-PGA (B-PGA) Uptake and Distribution in PND 23 Brain
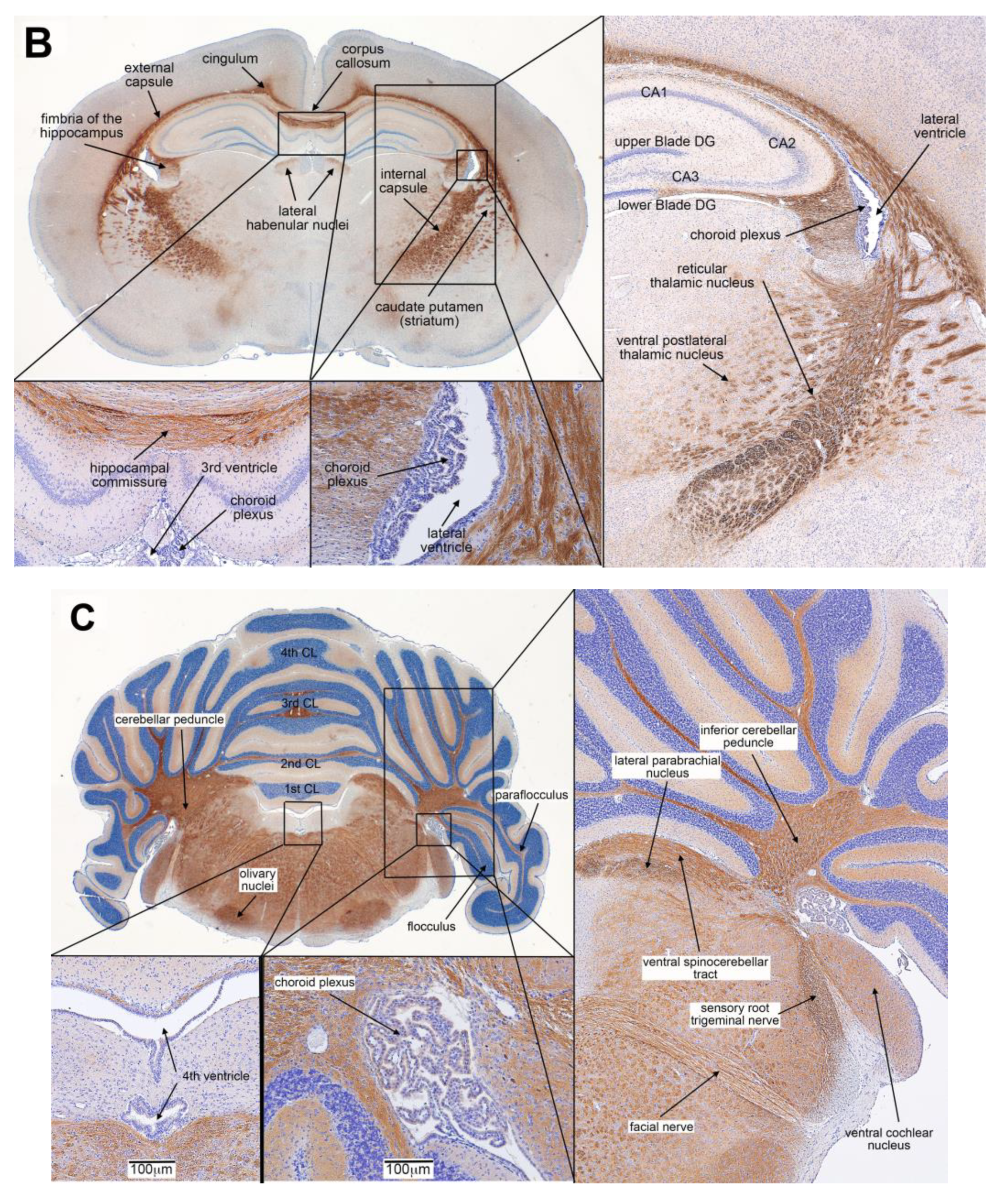
3.3. Distribution of IP Injected FRαAb in the Brain
3.4. Time Course of Folate Absorption, Tissue Distribution and the Effect of FRαAb on Distribution in the Brain
3.5. Tissue Distribution of Methylfolate Post Oral Dosing with Folate Forms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hepner, G.W.; Booth, C.C.; Cowan, J.; Hoffbrand, A.V.; Mollin, D.L. Absorption of crystalline folic acid in man. Lancet 1968, 2, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Olinger, E.J.; Bertino, J.R.; Binder, H.J. Intestinal folate absorption. II. Conversion and retention of pteroymonoglutamate by jejunum. J Clin. Investig. 1973, 52, 2138–2145. [Google Scholar] [CrossRef] [PubMed]
- Matherly, L.H.G.; Goldman, D.L. Membrane transport of folates. Vitam. Horm. 2003, 66, 403–456. [Google Scholar] [CrossRef] [PubMed]
- Duch, D.S.; Bigner, D.D.; Bowers, S.W.; Nichol, C.A. Dihydrofolate Reductase in Primary Brain Tumors, Cell Cultures of Central Nervous System Origin, and Normal Brain during Fetal and Neonatal Growth. Cancer Res. 1979, 39, 487–491. [Google Scholar] [PubMed]
- Ludwig, R.; Frei, E.; Kimmig, B.; Brandeis, W.E. Dihydrofolate reductase-activity in brain tissue. Effect of X-irradiation. Blut 1987, 55, 483–488. [Google Scholar] [CrossRef]
- Smith, A.D.; Kim, Y.I.; Refsum, H. Is folic acid good for everyone? Am. J. Clin. Nutr. 2008, 87, 517–533. [Google Scholar] [CrossRef]
- Reynolds, E.H. Benefits and risks of folic acid to the nervouse system. J. Neurol. Neurosurg. Psychiatry 2002, 72, 567–571. [Google Scholar] [CrossRef]
- Chu, D.; Li, L.; Jiang, Y.; Tan, J.; Ji, J.; Zhang, Y.; Jin, N.; Liu, F. Excess Folic Acid Supplementation Before and During Pregnancy and Lactation Activates Fos Gene Expression and Alters Behaviors in Male Mouse Offspring. Front. Neurosci. 2019, 13, 313. [Google Scholar] [CrossRef]
- Cosin-Tomas, M.; Luan, Y.; Leclerc, D.; Malysheva, O.V.; Lauzon, N.; Bahous, R.H.; Christensen, K.E.; Caudill, M.A.; Rozen, R. Moderate Folic Acid Supplementation in Pregnant Mice Results in Behavioral Alterations in Offspring with Sex-Specific Changes in Methyl Metabolism. Nutrients 2020, 12, 1716. [Google Scholar] [CrossRef]
- Harlan De Crescenzo, A.; Panoutsopoulos, A.A.; Tat, L.; Schaaf, Z.; Racherla, S.; Henderson, L.; Leung, K.Y.; Greene, N.D.E.; Green, R.; Zarbalis, K.S. Deficient or Excess Folic Acid Supply During Pregnancy Alter Cortical Neurodevelopment in Mouse Offspring. Cereb Cortex 2021, 31, 635–649. [Google Scholar] [CrossRef]
- Luan, Y.; Cosin-Tomas, M.; Leclerc, D.; Malysheva, O.V.; Caudill, M.A.; Rozen, R. Moderate Folic Acid Supplementation in Pregnant Mice Results in Altered Sex-Specific Gene Expression in Brain of Young Mice and Embryos. Nutrients 2022, 14, 1051. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Koletzko, B.; Pietrzik, K. The unresolved debate on lowering the recommended dietary intake for folate. Clin. Nutr. 2014, 33, 731–732. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R. Serum unmetabolized folic acid: The straw that broke dihydrofolate reductase’s back? J. Nutr. 2015, 145, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Hoffbrand, A.V.; Tripp, E.; Lavoie, A. Synthesis of folate polyglutamates in human cells. Clin. Sci. Mol. Med. 1976, 50, 61–68. [Google Scholar] [CrossRef]
- Obeid, R.; Schon, C.; Pietrzik, K.; Menzel, D.; Wilhelm, M.; Smulders, Y.; Knapp, J.P.; Bohni, R. Pharmacokinetics of Sodium and Calcium Salts of (6S)-5-Methyltetrahydrofolic Acid Compared to Folic Acid and Indirect Comparison of the Two Salts. Nutrients 2020, 12, 3623. [Google Scholar] [CrossRef]
- Hansen, F.J.; Blau, N. Cerebral folate deficiency: Life-changing supplementation with folinic acid. Mol. Genet. Metab. 2005, 84, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Wollack, J.B.; Makori, B.; Ahlawat, S.; Koneru, R.; Picinich, S.C.; Smith, A.; Goldman, I.D.; Qiu, A.; Cole, P.D.; Glod, J.; et al. Characterization of folate uptake by choroid plexus epithelial cells in a rat primary culture model. J. Neurochem. 2008, 104, 1494–1503. [Google Scholar] [CrossRef]
- Ramaekers, V.T.R.; Sheldon, P.; Sequeira, J.M.; Opladen, T.; Blau, N.; Quadros, E.V.; Selhub, J. Autoantibodies to Folate Receptors in the Cerebral Folate Deficiency Syndrome. N. Engl. J. Med. 2004, 352, 1985–1991. [Google Scholar] [CrossRef]
- Frye, R.E.; Sequeira, J.M.; Quadros, E.V.; James, S.J.; Rossignol, D.A. Cerebral folate receptor autoantibodies in autism spectrum disorder. Mol. Psychiatry 2013, 18, 369–381. [Google Scholar] [CrossRef]
- Sangha, V.; Hoque, M.T.; Henderson, J.; Bendayan, R. Localization of the Folate Transport Systems in the Murine Central Nervous System. Curr. Dev. Nutr. 2021, 5 (Suppl. 2), 922. [Google Scholar] [CrossRef]
- Sequeira, J.M.; Ramaekers, V.T.; Quadros, E.V. The diagnostic utility of folate receptor autoantibodies in blood. Clin. Chem. Lab. Med. 2013, 51, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Ramaekers, V.; Sequeira, J.M.; Quadros, E.V. Clinical recognition and aspects of the cerebral folate deficiency syndromes. Clin. Chem. Lab. Med. 2013, 51, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Levitt, M.; Mosher, M.B.; DeConti, R.C.; Farber, L.R.; Skeel, R.T.; Marsh, J.C.; Mitchell, M.S.; Papac, R.J.; Thomas, E.D.; Bertino, J.R. Improved therapeutic index of methotrexate with “leucovorin rescue”. Cancer Res. 1973, 33, 1729–1734. [Google Scholar] [PubMed]
- Rustum, Y.M.; Cao, S.; Zhang, Z. Rationale for treatment design: Biochemical modulation of 5-fluorouracil by leucovorin. Cancer J. Sci. Am. 1998, 4, 12–18. [Google Scholar] [PubMed]
- Bobrowski-Khoury, N.; Sequeira, J.M.; Arning, E.; Bottiglieri, T.; Quadros, E.V. Absorption and Tissue Distribution of Folate Forms in Rats: Indications for Specific Folate Form Supplementation during Pregnancy. Nutrients 2022, 14, 2397. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Sequeira, J.M.; Quadros, E.V. Prevention of behavioral deficits in rats exposed to folate receptor antibodies: Implication in autism. Mol. Psychiatry 2017, 22, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, J.M.; Desai, A.; Berrocal-Zaragoza, M.I.; Murphy, M.M.; Fernandez-Ballart, J.D.; Quadros, E.V. Exposure to Folate Receptor Alpha Antibodies during Gestation and Weaning Leads to Severe Behavioral Deficits in Rats: A Pilot Study. PLoS ONE 2016, 11, e0152249. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, S.P.; da Costa, M.P.; Rosenberg, Z. A Radioassay for Serum Folate: Use of a Two-Phase Sequential-Incubation, Ligand-Binding System. N. Engl. J. Med. 1972, 286, 1335–1339. [Google Scholar] [CrossRef]
- Rothenberg, S.P.; da Costa, M.P.; Lawson, J.; Rosenberg, Z. The Determination of Erythrocyte Folate Concentration Using a Two-Phase Ligand-binding Radioassay. Blood 1974, 43, 437–443. [Google Scholar] [CrossRef]
- Larkin, J.R.; Simard, M.A.; Khrapitchev, A.A.; Meakin, J.A.; Okell, T.W.; Craig, M.; Ray, K.J.; Jezzard, P.; Chappell, M.A.; Sibson, N.R. Quantitative blood flow measurement in rat brain with multiphase arterial spin labelling magnetic resonance imaging. J. Cereb. Blood Flow Metab. 2019, 39, 1557–1569. [Google Scholar] [CrossRef]
- Gilmore, J.H.; Knickmeyer, R.C.; Gao, W. Imaging structural and functional brain development in early childhood. Nat. Rev. Neurosci. 2018, 19, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Hodgetts, C.J.; Stefani, M.; Williams, A.N.; Kolarik, B.S.; Yonelinas, A.P.; Ekstrom, A.D.; Lawrence, A.D.; Zhang, J.; Graham, K.S. The role of the fornix in human navigational learning. Cortex 2020, 124, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.G.; Koumellis, P.; Dineen, R.A. The Fornix in Health and Disease: An Imaging Review. Radiographics 2011, 31, 1107–1121. [Google Scholar] [CrossRef] [PubMed]
- Safadi, Z.; Grisot, G.; Jbabdi, S.; Behrens, T.E.; Heilbronner, S.R.; McLaughlin, N.C.R.; Mandeville, J.; Versace, A.; Phillips, M.L.; Lehman, J.F.; et al. Functional Segmentation of the Anterior Limb of the Internal Capsule: Linking White Matter Abnormalities to Specific Connections. J. Neurosci. 2018, 38, 2106–2117. [Google Scholar] [CrossRef]
- Ribas, E.C.; Yagmurlu, K.; de Oliveira, E.; Ribas, G.C.; Rhoton, A. Microsurgical anatomy of the central core of the brain. J. Neurosurg. 2018, 129, 752–769. [Google Scholar] [CrossRef]
- Andersen, P.; Morris, R.; Amaral, D.; Bliss, T.; O‘Keefe, J. The Hippocampus Book; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Steinfeld, R.; Grapp, M.; Kraetzner, R.; Dreha-Kulaczewski, S.; Helms, G.; Dechent, P.; Wevers, R.; Grosso, S.; Gartner, J. Folate receptor alpha defect causes cerebral folate transport deficiency: A treatable neurodegenerative disorder associated with disturbed myelin metabolism. Am. J. Hum. Genet. 2009, 85, 354–363. [Google Scholar] [CrossRef]
- Nicolai, J.; van Kempen Marjan, J.A.; Postma, A.A. Teaching NeuroImages: White matter hyptomyelination and progressive calcifications in cerebral folate deficiency. Neurology 2016, 87, e4–e5. [Google Scholar] [CrossRef]
- Shiokawa, O.; Sadoshima, S.; Kusuda, K.; Nishmura, Y.; Ibayashi, S.; Fujishima, M. Cerebral and Cerebellar Blood Flow Autoregulations in Acutely Induced Cerebral Ischemia in Spontaneously Hypertensive Rats—Transtentorial Remote Effect. Stroke 1986, 17, 1309–1313. [Google Scholar] [CrossRef]
- Becker, E.B.; Stoodley, C.J. Autism spectrum disorder and the cerebellum. Int. Rev. Neurobiol. 2013, 113, 1–34. [Google Scholar] [CrossRef]
- Visentin, M.; Diop-Bove, N.; Zhao, R.; Goldman, I.D. The intestinal absorption of folates. Annu. Rev. Physiol. 2014, 76, 251–274. [Google Scholar] [CrossRef]
- Birn, H.; Spiegelstein, O.; Christensen, E.I.; Finnell, R.H. Renal tubular reabsorption of folate mediated by folate binding protein 1. J. Am. Soc. Nephrol. 2005, 16, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Grapp, M.; Just, I.A.; Linnankivi, T.; Wolf, P.; Lücke, T.; Häusler, M.; Gärtner, J.; Steinfeld, R. Molecular characterization of folate receptor 1 mutations delineates cerebral folate transport deficiency. Brain 2012, 135, 2022–2031. [Google Scholar] [CrossRef] [PubMed]
- Spector, R.; Lorenzo, A.V. Folate transport in the central nervous system. Am. J. Physiol. 1975, 229, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Spector, R. Nutrient transport systems in brain: 40 years of progress. J. Neurochem. 2009, 111, 315–320. [Google Scholar] [CrossRef]
- Reynolds, E.H.; Gallagher, B.B.; Mattson, R.H. Relationship between Serum and Cerebrospinal Fluid Folate. Nature 1972, 240, 155–157. [Google Scholar] [CrossRef]
- Zhao, R.; Diop-Bove, N.; Visentin, M.; Goldman, I.D. Mechanisms of membrane transport of folates into cells and across epithelia. Annu. Rev. Nutr. 2011, 31, 177–201. [Google Scholar] [CrossRef]
- Frye, R.E.; Slattery, J.; Delhey, L.; Furgerson, B.; Strickland, T.; Tippett, M.; Sailey, A.; Wynne, R.; Rose, S.; Melnyk, S.; et al. Folinic acid improves verbal communication in children with autism and language impairment: A randomized double-blind placebo-controlled trial. Mol. Psychiatry 2018, 23, 247–256. [Google Scholar] [CrossRef]
- Irwin, R.E.; Pentieva, K.; Cassidy, T.; Lees-Murdock, D.J.; McLaughlin, M.; Prasad, G.; McNulty, H.; Walsh, C.P. The interplay between DNA methylation, folate, and neurocognitive development. Epigenomics 2016, 8, 863–879. [Google Scholar] [CrossRef]
- Desai, A.; Sequeira, J.M.; Quadros, E.V. The metabolic basis for developmental disorders due to defective folate transport. Biochimie 2016, 126, 31–42. [Google Scholar] [CrossRef]
- Ramaekers, V.T.; Sequeira, J.M.; DiDuca, M.; Vrancken, G.; Thomas, A.; Philippe, C.; Peters, M.; Jadot, A.; Quadros, E.V. Improving Outcome in Infantile Autism with Folate Receptor Autoimmunity and Nutritional Derangements: A Self-Controlled Trial. Autism Res. Treat. 2019, 2019, 7486431. [Google Scholar] [CrossRef]
- Tam, C.; O‘Connor, D.; Koren, G. Circulating unmetabolized folic Acid: Relationship to folate status and effect of supplementation. Obstet. Gynecol. Int. 2012, 2012, 485179. [Google Scholar] [CrossRef] [PubMed]
- Straw, J.A.; Szapary, D.; Wynn, W.T. Pharmacokinetics of the diastereoisomers of leucovorin after intravenous and oral administration to normal subjects. Cancer Res. 1984, 44, 3114–3119. [Google Scholar] [PubMed]
- Schilsky, R.L.; Ratain, M.J. Clinical pharmacokinetics of high-dose leucovorin calcium after intravenous and oral administration. J. Natl. Cancer. Inst. 1990, 82, 1411–1415. [Google Scholar] [CrossRef] [PubMed]

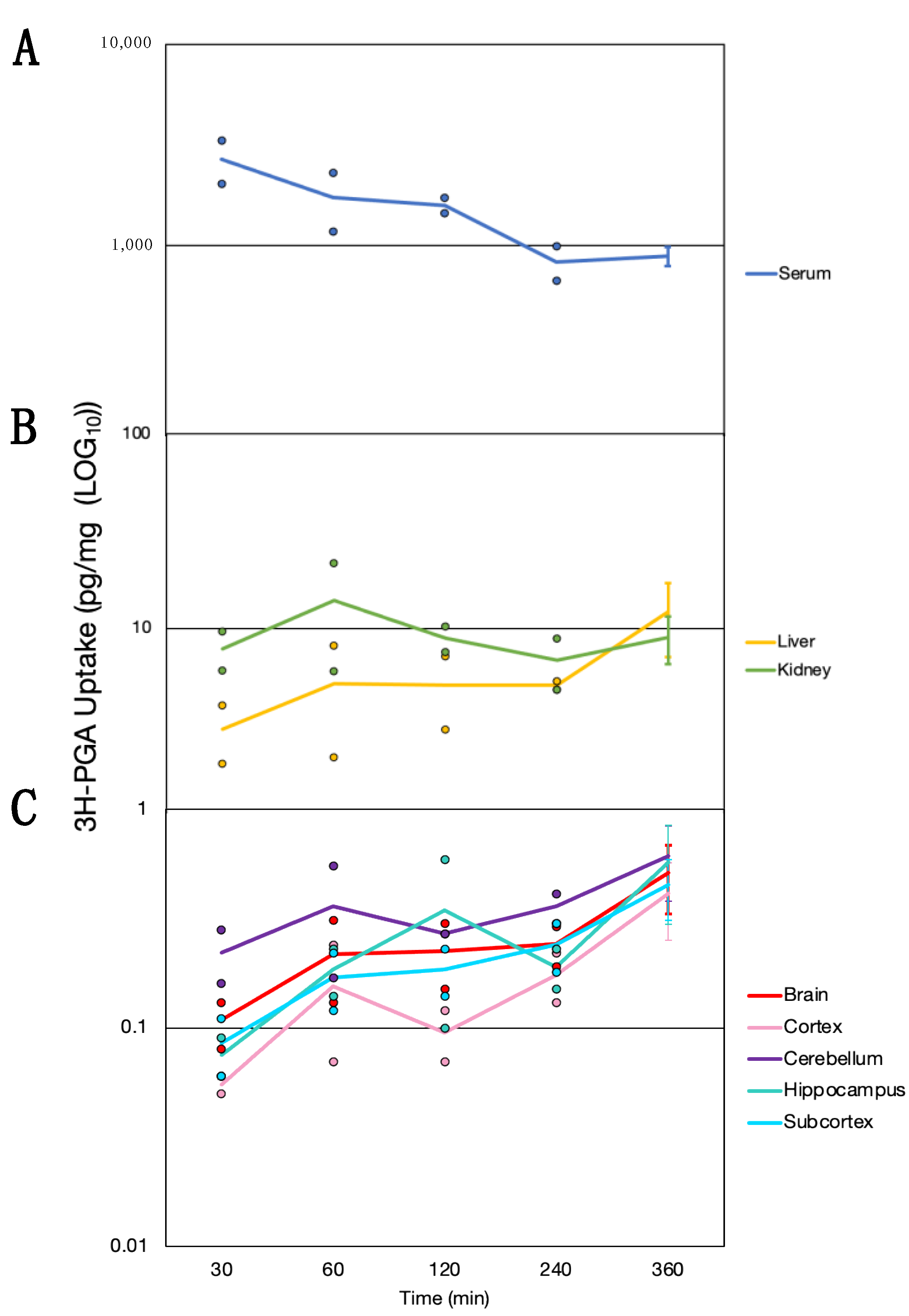
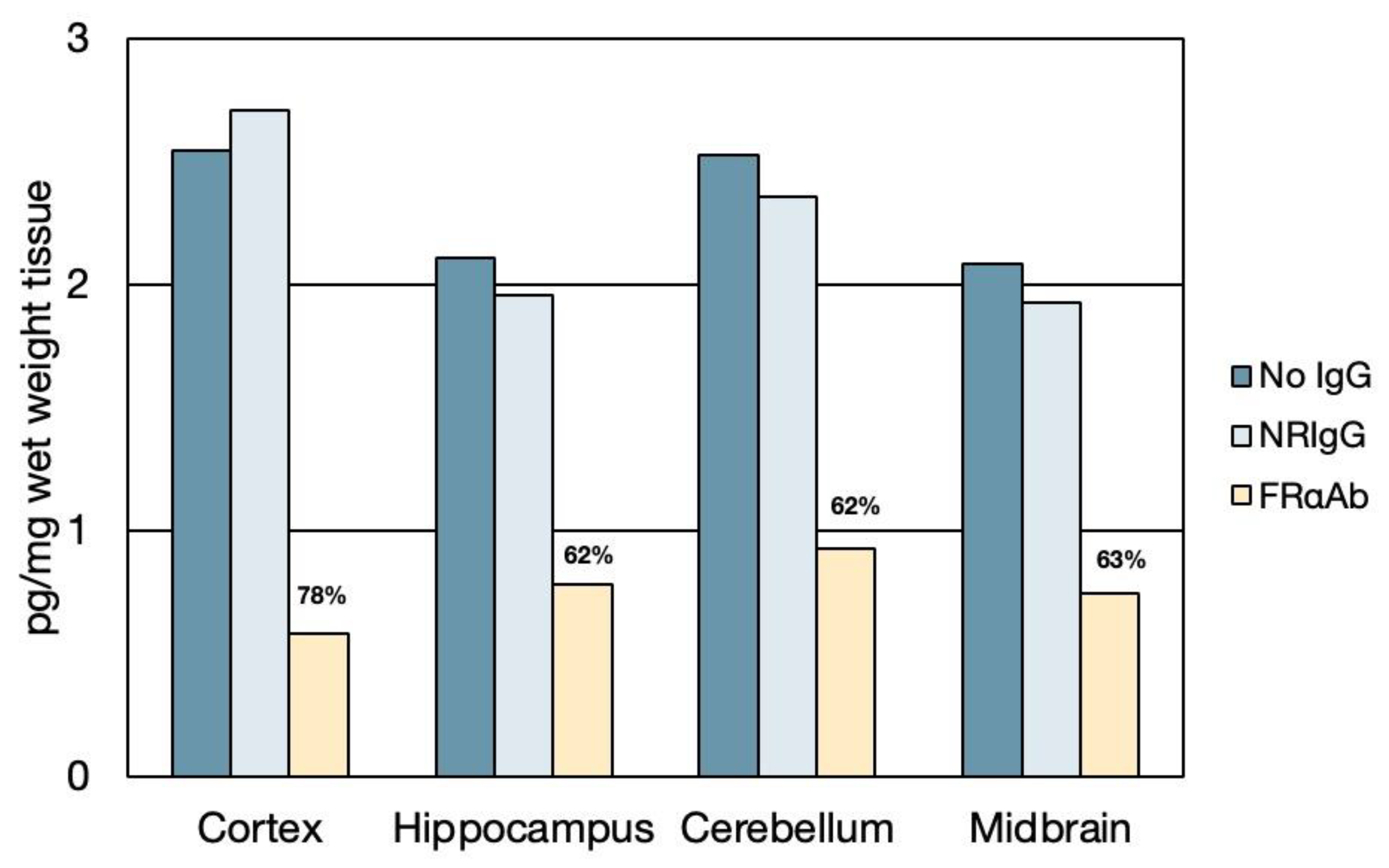
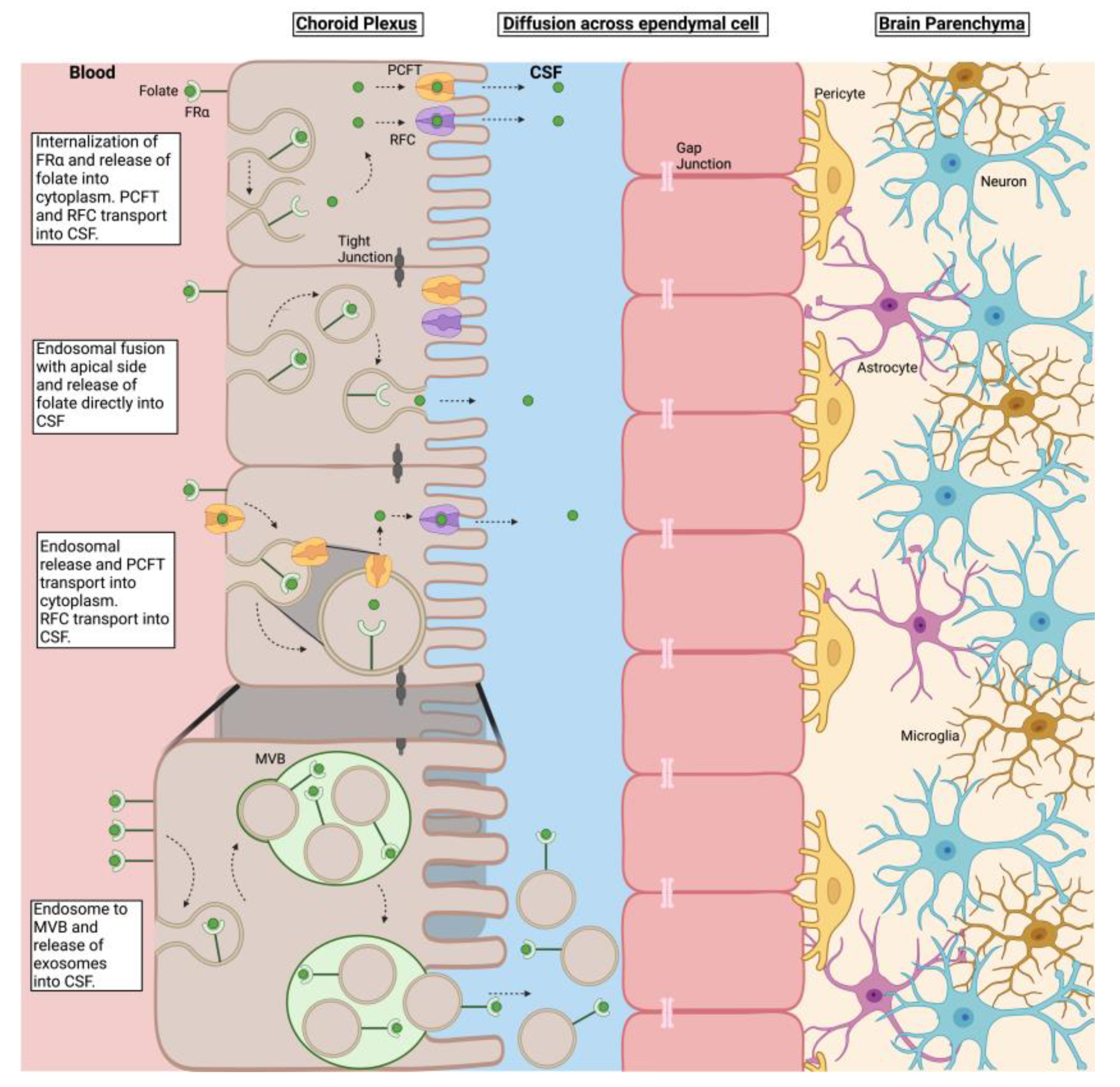
| Code | Condition | Methylfolate (ng/mg Wet Weight) | |||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Ratio | Mean ± SD | Mean ± SD | Ratio | ||
| Liver | Kidney | Liver | Cerebellum | Cerebrum | Cerebellum | ||
| Kidney | Cerebrum | ||||||
| AA | Control | 125 ± 5 | 46 ± 3 | 3 | 11 ± 4 | 4 ± 0.5 | 3 |
| AB | PGA + NRIgG | 150 ± 33 | 74 ± 28 | 2 | 38 ± 25 | 7 ± 2 | 6 |
| ABb | PGA + FRαAb | 160 ± 5 *** | 67 ± 18 | 2 | 21 ± 2 * | 8 ± 0.5 *** | 3 |
| AC | MTHF + NRIgG | 159 ± 6 ** | 87 ± 6 *** | 2 | 26 ± 10 | 4 ± 0.2 | 6 |
| ACb | MTHF + FRαAb | 250 ± 18 ** | 116 ± 13 *** | 2 | 61 ± 1 *** | 4 ± 0.5 | 16 |
| AD | Leucovorin + NRIgG | 92 ± 10 ** | 51 ± 8 | 2 | 48 ± 7 ** | 10 ± 0.5 *** | 5 |
| ADb | Leucovorin + FRαAb | 134 ± 41 | 60 ± 9 | 3 | 77 ± 31 | 14 ± 4 | 6 |
| AE | Levofolinate + NRIgG | 80 ± 19 * | 52 ± 11 | 2 | 93 ± 6 *** | 15 ± 2 ** | 6 |
| AEb | Levofolinate + FRαAb | 133 ± 8 | 49 ± 4 | 3 | 70 ± 2 *** | 13 ± 2 ** | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobrowski-Khoury, N.; Sequeira, J.M.; Quadros, E.V. Brain Uptake of Folate Forms in the Presence of Folate Receptor Alpha Antibodies in Young Rats: Folate and Antibody Distribution. Nutrients 2023, 15, 1167. https://doi.org/10.3390/nu15051167
Bobrowski-Khoury N, Sequeira JM, Quadros EV. Brain Uptake of Folate Forms in the Presence of Folate Receptor Alpha Antibodies in Young Rats: Folate and Antibody Distribution. Nutrients. 2023; 15(5):1167. https://doi.org/10.3390/nu15051167
Chicago/Turabian StyleBobrowski-Khoury, Natasha, Jeffrey M. Sequeira, and Edward V. Quadros. 2023. "Brain Uptake of Folate Forms in the Presence of Folate Receptor Alpha Antibodies in Young Rats: Folate and Antibody Distribution" Nutrients 15, no. 5: 1167. https://doi.org/10.3390/nu15051167
APA StyleBobrowski-Khoury, N., Sequeira, J. M., & Quadros, E. V. (2023). Brain Uptake of Folate Forms in the Presence of Folate Receptor Alpha Antibodies in Young Rats: Folate and Antibody Distribution. Nutrients, 15(5), 1167. https://doi.org/10.3390/nu15051167